Abstract
In this research, we aimed to assess antibacterial activity and develop oral care products from three natural plant extracts from the Thai highlands. The plants, including Camellia sinensis var. assamica, Zanthozylum limonella Alston, and Acorus calamus L., were extracted using two traditional extraction techniques: maceration and hydrodistillation methods. The extracts were characterized by percentage yield, total phenolic, and total flavonoid contents. Antibacterial activity against Staphylococcus aureus, which play a role in oral health and disease, was investigated. C. sinensis var. assamica extract had the highest content of phenolic acid (38.15 ± 4.12 mg GAE/g extract) and flavonoids (44.91 ± 2.76 mg QE/g extract). Interestingly, a combination of C. sinensis with Z. limonella and A. calamus provides a greater inhibitory effect against S. aureus. Furthermore, oral care products were prepared as a natural product mixture in two preparations: (i) oral ulcers gel and (ii) oral spray. Apart from antibacterial efficiency, volunteer satisfaction after the usage of oral care products containing traditional plant extracts was investigated via organoleptic evaluation. The findings of the volunteer surveys indicated positive feedback for both oral care products with high satisfaction levels. Hence, these oral care products could potentially be natural antimicrobial agents and can be further developed and applied for oral applications in the pharmaceutical and cosmetic industries.
1. Introduction
Natural plants have been rediscovered and studied extensively. Their role in several useful biological activities, including antibacterial and antifungal ones, have also been well documented [1,2]. They produce an abundance of biological actives, such as phenolics, flavonoids, alkaloids, and terpenoids. These have medicinal and pharmacological importance as well as therapeutic potential. Recently, research on natural plant products has greatly increased over recent decades with the investigation of the biological activities of several plants in different habitats [3,4]. Hence, in our study, we aimed to evaluate the activities of interesting plants in the Thai highlands and develop cost-effective products that are useful and satisfactory.
Over the years, many reports have demonstrated several properties of medicinal plants, and these have been used as traditional medicine in many areas.
(i) Camellia sinensis (L.), which is known as tea, belongs to the Theaceae family. It is the most popular plant that is used as a medicinal drink and non-alcoholic caffeine-containing beverage [5,6]. Leaves of C. sinensis var. assamica are consumed as a health beverage especially in the form of Miang, which is a traditional Northern Thai fermented tea leaf. Several studies reported that C. sinensis var. assamica has a positive effect on infection prevention and treatments [7,8]. In addition, these tea leaves have been reported in traditional medicine to exhibit anti-cardiovascular, antioxidant, anti-cancer and anti-diabetic, and immunomodulatory effects, as well as antibacterial activities [9]. Furthermore, it has been reported that C. sinensis (L.) leaves contain significant phytochemical compounds, including 30–40% polyphenols (catechins and gallocatechin, epicatechins and gallic acid esters, theaflavins, and polyphenols), 3% amino acids, enzymes, minerals, and volatile compounds [10]. However, they can vary widely due to differences in the soil quality, planting and climate conditions, and geographical region, as well as the processing methods [11].
(ii) Zanthoxylum limonella Alston or Ma-khwaen is also one of the medicinal plants used in Thai traditional medicine. It has been reported that spice essential oils containing aromatic organic compounds possess cytotoxic and anti-inflammatory effects [12]. Moreover, several studies showed that Z. limonella oil composed of three main chemical compounds, including limonene (31.09%), terpinen-4-ol (13.94%), and (+)-sabinene (9.13%), possesses biological activities with antimicrobial properties [13,14,15,16]. Another study showed the efficacy of alcohol-free mouthwash containing Z. limonella oil on dental biofilm, and it reduced the inflammation caused by gingivitis [17].
(iii) Acorus calamus L. or sweet flag belongs to the Acoraceae family and is a well-known traditional medicinal plant. Studies of the key bioactive compounds of A. calamus have revealed phenyl propanoids, alkaloids, monoterpenes, sesquiterpenes, flavonoids, polyphenols, and α-, and β-asarone as the major components in the extracts of different plant parts and essential oils [18,19,20]. Susanah et al. reported that the total phenolic and flavonoid contents in the ethanol extract of A. calamus rhizome totaled 23.98 mg GAE/g and 1.90 mg QE/g of the dry rhizome extract, respectively [21]. The bioactivity investigations indicated that it possesses potential for cardiovascular prevention and anti-cancer and antimicrobial activities [22,23,24,25,26,27]. It has been demonstrated that the alcohol extract of A. calamus exhibited strong anti-methicillin-resistant Staphylococcus aureus (MRSA) activity and showed growth inhibition against Escherichia coli [23,28].
(iv) Stevia rebaudiana Bertoni, a natural sweetener, is a brunched bushy shrub of the Asteraceae family, which is known as stevia or candy leaf, honey leaf, and sweet herb due to its sweet-tasting property. In the food industry, it is one of the most commonly used naturally sourced, zero-calorie sweeteners because of its steviol glycoside content [29]. The findings of some studies indicate that in addition to their sweetness, steviosides and their related compounds, including rebaudioside A and isosteviol, may also offer beneficial effects for human health, including an antibacterial role [2]. Moreover, it has been reported that chitosan-based polyherbal toothpaste, including S. rebaudiana extract, possesses the highest reduction rate of dental pathogens by significantly decreasing the plaque index and the bacterial count from 85% to 29% [30].
Staphylococci has been considered as part of the normal oral flora. In this genus, however, Staphylococcus aureus is a well-recognized pathogen associated with a variety of clinical syndromes. It is a transient and persistent part of resident flora, and approximately 30% of healthy people carry this bacterium [31]. The human anterior nares and throat are considered as a carriage site, which is the primary colonization site of S. aureus [31,32,33]. Oral host defenses and good oral health care play roles in controlling the overgrowth of bacteria, including S. aureus. Nevertheless, in the case of poor oral hygiene, the emergence of S. aureus as a cause of oral infections, such as tooth decay and gum disease, has been reported worldwide. It has become an increasing health care problem. Furthermore, the treatment options for bacterial infection are limited, complicated, and high-priced.
This study provides supporting scientific data for the use of natural plant extracts. Therefore, studying these Thai highlands plants and their biological activities could be beneficial for consumers who are looking for natural plant or organic products. As a result, plant extract oral care products could be employed for complete oral hygiene.
2. Materials and Methods
2.1. Plant Materials
The natural plant parts used were the fruit, stems, and leaves, according to their traditional use. All the plant samples were collected at the highland development project using the royal project system, Chiang Mai province, Thailand. Camellia sinensis var. assamica leaves were collected from Pang Ma-Oh village, Mae-Na sub-district, Chiang Dao district, Chiang Mai, Thailand. Zanthozylum limonella Alston fruits were collected from Papae sub-district, Mae Taeng district, Chiang Mai, Thailand. Acorus calamus L. stems and leaves were collected from Ban Huai Pong Pattana, Mae Thalop, Chai Prakarn district, Chiang Mai, Thailand. Stevia rebaudiana Bertoni leaves were collected from Baan Fah Suay, Chiang Dao district, Chiang Mai, Thailand. These plant samples were authenticated by the Herbarium of the Faculty of Pharmacy, Chiang Mai University, Thailand, and the voucher specimens’ numbers are shown in Table 1.

Table 1.
Herbarium vouchers of medicinal plants used in this study.
2.2. Preparation of Plant Extracts
2.2.1. Camellia sinensis var. assamica
C. sinensis var. assamica extract was prepared via a maceration method. Briefly, the leaves were dried at 50 °C for 8 h. Dried leaves were blended into a coarse powder to increase the surface area for the extraction process. One hundred grams of coarse powdered leaves were soaked with 1500 mL of 95% v/v ethanol for 72 h in an enclosed glass jar. The content was stirred periodically to ensure complete extraction. The process was repeated 3 times with the same solvent and pooled. The extract was then added with 10% activated charcoal and stirred using a magnetic stirrer for 1 min to remove the color. After that, the extract was further filtering through a filter paper (Whatman No.1, GE Healthcare, Buckinghamshire, UK) to remove the suspended solid particles. The solvents were separated from the extract with a rotary evaporator (Laborota 4010 digital, Heidolph, Schwabach, Germany) at 50 °C under vacuum pressure. The obtained crude extract was weighed, and the extraction yield (%) was calculated. The extract was stored at 4 °C in a tight container for further use.
2.2.2. Zanthozylum limonella Alston
Dried fruits of Z. limonella Alston were extracted via the hydrodistillation method. Briefly, 1000 g of dried fruits was cleaned, crushed, and added into a round-bottom flask. Water was added to the dried fruits at a 2:1 ratio and brought to boil on a heating mantle. Due to the influence of hot water and steam, the vapor mixture of water and oil was condensed by cooling with water. The extraction was completed in 5 h and was then transferred into a separator to separate essential oil from water. The obtained essential oil was dried with anhydrous sodium sulfate to remove residual water in the mixture. The yield of essential oil (%) was calculated as the volume of essential oil divided by the weight of dried fruits. The essential oil was stored at 4 °C in a tight container for further use.
2.2.3. Acorus calamus L.
A. calamus L. was extracted via the maceration method. Briefly, the rhizomes and leaves were cleaned and washed with distilled water, cut into small pieces, and allowed to air dry at 50 °C in a hot air oven for 8 h. A hundred grams of dried rhizomes and leaves were soaked with 1500 mL of 95% v/v ethanol for 72 h. The extract was then filtered through a filter paper (Whatman No.1, GE Healthcare, Buckinghamshire, UK), and the solvent was evaporated at 50 °C in a rotary evaporator under vacuum pressure. The obtained crude extract was weighed, and the extraction yield (%) was calculated. The extract was maintained in a tight container at 4 °C for further use.
2.3. Phytochemical Analysis
2.3.1. Screening of Phenolics
The total phenolic content of each traditional plant extract was measured using the Folin–Ciocalteu method [34]. Gallic acid was utilized as standard for calibration curves. In brief, 150 μL of each extract dissolved in distilled water was mixed with 50 μL of 2 N Folin–Ciocalteu reagent for 5 min. Then, 800 μL of 5% Sodium carbonate was added and further incubated at room temperature for 1 h in the dark. The absorbance of the reaction was measured with a UV–Vis spectrophotometer at 725 nm. The total phenolic content was calculated and is represented as milligram Gallic acid equivalent per gram extract (mg GAE/g extract) [35]. The experiments were carried out in triplicate.
2.3.2. Screening of Flavonoids
The total flavonoid content of all extracts was determined. Briefly, 0.5 mL of each extract was mixed with 1.5 mL of 95% ethanol, and then mixed with 100 μL of 10% (w/v) Aluminum chloride and 100 μL of 0.1 mM potassium acetate solution. After incubation at room temperature for 30 min, absorbance measurements were taken at a wavelength of 415 nm with a microplate reader. The total flavonoid content was calculated based on a standard curve of quercetin and reported as milligram Quercetin equivalent per gram of extract (mg QE/g extract) [35].
2.4. Biological Activity of Plant Extracts
2.4.1. Preparation of Inoculum
The antibacterial properties of plant extracts were tested against Gram-positive bacteria Staphylococcus aureus ATCC 25923 procured from the American Type Culture Collection. The bacteria were pre-cultured in Mueller–Hinton broth (MHB) overnight in a rotary shaker at 37 °C. Subsequently, bacterial cells were adjusted at a concentration of 108 CFU/mL using 0.5 McFarland standard, giving a final inoculum of 1.5 × 108 CFU/mL.
2.4.2. Anti-Bacterial Activity
Antimicrobial Screening
The agar well diffusion method was used to screen the antibacterial activity of different plant extracts [36]. One mL of fresh bacterial culture was pipetted in the center of sterile Mueller–Hinton Agar (MHA) plate. Wells were made using a sterile cork borer (6 mm in diameter) in agar plates containing inoculum. Then, each well was filled with 100 μL extracts from different plant extracts, positive control (Gentamicin 1 mg/mL) and negative/solvent control (vehicle A: 83% ethanol, 8.5% propylene glycol, and 8.5% glycerin; vehicle B: 50% ethanol and 50% polyethylene glycol (PEG) 400), respectively. The concentration of extracts (10% w/v) in vehicle solution was selected based on our pre-experiments and the existing literature. It was allowed to diffuse from the well into the agar for 30 min at room temperature and incubated for 18–24 h at 37 °C. After incubation, the antimicrobial activity of the tested extracts was observed by measuring the halo of inhibition (the formation of a clear zone around the well) that appeared after the incubation period.
Determination of Minimum Inhibitory Concentrations (MIC) and Minimum Bactericidal Concentration (MBC) of the Plant Extracts
All plant extracts demonstrated antimicrobial activity at a concentration of 10% (w/v). The broth microdilution method was used to determine the MIC according to CLSI. Serial two-fold dilutions of extracts (10, 5, 2.5, 1.25, 0.625, and 0.3125%) were prepared directly in a microtiter plate containing MHB to obtain various concentrations. The bacterial inoculum was added to give a final concentration of 108 CFU/mL, 0.5 McFarland’s standard. The positive control was determined using the well containing Gentamicin as a standard drug and the control well contained only inoculated broth. The plate was covered with a sterile sealer and incubated for 24 h at 37 °C. MIC was considered as the lowest concentration of the extract that completely inhibits bacterial growth. All assays were performed in triplicate. Vehicle A and B were served as a control for the extracts.
MBC was determined via the microtiter broth dilution method [37] and recorded as a lowest extract concentration that killed 99.9% of the bacterial inoculum after 24 h incubation at 37 °C. Briefly, ten microliters were taken from the well obtained from the MIC value and two wells above the MIC value well and spread on the MHA plates. The number of colonies was counted after 18–24 h at 37 °C incubation. The concentration of sample that produces <10 colonies was considered as the MBC value. All assays were performed in triplicate.
2.5. Formulation of the Oral Care Products
2.5.1. Materials
HPMC 4000 was purchased from Dow Chemical Co., Midland, MI, USA. Sorbitol, Glycerin, Propylene glycol, Phenoxyethanol, PEG 400, PEG 40 hydrogenated castor oil, EDTA, Menthol, Methyl Paraben, and Propyl Paraben were purchased from Namsiang Co., Ltd. (Chiang Mai, Thailand). Clove and Peppermint were purchased from Thai-China Flavors and Fragrances Industry (Nonthaburi, Thailand). Ethanol was purchased from the Liquor Distillery organization (Chachoengsao, Thailand).
2.5.2. Oral Ulcers Gel Preparation
Several formulations of oral ulcer gel with C. sinensis var. assamica Z. limonella Alston, A. calamus L., S. rebaudiana Bertoni, and hydroxypropyl methylcellulose (HPMC) were prepared. The final formulations are presented in Table 2. Briefly, the HPMC gel was prepared by dispersing 8 g of HPMC 4000 in the purified water (70 °C) to achieve homogenous dispersion. Then, the remaining amount of chilled water was slowly poured into the mixture and stirred well. After that, the HPMC solution was kept in the refrigerator for 24 h until a homogenous gel formed. A given amount of HPMC 4000 was added to mortar. Herbal extracts and the required quantity of sorbitol, glycerin, propylene glycol, phenoxyethanol, and 12% of menthol in ethanol were mixed separately in a beaker. All ingredients were then added to gel base and further triturated until the desired consistency was achieved.

Table 2.
Composition of oral ulcers gel preparation.
2.5.3. Oral Spray Preparation
Formulations of oral spray containing C. sinensis var. assamica, Z. limonella Alston, A. calamus L. and S. rebaudiana Bertoni were prepared. The final formulations are shown in Table 3. The oral spray solution was prepared via a simple solution method. An appropriate amount of PEG 40 hydrogenated castor oil was dissolved in 95% v/v ethanol. Subsequently, the required quantities of clove oil, peppermint oil, C. sinensis var. assamica, A. calamus L., and S. rebaudiana Bertoni were added into the solution and mixed well (the concentrations of all plants extract diluted in appropriate vehicle considering its MICs). Sorbitol, 5% EDTA, Parabens concentrate, and deionized water were added into the solutions and gently mixed. The obtained solution for oral spray was kept in a light-resistant container.

Table 3.
Composition of the oral spray preparation.
2.6. Evaluation of the Oral Product Formulations
2.6.1. Visual Appearances
The oral ulcer gel and oral spray were examined for their physical properties via the visual inspection of color, clarity, homogeneity, and phase separation.
2.6.2. pH
The pH of the prepared formulations was measured using a universal indicator. The pH strip was dipped into the oral ulcer gel or oral spray solution for 1 min and compared with the pH indicator’s color index. Determinations were carried out in triplicate.
2.7. Stability Testing
2.7.1. Accelerated Stability
The prepared formulations were first packed in glass bottles with samples of 5 g each. The oral ulcer gel and oral spray solution were submitted to accelerated stability tests. The samples were kept at 4 °C, room temperature (25–30 °C), and 45 °C to be characterized by physical appearance (color, odor, and clarity) and pH. The tests were performed for 30 days after the production of the formulation. Each test was conducted in triplicate.
2.7.2. Heating–Cooling Cycle
For the heating–cooling cycle, the samples were kept at temperatures of 4 °C and 45 °C for 6 cycles. In each cycle, the samples remained at a particular temperature for a period of 48 h. Periodically, the samples were removed and characterized by physical appearance and pH. Each test was conducted in triplicate.
2.7.3. Anti-Bacterial Testing of Oral Products
The MICs against S. aureus of oral ulcers gel and oral spray solutions were determined via serial dilution, such as in earlier studies on crude plant extracts. Gentamicin solution (1 mg/mL) was used as a positive control, and a gel-based and oral spray solution specially formulated without crude extracts were used as negative controls.
2.8. Organoleptic Evaluation
2.8.1. Ethics
The satisfaction of healthy volunteers in this study was approved by the Research Ethics Committee Panel 2, Faculty of Medicine, Chiang Mai University, Chiang Mai, Thailand (Study code: MIC-2565-09061). The efficiency evaluations were performed on 40 healthy volunteers (aged 20–80, N = 20 for oral ulcers gel, and N = 20 for oral spray). They were investigated for satisfaction on these 2 oral care products. Before enrolling in the study, each volunteer subject received protocol information that contained research-related information and terms and conditions of the clinical testing, and they signed an informed consent form.
2.8.2. Satisfaction Assessment
Information was collected via a self-administered questionnaire. Volunteers were asked to fill out the questionnaire paper related to their age, gender, and satisfaction with the oral care products. The organoleptic parameters of a group of volunteers aged between 30 and 75 years were recorded, and after informed consent, they were asked to use oral care products in their mouth. All the volunteers applied oral ulcer gel on their oral mucus membrane twice daily. For the oral spray, all the volunteers were applied 2–3 puffs in their mouth twice daily. All the volunteers were asked to rate their level of satisfaction regarding the color, consistency, odor, taste, and demulcent or mucoadhesive properties of the oral care products. The organoleptic parameters were also checked and a five-point rating scale (very satisfied, 5; satisfied, 4; uncertain, 3; dissatisfied, 2; very dissatisfied, 1) was used to evaluate their satisfaction with the oral ulcers gel and oral spray formulations.
2.9. Statistical Analysis
Data are reported as mean and standard deviation (S.D.). Statistical significance was assessed using one-way analysis of variance (ANOVA) using GraphPad Prism (version 8.0, GraphPad Software). The satisfaction assessment data were analyzed by using SPSS statistical software. Frequencies and proportions were calculated. In all cases, a p-value < 0.05 was considered to be significant.
3. Results and Discussion
3.1. Yields of Plant Extracts
Maceration and hydrodistillation were used for the extraction of active compounds from the plants. C. sinensis var. assamica leaves and rhizomes and the leaves of A. calamus were obtained via the maceration method. This process has the purpose of softening and breaking the plant’s cell walls to release soluble phytochemical compounds. C. sinensis var. assamica and A. calamus extracts showed a yellow-to-brown color, the extract yields were 2.70%, w/w, and 18.50%, w/w, respectively. In addition, a volatile aroma compound from Z. limonella dried fruit was isolated via hydrodistillation. The extraction yield was 4.67%, w/w. The percentage yields and appearances of plant extracts are shown in Table 4.

Table 4.
Yield of crude extracts from plants (means ± SD; n = 3).
3.2. Determination of Chemical Contents in Plant Extracts
The total phenolic content (TPC) and total flavonoid content (TFC) of three plant extracts are shown in Table 5. Among the extracts, C. sinensis var. assamica and Z. limonella produced the highest total phenolic contents, followed by A. calamus. Moreover, C. sinensis var. assamica also showed the highest total flavonoid content, followed by A. calamus, whereas Z. limonella extracts had the lowest total flavonoid content.

Table 5.
Phytochemical screening of plant extracts.
The results agreed well with a previous study, which reported that C. sinensis var. assamica extracts had the highest of both the total phenolic (10–50 mg GAE/g extract) and total flavonoids contents (3–10 mg QE/g extract). They contain several secondary metabolites, such as flavan-3-ols, phenolic acids, and flavonols [5,38]. As it is known, the phytochemical composition of plant extracts exhibit their various medicinal importance and pharmacological advantages. However, the variation in polyphenol contents in the natural plant extracts might occur due to several factors, such as the plant parts, varieties, the age of the harvested plants, climate, post-harvest processing, and methods of extraction.
3.3. Inhibitory Activity of Plant Extracts against S. aureus
The inhibition against S. aureus plant extracts was comparable to that of 1 mg/mL Gentamicin, a broad-spectrum, beta-lactam, naturally occurring penicillin antibiotic. The results noted that all the extracts possessed antibacterial activity by representing inhibition halos (Table 6). A small inhibition zone was found in the vehicle controls. However, 10% of C. sinensis var. assamica, Z. limonella, and A. calamus showed superior bactericidal activities against S. aureus. In relation to the minimum inhibitory concentrations, all the plant extracts showed growth inhibition (Table 7).

Table 6.
Zone diameter interpreting of plant extracts susceptibility against S. aureus via the Disk Diffusion method.

Table 7.
MICs (%) and MBCs (%) of natural plants extract against microorganisms related to oral health, S. aureus.
According to antimicrobial activity, C. sinensis var. assamica is related to the types of phenolic compounds which were present in the extract, such as flavon-3-ols and flavonols [39]. Previous studies have shown that these two compounds contribute to activity against microorganisms, such as the antibiofilm activity against Enterococcus faecalis ATCC 25212 and 19433 and Staphylococcus aureus ATCC 25923 [39,40]. In addition, synergistic interactions may act to provide greater antimicrobial protection from the combination of Z. limonella and A. calamus, which possess a strong and wide range of antimicrobial activities [41,42]. Limonene is one of the most well-studied monoterpenes related to the biological effects found in Z. limonella oil (30–40%), and it displays a variety of biological functions, especially as a potential antimicrobial agent with very minor adverse effects [13,43,44]. Furthermore, the presence of a phenolic compound in A. calamus might possibly be involved in this synergistic property. β-asarone (2, 4, 5-trimethoxy-(Z)-1-propenylbenzene) is one of the main active ingredients of the traditional medicinal herb A. calamus and exerts multiple pharmacological properties, including an antibacterial property [45,46,47,48]. Therefore, these results emphasize that all three plant extracts could be made available for product ingredients in the prevention or treatment of S. aureus infection and related disorders.
3.4. Evaluation of Oral Care Products
3.4.1. Characteristic of Oral Care Products
The oral ulcer gel (OG-9) and oral spray (MS-10) were prepared as shown in Figure 1 and assessed regarding their physical appearance, pH, and stability. OG-9 was prepared using hydroxypropyl methyl cellulose (HPMC) as a mucoadhesive gel. These polymers are water-soluble and useful in pharmaceutical industries [49]. Generally, water-based gels are easily microbial contaminated; hence, the use of a suitable preservative decreases the chance of microbial growth and a change in the formulation properties. In this study, phenoxyethanol was applied as a preservative agent to decrease the chance of contamination with the oral ulcer gel. OG-9 has suitable uniformity and physical properties. It has a smooth texture, light-brown color, a characteristic odor of mixed essential oils, and undergoes no phase separation. The pH value of this gel was found to be 5.0. The OG-9 characteristics remained the same throughout the study. There was no difference in the physical appearance and pH of OG-9 before and after the heating–cooling cycle, as shown in Table 8. The pH of the formulation must remain constant while it is being stored because changes in the pH can represent problems like microbial growth, ingredient incompatibilities, or ingredient decomposition [50]. Hence, the constant in physical appearance and pH after being freshly prepared and after the heating–cooling cycle indicates the stability of this formulation.
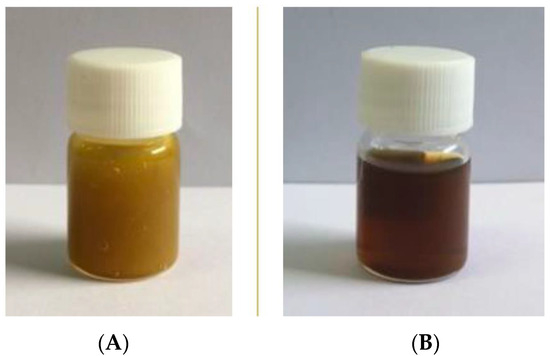
Figure 1.
Plant-based oral care products. (A) Oral ulcer gel (OG-9) and (B) oral spray (MS-10).

Table 8.
The stability testing results of oral ulcer gel and oral spray.
MS-10 was prepared via a simple solution method. The oral spray has a dark-brown solution, a characteristic odor of mixed essential oils, and undergoes no phase separation. The pH value of this spray was found to be 5.0. The MS-10 characteristics remained the same throughout the study. After the heating–cooling cycle, MS-10 showed no change in physical appearance and pH compared to those of the freshly prepared preparation. These results indicate that the oral spray formulation is stable.
3.4.2. Anti-Bacterial Activity of Oral Care Products
The effects of both the oral ulcer gel and oral spray were determined in terms of antibacterial activity as the values of MICs and MBCs. The results showed that these two oral care products still have a larger influence on the growth of S. aureus, as shown in Table 9.

Table 9.
MICs (%) and MBCs (%) of oral ulcer gel and oral spray against S. aureus.
3.5. Determination of Volunteer’s Satisfaction
Forty volunteers constituted the study sample, and questionnaires were distributed to 20 for the oral ulcer gel and 20 for oral spray. The ages of the respondents ranged from 24 to 67 years for the oral ulcer gel and from 30 to 74 years for the oral spray.
Healthy volunteers were asked to fill in a questionnaire after using the oral care products two times. The satisfaction level was determined using a 5-point scale, in which the point value represented the volunteers’ feelings about how well the product worked, from very satisfied (5) to very dissatisfied (1). The results revealed that the volunteers were satisfied with the oral ulcer gel, with the responses mostly being between uncertain and very satisfied for all the areas measured, as shown in Figure 2A. The most satisfying properties of the oral ulcer gel OG-9 were the color and the odor of the gel, which had means of 4.38 and 4.16, respectively, whereas the lowest score of the oral ulcer gel was the taste, with a mean of 3.55. Moreover, the results of the oral spray were also satisfying, with most responses being between uncertain and very satisfying for all the areas measured, as shown in Figure 2B. The most satisfying properties of the oral spray MS-10 were that it is an oral demulcent and the satisfactory effect of the spray, which had an equal mean of 4.25, while the lowest score of the spray was given for the color, with a mean of 3.18. Additionally, none of the healthy volunteers experienced oral or mouth irritation and had no allergic reactions during the test period.
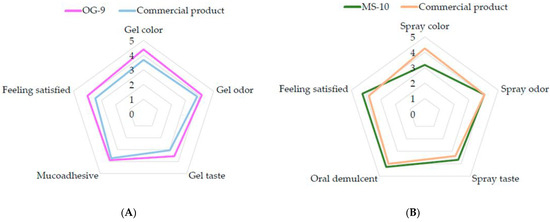
Figure 2.
The radar graph demonstrates the satisfaction of the volunteers with the (A) oral ulcer gel and (B) oral spray. A five-point rating scale represented: very satisfied, 5; satisfied, 4; uncertain, 3; dissatisfied, 2; and very dissatisfied, 1.
4. Conclusions
In conclusion, the findings from this research highlight that natural plant extracts from the Thai highlands, including C. sinensis var. assamica, Z. limonella, and A. calamus, could potentially be natural ingredients with an antibacterial property in oral care product development. The results reveal that the extract from C. sinensis var. assamica had the highest contents of phenolics and flavonoids, which are responsible for its antibacterial activity. With the formulation of an oral ulcer gel and oral spray, these two products had a stable physical appearance and pH. The products were found to be safe, with no reported irritation in the mouth or throat, and they were also found to be satisfactory to the volunteers. As a result, oral care products containing natural plant extracts from the Thai highlands might be effective alternative products used for the care of teeth and the mouth and could be produced on a large scale in the future. However, it is advised that the chemicals responsible for these antibacterial activities should be studied for further identification.
Author Contributions
Conceptualization, S.C. and P.T.; methodology, S.C. and P.T.; formal analysis, P.T.; investigation, S.C. and P.T.; resources, S.C., K.S. and K.K.; data curation, S.C. and P.T.; writing—original draft preparation, S.C. and P.T.; writing—review and editing, P.T.; funding acquisition, S.C. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by Highland Research and Development Institute (Public Organization).
Institutional Review Board Statement
Institutional Review Board Statement: The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board (or Ethics Committee) of the Research Ethics Committee Panel 2, Faculty of Medicine, Chiang Mai University, Chiang Mai, Thailand (Study code: MIC-2565-09061).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Not applicable.
Acknowledgments
The authors gratefully acknowledge the Highland Research and Development Institute (Public Organization), Department of Microbiology, Faculty of Medicine, Chiang Mai University and Department of pharmaceutical technology and biotechnology, Faculty of Pharmacy, Payap University, Chiang Mai, Thailand. English proof reading was kindly done conducted.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Phumthum, M.; Balslev, H. Anti-infectious plants of the Thai Karen: A meta-analysis. Antibiotics 2020, 9, 298. [Google Scholar] [CrossRef]
- Ferrazzano, G.F.; Cantile, T.; Alcidi, B.; Coda, M.; Ingenito, A.; Zarrelli, A.; Di Fabio, G.; Pollio, A. Is Stevia rebaudiana Bertoni a non cariogenic sweetener? A review. Molecules 2015, 21, 38. [Google Scholar] [CrossRef] [PubMed]
- De La Cruz-Jimenez, L.; Hernandez-Torres, M.A.; Monroy-Garcia, I.N.; Rivas-Morales, C.; Verde-Star, M.J.; Gonzalez-Villasana, V.; Viveros-Valdez, E. Biological activities of seven medicinal plants used in Chiapas, Mexico. Plants 2022, 11, 1790. [Google Scholar] [CrossRef] [PubMed]
- Ekiert, H.M.; Szopa, A. Biological activities of natural products. Molecules 2020, 25, 5769. [Google Scholar] [CrossRef]
- Zhang, L.; Ho, C.T.; Zhou, J.; Santos, J.S.; Armstrong, L.; Granato, D. Chemistry and biological activities of processed Camellia sinensis teas: A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1474–1495. [Google Scholar] [CrossRef] [PubMed]
- Graham, H.N. Green tea composition, consumption, and polyphenol chemistry. Prev. Med. 1992, 21, 334–350. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Yamada, H.; Takuma, N.; Niino, H.; Sagesaka, Y.M. Effects of green tea catechins and theanine on preventing influenza infection among healthcare workers: A randomized controlled trial. BMC Complement. Altern. Med. 2011, 11, 15. [Google Scholar] [CrossRef]
- Mora, A.; Pawa, J.; Chaverri, J.M.; Arias, M.L. Determination of the antimicrobial capacity of green tea (Camellia sinensis) against the potentially pathogenic microorganisms Escherichia coli, Salmonella enterica, Staphylococcus aureus, Listeria monocytogenes, Candida albicans and Aspergillus niger. Arch. Latinoam. Nutr. 2013, 63, 247–253. [Google Scholar]
- Dufresne, C.J.; Farnworth, E.R. A review of latest research findings on the health promotion properties of tea. J. Nutr. Biochem. 2001, 12, 404–421. [Google Scholar] [CrossRef]
- Samanta, S. Potential bioactive components and health promotional benefits of tea (Camellia sinensis). J. Am. Nutr. Assoc. 2022, 41, 65–93. [Google Scholar] [CrossRef]
- Zhang, X.; Dai, C.; You, Y.; He, L.; Chen, T. Tea regimen, a comprehensive assessment of antioxidant and antitumor activities of tea extract produced by Tie Guanyin hybridization. RSC Adv. 2018, 8, 11305–11315. [Google Scholar] [CrossRef] [PubMed]
- Oliver-Bever, B. Medicinal plants in tropical West Africa. III. Anti-infection therapy with higher plants. J. Ethnopharmacol. 1983, 9, 1–83. [Google Scholar] [CrossRef] [PubMed]
- Charoensup, R.; Duangyod, T.; Phuneerub, P.; Singharachai, C. Pharmacognostic specification of Zanthoxylum limonella (Dennst.) Alston: Fruits and seeds in Thailand. J. Adv. Pharm. Technol. Res. 2016, 7, 134–138. [Google Scholar] [CrossRef]
- Diao, W.R.; Hu, Q.P.; Feng, S.S.; Li, W.Q.; Xu, J.G. Chemical composition and antibacterial activity of the essential oil from green huajiao (Zanthoxylum schinifolium) against selected foodborne pathogens. J. Agric. Food Chem. 2013, 61, 6044–6049. [Google Scholar] [CrossRef] [PubMed]
- Sriwichai, T.; Wisetkomolmat, J.; Pusadee, T.; Sringarm, K.; Duangmal, K.; Prasad, S.K.; Chuttong, B.; Sommano, S.R. Aromatic profile variation of essential oil from dried Makwhaen fruit and related species. Plants 2021, 10, 803. [Google Scholar] [CrossRef]
- Itthipanichpong, C.; Ruangrungsi, N.; Pattanaautsahakit, C. Chemical compositions and pharmacological effects of essential oil from the fruit of Zanthoxylum limonella. J. Med. Assoc. Thail. 2002, 85 (Suppl. 1), S344–S354. [Google Scholar]
- Vachirarojpisan, T.; Karnchanarungroj, K.; Posiri, C.; Hongsamsipjet, P.; Charoensup, R.; Worapamorn, W. Efficacy of alcohol-free mouthwash containing essential oil from the fruits of Zanthoxylum limonella Alston on dental biofilm, gingivitis, and Streptococcus mutans controls. Mahidol Dent. J. 2021, 41, 83–90. [Google Scholar]
- Liu, X.C.; Zhou, L.G.; Liu, Z.L.; Du, S.S. Identification of insecticidal constituents of the essential oil of Acorus calamus rhizomes against Liposcelis bostrychophila Badonnel. Molecules 2013, 18, 5684–5696. [Google Scholar] [CrossRef]
- Hao, Z.; Zhang, Y.; Cao, Y.; Sun, Y.; Wang, Y.; Zhang, C.; Liang, D.; Liu, Y.; Feng, W. Chemical constituents from Acorus calamus with potent anti-diabetic and hepatoprotective activities. Fitoterapia 2023, 169, 105591. [Google Scholar] [CrossRef]
- Hao, Z.Y.; Liang, D.; Luo, H.; Liu, Y.F.; Ni, G.; Zhang, Q.J.; Li, L.; Si, Y.K.; Sun, H.; Chen, R.Y.; et al. Bioactive sesquiterpenoids from the rhizomes of Acorus calamus. J. Nat. Prod. 2012, 75, 1083–1089. [Google Scholar] [CrossRef] [PubMed]
- Rita, W.S.; Kawuri, R.; Swantara, I.M.D. Total phenolic and flavonoid contents and antimicrobial activity of Acorus calamus L. rhizome ethanol extract. Res. J. Chem. Environ. 2018, 22, 65–70. [Google Scholar]
- Olas, B.; Brys, M. Is it safe to use Acorus calamus as a source of promising bioactive compounds in prevention and treatment of cardiovascular diseases? Chem. Biol. Interact. 2018, 281, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Sudhakar, C.; Selvam, K.; Govarthanan, M.; Senthilkumar, B.; Sengottaiyan, A.; Stalin, M.; Selvankumar, T. Acorus calamus rhizome extract mediated biosynthesis of silver nanoparticles and their bactericidal activity against human pathogens. J. Genet. Eng. Biotechnol. 2015, 13, 93–99. [Google Scholar] [CrossRef]
- Rahamooz Haghighi, S.; Asadi, M.H.; Akrami, H.; Baghizadeh, A. Anti-carcinogenic and anti-angiogenic properties of the extracts of Acorus calamus on gastric cancer cells. Avicenna J. Phytomed. 2017, 7, 145–156. [Google Scholar]
- Das, B.K.; Swamy, A.V.; Koti, B.C.; Gadad, P.C. Experimental evidence for use of Acorus calamus (asarone) for cancer chemoprevention. Heliyon 2019, 5, e01585. [Google Scholar] [CrossRef] [PubMed]
- Joshi, R.K. Acorus calamus Linn.: Phytoconstituents and bactericidal property. World J. Microbiol. Biotechnol. 2016, 32, 164. [Google Scholar] [CrossRef] [PubMed]
- Parki, A.; Chaubey, P.; Prakash, O.; Kumar, R.; Pant, A.K. Seasonal variation in essential oil compositions and antioxidant properties of Acorus calamus L. accessions. Medicines 2017, 4, 81. [Google Scholar] [CrossRef]
- Aqil, F.; Ahmad, I.; Owais, M. Evaluation of anti-methicillin-resistant Staphylococcus aureus (MRSA) activity and synergy of some bioactive plant extracts. Biotechnol. J. 2006, 1, 1093–1102. [Google Scholar] [CrossRef]
- Saraiva, A.; Carrascosa, C.; Raheem, D.; Ramos, F.; Raposo, A. Natural sweeteners: The relevance of food naturalness for consumers, food security aspects, sustainability and health impacts. Int. J. Environ. Res. Public Health 2020, 17, 6285. [Google Scholar] [CrossRef] [PubMed]
- Mohire, N.C.; Yadav, A.V. Chitosan-based polyherbal toothpaste: As novel oral hygiene product. Indian J. Dent. Res. 2010, 21, 380–384. [Google Scholar] [CrossRef] [PubMed]
- Kluytmans, J.; van Belkum, A.; Verbrugh, H. Nasal carriage of Staphylococcus aureus: Epidemiology, underlying mechanisms, and associated risks. Clin. Microbiol. Rev. 1997, 10, 505–520. [Google Scholar] [CrossRef]
- Mertz, D.; Frei, R.; Periat, N.; Zimmerli, M.; Battegay, M.; Fluckiger, U.; Widmer, A.F. Exclusive Staphylococcus aureus throat carriage: At-risk populations. Arch. Intern. Med. 2009, 169, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Mertz, D.; Frei, R.; Jaussi, B.; Tietz, A.; Stebler, C.; Fluckiger, U.; Widmer, A.F. Throat swabs are necessary to reliably detect carriers of Staphylococcus aureus. Clin. Infect. Dis. 2007, 45, 475–477. [Google Scholar] [CrossRef]
- Sastry, C.S.; Rao, A.R. Application of Folin-Ciocalteu reagent for the spectrophotometric determination of some nonsteroidal antiinflammatory agents. J. Pharmacol. Methods 1988, 19, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, K.; Ghasemi, Y.; Ebrahimzadeh, M.A. Antioxidant activity, phenol and flavonoid contents of 13 citrus species peels and tissues. Pak. J. Pharm. Sci. 2009, 22, 277–281. [Google Scholar]
- Manandhar, S.; Luitel, S.; Dahal, R.K. In vitro antimicrobial activity of some medicinal plants against human pathogenic bacteria. J. Trop. Med. 2019, 2019, 1895340. [Google Scholar] [CrossRef] [PubMed]
- Mudzengi, C.P.; Murwira, A.; Tivapasi, M.; Murungweni, C.; Burumu, J.V.; Halimani, T. Antibacterial activity of aqueous and methanol extracts of selected species used in livestock health management. Pharm. Biol. 2017, 55, 1054–1060. [Google Scholar] [CrossRef]
- de Moura, C.; Kabbas Junior, T.; Pedreira, F.R.O.; Azevedo, L.; Furtado, M.M.; Sant’Ana, A.S.; Franchin, M.; Gonzaga, V.R.; Cui, Y.; Wen, M.; et al. Purple tea (Camellia sinensis var. assamica) leaves as a potential functional ingredient: From extraction of phenolic compounds to cell-based antioxidant/biological activities. Food Chem. Toxicol. 2022, 159, 112668. [Google Scholar] [CrossRef]
- Armstrong, L.; Araujo Vieira do Carmo, M.; Wu, Y.; Antonio Esmerino, L.; Azevedo, L.; Zhang, L.; Granato, D. Optimizing the extraction of bioactive compounds from pu-erh tea (Camellia sinensis var. assamica) and evaluation of antioxidant, cytotoxic, antimicrobial, antihemolytic, and inhibition of alpha-amylase and alpha-glucosidase activities. Food Res. Int. 2020, 137, 109430. [Google Scholar] [CrossRef]
- Reygaert, W.C. The antimicrobial possibilities of green tea. Front. Microbiol. 2014, 5, 434. [Google Scholar] [CrossRef]
- Khruengsai, S.; Sripahco, T.; Pripdeevech, P. Antibacterial activity and synergic effects of the essential oils of Amomum verum Blackw and Zanthoxylum limonella (Dennst.) Alston. Arch. Microbiol. 2023, 205, 102. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.J.; Hwang, K.H.; Park, D.G.; Kim, T.J.; Kim, D.W.; Choi, D.K.; Moon, W.K.; Lee, K.H. Major constituents and antimicrobial activity of Korean herb Acorus calamus. Nat. Prod. Res. 2011, 25, 1278–1281. [Google Scholar] [CrossRef]
- Gupta, A.; Jeyakumar, E.; Lawrence, R. Strategic approach of multifaceted antibacterial mechanism of limonene traced in Escherichia coli. Sci. Rep. 2021, 11, 13816. [Google Scholar] [CrossRef]
- Soonwera, M.; Moungthipmalai, T.; Aungtikun, J.; Sittichok, S. Combinations of plant essential oils and their major compositions inducing mortality and morphological abnormality of Aedes aegypti and Aedes albopictus. Heliyon 2022, 8, e09346. [Google Scholar] [CrossRef]
- Chellian, R.; Pandy, V.; Mohamed, Z. Pharmacology and toxicology of alpha- and beta-Asarone: A review of preclinical evidence. Phytomedicine 2017, 32, 41–58. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wang, J.; Shi, L.; Zhang, W.; Du, X.; Wang, Z.; Zhang, Y. beta-Asarone induces senescence in colorectal cancer cells by inducing lamin B1 expression. Phytomedicine 2013, 20, 512–520. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.L.; Ouyang, C.S.; Lin, L.Z. beta-Asarone suppresses Wnt/beta-catenin signaling to reduce viability, inhibit migration/invasion/adhesion and induce mitochondria-related apoptosis in lung cancer cells. Biomed. Pharmacother. 2018, 106, 821–830. [Google Scholar] [CrossRef]
- Shenvi, S.; Diwakar, L.; Reddy, G.C. Nitro derivatives of naturally occurring beta-Asarone and their anticancer activity. Int. J. Med. Chem. 2014, 2014, 835485. [Google Scholar] [CrossRef] [PubMed]
- Li, C.L.; Martini, L.G.; Ford, J.L.; Roberts, M. The use of hypromellose in oral drug delivery. J. Pharm. Pharmacol. 2005, 57, 533–546. [Google Scholar] [CrossRef]
- Aslani, A.; Zolfaghari, B.; Davoodvandi, F. Design, formulation and evaluation of an oral gel from Punica granatum flower extract for the treatment of recurrent aphthous stomatitis. Adv. Pharm. Bull. 2016, 6, 391–398. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).