Abstract
In submerged arc welding, evaluating elemental transfer behaviors is critical for selecting and designing welding materials. Accurate assessment of O, Si, and Mn transfer behavior is essential for ensuring process quality, particularly when silicon-manganese fluxes are applied. Traditional quantification methods, however, focus only on chemical reactions in the weld pool zone, potentially overlooking the cross-zone elemental transfer behavior and leading to significant predictive inaccuracies. This study investigates the CaO-SiO2-MnO flux, a prevalent silicon-manganese flux, focusing on O, Si, and Mn, which exhibit notable transfer behaviors of O, Si, and Mn. By employing a multi-zone approach and integrating various scientific principles, the research aims to improve the accuracy of predicting elemental transfer behaviors and deepen the understanding of the metallurgical processes in submerged arc welding when silicon-manganese fluxes are employed. The study proposes strategic enhancements to traditional quantification methods, which may offer valuable insights for the improvement of industry standards. This study demonstrates that considering only the local thermodynamic equilibrium of the weld pool zone when quantifying the transfer behavior of elements may lead to predictive errors, especially for easily evaporating metallic elements. By incorporating a cross-zone assessment for submerged arc welding process, i.e., introducing new quantifying parameters (Δd and Δw), the predictive accuracy of the transfer behavior of elements and their cross-zone actions can be enhanced.
1. Introduction
In the field of thick steel plate welding, the submerged arc welding (SAW) method stands out for its remarkable reliability and high deposition rate [1]. During such a welding process, the arc plasma and weld pool remain hidden, concealed by a layer of flux rendering them invisible [2]. Controlling the composition of the weld metal (WM) is vital for the overall SAW process as it directly influences the performance of the final welded joint during the service [3].
The final chemical composition of the submerged arc welded metal is predominantly dictated by the electrode, base metal (BM), and flux, thereby imposing technical requirements for the design and selection of welding materials [4]. In SAW engineering, the compositional contribution from the electrode and BM is evaluated through the nominal composition (MN), which specifically indicates the contents only considering the physical dilution of the electrode and BM [2]. The compositional contribution from the flux is quantified using a parameter known as the Δ value [3]. A positive Δ value indicates that the flux induces an elemental gain for the WM, while a negative Δ value indicates that the flux induces an elemental loss for the WM. The magnitude of Δ represents the amount of transition of the elements [2].
The evaluation of the Δ value is significant as it aids in matching or designing flux for the SAW process [5]. For example, Indacochea et al. [6] quantified the elemental transfer behavior when FeO-SiO2-MnO fluxes were applied and detailed the chemical reactions within the weld pool reaction zones in SAW. Burck et al. [7] studied the elemental transfer behaviors of O, Si, and Mn in SAW and found that metallurgical thermodynamic knowledge enables the evaluation of trends in elemental transfer behaviors induced by changing the flux formula. Zhang et al. [8], on the other hand, employed thermodynamic technology to evaluate the transfer behavior during SAW when CaO-SiO2-MnO fluxes were employed.
Investigations into the elemental transfer behavior of the SAW process, whether from an engineering or scientific standpoint, have been somewhat limited in scope [3]. That is, the current investigations primarily focus on the disparities between the final and initial compositions of the WM [3]. Similarly, concerning the transfer of elements, the focus has predominantly been on exploring the potential existence of local thermodynamic equilibrium only within the weld pool reaction zone [3]. For instance, the prevalent models concerning the basicity index model, slag-metal equilibrium model, and gas–slag-metal equilibrium model are all developed based on chemical reactions occurring within the weld pool zone [3,9,10,11].
However, the existing studies concerning SAW have focused solely on the chemical reactions within the molten pool zone, thus neglecting a more comprehensive understanding of the SAW process [3]. Many research results have shown that considering only the chemical reactions in the weld pool zone is insufficient for fully explaining the chemical reactions in the process of SAW because of the following points:
- The O content in the droplets is significantly higher, typically possessing an order of magnitude higher than that in the WM [12,13].
- Although the mechanism is not clear, the evaporation of metal elements (such as Mn and Si) does occur during SAW [1]. Numerous studies have indicated that only thermodynamics is insufficient for predicting the extent of such loss [2].
For SAW, when using silico-manganese flux for the welding process, transitions of O, Si, and Mn elements are likely to occur [6,8]. As such, the accurate prediction of the transfer behavior of O, Si, and Mn is essential for the welding design and the selection of welding materials [4]. As mentioned earlier, the prevailing method for assessing the transfer behavior of elements is currently the quantification represented by Δ [2]. However, traditional quantification methods are prone to significant predictive errors and cannot predict transitional characteristics in different zones of the SAW process since only the chemical interactions within the weld pool zone are taken into account [3]. Consequently, there is a need to introduce a new cross-zone quantification method to address such scientific and engineering challenges.
The present study has been undertaken to evaluate the limitations of conventional methods used to quantify elemental transfer behaviors in the SAW process, specifically the Δ value, and propose recommended refinements. The primary research focus of this study is on silicon-manganese-based flux, with a specific focus on the essential elements in low-carbon, low-alloy weld metals, namely O, Si, and Mn [8,14]. By employing scientific assumptions, the multi-zone consideration is utilized to facilitate predictions of elemental behaviors throughout the SAW process, thereby enhancing the predictive accuracy of elemental transition behaviors and improving the understanding of the SAW metallurgical process. Moreover, a quantitative scheme necessitating further optimization in subsequent endeavors is outlined.
2. Methodology
2.1. Experimental Design
The focus of this study is on the three elements of O, Si, and Mn. The reasons for selecting these three elements are stated below.
The CaO-SiO2-MnO flux system chosen for this study represents a classic example of silico-manganese type fluxes. The design of the flux adhered to the principles of welding metallurgy, following the guidelines stated as follows [8,14]:
- The SiO2 content remained unchanged since SiO2 is the slag network-builder (a pivotal factor in determining the physical properties of the flux/slag) and modifying SiO2 content could potentially disrupt welding stability.
- While maintaining a constant SiO2 content, gradual variations were introduced in the concentrations of MnO and CaO. The gradual changes in MnO and CaO concentrations would influence the activities of MnO and SiO2. Hence, these modifications were expected to induce alterations in the elemental transfer behavior of O, Si, and Mn.
When designing flux, it is essential to ensure that the flux melts at a lower temperature than the BM. As such, the flux is able to melt and cover the WM before the BM does, thus providing the necessary protection [5]. Figure 1 illustrates the ternary phase diagram of CaO-SiO2-MnO [8,14,15]. To ensure an appropriate flux melting point and a wider range for flux composition control, the SiO2 component has been set at 40 wt pct [8,14]. The formula of each flux has been plotted in Figure 1 (The deeper the green color the higher the content of CaO and the lower the content of MnO in the flux).
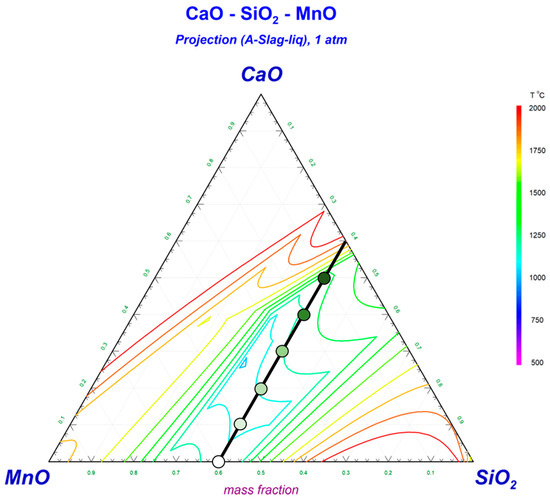
Figure 1.
A summary of flux formulas within this framework.
The details of the experiment have been detailed in previous studies [8,14]. The data from the previous articles, such as compositions and Δ values, will be cited in this paper for comparative analysis [8,14].
2.2. Thermodynamic Simulation of SAW Process
2.2.1. Modeling Mechanism and Scientific Hypotheses
Computer coupling of phase diagrams and thermochemistry (Calphad) technology plays an essential role in the metallurgical analysis of the SAW process [15,16]. Firstly, during the process of SAW, all slag and molten metals are concealed beneath flux particles, thereby hindering the ability of researchers to directly analyze these components [3]. Secondly, the temperature of the chemical reactions occurring in SAW exceeds 2000 °C, a level beyond the current capabilities of scientific detection methods [17]. The Calphad method employs thermodynamic models to enhance the precision of predictions about material properties [15]. As such, despite the impossibility of directly measuring thermodynamic data during the SAW process due to temperatures surpassing 2000 °C, the thermodynamic models in the Calphad technique have demonstrated their reliability in gathering thermodynamic data beyond such a temperature limit [3]. Therefore, by integrating Calphad with specific thermodynamic models (slag-metal or gas–slag-metal equilibrium models), it becomes feasible to assess and predict the transfer behavior of elements [15,16].
It is well known that equilibrium is not attained in the process of SAW due to significant temperature differentials, pronounced density gradients, brief reaction intervals, and substantial electric currents [6]. Despite such deviations from equilibrium, it is possible to employ thermodynamic principles by hypothesizing that thermodynamic equilibrium is established locally [4]. This assumption accounts for the elevated temperatures and substantial surface-to-volume ratios, effectively mitigating the limited time for chemical reactions [3].
Figure 2 illustrates a schematic diagram of the SAW process and the primary chemical reaction interfaces that determine the transfer behavior of the elements of O, Si, and Mn. In the context of SAW, the process is typically divided into three fundamental zones according to the temperatures at which the chemical reactions take place [18,19,20].
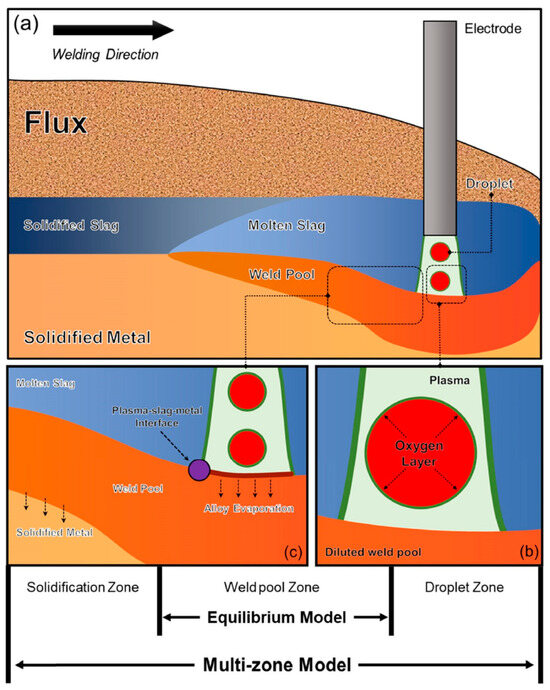
Figure 2.
Zones that govern the transfer behavior in the SAW [20]. (a) Schematic representation of SAW, (b) chemical processes within the droplet zone, and (c) chemical reactions occurring in the weld pool and solidification zones.
Within the framework of thermodynamic equilibrium models, emphasis is placed upon the chemical reactions occurring within the reaction zone of the weld pool (indicated through the purple dot in Figure 2) [3]. Scholars have formulated hypotheses pertaining to this interface, allowing for predictions of the transfer behaviors of elements from both the slag-metal and gas–slag-metal perspectives [3,18]. Nonetheless, a limitation of such an assessment approach emerges as it neglects the fact of O enrichment within the droplet reaction zone (see Figure 2b) and the loss of alloying elements induced by the arc plasma [1].
In this study, multi-zone model is employed to predict the transfer behavior of essential elements, namely O, Si, and Mn, and conduct a comparative analysis with predictions from thermodynamic equilibrium models [20]. The scientific assumptions applied in the model as well as the employed databases are written out below.
- The droplet zone.
A typical characteristic of SAW is the use of flux. During the welding process, the oxides in the flux decompose and release O2 under the high temperature of the arc plasma, as detailed in several references [3,13]. The high O content forms an oxygen-rich layer on the surface of the molten droplet, hindering the redox reactions of metal elements such as Si, Mn, and Ti [3,18,19,21].
In this zone, the temperature soars to an astounding 2500 °C as the droplet separates from the electrode tip and traverses through the arc cavity [3]. It has been postulated that the O in the droplet metal primarily originates from the O2 generated through the decomposition of the oxide in the flux [13,22]. Preliminary experiments have revealed that there is an insignificant transfer of alloy elements within this zone [12,13]. Mitra et al. [18,19,21] have suggested that a dynamic O layer forms at the metal–plasma interface, effectively blocking the alloy elements from reaching the interface, as illustrated in Figure 2b.
- The weld pool zone.
When molten droplets become diluted through the molten pool, chemical reactions ensue at the gas–slag-metal interface, giving rise to elemental transitions [6]. It is noted that the previously mentioned thermodynamic equilibrium model is primarily designed to model this specific zone [3]. However, thermodynamic equilibrium models do not account for the substantial increase in O level within the droplet reaction zone [3].
Furthermore, extensive research has highlighted significant losses of alloy elements during arc welding, particularly of Mn and Si [1]. In this work, the recently developed Zhu’s model is utilized to estimate the evaporation of Mn and Si during the arc welding process as previous studies have validated the model’s efficacy in predicting the evaporation of Mn and Si in arc welding processes [20,23].
It is worth noting that during the solidification process following the weld pool reaction zone, there is a small reduction in element content at the slag–metal interface attributed to the adsorption of the oxides into the slag [24]. As this study predominantly explores the bulk composition of the metal, the reduction of element at the slag–metal interface is not considered [18,19,21].
Figure 3 illustrates the detailed flowchart of the modeling process, including the scientific assumptions, thermodynamic data, and computational modules utilized. Data from previous studies (the measured compositions, predicted compositions, and model data, etc.) will be referred in this paper for the purpose of comparative analysis [8,14]. Compositions of metals and fluxes have been summarized in Table 1 and Table 2 [8,14].
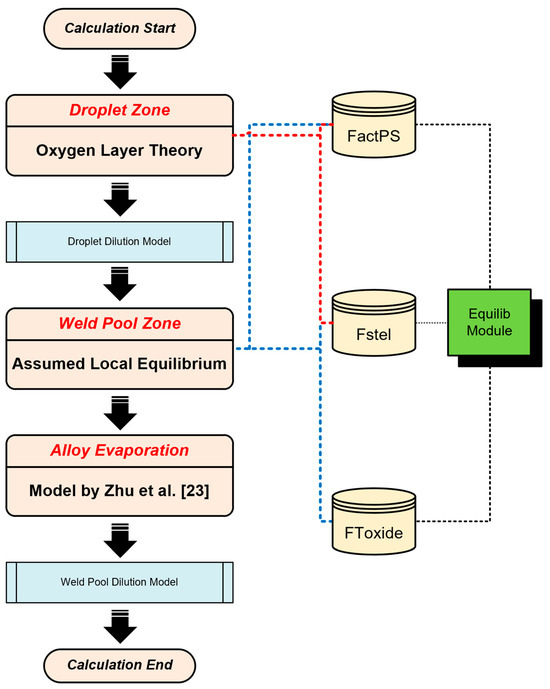
Figure 3.
Modeling process diagram.

Table 1.
Flux formula and label of the weldment (wt pct) [8,14].

Table 2.
Measured compositions of the BM and electrode (wt pct) [8,14].
2.2.2. Modeling Process
The thermodynamic simulation process in the droplet zone is as follows [3]:
- Step 1: database selection and phase simulation.
- Three critical databases were chosen as follows: FToxid, Fstel, and FactPS.
- These databases were configured within the Equilib module for subsequent phase simulations.
- To simulate the molten slag and steel phases, the following solution phases were selected: FToxid-SLAGA and FStel-Liqu.
- Step 2: setting the equilibrium temperature for the SAW process.
- To model the SAW process accurately, the equilibrium temperature was set at 2500 °C, corresponding to the temperature of the arc plasma.
- Input metal chemistries were acquired from the BM compositions.
- Step 3: predicting O concentration in droplets.
Equilibrium calculations were performed using Fe and O as input metal constituents.
For the weld pool zone, the previously established thermodynamic equilibrium model will be utilized to predict the transfer behavior and metal composition [3]. However, unlike previous cases, the increase in O content in the droplet will be taken into consideration when setting the input stream. Considering the dilution of the droplet, the input stream is set for the equilibrium in this part [20]. The setting details have been extensively stated in our previous studies [3]. Then, the evaporation level of the metal is estimated using Formulas (1) and (2) proposed by Zhu et al. [23]
Δd and Δw are used to measure the extent of the material transfer within the droplet and weld pool regions in this investigation. For a specific element, the Δd and Δw values are computed using Equations (3) and (4) where Δd signifies the extent of elemental transfer in the droplet area, Md represents the content in the droplet, ΔW signifies the extent of elemental transfer in the weld pool region, MW represents the content in the weld metal, and MN represents the nominal composition. The MN value is subsequently determined using Equation (5), which is derived by combining the measured compositions of the base metal (MBM) and the electrode (MEL) in conjunction with the dilution factor of the BM (d). Considering the minor difference in composition between the BM and the electrode, the value of d is set at 0.5 [25].
To facilitate the reference, Table 3 summarizes the models and theories mentioned in the article.

Table 3.
Model and theory employed in this study [8,14].
The main symbols used in the model and their definitions are summarized in Table 4.

Table 4.
Main symbols used in the model and their definitions [8,14].
Here are examples of the key operations/processes.
(1) According to the model setting subjected to the droplet zone, the calculation utilized the PO2 value in Table 5 to simulate the O content in the droplet.

Table 5.
Simulated droplet O content and PO2.
(2) According to the model setting subjected to the droplet zone, the metal compositions after the dilution calculated from Equation (5) are set as the input stream. The output stream of this stage has been given in Table 6.

Table 6.
The metal compositions calculated using the (Calphad) thermodynamic model.
(3) After consideration of the chemical interaction within the weld pool zone, the metal evaporation is evaluated using Equations (1) and (2) with the output given in Table 7.

Table 7.
The metal compositions calculated using the metal evaporation model.
(4) Then, Equations (3) and (4) are used to quantify the level of elemental transfer within the droplet zone and weld pool zone, respectively. Data will be detailed next.
3. Results and Discussion
3.1. Transfer Behavior of O
Predicted values of ΔO content in the metal across various reaction zones are depicted in Figure 4. In Figure 4a, ΔO is predicted considering multi-zone chemical reactions, while Figure 4b represents ΔO predicted using only the thermodynamic equilibrium model of the weld pool zone.
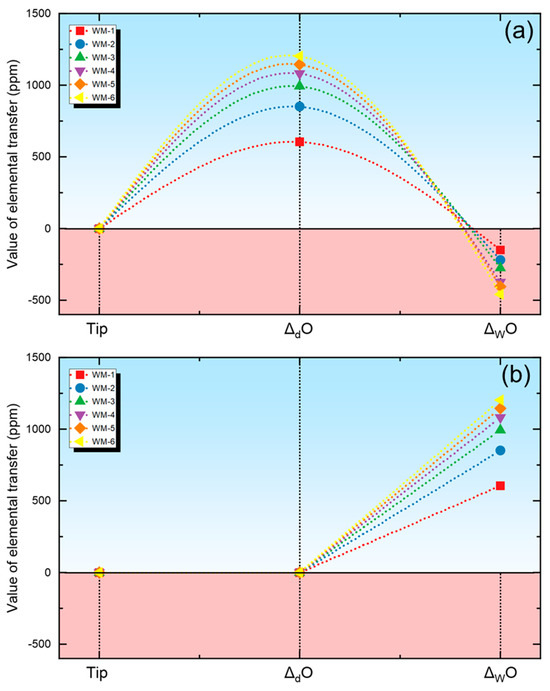
Figure 4.
Quantification of O transfer behavior using different quantification methods. (a) Evaluation results using new quantification methods, (b) evaluation results using traditional methods.
By observing Figure 4, it can be noted that the oxidation–reduction of the metal occurs in both the droplet zone and the melt pool zone during the Saw process when using the new quantification method. In contrast, the traditional quantification method only considers the increase of O in the droplet zone and does not account for the cross-zone transition behavior of oxygen.
Through observation, it is evident that O enrichment in the metal primarily manifests within the droplet reaction zone when employing a multi-zone consideration for estimation. In this zone, the O content in the molten metal can even reach levels exceeding 1000 ppm, aligning with previous experimental findings [12,13].
Conversely, within the weld pool zone, a deoxidation process predominantly occurs. Remarkably, when exclusively considering the chemical interactions within the confines of the weld pool zone, oxygen enrichment in the metal solely occurs during the weld pool zone, deviating from the metallurgical phenomena observed in the SAW processes [8].
As is well known, the O content of the metal depends on the flux’s O potential [13,22]. This is because the oxides present in the flux tend to decompose under the influence of the arc plasma, releasing O2 and thereby increasing the O content in hot metal [1]. Although researchers have proposed models such as the basicity index model or thermodynamic equilibrium model to predict the flux’s O potential (i.e., O content in WM) in SAW, these models typically only consider chemical reactions in the weld pool zone [3]. However, extensive scholars have presented a differing viewpoint suggesting that the O enrichment in the WM primarily occurs within the droplet reaction zone rather than in the weld pool zone [12,13,26]. Therefore, by introducing cross-zone analysis, the behavior of O has been more accurately predicted.
3.2. Transfer Behavio of Mn
The transfer behavior of Mn is depicted in Figure 5. When examining Figure 5a, it is observed that the transfer behavior of Mn can be dissected into three zones when using the multi-zone chemical reaction consideration. Initially, in the droplet reaction zone, Mn experiences negligible transition due to the impediment posed by the oxygen-rich layer of the droplet, as shown through the positive value in Figure 5a [18]. Within the molten pool reaction zone, being influenced by the slag, Mn achieves a local thermodynamic equilibrium at the gas–slag-metal interface. Subsequently, Mn undergoes evaporation under the influence of the arc plasma, leading to the depletion of Mn elements within the molten pool [3]. Such process can be predicted using the multi-zone model in Figure 5a, as demonstrated through the changes in ΔDMn, ΔWMn, and ΔSMn.
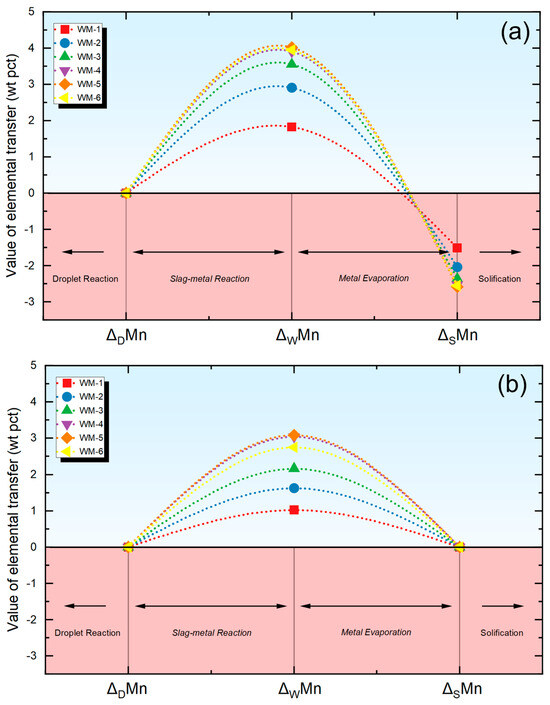
Figure 5.
Quantification of Mn transfer behavior using different quantification methods. (a) Evaluation results using new quantification methods, (b) evaluation results using traditional methods.
Mn is essential alloying element that determine the microstructure and mechanical properties of WM [20]. Predicting the transition behavior of Mn is of paramount significance for flux design and the matching of welding material [2]. According to the theory of the enriched oxygen layer, the transfer behavior of Mn in the molten droplet reaction zone can be negligible [18].
To save experimental consumption, predictions are usually made through the slag-metal model or the gas–slag-metal thermodynamic model, focusing on the local thermodynamic equilibrium in the weld pool area [3]. The evaluation of ΔMn is key to establishing welding material matching strategies. However, predicting ΔMn has always been a technical challenge since Mn is a metal that evaporates easily [3]. Although many researchers have tried to estimate the evaporation of Mn using thermodynamic methods, this has led to significant prediction errors [2].
As shown through Figure 5, if the assessment of Mn’s transfer behavior is confined to the thermodynamic model within the weld pool zone, the transition of Mn appears to be solely restricted to the weld pool reaction zone, which contradicts the real metallurgical processes subject to SAW [1], as shown through the positive value in Figure 5b. Clearly, neglecting the strong oxidizing nature of the droplet and the subsequent evaporation of Mn can lead to an overestimation of the elemental transfer level of Mn [20].
3.3. Transfer Behavior of Si
As depicted in Figure 6a, when comprehensively considering the droplet reaction zone, the molten pool zone, and the evaporation of Si, the transfer behavior of Si aligns with the actual metallurgical process of SAW [1]. Given the high content of SiO2 in the flux and its high activity, the transfer of Si to the molten pool occurs in the weld pool reaction zone, as previously described [8,14].
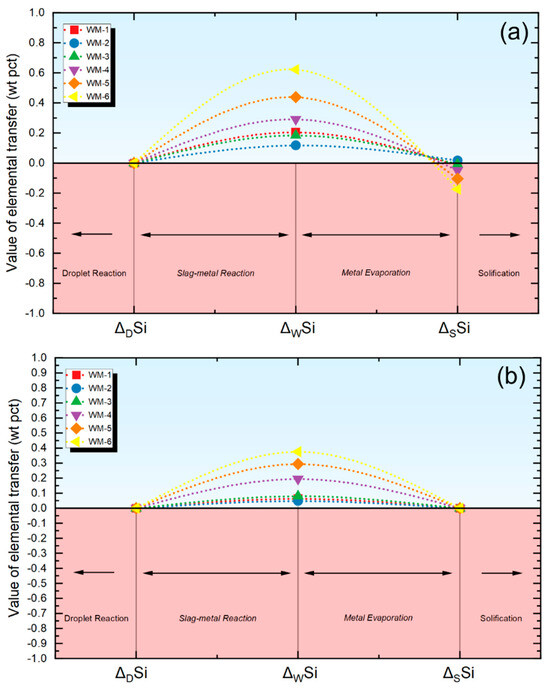
Figure 6.
Quantification of Si transfer behavior using different quantification methods. (a) Evaluation results using new quantification methods, (b) evaluation results using traditional methods.
It is well known that the evaporation level of Si during the arc welding process is not as pronounced as that of Mn [2]. As such, comparing Figure 5a with Figure 6a, it is evident that the evaporation of Si is significantly less than that of Mn, a characteristic consistent with the metallurgical features of SAW [1]. However, if only the chemical interactions within the molten pool reaction zone are considered, Si undergoes transition exclusively in the molten pool area, as shown in Figure 6b, which does not align with the actual metallurgical process of SAW [1,5].
Therefore, the novel quantification method proposed in this study accurately simulates the cross-zone transition behavior of the Si element. In contrast, traditional quantification methods only consider local thermodynamic equilibrium during the submerged arc welding process.
3.4. Accuracy of Quantitative Methods
As illustrated in Figure 7 of this study, a comparative analysis is presented between the ΔMn values (the overall transfer level of Mn) estimated using traditional quantification methods and those calculated using the quantification approach proposed in this paper. This comparison elucidates a crucial insight as follows:
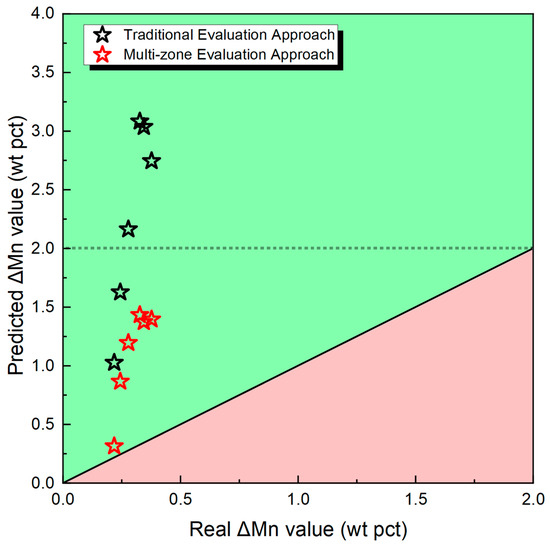
Figure 7.
Predictive and real ΔMn value.
- Solely considering local thermodynamic equilibrium within the weld pool zone leads to a substantial overestimation of ΔMn. However, the real scenario is more complex.
- In the melt pool zone, Mn undergoes evaporation, primarily influenced by the arc plasma, resulting in a notable loss of Mn elements as referenced in [3,5].
Therefore, it is imperative that the assessment of ΔMn levels incorporates this Mn loss, transitioning from the weld pool to the solidification reaction zone. The paper introduces the concept of ΔD and ΔW, advocating for its consideration in attaining a more accurate understanding of Mn behavior during SAW processes.
However, from the perspective of predicting Δ values, the quantitative method proposed in this study does not significantly improve the accuracy of ΔO and ΔSi. As described in previous articles, the transfer behavior of O primarily depends on the oxygen potential of the flux used [8,13,14]. The study focused on the mildly acidic silicon-manganese flux (CaO-SiO2-MnO flux). When using such a type of flux, the transfer behavior of O and Si primarily depends on the activity of SiO2 in the flux (slag); specific mechanisms can be found in earlier articles [22,27].
To further validate such inference, the values of ΔO and ΔSi were plotted in Figure 8. By observing Figure 8, it is evident that the use of different assessment methods does not significantly affect the overall accuracy of predictions for the total transition quantities of Si and O that are denoted as ΔO and ΔSi.
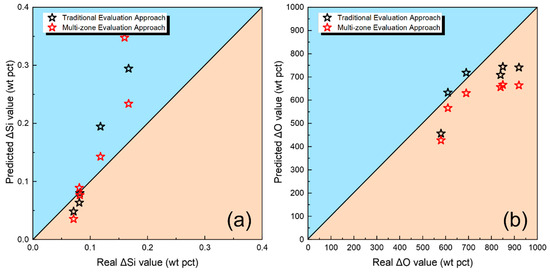
Figure 8.
Predictive and real ΔSi and ΔO value.
As previously discussed in previouse article, when using silicon-manganese type fluxes, the overall transfer of Si and O is more dependent on the activity of SiO2 in the flux and slag, particularly acidic fluxes [27]. Such an observation further underscores the pivotal role of SiO2 activity in dictating the transfer behaviors of O and Si in the case of acidic fluxes. Notably, the quantitative approach introduced in this study, as opposed to traditional methods, accurately predicts the variation trends of O and Si at different stages of the SAW process, which may enhance our understanding of the intricacies of elemental transfer behavior. However, the improvement in predicting the total quantities of Si and O using the new quantitative method proposed in this study is not significantly pronounced.
It should be noted that the use of chemistry-based assessment methods still has certain limitations. As is well known, this approach mainly takes into consideration the chemical interactions during the SAW process [28,29]. However, physical interactions during the SAW process can also have an impact on the transition behavior of elements [30,31,32]. For instance, oxide inclusions may be entrapped in the molten droplets or WM and , elements may undergo redistribution within the metal during the solidification process, thereby influencing the final bulk composition [18,19,21,24]. Therefore, to further enhance overall predictive accuracy, considerations regarding the physical aspects are necessary, which will be detailed in our future work.
4. Conclusions
In conclusion, this study undertakes a comprehensive cross-zone assessment of elemental transfer behavior facilitated by a typical silicon-manganese-based flux, namely, the CaO-SiO2-MnO flux. The limitations associated with conventional quantitative methodologies are addressed and corrective strategies are proposed. The main findings of this investigation are summarized as follows:
- The consideration of cross-zone chemical reactions enhances the precision of describing metallurgical processes in SAW. This improvement is not only evident in the transfer behavior of elements across various welding zones but also in the accuracy of predicting the overall transfer quantities.
- The proposed assessment method takes into full account the impact of reactions within the molten droplet zone and the influence of metal evaporation on the transfer behavior of elements. Notably, the accuracy of predicting the transfer behavior of alloying elements, such as Mn, that are susceptible to oxidation and evaporation is significantly improved.
- The overall transitional quantities of O and Si are still governed by the slag SiO2 activity within the weld pool reaction zone. The multi-zone consideration does not lead itself to a significant improvement in the overall predictive accuracy of ΔO and ΔSi. Nevertheless, the new quantification method is able to describe the cross-zone transfer behavior of O and Si, thus aiding in the analysis of the metallurgical mechanisms in SAW processes.
Author Contributions
Conceptualization, J.Z., J.F. and D.Z.; funding acquisition, J.F. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the National Natural Science Foundation of China (No.50474085), the Initial Fund of Suqian University (No. 2022XRC040), and the Suqian Science & Technology Project (No. K202239).
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
On behalf of all the authors, the corresponding author states that there are no conflicts of interest.
References
- Sengupta, V.; Havrylov, D.; Mendez, P. Physical Phenomena in the Weld Zone of Submerged Arc Welding—A Review. Weld. J. 2019, 98, 283–313. [Google Scholar]
- Kou, S. Welding Metallurgy, 3rd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2003; pp. 22–122. [Google Scholar]
- Zhang, J.; Shao, G.; Fan, J.; Wang, L.; Zhang, D. A Review on Parallel Development of Flux Design and Thermodynamics Subject to Submerged Arc Welding. Processes 2022, 10, 2305. [Google Scholar] [CrossRef]
- Olson, D.; Liu, S.; Frost, R.; Edwards, G.; Fleming, D. Nature and Behavior of Fluxes Used for Welding. In ASM Handbook; ASM International: Almere, The Netherlands, 1993; Volume 6, pp. 55–63. [Google Scholar]
- Natalie, C.A.; Olson, D.L.; Blander, M. Physical and Chemical Behavior of Welding Fluxes. Annu. Rev. Mater. Sci. 1986, 16, 389–413. [Google Scholar] [CrossRef]
- Indacochea, J.E.; Blander, M.; Christensen, N.; Olson, D.L. Chemical Reactions During Submerged Arc Welding with FeO-MnO-SiO2 Fluxes. Metall. Trans. B 1985, 16, 237–245. [Google Scholar] [CrossRef]
- Burck, P.; Indacochea, J.; Olson, D. Effects of Welding Flux Additions on 4340 Steel Weld Metal Composition. Weld. J. 1990, 3, 115–122. [Google Scholar]
- Zhang, J.; Wang, C.; Coetsee, T. Thermodynamic Evaluation of Element Transfer Behaviors for Fused CaO-SiO2-MnO Fluxes Subjected to High Heat Input Submerged Arc Welding. Metall. Mater. Trans. B 2021, 52, 1937–1944. [Google Scholar] [CrossRef]
- Tuliani, S.; Boniszewski, T.; Eaton, N. Notch Toughness of Commercial Submerged Arc Weld Metal. Weld. Met. Fabr. 1969, 37, 327–339. [Google Scholar]
- Chai, C.; Eagar, T. Slag-metal Equilibrium during Submerged Arc Welding. Metall. Trans. B 1981, 12, 539–547. [Google Scholar] [CrossRef]
- Chai, C.-S. Slag-Metal Reactions during Flux Shielded Arc Welding. Ph.D. Thesis, Massachusetts Institute of Technology, Cambridge, MA, USA, 1980. [Google Scholar]
- Lau, T.; Weatherly, G.; McLean, A. Gas/metal/slag Reactions in Submerged Arc Welding Using CaO-Al2O3 Based Fluxes. Weld. J. 1986, 65, 31–38. [Google Scholar]
- Lau, T.; Weatherly, G.; McLean, A. The Sources of Oxygen and Nitrogen Contamination in Submerged Arc Welding using CaO-Al2O3 Based Fluxes. Weld. J. 1985, 64, 343–347. [Google Scholar]
- Zhang, J.; Wang, C.; Coetsee, T. Assessment of Weld Metal Compositional Prediction Models Geared Towards Submerged Arc Welding: Case Studies Involving CaF2-SiO2-MnO and CaO-SiO2-MnO Fluxes. Mater. Trans. B 2021, 52, 2404–2415. [Google Scholar] [CrossRef]
- Bale, C.W.; Bélisle, E.; Chartrand, P.; Decterov, S.; Eriksson, G.; Gheribi, A.; Hack, K.; Jung, I.-H.; Kang, Y.-B.; Melançon, J. Reprint of: FactSage Thermochemical Software and Databases, 2010–2016. Calphad 2016, 55, 1–19. [Google Scholar] [CrossRef]
- Park, J.H. Formation of CaZrO3 at the Interface between CaO–SiO2–MgO–CaF2 (–ZrO2) Slags and Magnesia Refractories: Computational and Experimental Study. Calphad 2007, 31, 149–154. [Google Scholar] [CrossRef]
- Abson, D.; Pargeter, R. Factors Influencing As-deposited Strength, Microstructure, and Toughness of Manual Metal Arc Welds Suitable for C-Mn Steel Fabrications. Int. Met. Rev. 1986, 31, 141–196. [Google Scholar] [CrossRef]
- Mitra, U.; Eagar, T. Slag-metal Reactions during Welding: Part I. Evaluation and Reassessment of Existing Theories. Metall. Trans. B 1991, 22, 65–71. [Google Scholar] [CrossRef]
- Mitra, U.; Eagar, T. Slag-metal Reactions During Welding: Part II. Theory. Metall. Trans. B 1991, 22, 73–81. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, P.; Zhang, D. Advancing Manganese Content Prediction in Submerged Arc Welded Metal: Development of a Multi-Zone Model via the Calphad Technique. Processes 2023, 11, 1265. [Google Scholar] [CrossRef]
- Mitra, U.; Eagar, T. Slag-metal Reactions during Welding: Part III. Verification of the Theory. Metall. Trans. B 1991, 22, 83–100. [Google Scholar] [CrossRef]
- Eagar, T. Sources of Weld Metal Oxygen Contamination during Submerged Arc Welding. Weld. J. 1978, 57, 76–80. [Google Scholar]
- Zhu, J.; Wang, Y.; Shi, H.; Huang, L.; Mao, Z. Element Loss Behavior and Compensation in Additive Manufacturing of Memory Alloys. Trans. China Weld. Inst. 2022, 43, 50–55. [Google Scholar]
- Mitra, U. Kinetics of Sslag Metal Reactions during Submerged Arc Welding of Steel. Ph.D. Thesis, Massachusetts Institute of Technology, Cambridge, MA, USA, 1984. [Google Scholar]
- Chai, C.; Eagar, T. Prediction of Weld-metal Composition during Flux-shielded Welding. J. Mater. Energy Syst. 1983, 5, 160–164. [Google Scholar] [CrossRef]
- Polar, A.; Indacochea, J.; Blander, M. Electrochemically Generated Oxygen Contamination in Submerged Arc Welding. Weld. J. 1990, 69, 69–74. [Google Scholar]
- Jackson, C. Flux and Slag in Welding. Weld Res. Bull. 1973. [Google Scholar]
- Robin, I.K.; Gräning, T.; Yang, Y.; Haider, S.B.; Lass, E.A.; Katoh, Y.; Zinkle, S.J. Evaluation of Tungsten—Steel Solid-State Bonding: Options and the Role of CALPHAD to Screen Diffusion Bonding Interlayers. Metals 2023, 13, 1438. [Google Scholar]
- Liu, Z.-K. Thermodynamics and its prediction and CALPHAD modeling: Review, state of the art, and perspectives. Calphad 2023, 82, 102580. [Google Scholar] [CrossRef]
- Sharma, L.; Chhibber, R.; Kumar, V.; Khan, W.N. Element transfer investigations on silica based submerged arc welding fluxes. Silicon 2023, 15, 305–319. [Google Scholar] [CrossRef]
- Bui, H.V.; Trinh, N.Q.; Tashiro, S.; Suga, T.; Kakizaki, T.; Yamazaki, K.; Lersvanichkool, A.; Murphy, A.B.; Tanaka, M. Individual Effects of Alkali Element and Wire Structure on Metal Transfer Process in Argon Metal-Cored Arc Welding. Materials 2023, 16, 3053. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, C.; Hu, H.; Zhang, D.; Gong, S.; Peng, D.; Wu, S. Phase change heat transfer analysis of anisotropic structures with moving heat source using the element-free Galerkin method. Numer. Heat Transf. Part B Fundam. 2023, 84, 392–414. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).