Optimization of Batch Crystallization of Magnetic Lysozyme Crystals and Study of the Continuous Crystallization Process
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of PASP-Modified Fe3O4 nanoparticles
2.2.2. Screening of Batch Crystallization Conditions
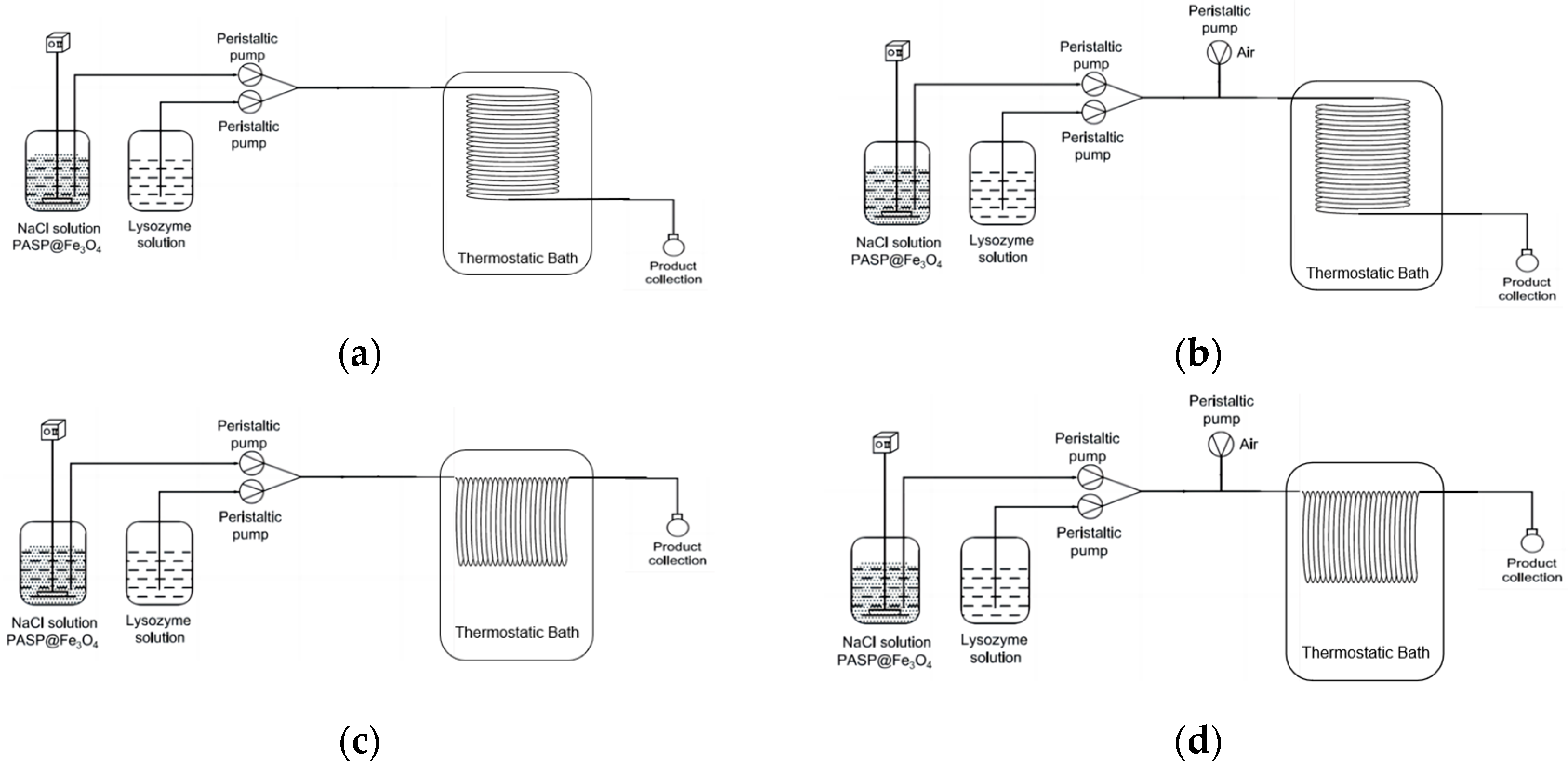
2.2.3. Selection of Continuous Crystallizer
2.2.4. Push Flow Crystallization (PFC)
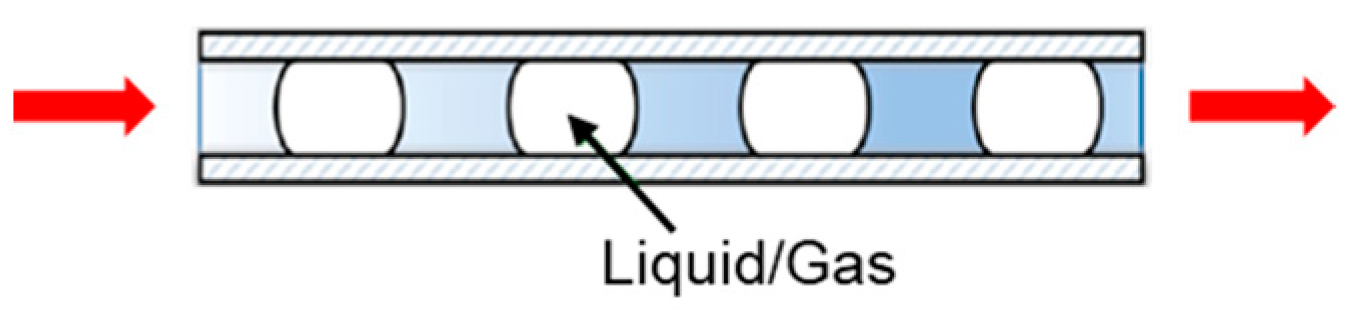
2.2.5. Slug Flow Crystallization (SFC)
3. Results
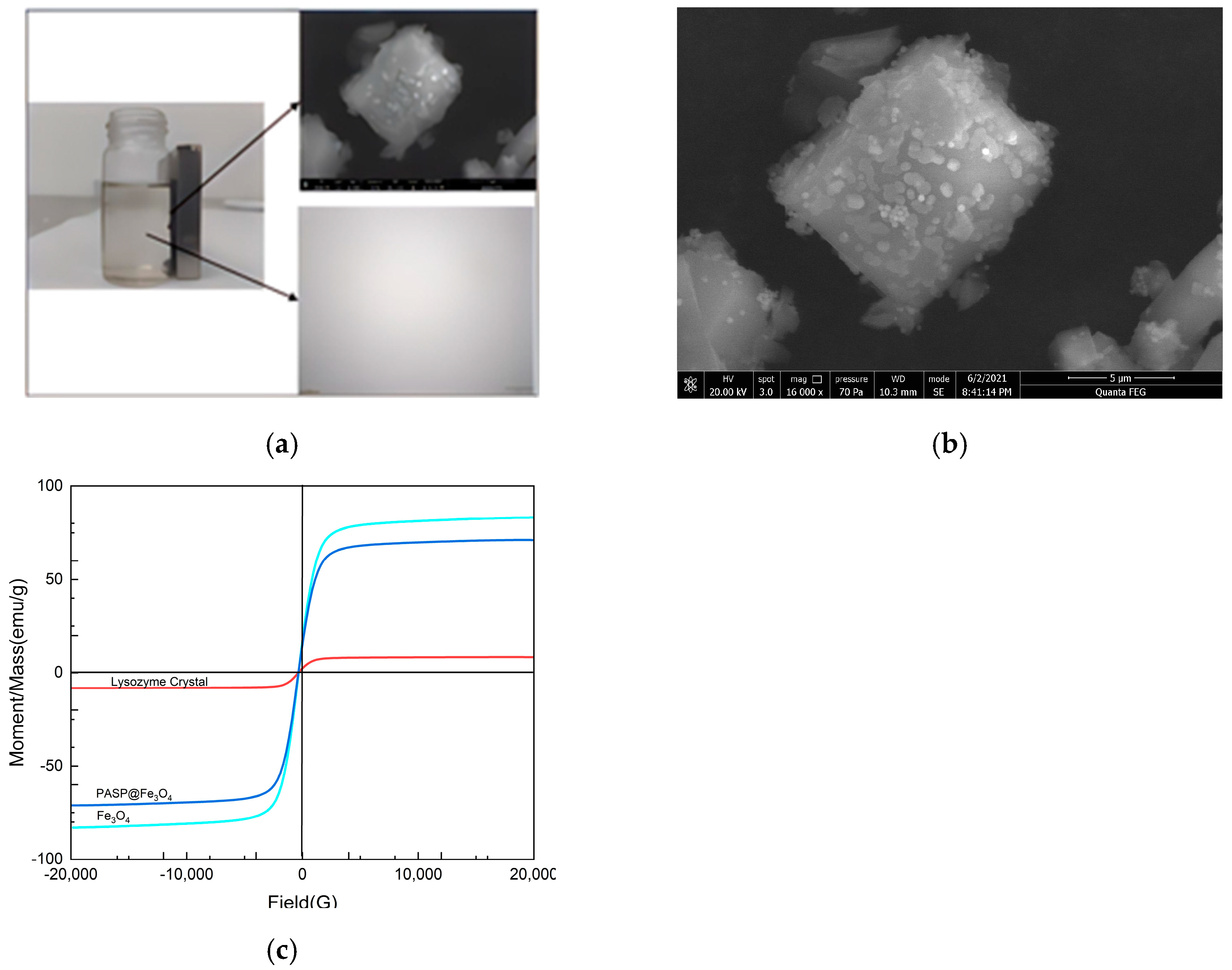
3.1. Characterization of PASP-Modified Fe3O4 Nanoparticles
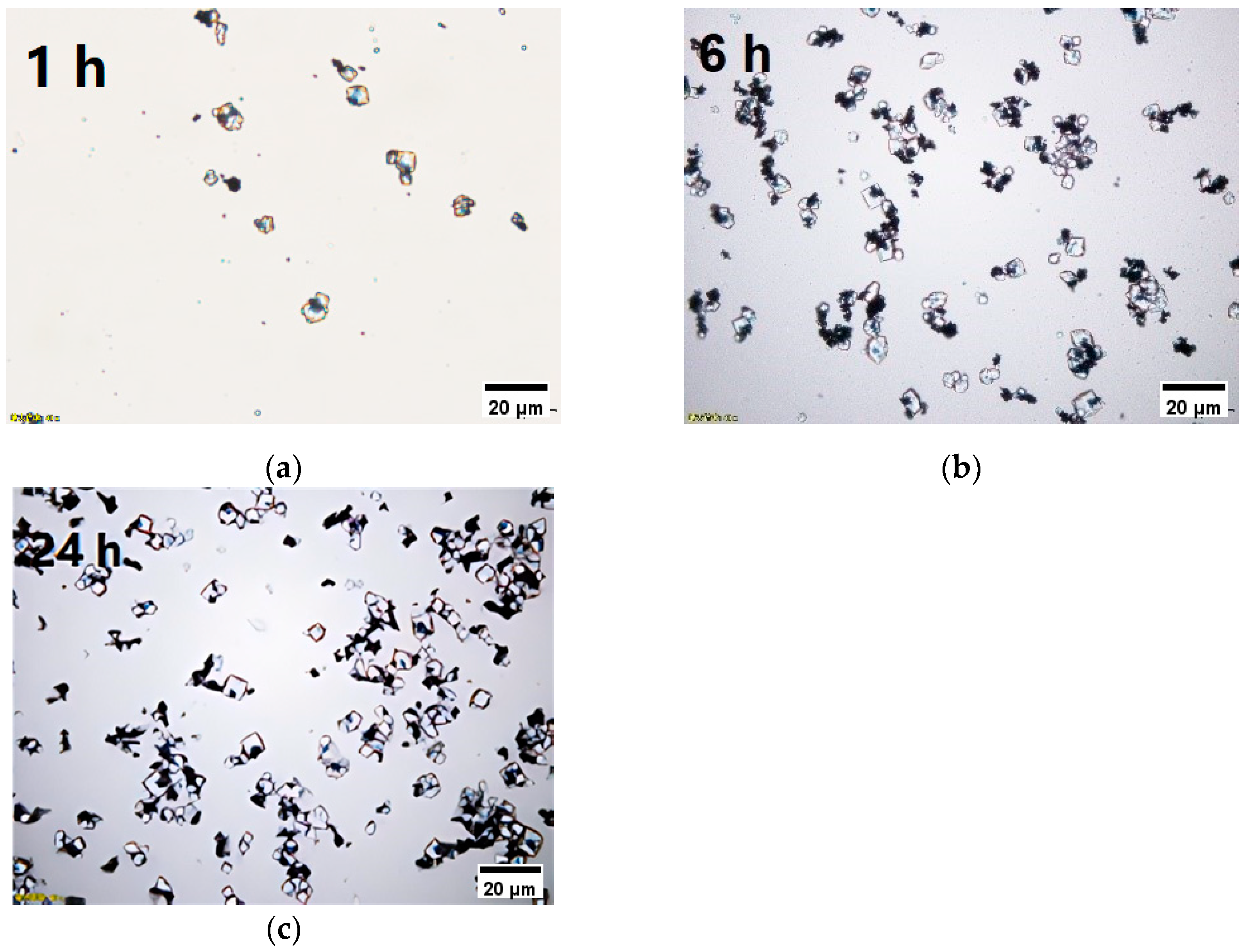
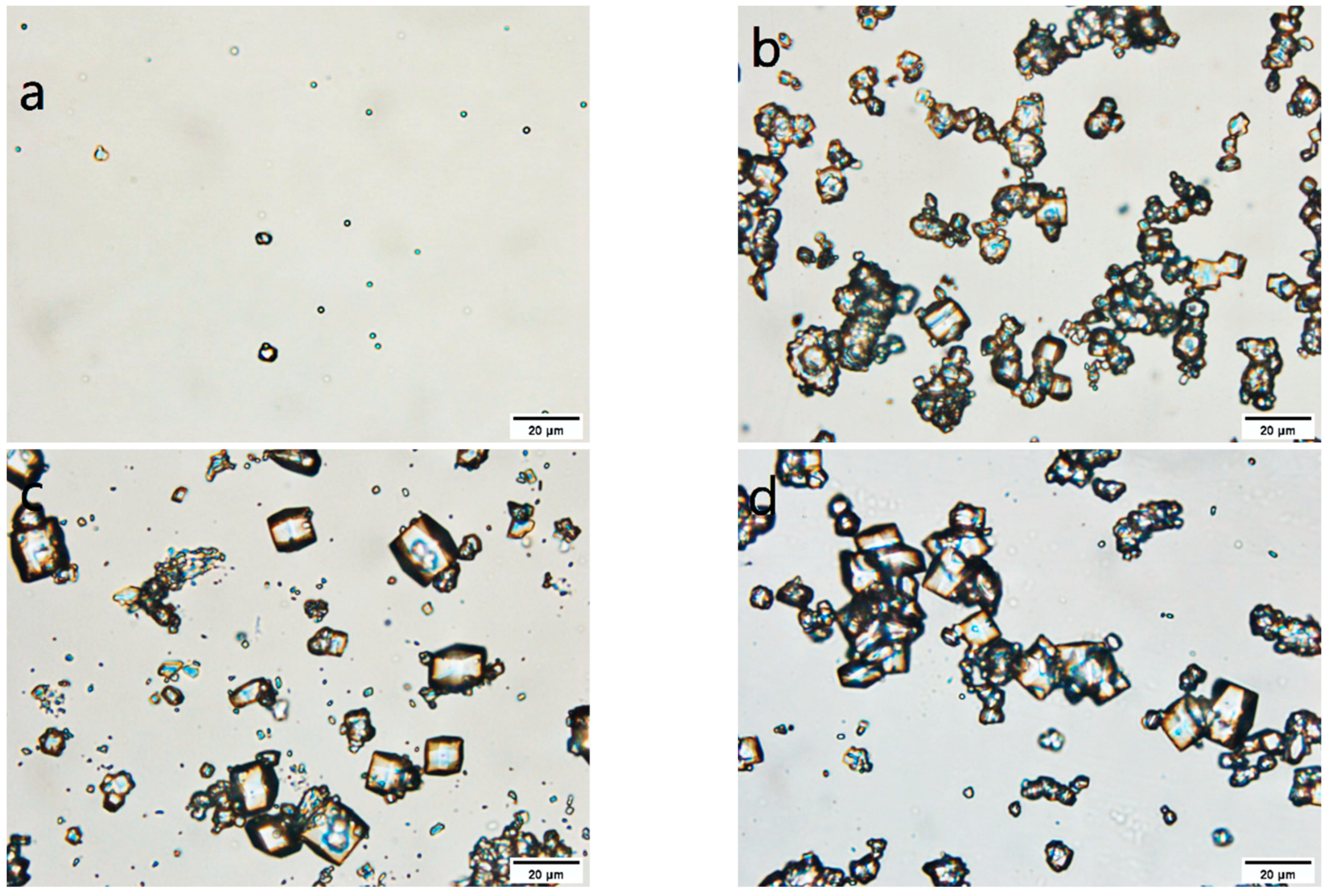
3.2. Screening of Intermittent Crystallization Conditions
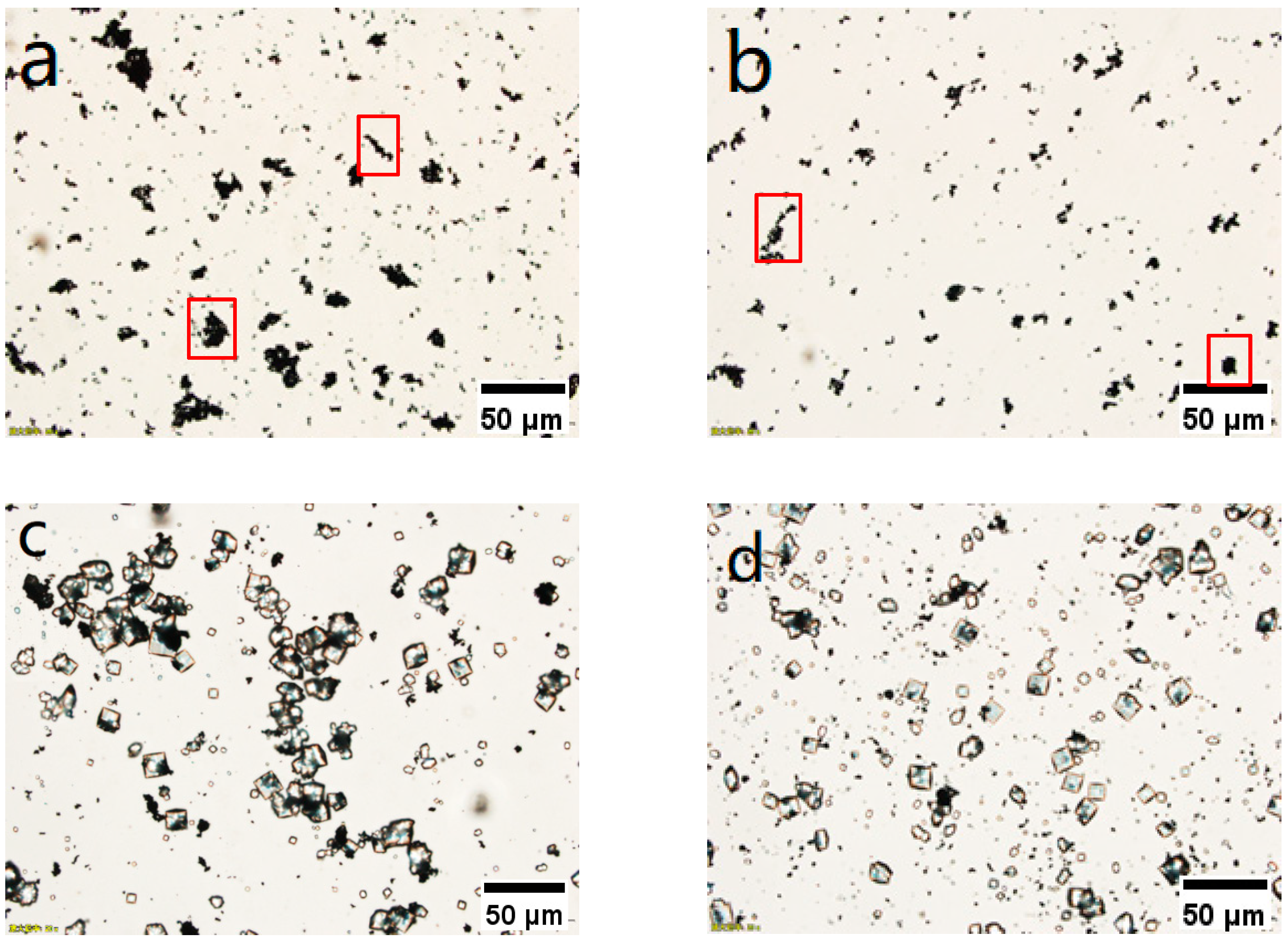
3.3. Selection of Continuous Crystallizer
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Puhl, S.; Meinel, L.; Germershaus, O. Recent advances in crystalline and amorphous particulate protein formulations for controlled delivery. Asian J. Pharm. Sci. 2016, 11, 469–477. [Google Scholar] [CrossRef]
- Zhu, M.M.; Mollet, M.; Hubert, R.S.; Kyung, Y.S.; Zhang, G.G. Industrial production of therapeutic proteins: Cell lines, cell culture, and purification. In Handbook of Industrial Chemistry and Biotechnology; Springer: Berlin/Heidelberg, Germany, 2017; pp. 1639–1669. [Google Scholar]
- Neugebauer, P.; Khinast, J.G. Continuous crystallization of proteins in a tubular plug-flow crystallizer. Cryst. Growth Des. 2015, 15, 1089–1095. [Google Scholar] [CrossRef]
- Dos Santos, R.; Carvalho, A.L.; Roque, A.C.A. Renaissance of protein crystallization and precipitation in biopharmaceuticals purification. Biotechnol. Adv. 2017, 35, 41–50. [Google Scholar] [CrossRef]
- Dafnomilis, A.; Diab, S.; Rodman, A.D.; Boudouvis, A.G.; Gerogiorgis, D.I. Multiobjective Dynamic Optimization of Ampicillin Batch Crystallization: Sensitivity Analysis of Attainable Performance vs Product Quality Constraints. Ind. Eng. Chem. Res. 2019, 58, 18756–18771. [Google Scholar] [CrossRef]
- Barros Groβ, M.; Kind, M. Comparative Study on Seeded and Unseeded Bulk Evaporative Batch Crystallization of Tetragonal Lysozyme. Cryst. Growth Des. 2017, 17, 3491–3501. [Google Scholar] [CrossRef]
- Hemalatha, K.; Nagveni, P.; Kumar, P.N.; Rani, K.Y. Multiobjective optimization and experimental validation for batch cooling crystallization of citric acid anhydrate. Comput. Chem. Eng. 2018, 112, 292–303. [Google Scholar] [CrossRef]
- Jiang, M.; Braiek, M.; Farre, C.; Bonhomme, A.; Chaix, C.; Chateaux, J.; Zhang, A.; Jaffrezic-Renault, N. Effect of perfluorinated-hexamethylene glycol functionalization of gold nanoparticles on the enhancement of the response of an enzymatic conductometric biosensor for urea detection. Curr. Bionanotechnol. 2015, 1, 110–115. [Google Scholar] [CrossRef]
- Hong, M.S.; Severson, K.A.; Jiang, M.; Lu, A.E.; Love, J.C.; Braatz, R.D. Challenges and opportunities in biopharmaceutical manufacturing control. Comput. Chem. Eng. 2018, 110, 106–114. [Google Scholar] [CrossRef]
- Pu, S.; Hadinoto, K. Improving the reproducibility of the size distribution of protein crystals produced in continuous slug flow crystallizer operated at short residence time. Chem. Eng. Sci. 2021, 230, 116181. [Google Scholar] [CrossRef]
- Pu, S.; Hadinoto, K. Continuous crystallization as a downstream processing step of pharmaceutical proteins: A review. Chem. Eng. Res. Des. 2020, 160, 89–104. [Google Scholar] [CrossRef]
- Thomas, K.M.; Kwon, S.; Lakerveld, R. Continuous Protein Crystallization in Mixed-Suspension Mixed-Product-Removal Crystallizers. Cryst. Growth Des. 2021, 21, 757–769. [Google Scholar] [CrossRef]
- Cole, K.P.; Groh, J.M.; Johnson, M.D.; Burcham, C.L.; Campbell, B.M.; Diseroad, W.D.; Heller, M.R.; Howell, J.R.; Kallman, N.J.; Koenig, T.M.; et al. Kilogram-scale prexasertib mono lactate monohydrate synthesis under continuous-flow CGMP conditions. Science 2017, 356, 1144–1150. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Lai TT, C.; Trout, B.L.; Myerson, A.S. Continuous crystallization of cyclosporine: Effect of operating conditions on yield and purity. Cryst. Growth Des. 2017, 17, 1000–1007. [Google Scholar] [CrossRef]
- Wong, S.Y.; Tatusko, A.P.; Trout, B.L.; Myerson, A.S. Development of continuous crystallization processes using a single-stage mixed-suspension, mixed-product removal crystallizer with recycle. Cryst. Growth Des. 2012, 12, 5701–5707. [Google Scholar] [CrossRef]
- Poechlauer, P.; Manley, J.; Broxterman, R.; Gregertsen, B.; Ridemark, M. Continuous processing in the manufacture of active pharmaceutical ingredients and finished dosage forms: An industry perspective. Org. Process Res. Dev. 2012, 16, 1586–1590. [Google Scholar] [CrossRef]
- Peña, R.; Nagy, Z.K. Process intensification through continuous spherical crystallization using a two-stage mixed suspension mixed product removal (MSMPR) system. Cryst. Growth Des. 2015, 15, 4225–4236. [Google Scholar] [CrossRef]
- Yang, X.; Acevedo, D.; Mohammad, A.; Pavurala, N.; Wu, H.; Brayton, A.L.; Shaw, R.A.; Goldman, M.J.; He, F.; Li, S.; et al. Risk considerations on developing a continuous crystallization system for carbamazepine. Org. Process Res. Dev. 2017, 21, 1021–1033. [Google Scholar] [CrossRef]
- Jiang, M.; Braatz, R.D. Designs of continuous-flow pharmaceutical crystallizers: Developments and practice. CrystEngComm 2019, 21, 3534–3551. [Google Scholar] [CrossRef]
- Pu, S.; Hadinoto, K. Comparative evaluations of bulk seeded protein crystallization in batch versus continuous slug flow crystallizers. Chem. Eng. Res. Des. 2021, 171, 139–149. [Google Scholar] [CrossRef]
- Thomas, K.M.; Nyande, B.W.; Lakerveld, R. Design and characterization of Kenics static mixer crystallizers. Chem. Eng. Res. Des. 2022, 179, 549–563. [Google Scholar] [CrossRef]
- Besenhard, M.O.; Neugebauer, P.; Scheibelhofer, O.; Khinast, J.G. Crystal engineering in continuous plug-flow crystallizers. Cryst. Growth Des. 2017, 17, 6432–6444. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Su, M.; Bai, Y. Mechanism of Glycine Crystal Adhesion and Clogging in a Continuous Tubular Crystallizer. Ind. Eng. Chem. Res. 2019, 59, 25–33. [Google Scholar] [CrossRef]
- Zhang, Q.; Su, M.; Yu, L.; Zhang, Y. Self-Assembly of Magnetic Lysozyme Crystals with a Medium of Polymers. Cryst. Growth Des. 2021, 21, 6727–6736. [Google Scholar] [CrossRef]
- Su, M.; Gao, Y. Air–Liquid Segmented Continuous Crystallization Process Optimization of the Flow Field, Growth Rate, and Size Distribution of Crystals. Ind. Eng. Chem. Res. 2018, 57, 3781–3791. [Google Scholar] [CrossRef]
- Prathibha, V.; Karthika, S.; Cyriac, J.; Sudarasanakumar, C.; Unnikrishnan, N.V. Synthesis of pure anatase TiO2 nanocrystals in SiO2 host and the determination of crystal planes by ImageJ. Mater. Lett. 2011, 65, 664–666. [Google Scholar] [CrossRef]
- Gavira, J.A.; García-Ruiz, J.M. Effects of a Magnetic Field on Lysozyme Crystal Nucleation and Growth in a Diffusive Environment. Cryst. Growth Des. 2009, 9, 2610–2615. [Google Scholar] [CrossRef]



| NaCl (%) | Lysozyme (mg/mL) | S | T/min | Yield (%) |
|---|---|---|---|---|
| 5.84 | 18.75 | 10.31 | 360 | 57.13 ± 1.13 |
| 5.84 | 25.00 | 13.74 | 40 | 74.20 ± 1.41 |
| 5.84 | 30.00 | 16.48 | 60 | 79.86 ± 2.03 |
| 5.00 | 30.00 | 15.31 | 90 | 58.62 ± 3.49 |
| 4.00 | 30.00 | 9.81 | 180 | 35.61 ± 2.82 |
| Ω (%) | Yield (%) | Ms (emu/g) | Mr (emu/g) |
|---|---|---|---|
| 10 | 76.18 ± 3.17 | 7.79 ± 0.31 | 2.21 ± 0.10 |
| 15 | 78.33 ± 4.70 | 9.89 ± 0.44 | 2.78 ± 0.13 |
| 20 | 79.69 ± 2.90 | 11.01 ± 0.49 | 3.09 ± 0.14 |
| No | mL/min | mL/min | RSD | |
|---|---|---|---|---|
| 1 | 1.79 | 0.91 | 1.96 | 9.64 |
| 2 | 1.79 | 1.28 | 1.40 | 8.05 |
| 3 | 1.79 | 1.64 | 1.09 | 3.17 |
| 4 | 1.79 | 2.00 | 0.89 | 6.23 |
| 5 | 1.79 | 2.37 | 0.75 | 3.83 |
| 6 | 3.22 | 1.82 | 1.77 | 5.05 |
| 7 | 3.22 | 2.55 | 1.26 | 4.19 |
| 8 | 3.22 | 3.28 | 0.98 | 3.30 |
| 9 | 1.07 | 0.91 | 1.17 | 2.43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, S.; Hou, L.; Su, M. Optimization of Batch Crystallization of Magnetic Lysozyme Crystals and Study of the Continuous Crystallization Process. Processes 2023, 11, 2644. https://doi.org/10.3390/pr11092644
Yang S, Hou L, Su M. Optimization of Batch Crystallization of Magnetic Lysozyme Crystals and Study of the Continuous Crystallization Process. Processes. 2023; 11(9):2644. https://doi.org/10.3390/pr11092644
Chicago/Turabian StyleYang, Shanshan, Lixia Hou, and Min Su. 2023. "Optimization of Batch Crystallization of Magnetic Lysozyme Crystals and Study of the Continuous Crystallization Process" Processes 11, no. 9: 2644. https://doi.org/10.3390/pr11092644
APA StyleYang, S., Hou, L., & Su, M. (2023). Optimization of Batch Crystallization of Magnetic Lysozyme Crystals and Study of the Continuous Crystallization Process. Processes, 11(9), 2644. https://doi.org/10.3390/pr11092644





