The Improved Cytotoxic Capacity of Functionalized Nanodiamonds with Metformin in Breast and Ovarian Cancer Cell Lines
Abstract
:1. Introduction
2. Materials and Methods
2.1. Regents
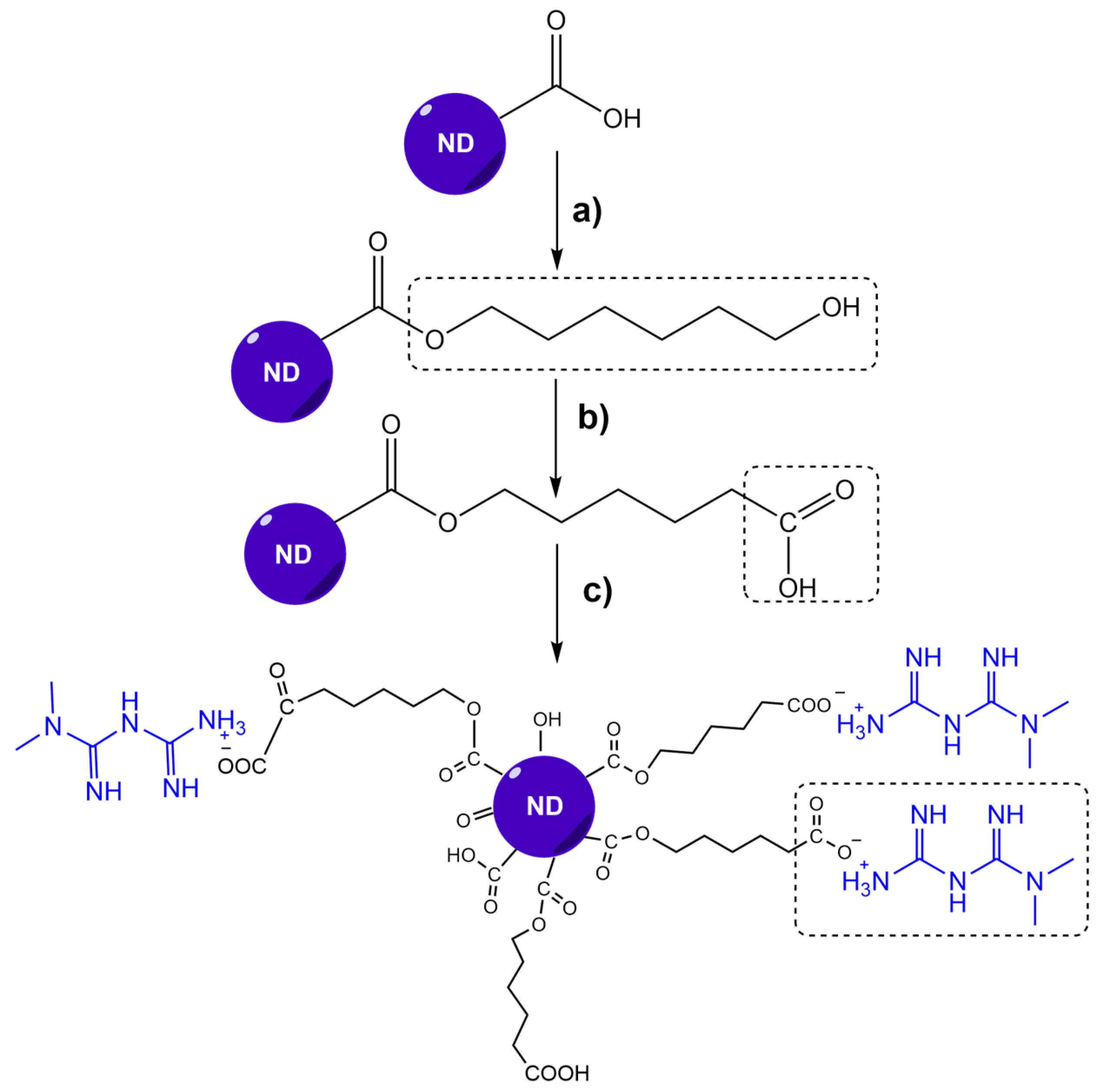
2.2. ND-MET Synthesis
2.2.1. Metformin Hydrochloride Extraction (MET*HCl)
2.2.2. Obtention of ND-MET Complex
2.3. Characterization
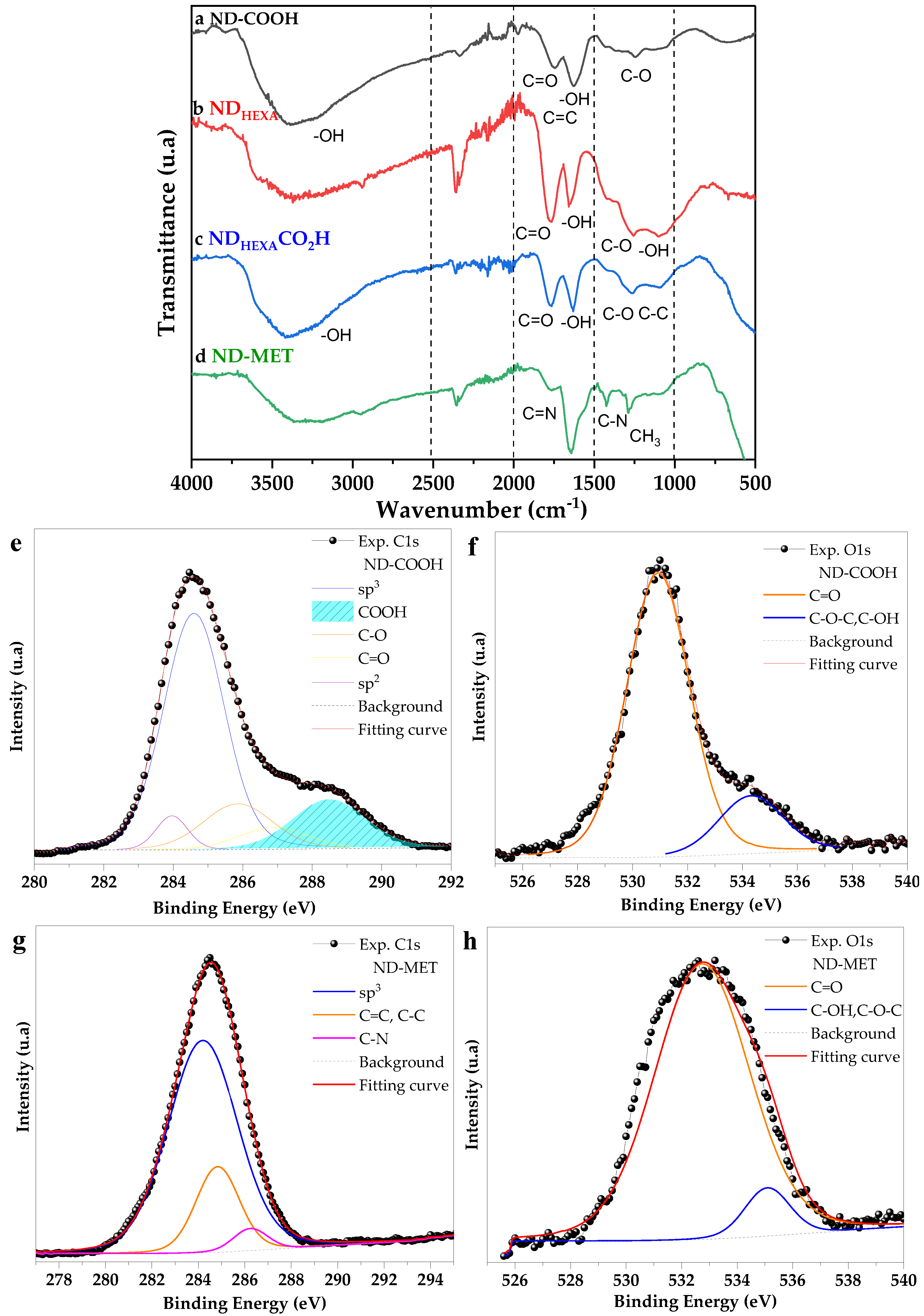
2.3.1. Fourier Transform Infrared Spectroscopy
2.3.2. X-ray Photoelectron Spectroscopy
2.3.3. Bohem Titration
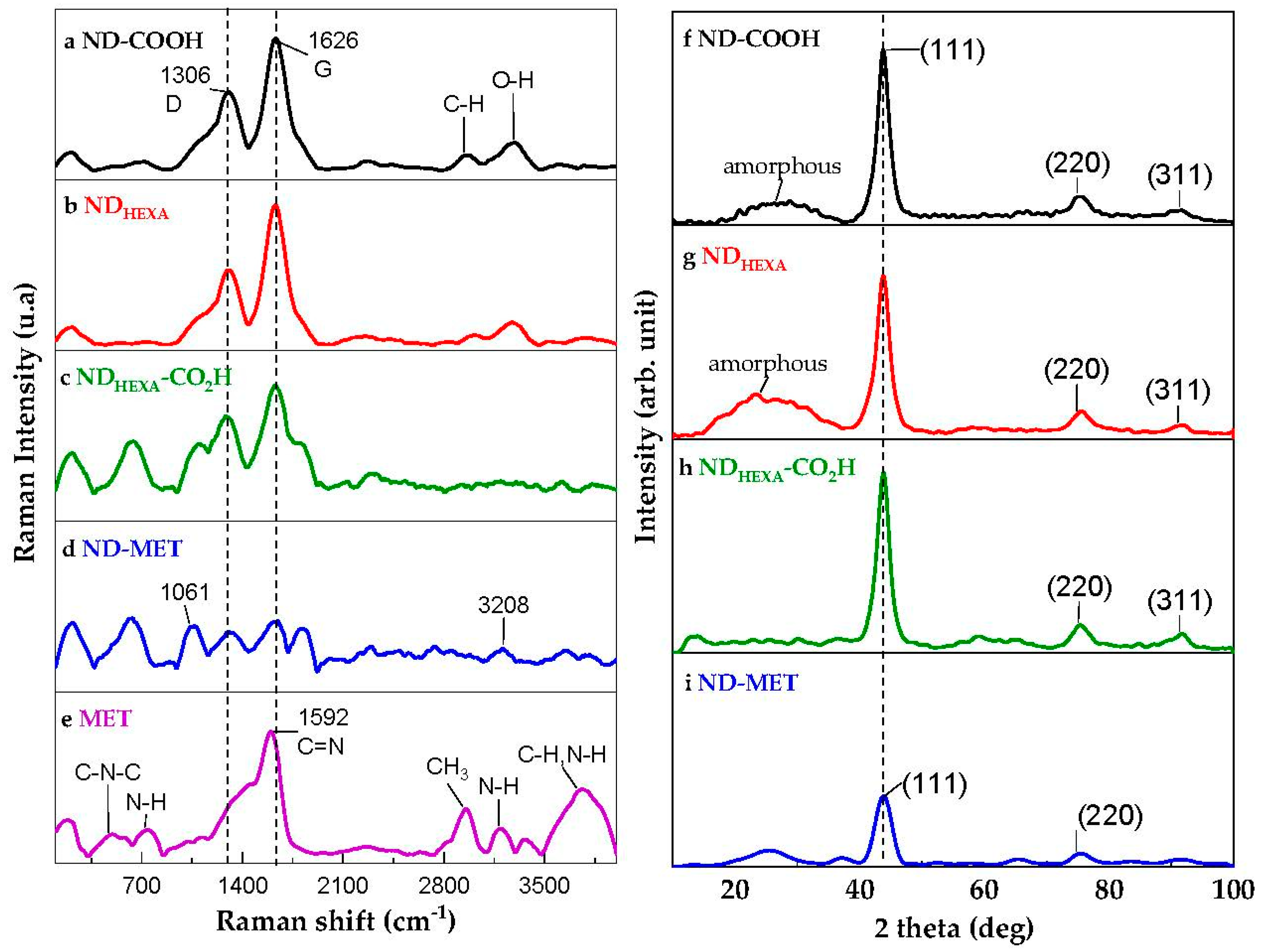
2.3.4. RAMAN Spectroscopy
2.3.5. X-ray Diffraction Analysis
2.3.6. Transmission Electron Microscopy
2.3.7. Dynamic Light Scattering
2.4. Biological Assays
2.4.1. Cell Culture
2.4.2. Cytotoxicity Assay
3. Results and Discussion
3.1. ND-MET Characterization
3.2. Cytotoxicity Evaluation of ND-MET in Breast and Ovarian Cancer Cell Lines
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, Y.; Li, J.; Li, W.; Zhang, Y.; Yang, X.; Chen, N.; Sun, Y.; Zhao, Y.; Fan, C.; Huang, Q. The Biocompatibility of Nanodiamonds and Their Application in Drug Delivery Systems. Theranostics 2012, 2, 302–312. [Google Scholar] [CrossRef]
- Gvozdev, D.A.; Ramonova, A.A.; Slonimskiy, Y.B.; Gudkova, V.R.; Nikelshparg, E.I.; Moisenovich, A.M.; Moisenovich, M.M.; Zaitsev, A.V.; Olshevskaya, V.A.; Paschenko, V.Z.; et al. Nanodiamonds as a Platform for Targeted Delivery of Chlorin-Based Photosensitizers to Cancer Cells. Diam. Relat. Mater. 2021, 120, 108676. [Google Scholar] [CrossRef]
- Chow, E.K.; Zhang, X.-Q.; Chen, M.; Lam, R.; Robinson, E.; Huang, H.; Schaffer, D.; Osawa, E.; Goga, A.; Ho, D. Nanodiamond Therapeutic Delivery Agents Mediate Enhanced Chemoresistant Tumor Treatment. Sci. Transl. Med. 2011, 3, 73. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Chow, E.K.-H. Biomedical Applications of Nanodiamonds: From Drug-Delivery to Diagnostics. SLAS Technol. 2023, 4, S2472. [Google Scholar] [CrossRef] [PubMed]
- Perevedentseva, E.; Hong, S.-F.; Huang, K.-J.; Chiang, I.-T.; Lee, C.-Y.; Tseng, Y.-T.; Cheng, C.-L. Nanodiamond Internalization in Cells and the Cell Uptake Mechanism. J. Nanoparticle Res. 2013, 15, 1834. [Google Scholar] [CrossRef]
- Chipaux, M.; van der Laan, K.J.; Hemelaar, S.R.; Hasani, M.; Zheng, T.; Schirhagl, R. Nanodiamonds and Their Applications in Cells. Small 2018, 14, 1704263. [Google Scholar] [CrossRef]
- Dai, L.; Liu, M.; Long, W.; Hu, X.; Ouyang, H.; Feng, Y.; Deng, F.; Wen, Y.; Zhang, X.; Wei, Y. Synthesis of Water Dispersible and Biocompatible Nanodiamond Composite via Photocatalytic Surface Grafting of Zwitterionic Polymers for Intracellular Delivery of DOX. Mater. Today Commun. 2022, 30, 103010. [Google Scholar] [CrossRef]
- Perevedentseva, E.; Lin, Y.-C.; Cheng, C.-L. A Review of Recent Advances in Nanodiamond-Mediated Drug Delivery in Cancer. Expert Opin. Drug Deliv. 2021, 18, 369–382. [Google Scholar] [CrossRef]
- Szymczak-Pajor, I.; Wenclewska, S.; Śliwińska, A. Metabolic Action of Metformin. Pharmaceuticals 2022, 15, 810. [Google Scholar] [CrossRef]
- Tądel, K.; Wiatrak, B.; Bodetko, D.; Barg, E. Metformin and Proliferation of Cancer Cell Lines. Pediatr. Endocrinol. Diabetes Metab. 2020, 26, 159–166. [Google Scholar] [CrossRef]
- El-khayat, S.M.; Abouegylah, M.; Abdallah, D.; Geweil, A.G.; Elenbaby, A.M.; Zahra, O.S. The Effect of Metformin When Combined with Neoadjuvant Chemotherapy in Breast Cancer Patients. Med. Oncol. 2022, 39, 1. [Google Scholar] [CrossRef]
- Koh, M.; Lee, J.-C.; Min, C.; Moon, A. A Novel Metformin Derivative, HL010183, Inhibits Proliferation and Invasion of Triple-Negative Breast Cancer Cells. Bioorg. Med. Chem. 2013, 21, 2305–2313. [Google Scholar] [CrossRef] [PubMed]
- Amable, G.; Martínez-León, E.; Picco, M.E.; Rey, O. Metformin and Colorectal Cancer. Biocell 2021, 46, 51–59. [Google Scholar] [CrossRef]
- Joentausta, R.M.; Rannikko, A.; Murtola, T.J. Prostate Cancer–Specific Survival after Radical Prostatectomy Is Improved among Metformin Users but Not among Other Antidiabetic Drug Users. Eur. Urol. Open Sci. 2021, 34, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Mogheri, F.; Jokar, E.; Afshin, R.; Akbari, A.A.; Dadashpour, M.; Firouzi-amandi, A.; Serati-Nouri, H.; Zarghami, N. Co-Delivery of Metformin and Silibinin in Dual-Drug Loaded Nanoparticles Synergistically Improves Chemotherapy in Human Non-Small Cell Lung Cancer A549 Cells. J. Drug Deliv. Sci. Technol. 2021, 66, 102752. [Google Scholar] [CrossRef]
- Faramarzi, L.; Dadashpour, M.; Sadeghzadeh, H.; Mahdavi, M.; Zarghami, N. Enhanced Anti-Proliferative and pro-Apoptotic Effects of Metformin Encapsulated PLGA-PEG Nanoparticles on SKOV3 Human Ovarian Carcinoma Cells. Artif. Cells Nanomed. Biotechnol. 2019, 47, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Yenmis, G.; Yaprak Sarac, E.; Besli, N.; Soydas, T.; Tastan, C.; Dilek Kancagi, D.; Yilanci, M.; Senol, K.; Karagulle, O.O.; Ekmekci, C.G.; et al. Anti-Cancer Effect of Metformin on the Metastasis and Invasion of Primary Breast Cancer Cells through Mediating NF-KB Activity. Acta Histochem. 2021, 123, 151709. [Google Scholar] [CrossRef]
- Basu, A.; Upadhyay, P.; Ghosh, A.; Bose, A.; Gupta, P.; Chattopadhyay, S.; Chattopadhyay, D.; Adhikary, A. Hyaluronic Acid Engrafted Metformin Loaded Graphene Oxide Nanoparticle as CD44 Targeted Anti-Cancer Therapy for Triple Negative Breast Cancer. Biochim. Biophys. Acta (BBA) Gen. Subj. 2021, 1865, 129841. [Google Scholar] [CrossRef]
- Lee, K.-M.; Lee, M.; Lee, J.; Kim, S.W.; Moon, H.-G.; Noh, D.-Y.; Han, W. Enhanced Antitumor Activity and Cytotoxic Effect on Cancer Stem Cell Population of Metformin-Butyrate Compared with Metformin HCl in Breast Cancer. Oncotarget 2016, 7, 38500–38512. [Google Scholar] [CrossRef]
- Chen, Y.; Shan, X.; Luo, C.; He, Z. Emerging Nanoparticulate Drug Delivery Systems of Metformin. J. Pharm. Investig. 2020, 50, 219–230. [Google Scholar] [CrossRef]
- Jariwala, D.H.; Patel, D.; Wairkar, S. Surface Functionalization of Nanodiamonds for Biomedical Applications. Mater. Sci. Eng. C 2020, 113, 110996. [Google Scholar] [CrossRef] [PubMed]
- Reina, G.; Zhao, L.; Bianco, A.; Komatsu, N. Chemical Functionalization of Nanodiamonds: Opportunities and Challenges Ahead. Angew. Chem. Int. Ed. 2019, 58, 17918–17929. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Ni, J.; Beretov, J.; Graham, P.; Li, Y. Triple-negative breast cancer therapeutic resistance: Where is the Achilles’ heel? Cancer Lett. 2021, 497, 100–111. [Google Scholar] [CrossRef] [PubMed]
- Dawson, S.J.; Provenzano, E.; Caldas, C. Triple negative breast cancers: Clinical and prognostic implications. Eur. J. Cancer 2009, 45, 27–40. [Google Scholar] [CrossRef]
- Waks, A.G.; Winer, E.P. Breast Cancer Treatment: A review. JAMA 2019, 321, 288. [Google Scholar] [CrossRef]
- Bianchini, G.; Balko, J.M.; Mayer, I.A.; Sanders, M.E.; Gianni, L. Triple-negative breast cancer: Challenges and opportunities of a heterogeneous disease. Nat. Rev. Clin. Oncol. 2016, 13, 674–690. [Google Scholar] [CrossRef]
- Hirst, J.; Crow, J.; Godwin, A. Ovarian Cancer Genetics: Subtypes and Risk Factors. In Ovarian Cancer—From Pathogenesis to Treatment; InTech Open: London, UK, 2018. [Google Scholar]
- Elias, K.M.; Emori, M.M.; Papp, E.; MacDuffie, E.; Konecny, G.E.; Velculescu, V.E.; Drapkin, R. Beyond genomics: Critical evaluation of cell line utility for ovarian cancer research. Gynecol. Oncol. 2015, 139, 97–103. [Google Scholar] [CrossRef]
- Liu, R.; Zhao, F.; Yu, X.; Naito, K.; Ding, H.; Qu, X.; Zhang, Q. Synthesis of Biopolymer-Grafted Nanodiamond by Ring-Opening Polymerization. Diam. Relat. Mater. 2014, 50, 26–32. [Google Scholar] [CrossRef]
- Ciufolini, M.A.; Swaminathan, S. Synthesis of a Model Depsipeptide Segment of Luzopeptins (BBM 928), Potent Antitumor and Antiretroviral Antibiotics. Tetrahedron. Lett. 1989, 30, 3027–3028. [Google Scholar] [CrossRef]
- AAT Bioquest, Inc. Quest Graph IC50 Calculator. AAT Bioquest. Available online: https://www.aatbio.com/tools/ic50-calculator (accessed on 15 August 2023).
- Wawruszak, A.; Luszczki, J.J.; Grabarska, A.; Gumbarewicz, E.; Dmoszynska-Graniczka, M.; Polberg, K.; Stepulak, A. Assessment of Interactions between Cisplatin and Two Histone Deacetylase Inhibitors in MCF7, T47D and MDA-MB-231 Human Breast Cancer Cell Lines—An Isobolographic Analysis. PLoS ONE 2015, 10, e0143013. [Google Scholar] [CrossRef]
- Jeong, S.H.; Lim, D.; Kim, K.; Kang, E.; Lim, S.; Ricci, J.; Sung, S.; Kwon, M. Comprehensive Evaluation of Carboxylated Nanodiamond as a Topical Drug Delivery System. Int. J. Nanomed. 2016, 11, 2381. [Google Scholar] [CrossRef] [PubMed]
- Arnault, J.C. Surface Modifications of Nanodiamonds and Current Issues for Their Biomedical Applications. In Novel Aspects of Diamond. Topics in Applied Physics; Yang, N., Ed.; Springer: Cham, Switzerland, 2015; Volume 121, pp. 85–122. [Google Scholar] [CrossRef]
- Ullah, M.; Kausar, A.; Siddiq, M.; Subhan, M.; Abid Zia, M. Reinforcing Effects of Modified Nanodiamonds on the Physical Properties of Polymer-Based Nanocomposites: A Review. Polym. Plast. Technol. Eng. 2015, 54, 861–879. [Google Scholar] [CrossRef]
- Mermoux, M.; Chang, S.; Girard, H.A.; Arnault, J.-C. Raman Spectroscopy Study of Detonation Nanodiamond. Diam. Relat. Mater. 2018, 87, 248–260. [Google Scholar] [CrossRef]
- Mochalin, V.; Osswald, S.; Gogotsi, Y. Contribution of Functional Groups to the Raman Spectrum of Nanodiamond Powders. Chem. Mater. 2009, 21, 273–279. [Google Scholar] [CrossRef]
- Espectroscopía Vibracional. Available online: https://independent.academia.edu/MauricioSandy (accessed on 28 May 2023).
- Srinivasan, S.; Renganayaki, V. Computational Studies of Vibration Spectra and Thermodynamic Properties of Metformin Using HF, DFT Methods. Mater. Sci. Res. India 2011, 8, 165–172. [Google Scholar] [CrossRef]
- Bayramov, F.B.; Toporov, V.V.; Chakchir, O.B.; Anisimov, V.N.; Rud, V.Y.; Glinushkin, A.P.; Bairamov, B.H. Raman Spectroscopic Characterization of Anti-Diabetic Drug Metformin Hydrochloride. J. Phys. Conf. Ser. 2019, 1400, 033010. [Google Scholar] [CrossRef]
- Arafat, M.; Sakkal, M.; Yuvaraju, P.; Esmaeil, A.; Poulose, V.; Aburuz, S. Effect of Excipients on the Quality of Drug Formulation and Immediate Release of Generic Metformin HCl Tablets. Pharmaceuticals 2023, 16, 539. [Google Scholar] [CrossRef]
- Fan, J.; Li, T.; Gao, Y.; Wang, J.; Ding, H.; Heng, H. Comprehensive Study of Graphene Grown by Chemical Vapor Deposition. J. Mater. Sci. Mater. Electron. 2014, 25, 4333–4338. [Google Scholar] [CrossRef]
- Johra, F.T.; Lee, J.-W.; Jung, W.-G. Facile and Safe Graphene Preparation on Solution Based Platform. J. Ind. Eng. Chem. 2014, 20, 2883–2887. [Google Scholar] [CrossRef]
- Cabral, H.; Miyata, K.; Osada, K.; Kataoka, K. Block Copolymer Micelles in Nanomedicine Applications. Chem. Rev. 2018, 118, 6844–6892. [Google Scholar] [CrossRef]
- Riddick, T.M. Control of Colloid Stability Through Zeta Potential; With a Closing Chapter on Its Relationship to Cardiovascular, Disease; Zeta-Meter, Incorporated; Livingston Publishing Company: Wynnewood, PA, USA, 1968; Volume 1, p. 172. [Google Scholar]
- Saed, M.E. The Use of Nanotechnology for Treatment of Multidrug Resistant Ovarian Cancer Cells. Ph.D. Thesis, Trinity College Dublin, Dublin, Germany, 2019. [Google Scholar]


| Sample | COOH Sites (COOH/nm2) |
|---|---|
| ND-COOH | 1.10 ± 0.04 |
| NDHEXA-CO2H | 9.36 ± 1.42 |
| Cancer Cell Lines | Time | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 24 h | 48 h | 72 h | |||||||
| SKOV3 | MET | ND-COOH | ND-MET | MET | ND-COOH | ND-MET | MET | ND-COOH | ND-MET |
| 914 ± 127 | 458 ± 139 | 24 ± 7 ** | 632 ± 116 | 157 ± 64 | 35 ± 14 ** | UD | UD | UD | |
| MDA-MB-231 | UD | UD | 842 ± 81 | UD | UD | 801 ± 37 | UD | UD | 454 ± 49 |
| Hs578T | UD | UD | UD | UD | UD | UD | UD | UD | 759 ± 44 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Acuña-Aguilar, L.E.; Conejo-Dávila, A.S.; Miki-Yoshida, M.; Hernández-de la Cruz, O.N.; Sánchez-Sánchez, G.; López-Camarillo, C.; Salas-Leiva, J.S.; Zaragoza-Contreras, E.A.; Reyes-Martínez, R.; Orrantia-Borunda, E. The Improved Cytotoxic Capacity of Functionalized Nanodiamonds with Metformin in Breast and Ovarian Cancer Cell Lines. Processes 2023, 11, 2616. https://doi.org/10.3390/pr11092616
Acuña-Aguilar LE, Conejo-Dávila AS, Miki-Yoshida M, Hernández-de la Cruz ON, Sánchez-Sánchez G, López-Camarillo C, Salas-Leiva JS, Zaragoza-Contreras EA, Reyes-Martínez R, Orrantia-Borunda E. The Improved Cytotoxic Capacity of Functionalized Nanodiamonds with Metformin in Breast and Ovarian Cancer Cell Lines. Processes. 2023; 11(9):2616. https://doi.org/10.3390/pr11092616
Chicago/Turabian StyleAcuña-Aguilar, Lucero Evelia, Alain Salvador Conejo-Dávila, Mario Miki-Yoshida, Olga N. Hernández-de la Cruz, Gricelda Sánchez-Sánchez, César López-Camarillo, Joan Sebastian Salas-Leiva, Erasto Armando Zaragoza-Contreras, Reyna Reyes-Martínez, and Erasmo Orrantia-Borunda. 2023. "The Improved Cytotoxic Capacity of Functionalized Nanodiamonds with Metformin in Breast and Ovarian Cancer Cell Lines" Processes 11, no. 9: 2616. https://doi.org/10.3390/pr11092616
APA StyleAcuña-Aguilar, L. E., Conejo-Dávila, A. S., Miki-Yoshida, M., Hernández-de la Cruz, O. N., Sánchez-Sánchez, G., López-Camarillo, C., Salas-Leiva, J. S., Zaragoza-Contreras, E. A., Reyes-Martínez, R., & Orrantia-Borunda, E. (2023). The Improved Cytotoxic Capacity of Functionalized Nanodiamonds with Metformin in Breast and Ovarian Cancer Cell Lines. Processes, 11(9), 2616. https://doi.org/10.3390/pr11092616







