Research on Classified Treatment of Electrolytic Zn Anode Slime Based on μ-XRF and Cluster Heatmap
Abstract
:1. Introduction
2. Materials and Methods
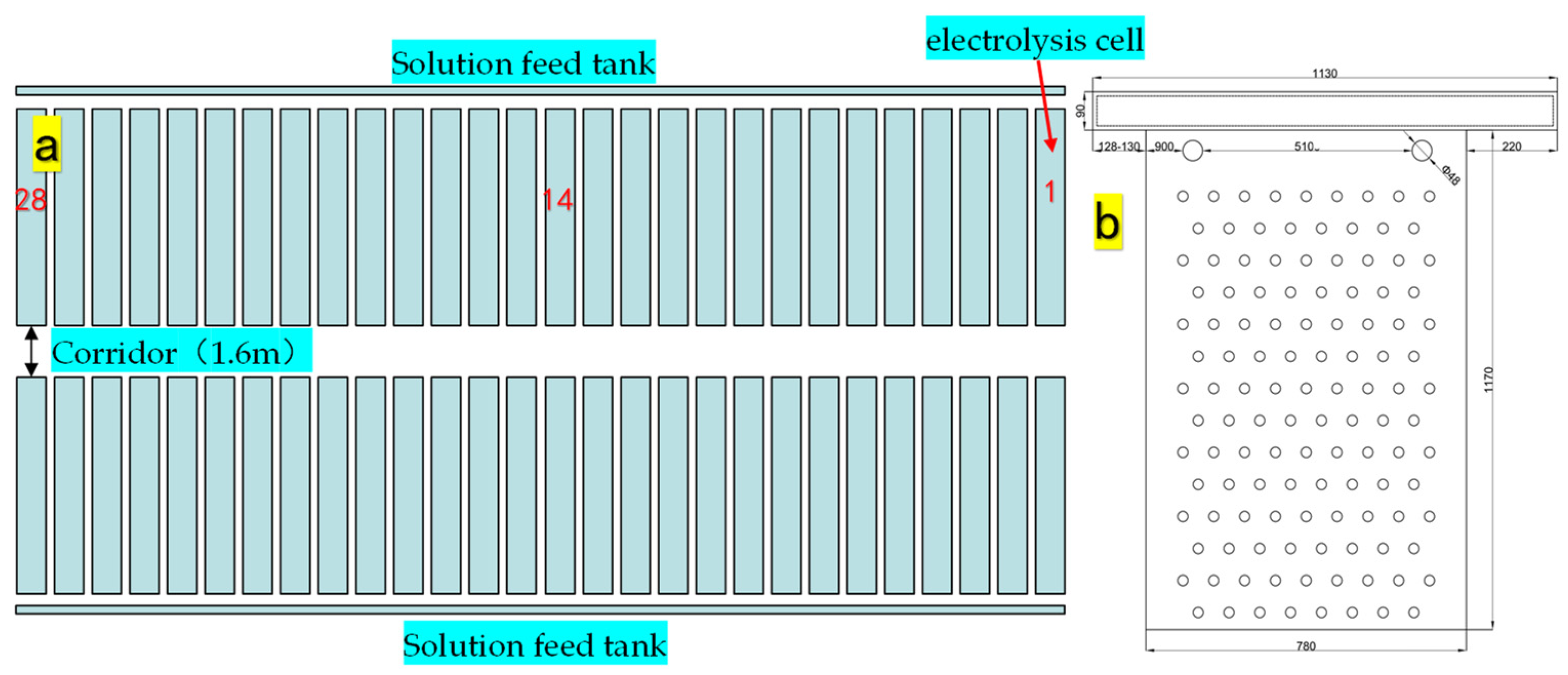
2.1. The Zinc Production Process Conditions of Sampling Enterprises
2.2. Test Sample Collection and Test Method
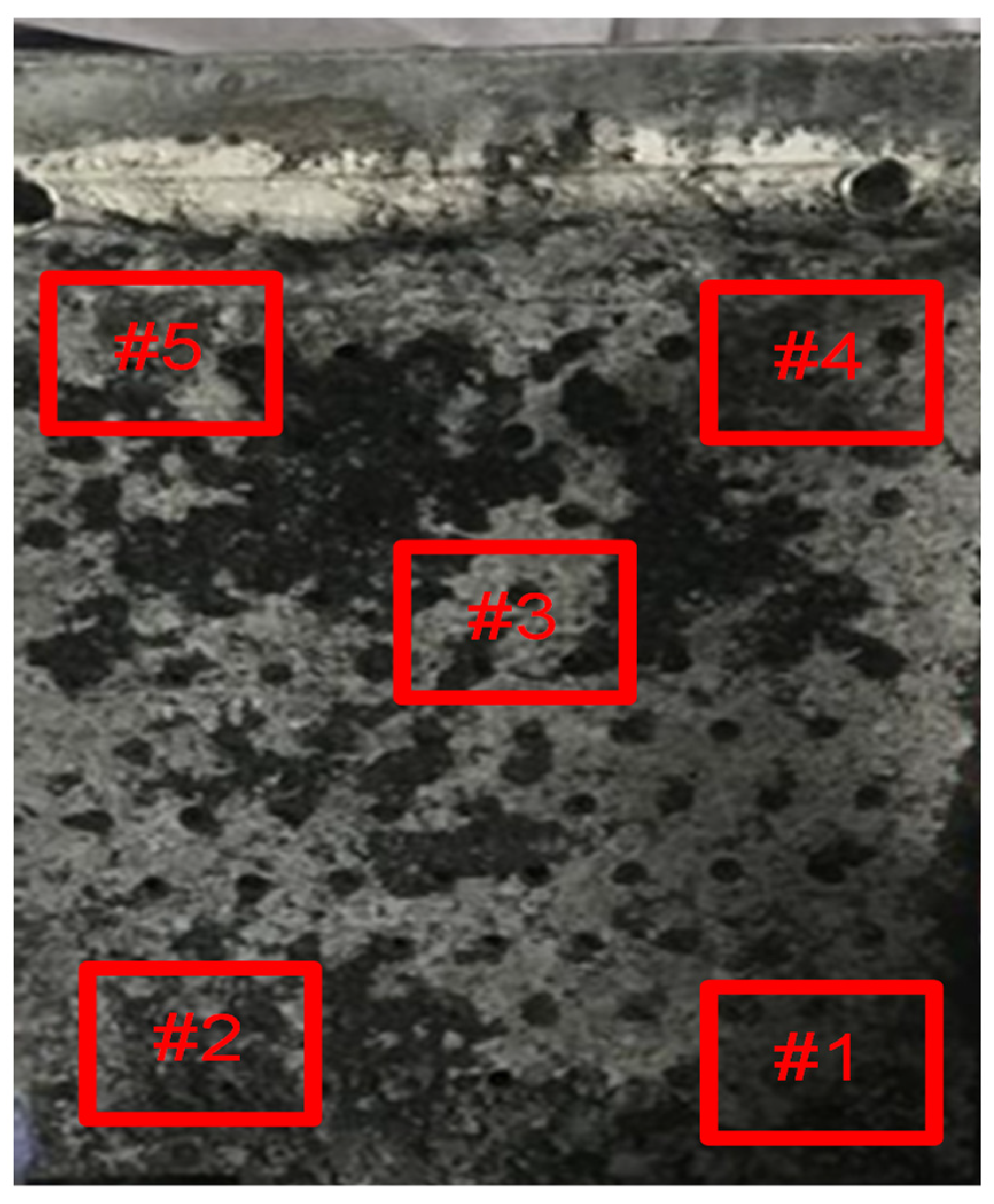
2.2.1. Sampling
2.2.2. Preparation of Sealant Samples
2.2.3. μ-XRF and mm-XRF Tests
3. Results and Discussion
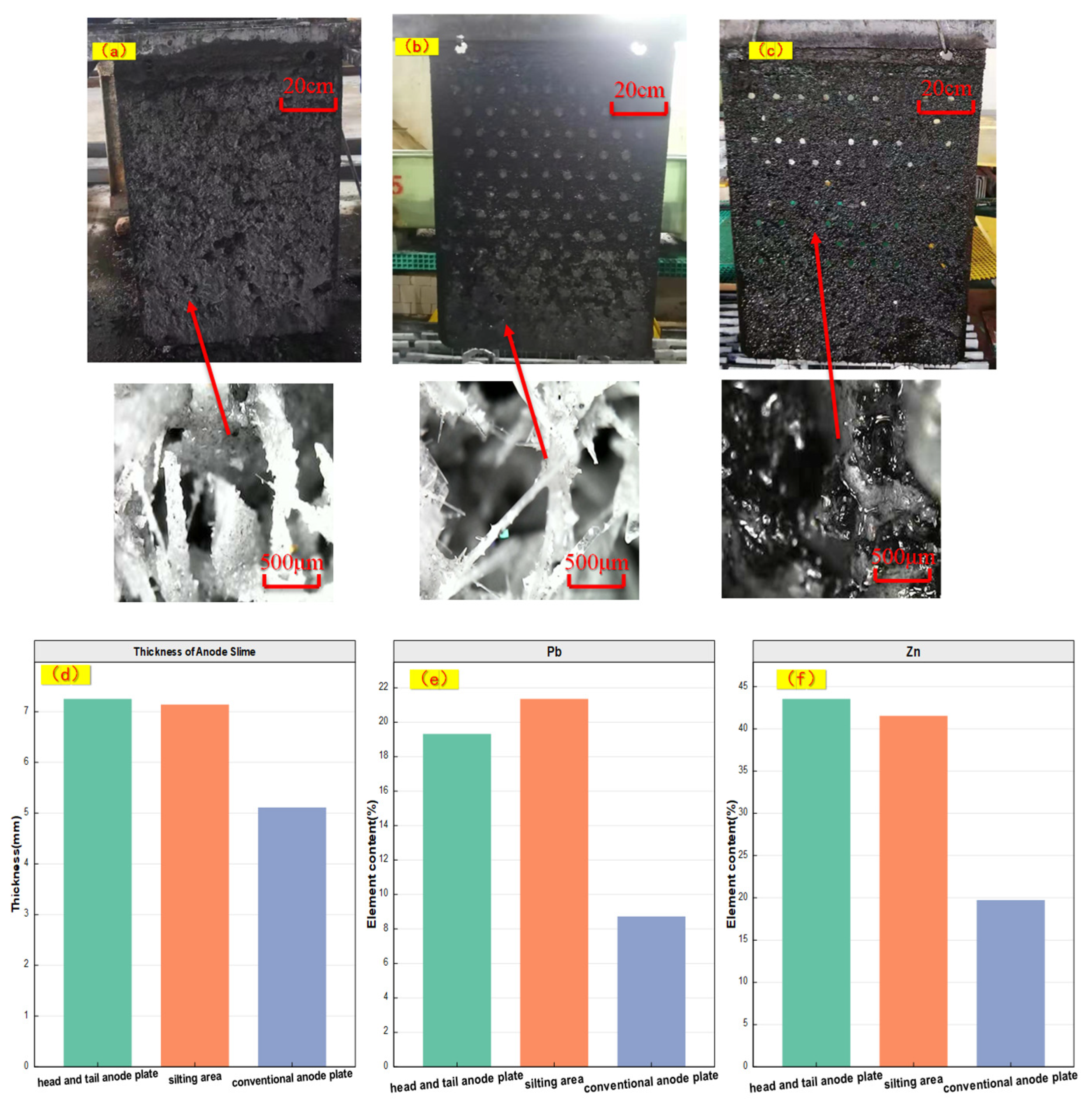
3.1. Analysis of Morphology and Element Distribution of Tested Anode Slime
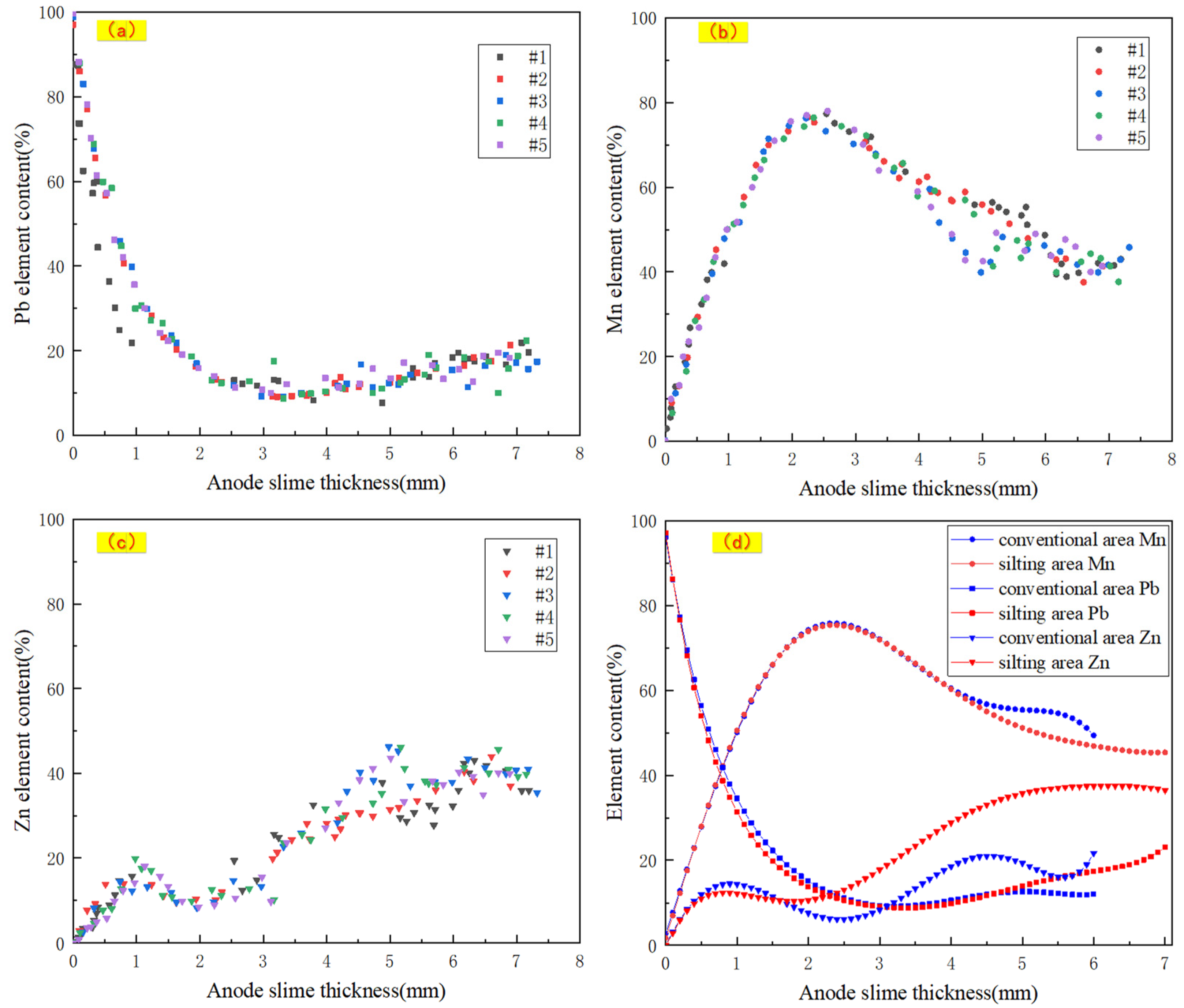
3.2. Spatial Distribution of Pb, Mn and Zn in Micro-Zone of Anode Slime Detected by μ-XRF
3.3. mm-XRF Test and Cluster Analysis
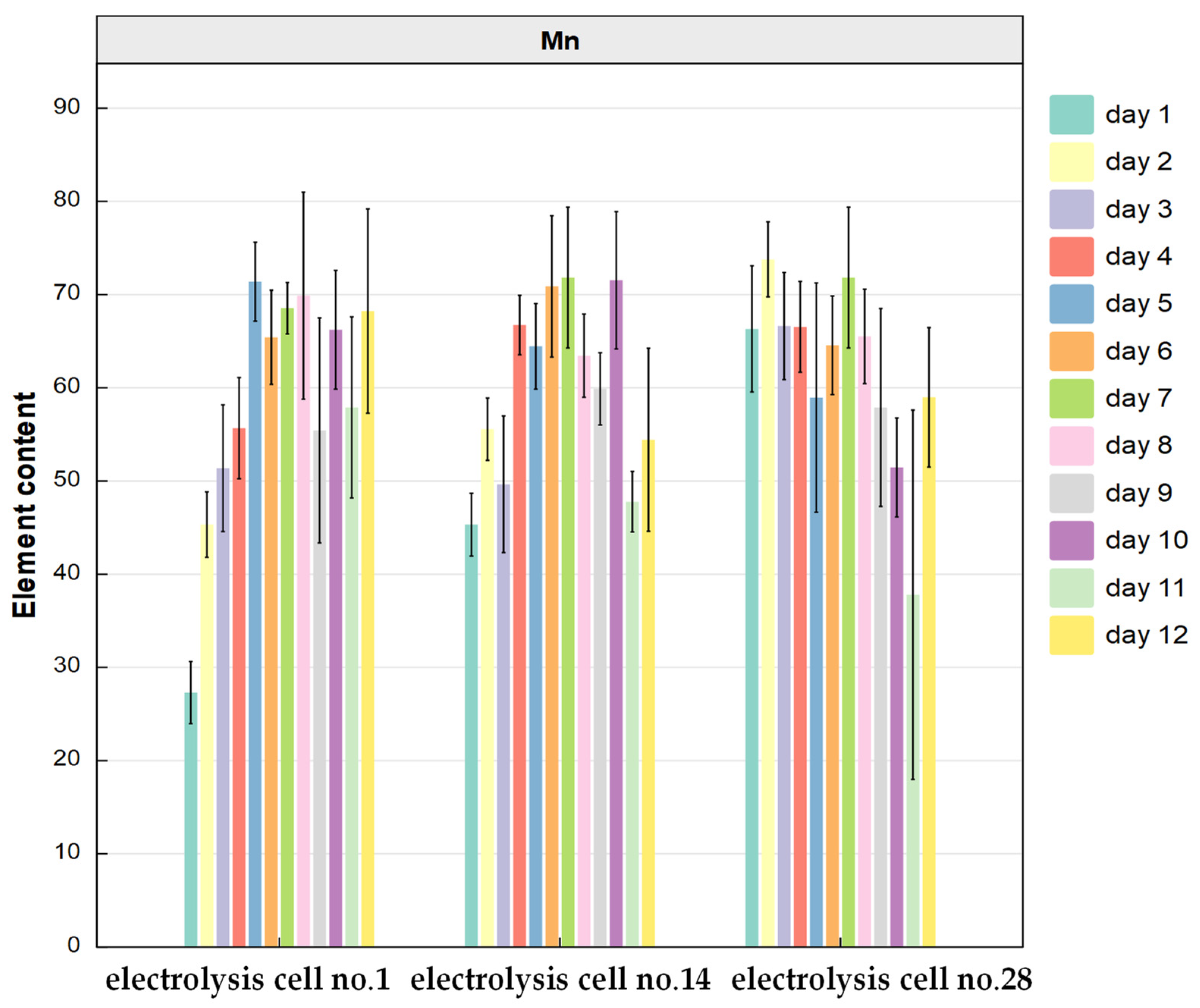
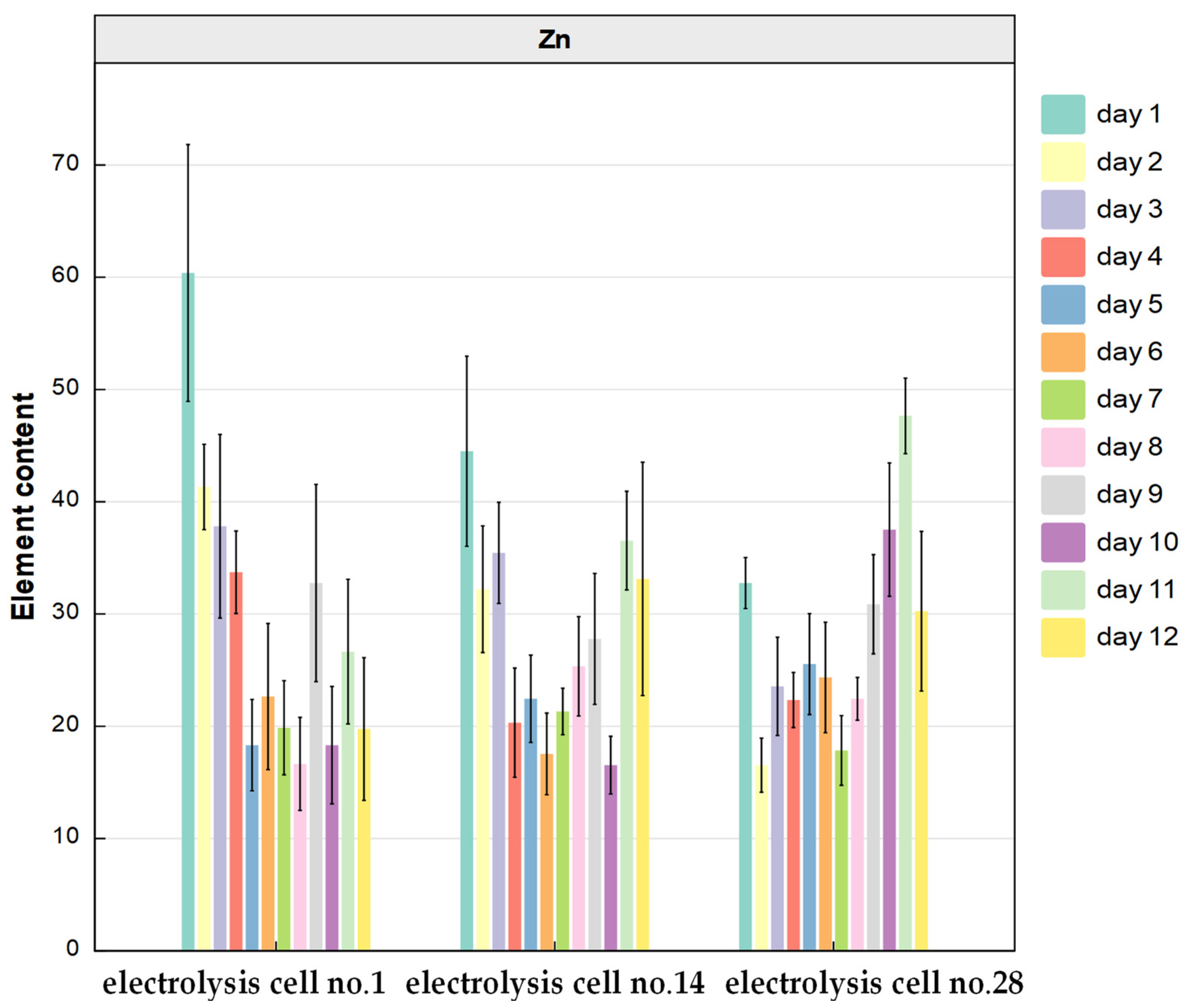
3.3.1. Spatial Distribution of Target Elements in Anode Slime on the Silting Plate and the Conventional Area
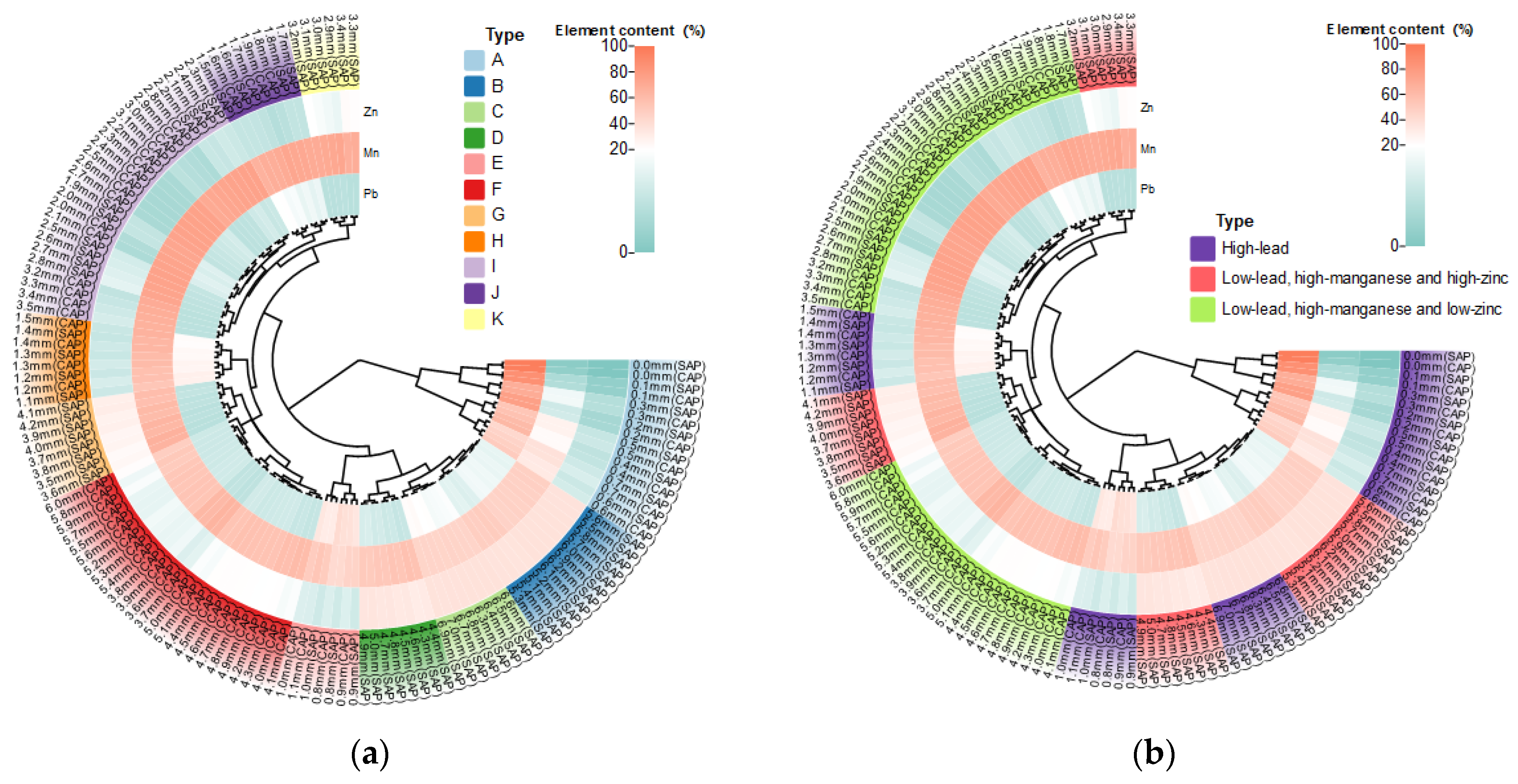
3.3.2. Cluster Analysis of Elements in Anode Slime Resources on the Silting Plate and in the Conventional Area
4. Conclusions
- (1)
- Based on mm-XRF, thickness measurement, morphology observation and liquid content detection, it is preliminarily determined that there were two different forms of electrolytic Zn anode slime, namely, silting area and conventional area, and the silting area accounted for about 15% of the total anode plates. Based on μ-XRF in-situ analysis of micro-area elements, it was found that there were lumps with fluorescence counting intensity that was close to pure Pb in anode slime in the silting area 3.9 mm away from the Pb-based surface.
- (2)
- Through mm-XRF analysis of a large number of data on anode slime in the silting area and the conventional area, anode slime with a thickness of 0.1 mm in conventional and silting areas could be regarded as a kind of resource, and we categorized the resource with the use of a complete-linkage algorithm for clustering division (to supplement the conclusion on linear relationship between elements and thickness).
- (3)
- High-Pb resource at 6.3–7.0 mm (SAP); low-Pb, high-Mn and high-Zn resource at 2.9–6.0 mm (SAP) and low-Pb, high-Mn and low-Zn resources including 1.6–6.0 mm (CAP), 1.5–2.8 mm (SAP) and 6.1–6.2 mm (SAP) were scraped out and collected. High-Pb resources 0–1.5 mm (CAP) and 0–1.4 mm (SAP) were retained on the anode plate surface, to realize the Pb-sealed anode plate surface and to classify the resource recovery of anode slime. This provides a theoretical basis for the efficient resource utilization of anode slime in the Zn hydrometallurgy industry.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, F.Y.; Jiang, L.H.; Li, J.H.; Zou, C.; Wen, Y.C.; Zhang, G.; Li, Z.Q.; Duan, N. Mass balance and quantitative analysis of cleaner production potential in a Zn electrolysis cellhouse. J. Clean. Prod. 2016, 135, 712–720. [Google Scholar] [CrossRef]
- Karbasi, M.; Alamdari, E.K. Investigation of Pb base composite anodes produced by accumulative roll bonding. Mater. Des. 2015, 67, 118–129. [Google Scholar] [CrossRef]
- Zhang, C.; Jiang, L.; Xu, F.; Duan, N.; Xin, B.; Han, G.; Zhang, G.; Wen, Y. New insight into cleaner control of heavy metal anode slime from aqueous sulfate electrolytes containing Mn (II): Preliminary characterization and mechanism analysis. J. Clean. Prod. 2018, 177, 276–283. [Google Scholar] [CrossRef]
- Ye, W.; Xu, F.; Jiang, L.; Duan, N.; Li, J.; Ma, Z.; Zhang, F.; Chen, L. Pb release kinetics and film transformation of Pb-MnO2 pre-coated anode in long-term Zn electrowinning. J. Hazard. Mater. 2021, 408, 124931. [Google Scholar] [CrossRef]
- Ye, W.; Xu, F.; Jiang, L.; Duan, N.; Li, J.; Ma, Z.; Zhang, F.; Chen, L. A novel functional Pb-based anode for efficient Pb dissolution inhibition and slime generation reduction in Zn electrowinning. J. Clean. Prod. 2021, 284, 9776. [Google Scholar] [CrossRef]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.; Beeregowda, B.; Krishnamurthy, N. Toxicity, mechanism and health effects of some heavy metals. Interdiscipl. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Jiang, J.; Duan, L.; Li, Z.; Deng, J.; Li, J.; Zhang, R.; Zhou, C.; Xu, F.; Jiang, L.; et al. Ultrasonication to reduce particulate matter generated from bursting bubbles: A case study on Zn electrolysis. J. Clean. Prod. 2021, 272, 122697. [Google Scholar] [CrossRef]
- Devoz, P.P.; Reis, M.B.D.; Gomes, W.R.; Maraslis, F.T.; Ribeiro, D.L.; Antunes, L.M.G.; Batista, B.L.; Grotto, D.; Reis, R.M.; Barbosa, F., Jr.; et al. Adaptive epigenetic response of glutathione (GSH)-related genes against Pb (Pb)-induced toxicity, in individuals chronically exposed to the metal. Chemosphere 2021, 269, 128758. [Google Scholar] [CrossRef]
- Liu, W.; Cui, Z.J.; Tian, J.P.; Chen, L.J. Dynamic analysis of Pb stocks and flows in China from 1990 to 2015. J. Clean. Prod. 2018, 205, 86–94. [Google Scholar] [CrossRef]
- Cao, J.L.; Zhao, H.Y.; Cao, F.H.; Zhang, J.Q. The influence of F−doping on the activity of PbO2 film electrodes in oxygen evolution reaction. Electrochim. Acta 2007, 52, 7870–7876. [Google Scholar] [CrossRef]
- Wang, S.; Zhou, X.; Ma, C.; Long, B.; Wang, H.; Tang, J.; Yang, J. Electrochemical properties of Pb-0.6 wt% Ag powder-pressed alloy in sulfuric acid electrolyte containing Cl/Mn2þ ions. Hydrometallurgy 2018, 177, 218–226. [Google Scholar] [CrossRef]
- Nijjer, S.; Thonstad, J.; Haarberg, G.M. Oxidation of Mn(II) and reduction of Mn dioxide in sulphuric acid. Electrochim. Acta 2000, 46, 395–399. [Google Scholar] [CrossRef]
- Mohammadi, M.; Alfantazi, A. Evaluation of Mn dioxide siltation on Pb-based electrowinning anodes. Hydrometallurgy 2016, 159, 28–39. [Google Scholar] [CrossRef]
- Tunnicliffe, M.; Mohammadi, F.; Alfantazi, A. Polarization behavior of Pb-silver anodes in Zn electrowinning electrolytes. J. Electrochem. Soc. 2012, 159, C170–C180. [Google Scholar] [CrossRef]
- Ivanov, I.; Stefanov, Y. Electroextraction of Zn from sulphate electrolytes containing antimony ions and hydroxyethylated-butyne-2-diol-1,4: Part 3. The influence of Mn ions and a divided cell. Hydrometallurgy 2015, 6, 181–186. [Google Scholar]
- Devilliers, D.; Thi, M.T.D.; Mahe, E.; Daruiac, V.; Lequeux, N. Electroanalytical investigations on electrodeposited Pb dioxide. J. Electroanal. Chem. 2004, 573, 227–239. [Google Scholar] [CrossRef]
- Tang, L.; Tang, C.; Xiao, J.; Zeng, P.; Tang, M. A cleaner process for valuable metals recovery from hydrometallurgical Zn residue. J. Clean. Prod. 2018, 201, 764–773. [Google Scholar] [CrossRef]
- Yang, H.T.; Liu, H.R.; Guo, Z.C.; Chen, B.M.; Zhang, Y.C.; Huang, H.; Li, X.L.; Fu, R.C.; Xu, R.D. Electrochemical behavior of rolled Pb-0.8% Ag anodes. Hydrometallurgy 2013, 140, 144–150. [Google Scholar] [CrossRef]
- Jaimes, R.; Miranda-Hernández, M.; Lartundo-Rojas, L.; González, I. Characterization of anodic deposits formed on Pb-Ag electrodes during electrolysis in mimic Zn electrowinning solutions with different concentrations of Mn(II). Hydrometallurgy 2015, 156, 53–62. [Google Scholar] [CrossRef]
- Xu, R.C.; Jiang, L.H.; Duan, N.; Xu, F.Y.; Zhang, F.L.; Zhou, C.; Li, W.D.; Li, Z.Q. Research on microstructure of membrane-slime layer on lead-based anode surface in zinc hydrometallurgy by combining μ-XRF with mm-XRF. J. Clean. Prod. 2022, 379, 1. [Google Scholar] [CrossRef]
- Terzano, R.; Al Chami, Z.; Vekemans, B.; Janssens, K.; Miano, T.; Ruggiero, P. Zn distribution and speciation within rocket plants (Eruca vesicaria L. Cavalieri) grown on a polluted soil amended with compost as determined by XRF microtomography and micro-XANES. J. Agric. Food Chem. 2008, 56, 3222–3231. [Google Scholar] [CrossRef]
- Peng, C.; Xu, C.; Liu, Q.; Sun, L.; Luo, Y.; Shi, J. Fate and Transformation of CuO Nanoparticles in the Soil–Rice System during the Life Cycle of Rice Plants. Environ. Sci. Technol. 2017, 51, 4907–4917. [Google Scholar] [CrossRef] [PubMed]
- Xing, L.D.; O’Connor, J.K.; McKellar, R.C.; Chiappe, L.M.; Bai, M.; Tseng, K.W.; Zhang, J.; Yang, H.D.; Fang, J.; Li, G. A flattened enantiornithine in mid-Cretaceous Burmese amber: Morphology and preservation. Sci. Bull. 2018, 63, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Rajabi, A.; Eskandari, M.; Ghadi, M.J.; Li, L.; Zhang, J.F.; Siano, P.A. Comparative study of clustering techniques for electrical load pattern segmentation. Renew. Sust. Energ. Rev. 2020, 120, 109628. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, R.; Jiang, L.; Duan, N.; Zhu, G.; Liu, Y.; Zhou, C.; Li, W.; Li, Z. Research on Classified Treatment of Electrolytic Zn Anode Slime Based on μ-XRF and Cluster Heatmap. Processes 2023, 11, 2585. https://doi.org/10.3390/pr11092585
Xu R, Jiang L, Duan N, Zhu G, Liu Y, Zhou C, Li W, Li Z. Research on Classified Treatment of Electrolytic Zn Anode Slime Based on μ-XRF and Cluster Heatmap. Processes. 2023; 11(9):2585. https://doi.org/10.3390/pr11092585
Chicago/Turabian StyleXu, Ruichao, Linhua Jiang, Ning Duan, Guangbin Zhu, Yong Liu, Chao Zhou, Weidong Li, and Zhiqiang Li. 2023. "Research on Classified Treatment of Electrolytic Zn Anode Slime Based on μ-XRF and Cluster Heatmap" Processes 11, no. 9: 2585. https://doi.org/10.3390/pr11092585
APA StyleXu, R., Jiang, L., Duan, N., Zhu, G., Liu, Y., Zhou, C., Li, W., & Li, Z. (2023). Research on Classified Treatment of Electrolytic Zn Anode Slime Based on μ-XRF and Cluster Heatmap. Processes, 11(9), 2585. https://doi.org/10.3390/pr11092585




