Evaluation of Polyphenolic Profile and Antioxidant Activity of Sea Buckthorn (Elaeagnus rhamnoides (L.) A. Nelson) Leaf and Berry Extracts Obtained via Optimized Microwave-Assisted and Accelerated Solvent Extraction
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Chemicals and Reagents
2.3. Microwave-Assisted Extraction (MAE)
2.4. Accelerated Solvent Extraction (ASE)
2.5. Conventional Extraction
2.6. Analysis of Polyphenols
2.6.1. Total Phenolic Content (TPC) Determination
2.6.2. Polyphenol Characterization by UPLC/ESI-MS2 Analysis
2.6.3. Oxygen Radical Absorbance Capacity (ORAC) Assay
2.7. Statistical Analysis
3. Results and Discussion
3.1. Effect of MAE on the TPC of Sea Buckthorn Leaves and Berries
3.2. Effect of ASE on the TPC of Sea Buckthorn Leaves and Fruits
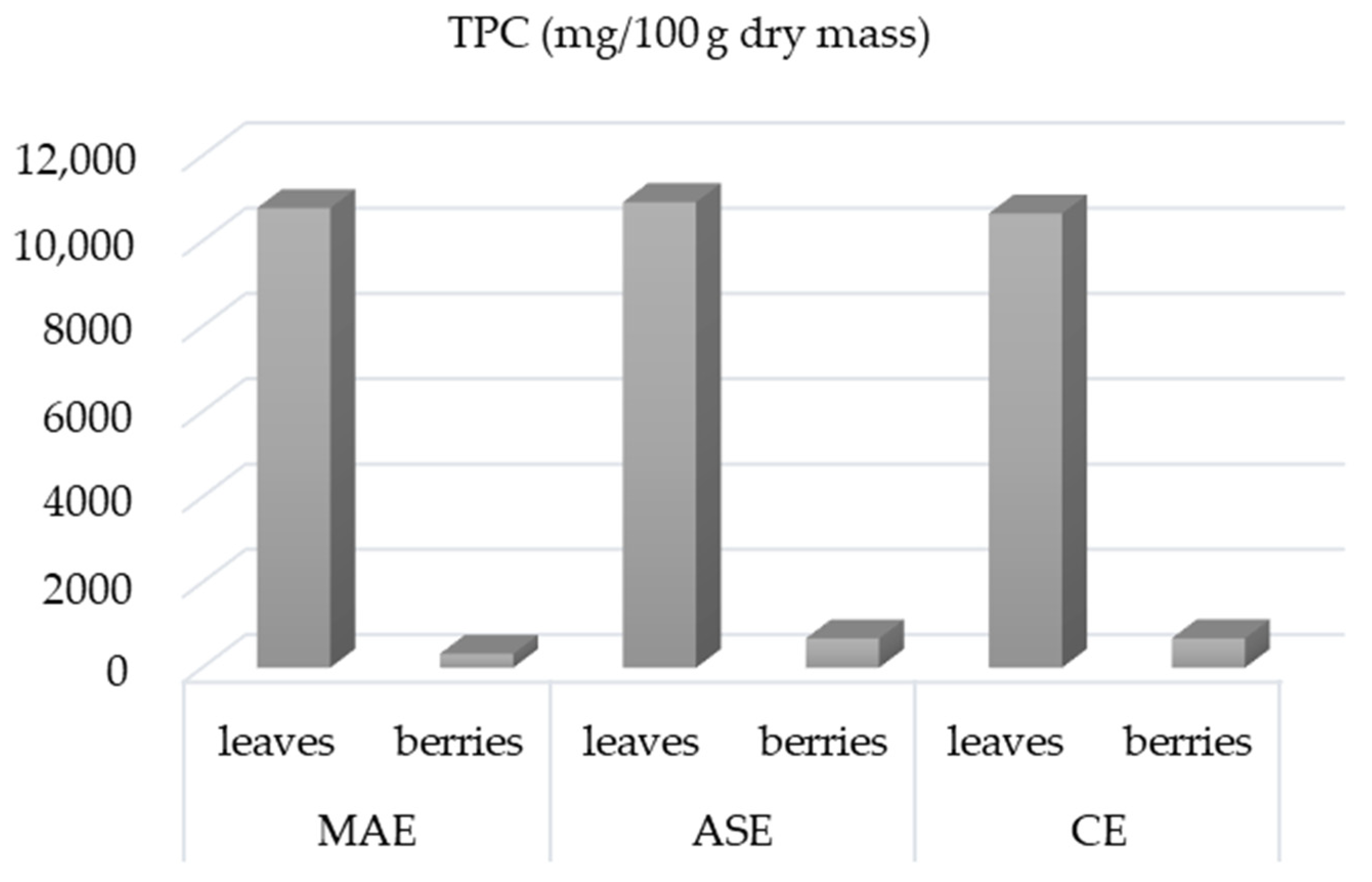
3.3. Comparison between CE, MAE and ASE
3.4. UPLC/ESI-MS2
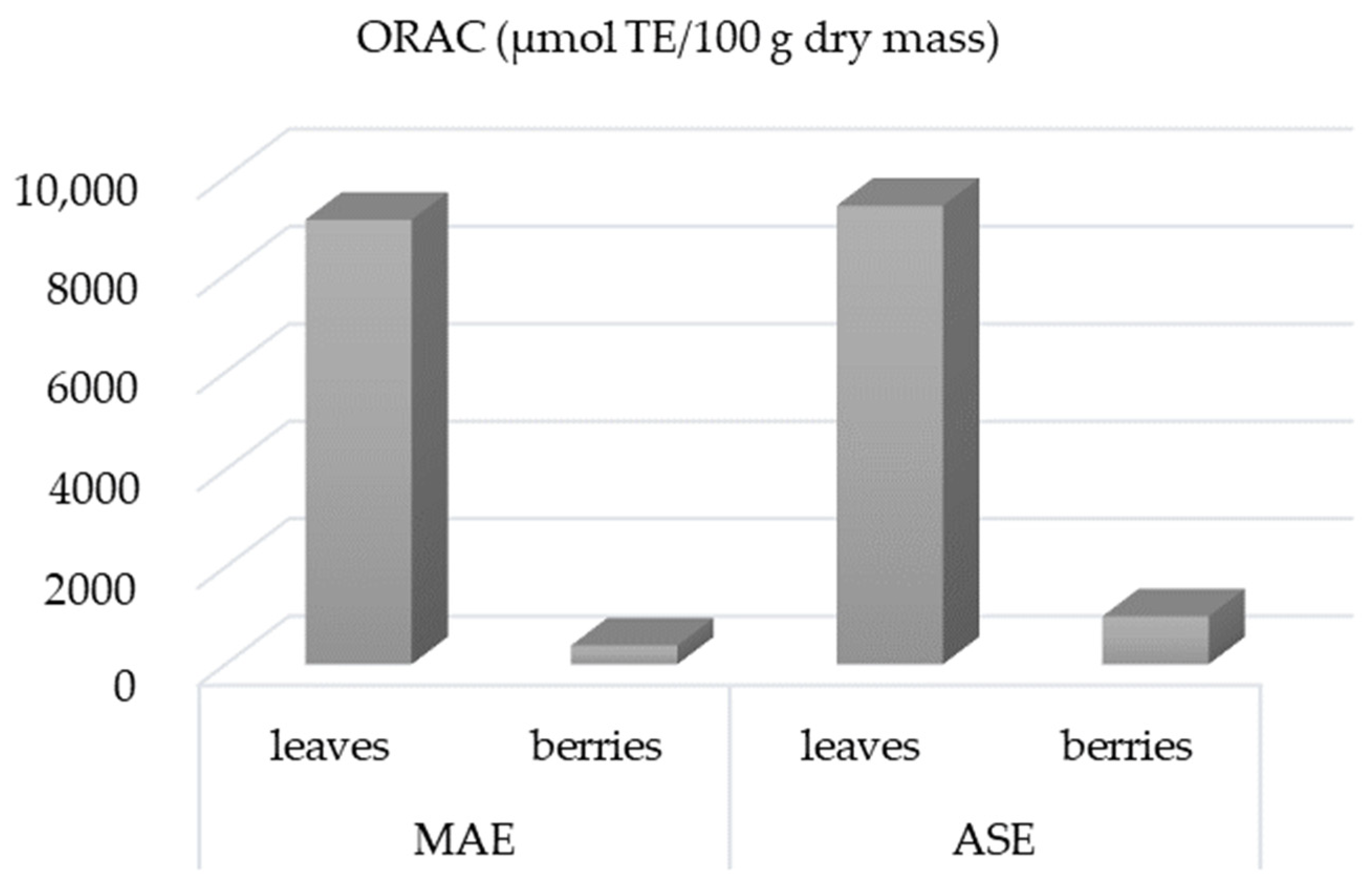
3.5. Antioxidant Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tiitinen, K.M.; Hakala, M.A.; Kallio, H.P. Quality components of sea buckthorn (Hippophae rhamnoides L.) varieties. J. Agric. Food Chem. 2005, 53, 1692–1699. [Google Scholar] [CrossRef] [PubMed]
- Jain, M.; Ganju, L.; Katiyal, A.; Padwad, Y.; Mishra, K.P.; Chanda, S.; Karan, D.; Yogendra, K.M.S.; Sawhney, R.C. Effect of leaf extract against Dengue virus infection in human blood-derived macrophages. Phytomedicine 2008, 15, 793–799. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.S.Y.; Dutta, R.; Prasad, D.; Misra, K. Subcritical water extraction of antioxidant compounds from Seabuckthorn (Hippophae rhamnoides L.) leaves for the comparative evaluation of antioxidant activity. Food Chem. 2011, 127, 1309–1316. [Google Scholar] [CrossRef] [PubMed]
- Saggu, S.; Divekar, H.M.; Gupta, V.; Sawhney, R.C.; Banerjee, P.K.; Kumar, R. Adaptogenic and safety evaluation of seabuckthorn (Hippophae rhamnoides L.) leaf extract: A dose dependent study. Food Chem. Toxicol. 2007, 45, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Ciesarová, Z.; Murkovic, M.; Cejpek, K.; Kreps, F.; Tobolková, B.; Koplík, R.; Belajová, E.; Kukurová, K.; Daško, K.; Panovská, Z.; et al. Why is sea buckthorn (Hippophae rhamnoides L.) so exceptional? A review. Food Res. Int. 2020, 133, 109170. [Google Scholar] [CrossRef] [PubMed]
- Tkacz, K.; Wojdylo, A.; Turkiewicz, I.P.; Bobak, L.; Nowicka, P. Anti-Oxidant and Anti-Enzymatic Activities of Sea Buckthorn (Hippophae rhamnoides L.) Fruits Modulated by Chemical Components. Antioxidants 2019, 8, 618. [Google Scholar] [CrossRef]
- Cao, J.G.; Zheng, Y.X.; Xia, X.; Wang, Q.X.; Xiao, J.B. Total flavonoid contents, antioxidant potential and acetylcholinesterase inhibition activity of the extracts from 15 ferns in China. Ind. Crop Prod. 2015, 75, 135–140. [Google Scholar] [CrossRef]
- Chen, C.; Xu, X.M.; Chen, Y.; Yu, M.Y.; Wen, F.Y.; Zhang, H. Identification, quantification and antioxidant activity of acylated flavonol glycosides from sea buckthorn (Hippophae rhamnoides ssp.). Food Chem. 2013, 141, 1573–1579. [Google Scholar] [CrossRef]
- Kumar, M.S.Y.; Tirpude, R.J.; Maheshwari, D.T.; Bansal, A.; Misra, K. Antioxidant and antimicrobial properties of phenolic rich fraction of Seabuckthorn (Hippophae rhamnoides L.) leaves. Food Chem. 2013, 141, 3443–3450. [Google Scholar] [CrossRef]
- Arimboor, R.; Arumughan, C. HPLC-DAD-MS/MS profiling of antioxidant flavonoid glycosides in sea buckthorn (Hippophae rhamnoides L.) seeds. Int. J. Food Sci. Nutr. 2012, 63, 730–738. [Google Scholar] [CrossRef]
- Fatima, T.; Kesari, V.; Watt, I.; Wishart, D.; Todd, J.F.; Schroeder, W.R.; Paliyath, G.; Krishna, P. Metabolite profiling and expression analysis of flavonoid, vitamin C and tocopherol biosynthesis genes in the antioxidant-rich sea buckthorn (Hippophae rhamnoides L.). Phytochemistry 2015, 118, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Cosmulescu, S.; Trandafir, I.; Nour, V. Phenolic acids and flavonoids profiles of extracts from edible wild fruits and their antioxidant properties. Int. J. Food Prop. 2017, 20, 3124–3134. [Google Scholar] [CrossRef]
- Olas, B. Sea buckthorn as a source of important bioactive compounds in cardiovascular diseases. Food Chem. Toxicol. 2016, 97, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Teleszko, M.; Wojdylo, A.; Rudzinska, M.; Oszmianski, J.; Golis, T. Analysis of Lipophilic and Hydrophilic Bioactive Compounds Content in Sea Buckthorn (Hippophae rhamnoides L.) Berries. J. Agr. Food Chem. 2015, 63, 4120–4129. [Google Scholar] [CrossRef]
- Ji, M.Y.; Gong, X.; Li, X.; Wang, C.C.; Li, M.H. Advanced Research on the Antioxidant Activity and Mechanism of Polyphenols from Species—A Review. Molecules 2020, 25, 917. [Google Scholar] [CrossRef]
- Uttara, B.; Singh, A.V.; Zamboni, P.; Mahajan, R.T. Oxidative Stress and Neurodegenerative Diseases: A Review of Upstream and Downstream Antioxidant Therapeutic Options. Curr. Neuropharmacol. 2009, 7, 65–74. [Google Scholar] [CrossRef]
- Wannes, W.A.; Mhamdi, B.; Sriti, J.; Ben Jemia, M.; Ouchikh, O.; Hamdaoui, G.; Kchouk, M.E.; Marzouk, B. Antioxidant activities of the essential oils and methanol extracts from myrtle (Myrtus communis var. L.) leaf, stem and flower. Food Chem. Toxicol. 2010, 48, 1362–1370. [Google Scholar] [CrossRef]
- Garofulic, I.E.; Kruk, V.; Martic, A.; Martic, I.; Zoric, Z.; Pedisic, S.; Dragovic, S.; Dragovic-Uzelac, V. Evaluation of Polyphenolic Profile and Antioxidant Activity of Pistacia lentiscus L. Leaves and Fruit Extract Obtained by Optimized Microwave-Assisted Extraction. Foods 2020, 9, 1556. [Google Scholar] [CrossRef]
- Repajic, M.; Cegledi, E.; Kruk, V.; Pedisic, S.; Çinar, F.; Kovacevic, D.B.; Zutic, I.; Dragovic-Uzelac, V. Accelerated Solvent Extraction as a Green Tool for the Recovery of Polyphenols and Pigments from Wild Nettle Leaves. Processes 2020, 8, 803. [Google Scholar] [CrossRef]
- Chen, Y.L.; Duan, G.L.; Xie, M.F.; Chen, B.; Li, Y. Infrared-assisted extraction coupled with high-performance liquid chromatography for simultaneous determination of eight active compounds in. J. Sep. Sci. 2010, 33, 2888–2897. [Google Scholar] [CrossRef]
- Barba, F.J.; Zhu, Z.Z.; Koubaa, M.; Sant’Ana, A.S.; Orlien, V. Green alternative methods for the extraction of antioxidant bioactive compounds from winery wastes and by-products: A review. Trends Food Sci. Technol. 2016, 49, 96–109. [Google Scholar] [CrossRef]
- Krishnaswamy, K.; Orsat, V.; Gariépy, Y.; Thangavel, K. Optimization of Microwave-Assisted Extraction of Phenolic Antioxidants from Grape Seeds. Food Bioprocess. Technol. 2013, 6, 441–455. [Google Scholar] [CrossRef]
- Périno-Issartier, S.; Zill-e-Huma; Abert-Vian, M.; Chemat, F. Solvent Free Microwave-Assisted Extraction of Antioxidants from Sea Buckthorn (Hippophae rhamnoides) Food By-Products. Food Bioprocess. Technol. 2011, 4, 1020–1028. [Google Scholar] [CrossRef]
- Dragovic-Uzelac, V.; Garofulic, I.E.; Jukic, M.; Penic, M.; Dent, M. The Influence of Microwave-Assisted Extraction on the Isolation of Sage (Salvia officinalis L.) Polyphenols. Food Technol. BioTechnol. 2012, 50, 377–383. [Google Scholar]
- Herrero, M.; Cifuentes, A.; Ibáñez, E. Extraction techniques for the determination of carotenoids and vitamins in food. In Comprehensive Sampling and Sample Preparation; Pawliszyn, J., Ed.; Elsevier: Amsterdam, The Netherlands, 2012; Volume 4, pp. 181–201. [Google Scholar]
- Olas, B. The beneficial health aspects of sea buckthorn (Eleagnus Rhamnoides (L.) A.Nelson) oil. J. Ethnopharmacol. 2018, 213, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Osorio-Tobón, J.F. Recent advances and comparisons of conventional and alternative extraction techniques of phenolic compounds. J. Food Sci. Technol. 2020, 57, 4299–4315. [Google Scholar] [CrossRef] [PubMed]
- Nour, A.H.; Oluwaseun, A.R.; Nour, A.H.; Omer, M.S.; Ahmed, N. Microwave-Assisted Extraction of Bioactive Compounds. In Microwave Heating—Electromagnetic Fields Causing Thermal and Non-Thermal Effects; Churyumov, G.I., Ed.; IntechOpen: Rijeka, Croatia, 2021. [Google Scholar]
- Fan, J.F.; Huang, Y.F.; Zhang, H.R. Extraction of flavonoids from fresh sea buckthorn leaves by microwave-assisted extraction. China Brew. 2009, 8, 94–96. [Google Scholar]
- Asofiei, I.; Calinescu, I.; Trifan, A.; David, I.G.; Gavrila, A.I. Microwave-Assisted Batch Extraction of Polyphenols from Sea Buckthorn Leaves. Chem. Eng. Commun. 2016, 203, 1547–1553. [Google Scholar] [CrossRef]
- Bitwell, C.; Sen Indra, S.; Luke, C.; Kakoma, M.K. A review of modern and conventional extraction techniques and their applications for extracting phytochemicals from plants. Sci. Afr. 2023, 19, e01585. [Google Scholar] [CrossRef]
- Ameer, K.; Shahbaz, H.M.; Kwon, J.H. Green Extraction Methods for Polyphenols from Plant Matrices and Their Byproducts: A Review. Compr. Rev. Food Sci. Food Saf. 2017, 16, 295–315. [Google Scholar] [CrossRef]
- Culina, P.; Cvitkovic, D.; Pfeifer, D.; Zoric, Z.; Repajic, M.; Garofulic, I.E.; Balbino, S.; Pedisic, S. Phenolic Profile and Antioxidant Capacity of Selected Medicinal and Aromatic Plants: Diversity upon Plant Species and Extraction Technique. Processes 2021, 9, 2207. [Google Scholar] [CrossRef]
- Shortle, E.; O’Grady, M.N.; Gilroy, D.; Furey, A.; Quinn, N.; Kerry, J.P. Influence of extraction technique on the anti-oxidative potential of hawthorn extracts in bovine muscle homogenates. Meat Sci. 2014, 98, 828–834. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.N.; Wen, X.F.; Gao, Y.; Lu, S.G.; Li, Y.M.; Shi, Y.B.; Yang, Z.G. Identification and Characterization of the Bioactive Polyphenols and Volatile Compounds in Sea Buckthorn Leaves Tea Together With Antioxidant and α-Glucosidase Inhibitory Activities. Front. Nutr. 2022, 9, 890486. [Google Scholar] [CrossRef] [PubMed]
- Rösch, D.; Krumbein, A.; Mügge, C.; Kroh, L.W. Structural investigations of flavonol glycosides from sea buckthorn (Hippophae rhamnoides L.) pomace by NMR Spectroscopy and HPLC-ESI-MSn. J. Agric. Food Chem. 2004, 52, 4039–4046. [Google Scholar] [CrossRef] [PubMed]
- Pop, R.M.; Socaciu, C.; Pintea, A.; Buzoianu, A.D.; Sanders, M.G.; Gruppen, H.; Vincken, J.P. UHPLC/PDA-ESI/MS Analysis of the Main Berry and Leaf Flavonol Glycosides from Different Carpathian Hippophae rhamnoides L. Varieties. Phytochem. Analysis 2013, 24, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.B.; Rai, D.K.; Brunton, N.P.; Martin-Diana, A.B.; Barry-Ryan, C. Characterization of Phenolic Composition in Lamiaceae Spices by LC-ESI-MS/MS. J. Agric. Food Chem. 2010, 58, 10576–10581. [Google Scholar] [CrossRef] [PubMed]
- March, R.E.; Miao, X.S. A fragmentation study of kaempferol using electrospray quadrupole time-of-flight mass spectrometry at high mass resolution. Int. J. Mass. Spectrom. 2004, 231, 157–167. [Google Scholar] [CrossRef]
- Galan, A.M.; Calinescu, J.; Trifan, A.; Winkworth-Smith, C.; Calvo-Carrascal, M.; Dodds, C.; Binner, E. New insights into the role of selective and volumetric heating during microwave extraction: Investigation of the extraction of polyphenolic compounds from sea buckthorn leaves using microwave-assisted extraction and conventional solvent extraction. Chem. Eng. Process 2017, 116, 29–39. [Google Scholar] [CrossRef]
- Michel, T.; Destandau, E.; Le Floch, G.; Lucchesi, M.E.; Elfakir, C. Antimicrobial, antioxidant and phytochemical investigations of sea buckthorn (Hippophae rhamnoides L.) leaf, stem, root and seed. Food Chem. 2012, 131, 754–760. [Google Scholar] [CrossRef]
- Camel, V. Recent extraction techniques for solid matrices-supercritical fluid extraction, pressurized fluid extraction and microwave-assisted extraction: Their potential and pitfalls. Analyst 2001, 126, 1182–1193. [Google Scholar] [CrossRef]
- Rodríguez-Pérez, C.; Gilbert-López, B.; Mendiola, J.A.; Quirantes-Piné, R.; Segura-Carretero, A.; Ibáñez, E. Optimization of microwave-assisted extraction and pressurized liquid extraction of phenolic compounds from Moringa oleifera leaves by multiresponse surface methodology. Electrophoresis 2016, 37, 1938–1946. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, A.; Turner, C. Pressurized liquid extraction as a green approach in food and herbal plants extraction: A review. Anal. Chim. Acta 2011, 703, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Bouras, M.; Chadni, M.; Barba, F.J.; Grimi, N.; Bals, O.; Vorobiev, E. Optimization of microwave-assisted extraction of polyphenols from bark. Ind. Crop Prod. 2015, 77, 590–601. [Google Scholar] [CrossRef]
- Wu, J.Y.; Lin, L.D.; Chau, F.T. Ultrasound-assisted extraction of ginseng saponins from ginseng roots and cultured ginseng cells. Ultrason. Sonochem. 2001, 8, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Moret, S.; Conchione, C.; Srbinovska, A.; Lucci, P. Microwave-Based Technique for Fast and Reliable Extraction of Organic Contaminants from Food, with a Special Focus on Hydrocarbon Contaminants. Foods 2019, 8, 503. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Mumper, R.J. Plant Phenolics: Extraction, Analysis and Their Antioxidant and Anticancer Properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.; Gramza-Michalowska, A. Recent Development on the Chemical Composition and Phenolic Extraction Methods of Apple (Malus domestica)—A Review. Food Bioprocess. Technol. 2023. [Google Scholar] [CrossRef]
- Gahruie, H.H.; Parastouei, K.; Mokhtarian, M.; Rostami, H.; Niakousari, M.; Mohsenpour, Z. Application of innovative processing methods for the extraction of bioactive compounds from saffron petals. J. Appl. Res. Med. Aroma 2020, 19, 100264. [Google Scholar] [CrossRef]
- Flórez, N.; Conde, E.; Domínguez, H. Microwave assisted water extraction of plant compounds. J. Chem. Technol. Biot. 2015, 90, 590–607. [Google Scholar] [CrossRef]
- Dahmoune, F.; Nayak, B.; Moussi, K.; Remini, H.; Madani, K. Optimization of microwave-assisted extraction of polyphenols from Hippophae rhamnoides L. leaves. Food Chem. 2015, 166, 585–595. [Google Scholar] [CrossRef]
- Alara, O.R.; Abdurahman, N.H.; Olalere, O.A. Optimization of microwave-assisted extraction of flavonoids and antioxidants from leaf using response surface methodology. Food Bioprod. Process. 2018, 107, 36–48. [Google Scholar] [CrossRef]
- Rafiee, Z.; Jafari, S.M.; Alami, M.; Khomeiri, M. Microwave-Assisted Extraction of Phenolic Compounds from Olive Leaves; a Comparison with Maceration. J. Anim. Plant Sci. 2011, 21, 738–745. [Google Scholar]
- Shi, L.H.; Zhao, W.R.; Yang, Z.H.; Subbiah, V.; Suleria, H.A.R. Extraction and characterization of phenolic compounds and their potential antioxidant activities. Environ. Sci. Pollut. Res. 2022, 29, 81112–81129. [Google Scholar] [CrossRef] [PubMed]
- Khoddami, A.; Wilkes, M.A.; Roberts, T.H. Techniques for Analysis of Plant Phenolic Compounds. Molecules 2013, 18, 2328–2375. [Google Scholar] [CrossRef] [PubMed]
- Mottaleb, M.A.; Sarker, S.D. Accelerated Solvent Extraction for Natural Products Isolation. In Natural Products Isolation, Methods in Molecular Biology, 3rd ed.; Sarker, S.D., Nahar, L., Eds.; Springer: New York, NY, USA, 2012; pp. 75–88. [Google Scholar]
- Li, J.; Zhang, S.T.; Zhang, M.N.; Sun, B.S. Novel approach for extraction of grape skin antioxidants by accelerated solvent extraction: Box-Behnken design optimization. J. Food Sci. Technol. 2019, 56, 4879–4890. [Google Scholar] [CrossRef] [PubMed]
- Gomes, S.V.F.; Portugal, L.A.; dos Anjos, J.P.; de Jesus, O.N.; de Oliveira, E.J.; David, J.P.; David, J.M. Accelerated solvent extraction of phenolic compounds exploiting a Box-Behnken design and quantification of five flavonoids by HPLC-DAD in Passiflora species. Microchem. J. 2017, 132, 28–35. [Google Scholar] [CrossRef]
- Chaves, J.O.; de Souza, M.C.; da Silva, L.C.; Lachos-Perez, D.; Torres-Mayanga, P.C.; Machado, A.P.D.; Forster-Carneiro, T.; Vázquez-Espinosa, M.; González-de-Peredo, A.V.; Barbero, G.F.; et al. Extraction of Flavonoids From Natural Sources Using Modern Techniques. Front. Chem. 2020, 8, 507887. [Google Scholar] [CrossRef]
- Ince, A.E.; Sahin, S.; Sumnu, G. Comparison of microwave and ultrasound-assisted extraction techniques for leaching of phenolic compounds from nettle. J. Food Sci. Technol. 2014, 51, 2776–2782. [Google Scholar] [CrossRef]
- Georgiopoulou, I.; Tzima, S.; Louli, V.; Magoulas, K. Process Optimization of Microwave-Assisted Extraction of Chlorophyll, Carotenoid and Phenolic Compounds from Chlorella vulgaris and Comparison with Conventional and Supercritical Fluid Extraction. Appl. Sci. 2023, 13, 2740. [Google Scholar] [CrossRef]
- Alhallaf, W.; Bishop, K.; Perkins, L.B. Optimization of Accelerated Solvent Extraction of Phenolic Compounds from Chaga Using Response Surface Methodology. Food Anal. Methods 2022, 15, 2777–2790. [Google Scholar] [CrossRef]
- Mcnab, H.; Ferreira, E.S.B.; Hulme, A.N.; Quye, A. Negative ion ESI-MS analysis of natural yellow dye flavonoids—An isotopic labelling study. Int. J. Mass. Spectrom. 2009, 284, 57–65. [Google Scholar] [CrossRef]
- Wang, H.; Chen, L.J.; Yang, B.R.; Du, J.; Chen, L.; Li, Y.M.; Guo, F.J. Structures, Sources, Identification/Quantification Methods, Health Benefits, Bioaccessibility, and Products of Isorhamnetin Glycosides as Phytonutrients. Nutrients 2023, 15, 1947. [Google Scholar] [CrossRef] [PubMed]
- Perk, A.A.; Ceylan, F.D.; Yanar, O.; Boztas, K.; Capanoglu, E. Investigating the antioxidant properties and rutin content of Sea buckthorn (Hippophae rhamnoides L.) leaves and branches. Afr. J. Biotechnol. 2016, 15, 118–124. [Google Scholar]
- Li, Y.; Li, P.; Yang, K.L.; He, Q.; Wang, Y.; Sun, Y.H.; He, C.N.; Xiao, P.G. Impact of Drying Methods on Phenolic Components and Antioxidant Activity of Sea Buckthorn (Hippophae rhamnoides L.) Berries from Different Varieties in China. Molecules 2021, 26, 7189. [Google Scholar] [CrossRef]
- Arimboor, R.; Kumar, K.S.; Arumughan, C. Simultaneous estimation of phenolic acids in sea buckthorn (Hippophae rhamnoides L.) using RP-HPLC with DAD. J. Pharmaceut. Biomed. 2008, 47, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Cai, Y.F.; Wang, K.; Wang, Y.S. Bioactive Compounds in Sea Buckthorn and their Efficacy in Preventing and Treating Metabolic Syndrome. Foods 2023, 12, 1985. [Google Scholar] [CrossRef]
- Irakli, M.; Skendi, A.; Bouloumpasi, E.; Christaki, S.; Biliaderis, C.G.; Chatzopoulou, P. Sustainable Recovery of Phenolic Compounds from Distilled Rosemary By-Product Using Green Extraction Methods: Optimization, Comparison, and Antioxidant Activity. Molecules 2023, 28, 6669. [Google Scholar] [CrossRef]
- Alara, O.R.; Abdurahman, N.H.; Ukaegbu, C.I. Extraction of phenolic compounds: A review. Curr. Res. Food Sci. 2021, 4, 200–214. [Google Scholar] [CrossRef]
- Biesaga, M. Influence of extraction methods on stability of flavonoids. J. Chromatogr. A 2011, 1218, 2505–2512. [Google Scholar] [CrossRef]
- Liazid, A.; Palma, M.; Brigui, J.; Barroso, C.G. Investigation on phenolic compounds stability during microwave-assisted extraction. J. Chromatogr. A 2007, 1140, 29–34. [Google Scholar] [CrossRef]
- Prior, R.L. Oxygen radical absorbance capacity (ORAC): New horizons in relating dietary antioxidants/bioactives and health benefits. J. Funct. Foods 2015, 18, 797–810. [Google Scholar] [CrossRef]
| Temperature (°C) | Power (W) | Time (min) | Total Phenolic Content (mg GAE/100 g dm) | |
|---|---|---|---|---|
| Leaves | Berries | |||
| 40 | 300 | 5 | 7208 ± 32 | 278 ± 5 |
| 10 | 6650 ± 74 | 271 ± 9 | ||
| 15 | 7255 ± 302 | 306 ± 2 | ||
| 500 | 5 | 8197 ± 684 | 309 ± 1 | |
| 10 | 7888 ± 656 | 332 ± 22 | ||
| 15 | 9084 ± 506 | 270 ± 13 | ||
| 700 | 5 | 6514 ± 225 | 246 ± 1 | |
| 10 | 9671 ± 812 | 328 ± 9 | ||
| 15 | 9391 ± 177 | 318 ± 16 | ||
| 60 | 300 | 5 | 9477 ± 657 | 373 ± 0 |
| 10 | 7957 ± 155 | 333 ± 4 | ||
| 15 | 7756 ± 115 | 328 ± 13 | ||
| 500 | 5 | 7719 ± 417 | 320 ± 16 | |
| 10 | 8488 ± 263 | 329 ± 9 | ||
| 15 | 10,779 ± 557 | 355 ± 21 | ||
| 700 | 5 | 10,545 ± 660 | 319 ± 1 | |
| 10 | 7869 ± 31 | 344 ± 17 | ||
| 15 | 7935 ± 330 | 253 ± 7 | ||
| 80 | 300 | 5 | 8666 ± 431 | 358 ± 12 |
| 10 | 9192 ± 42 | 377 ± 13 | ||
| 15 | 9364 ± 206 | 360 ± 8 | ||
| 500 | 5 | 9707 ± 500 | 311 ± 4 | |
| 10 | 8647 ± 625 | 406 ± 20 | ||
| 15 | 9955 ± 258 | 372 ± 7 | ||
| 700 | 5 | 8198 ± 652 | 333 ± 5 | |
| 10 | 7796 ± 189 | 307 ± 15 | ||
| 15 | 8492 ± 531 | 283 ± 7 | ||
| Temperature (°C) | Static Time (min) | Cycle Number | Total Phenolic Content (mg GAE/100 g dm) | |
|---|---|---|---|---|
| Leaves | Berries | |||
| 80 | 5 | 1 | 7958 ± 169 | 354 ± 11 |
| 2 | 8151 ± 253 | 390 ± 6 | ||
| 3 | 8898 ± 230 | 406 ± 1 | ||
| 10 | 1 | 8678 ± 52 | 352 ± 10 | |
| 2 | 8928 ± 171 | 539 ± 22 | ||
| 3 | 9207 ± 137 | 573 ± 4 | ||
| 15 | 1 | 8605 ± 147 | 401 ± 5 | |
| 2 | 9036 ± 327 | 442 ± 21 | ||
| 3 | 9478 ± 253 | 494 ± 2 | ||
| 100 | 5 | 1 | 6905 ± 0 | 220 ± 17 |
| 2 | 8587 ± 451 | 428 ± 7 | ||
| 3 | 10,163 ± 232 | 436 ± 13 | ||
| 10 | 1 | 9035 ± 63 | 427 ± 21 | |
| 2 | 9614 ± 504 | 475 ± 8 | ||
| 3 | 9833 ± 52 | 434 ± 3 | ||
| 15 | 1 | 9092 ± 85 | 478 ± 13 | |
| 2 | 9626 ± 179 | 539 ± 6 | ||
| 3 | 1028 ± 11 | 532 ± 6 | ||
| 120 | 5 | 1 | 9840 ± 242 | 365 ± 12 |
| 2 | 9879 ± 211 | 452 ± 3 | ||
| 3 | 10,235 ± 169 | 459 ± 21 | ||
| 10 | 1 | 9664 ± 84 | 422 ± 13 | |
| 2 | 9712 ± 190 | 525 ± 7 | ||
| 3 | 10,238 ± 535 | 642 ± 21 | ||
| 15 | 1 | 9295 ± 315 | 470 ± 21 | |
| 2 | 10,497 ± 497 | 580 ± 18 | ||
| 3 | 10,918 ± 378 | 688 ± 9 | ||
| Source of Variation | Total Phenolic Content (mg GAE/100 g dm) | |
|---|---|---|
| Leaves | Berries | |
| Temperature (°C) | p = 0.043 * | p < 0.001 * |
| 40 | 7984 ± 282 a | 295 ± 7 a |
| 60 | 8725 ± 290 ab | 328 ± 8 b |
| 80 | 8891 ± 179 b | 345 ± 9 b |
| Power (W) | p = 0.139 | p = 0.059 |
| 300 | 810 ± 244 a | 331 ± 9 a |
| 500 | 8915 ± 242 a | 331 ± 11 a |
| 700 | 8516 ± 298 a | 306 ± 7 a |
| Time (min) | p = 0.243 | p = 0.088 |
| 5 | 8496 ± 315 a | 319 ± 8 a |
| 10 | 8240 ± 215 a | 336 ± 9 a |
| 15 | 8864 ± 257 a | 313 ± 11 a |
| Source of Variation | Total Phenolic Content (mg GAE/100 g dm) | |
|---|---|---|
| Leaves | Berries | |
| Temperature (°C) | p < 0.001 * | p < 0.001 * |
| 80 | 8771 ± 117 a | 439 ± 18 a |
| 100 | 9238 ± 240 b | 441 ± 21 a |
| 120 | 10031 ± 125 c | 511 ± 25 b |
| Static time (min) | p = 0.271 | p < 0.001 * |
| 5 | 8957 ± 268 a | 390 ± 17 a |
| 10 | 9434 ± 123 a | 488 ± 21 b |
| 15 | 9648 ± 181 a | 514 ± 20 b |
| Cycle number | p < 0.001 * | p < 0.001 * |
| 1 | 8786 ± 209 a | 388 ± 18 a |
| 2 | 9337 ± 175 a | 486 ± 14 b |
| 3 | 9917 ± 151 b | 518 ± 23 b |
| Phenolic Compounds | Precursor Ion (m/z) | Fragment Ions (m/z) | Ionization Mode | Mass Concentration (mg/100 g dm) | ||||
|---|---|---|---|---|---|---|---|---|
| ASE | MAE | |||||||
| Leaves | Berries | Leaves | Berries | |||||
| FLAVONOLS | ||||||||
| 1 | Isorhamnetin | 317 | 201 | positive | nd | 2.3 ± 0.1 | 0.9 ± 0.0 | nd |
| 2 | Isorhamnetin-3-sinapoyglucose-glucoside-7-rhamnoside | 993 | 463, 317 | positive | 1.4 ± 0.1 | 1.1 ± 0.2 | 0.9 ± 0.1 | 1.2 ± 0.1 |
| 3 | Ishorhamnetin-3-sophoroside-7-rhamnoside | 787 | 463, 317 | positive | 5.3 ± 0.1 | 1.9 ± 0.1 | 1.2 ± 0.1 | 7.5 ± 0.2 |
| 4 | Isorhamnetin-3-rutinoside-7-glucoside | 787 | 479, 317 | positive | 6.1 ± 0.2 | 1.9 ± 0.1 | 0.8 ± 0.1 | 4.6 ± 0.5 |
| 5 | Isorhamnetin-3-hexoside | 479 | 317 | positive | 32.7 ± 1.5 | 23.6 ± 1.1 | 12.8 ± 1.54 | 45.4 ± 1.5 |
| 6 | Isorhamnetin-3-rhamnoside | 463 | 317 | positive | 31.8 ± 1.8 | 10.3 ± 0.9 | 3.1 ± 0.2 | 18.8 ± 0.9 |
| 7 | Isorhamnetin-3,7-dihexoside | 641 | 479, 317 | positive | 40.4 ± 2.43 | 8.6 ± 1.0 | 4.3 ± 0.1 | 12.4 ± 1.5 |
| 8 | Isorhamnetin-3-rutinoside | 625 | 479, 317 | positive | 13.7 ± 0.8 | 19.1 ± 1.2 | 10.9 ± 1.1 | 4.3 ± 0.1 |
| 9 | Kaempferol | 287 | 145 | positive | 29.0 ± 2.4 | 51.3 ± 2.4 | 4.8 ± 0.1 | 81.2 ± 2.8 |
| 10 | Kaempferol-3-O-sophorose-7-O-rhamnoside | 757 | 287 | positive | 23.9 ± 2.8 | 8.2 ± 0.0 | 1.2 ± 0.1 | 11.5 ± 1.7 |
| 11 | Kaemferol-3-O-glucoside-7-O-rhamnoside | 595 | 433, 287 | positive | 11.9 ± 0.8 | 10.3 ± 1.1 | 4.4 ± 0.1 | 11.0 ± 1.5 |
| 12 | Kaempferol-3-rutinoside * | 595 | 287 | positive | 300.0 ± 12.4 | nd | 110.0 ± 20.1 | nd |
| 13 | Kaempferol-rhamnoside | 433 | 287 | positive | 50.4 ± 2.5 | nd | nd | nd |
| 14 | Quercetin-3-sophoroside-7-rhamnoside | 773 | 611, 303 | positive | 6.2 ± 0.1 | 2.5 ± 0.5 | 0.7 ± 0.1 | 4.1 ± 0.1 |
| 15 | Quercetin-3-rhamnosylglucoside-7-rhamnoside | 757 | 303 | positive | 3.9 ± 0.0 | 2.7 ± 0.1 | 1.3 ± 0.00 | 1.9 ± 0.0 |
| 16 | Quercetin-3-glucoside * | 465 | 303 | positive | 12.5 ± 1.1 | 8.8 ± 0.5 | 5.6 ± 0.1 | 19.7 ± 1.1 |
| 17 | Quercetin-3-rutinoside (rutin) | 611 | 303 | positive | 185.7 ± 1.4 | 15.6 ± 2.4 | 76.6 ± 1.4 | 12.6 ± 0.2 |
| 18 | Quercetin-3-rhamnoside (quercitrin) | 449 | 303 | positive | 18.6 ± 2.4 | 1.6 ± 0.2 | 0.8 ± 0.1 | 15.5 ± 1.6 |
| 19 | Quercetin-3-pentoside | 435 | 303 | positive | 8.1 ± 0.5 | nd | nd | 1.5 ± 0.1 |
| FLAVAN-3-OLS | ||||||||
| 20 | Catechin * | 291 | 139 | positive | 4.2 ± 0.1 | nd | 0.8 ± 0.0 | nd |
| 21 | Epicatechin * | 291 | 165 | positive | 10.3 ± 1.7 | nd | 2.1 ± 0.1 | nd |
| PHENOLIC ACIDS | ||||||||
| 22 | Caffeic acid * | 179 | 135 | negative | 14.1 ± 4.2 | 22.5 ± 1.4 | 9.4 ± 0.9 | 2.5 ± 0.0 |
| 23 | Chlorogenic acid * | 353 | 191 | negative | nd | 0.30 ± 0.00 | nd | nd |
| 24 | Ellagic acid | 301 | 257 | negative | 29.9 ± 7.1 | nd | nd | nd |
| 25 | Gallic acid * | 169 | 125 | negative | 87.1 ± 5.6 | nd | nd | nd |
| 26 | p-hydroxybenzoic acid | 137 | 93 | negative | 22.7 ± 2.4 | 30.6 ± 3.8 | 188.9 ± 0.2 | 8.1 ± 0.1 |
| 27 | p-coumaric acid * | 163 | 119 | negative | 21.4 ± 1.1 | 10.1 ± 1.5 | 5.8 ± 0.2 | 2.7 ± 0.0 |
| 28 | Protocatechuic acid | 153 | 109 | negative | nd | 35.1 ± 2.9 | nd | nd |
| 29 | Vanillic acid * | 169 | 125 | positive | 37.5 ± 2.8 | 52.8 ± 10.4 | 20.8 ± 5.4 | 22.1 ± 1.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Čulina, P.; Repajić, M.; Elez Garofulić, I.; Dragović-Uzelac, V.; Pedisić, S. Evaluation of Polyphenolic Profile and Antioxidant Activity of Sea Buckthorn (Elaeagnus rhamnoides (L.) A. Nelson) Leaf and Berry Extracts Obtained via Optimized Microwave-Assisted and Accelerated Solvent Extraction. Processes 2024, 12, 126. https://doi.org/10.3390/pr12010126
Čulina P, Repajić M, Elez Garofulić I, Dragović-Uzelac V, Pedisić S. Evaluation of Polyphenolic Profile and Antioxidant Activity of Sea Buckthorn (Elaeagnus rhamnoides (L.) A. Nelson) Leaf and Berry Extracts Obtained via Optimized Microwave-Assisted and Accelerated Solvent Extraction. Processes. 2024; 12(1):126. https://doi.org/10.3390/pr12010126
Chicago/Turabian StyleČulina, Patricija, Maja Repajić, Ivona Elez Garofulić, Verica Dragović-Uzelac, and Sandra Pedisić. 2024. "Evaluation of Polyphenolic Profile and Antioxidant Activity of Sea Buckthorn (Elaeagnus rhamnoides (L.) A. Nelson) Leaf and Berry Extracts Obtained via Optimized Microwave-Assisted and Accelerated Solvent Extraction" Processes 12, no. 1: 126. https://doi.org/10.3390/pr12010126
APA StyleČulina, P., Repajić, M., Elez Garofulić, I., Dragović-Uzelac, V., & Pedisić, S. (2024). Evaluation of Polyphenolic Profile and Antioxidant Activity of Sea Buckthorn (Elaeagnus rhamnoides (L.) A. Nelson) Leaf and Berry Extracts Obtained via Optimized Microwave-Assisted and Accelerated Solvent Extraction. Processes, 12(1), 126. https://doi.org/10.3390/pr12010126









