Abstract
The antioxidant activities (in vitro) of vacuum-packaged dry-cured sausages stored for 1 year, which were treated with varying concentrations of extracts from orange peel (EFOP) in a modification solution, were evaluated using a central composite design. The individual variables: soy lecithin concentration, soy oil concentration, treated time, lactic acid addition, EFOP addition, and dependent variable [i.e., in vitro antioxidant activity] were analyzed by response surface methodology. Among the 32 treatment combinations, treatment 26 (central point) exhibited a higher 2,2-Diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity compared to the control group (natural hog casing without modification). Spectral pre-treatments were applied to enhance the robustness of the model, and a partial least squares regression model (PLSRM) was fitted. The results of the response surface methodology indicated that the interactive effects of a high [soy lecithin concentration] × a high [EFOP addition] yielded a DPPH assay result of over 35%. The determination coefficients (R2 value) of the second-order polynomial model for the simultaneous effects on in vitro antioxidant activity reached 65.28%. The PLSRM developed using average reflectance data after the first derivation pre-treatment demonstrated a higher R2 value in the calibration group compared to the untreated data. The first principal component accounted for 77.39% of the overall attributes and effectively differentiated the sausages’ antioxidant activity from 31.88%.
1. Introduction
For thousands of years, sausages have been a popular food made from minced meats. While the exact origin of the first sausage remains unclear, these delectable sausage products were traditionally enjoyed during annual festive and sacrificial occasions. The sausage casings, used to encase the minced meats, should possess both strengths to withstand the pressure during stuffing or cooking and the ability to preserve their tender texture [1,2]. Sausage manufacturers face challenges when casings burst, as it leads to significant food waste and hampers efficient sausage production. To address this issue, Feng et al. (2014) explored the modification of casings using varying concentrations of surfactant solution and storage in slush salt [3]. The modified casings’ interior structure was observed to be more permeable, which reduced the incidence of bursting [3]. However, processed meat products are prone to experiencing a decline in redness, loss of lipid and protein functionality, and the development of a rancid flavor. These undesirable effects are attributed to the formation of highly reactive intermediate compounds during oxidation. Notably, processing procedures such as meat grinding can accelerate the rate of lipid oxidation due to the increased surface area exposed to oxygen [4]. Because the casing becomes more porous, it may easily lead to lipid oxidation or other oxidative reactions. It is common to add nitrites as a food additive during sausage production. Nitrites can actually enhance the antioxidant activity of sausages, particularly in vitro (laboratory) conditions. Nitrites, when added to sausages as a curing agent, play a crucial role in preventing bacterial growth, extending shelf life, and giving the meat its characteristic color and flavor. However, their impact on antioxidant activity is slightly complex. Nitrites have been found to exhibit antioxidant properties themselves, as they can scavenge free radicals and prevent oxidative damage. In this sense, they can contribute to the overall antioxidant capacity of sausages. Additionally, nitrites can help to stabilize and protect certain endogenous antioxidants, such as vitamin C and vitamin E, present in the meat. These antioxidants are susceptible to degradation during processing and storage, and nitrites can help preserve their activity.
However, the formation of nitrosamines, which can occur when nitrites react with amino acids and other components of the meat, has been a concern in terms of potential health risks. Nitrosamines are considered carcinogenic, and their presence in processed meats has raised concerns. Therefore, the use of nitrites in food processing is regulated and limited to specific levels to minimize the formation of nitrosamines. Owing to the potential health risk, numerous researchers are interested in exploiting new natural and healthy food preservatives or developing nitrites-free meat products. Serdaroğlu et al. (2023) employed arugula and barberry extracts to heat-treated fermented sausage. The inclusion of barberry extract resulted in a reduction in lipid oxidation compared with other alternatives [5]. The utilization of arugula extract or pre-converted arugula extract led to a decrease in carbonylation when compared to samples without nitrite [5]. Cenci-Goga et al. (2012) investigated the selected lactic acid bacteria starter culture of dairy origin in the nitrite-free low-acid fermented sausage [6]. The results show that lactococci and lactobacilli strains derived from dairy sources have the potential to effectively inhibit pathogens, enhance sensory properties, and simultaneously ensure that the final pH values of non-acid/low-acid fermented sausages remain within the appropriate range [6].
Citrus fruits, which are widely cultivated worldwide for commercial purposes, serve as a vital source for the fruit juice, jams, and jellies industry. These industries play a significant role in creating millions of jobs globally and offer substantial benefits [7,8]. The citrus-processing industry has been reported to generate over forty million tons of waste annually [9]. For instance, orange (Citrus sinensis) alone produces more than sixteen million tons of waste, including orange peel (60–65%) and pulp and seeds (30–35%) [10]. In Japan, it is estimated that around 400 thousand tons of fruit juice are consumed annually. At the JA (Japan Agricultural Cooperative) Foods Factory in Shizuoka prefecture alone, approximately 2000 tons of oranges are processed each year, resulting in the generation of 1000 tons of waste [11]. Considering that Shizuoka prefecture’s orange production accounts for only about 11% of Japan’s total, it suggests the potential for approximately 10 times more waste production [11]. However, managing this waste in compliance with the Food Recycling Law in Japan comes with significant costs. The waste orange peels are typically handled by either being directly discarded in landfills as fertilizer, used as animal feed, or sold as dried orange peels to China for incorporation into Chinese herbal medicine. From an economic perspective, the recycling of waste orange peels can address environmental concerns while also serving as a valuable resource for extracting flavonoids for the pharmaceutical industry [12]. Hesperidin, a flavonoid compound predominantly found in orange peels, processes antioxidative, anti-inflammatory, and anti-cancer properties [13] and shows potential in the treatment of SARS-CoV-2 [14]. Hesperidin has demonstrated effectiveness against various types of cancer, including liver cancer, breast cancer, lung cancer, and others [15]. Recent literature reveals that flavonoids such as hesperidin and rutin show stronger binding affinity to the main protease of COVID-19 compared to nelfinavir [14,16]. Therefore, they could serve as promising starting points for the development of therapeutics against COVID-19. In addition, as the orange peels contain flavonoids that are antioxidative, it may be interesting to investigate the antioxidant effects of orange peel extracts after adding to a casing modification solution on sausage during 1-year long-term storage. Although several studies have addressed applying plant extracts to processed meat products [17,18,19], the addition of orange extracts to casing modification solutions has not been exploited.
Hyperspectral imaging (HSI) is extensively utilized in the analysis of various food items, such as grapes [20,21,22], blueberries [23,24], pork [25,26,27], beef [28,29,30], lamb [31,32,33], and processed meat products [34], enabling the visualization of measured parameters on a pixel-by-pixel basis. In the case of dry-cured sausages (vacuum-packed), HSI has been employed to evaluate their physicochemical attributes, microbiological attributes, and sensory attributes [27]. Aheto et al. (2019) utilized HSI to assess the 2-thiobarbituric acid reactive substances of dry-cured pork meat, achieving prediction determination coefficients of 0.896 with a root mean square error of prediction of 1.034, using the PLSRM [35]. The antioxidant properties of tan mutton were evaluated by HSI coupled with the entropy weight method [36]. These findings clearly demonstrate that HSI is an innovative and efficient analytical technology that surpasses traditional spectroscopic methods.
Previous studies have explored the application of HSI and chemometrics to estimate the color [37,38], pH [39,40], and ATP [41] of sausages. However, limited research has been conducted on the impact of extracts from orange peel added to modified casing solutions on the in vitro antioxidant activity of sausages stuffed in various combinations of modified hog casings. Although sausages may not be commonly associated with being a source of dietary antioxidants or consumed for their antioxidant properties, studying the antioxidant activity of sausages can have several potential benefits:
- (1)
- Research and analysis in the food industry often explore various aspects of food quality, including the stability and preservation of food products over time.
- (2)
- Investigating the antioxidant activity of sausages stored for an extended period can provide insights into the oxidative stability of the product and its potential shelf life.
- (3)
- Antioxidants play a role in inhibiting or slowing down oxidative processes that can lead to changes in flavor, color, texture, and overall quality of food products. By evaluating the antioxidant activity of sausages, researchers can gain a better understanding of the oxidative processes occurring during storage and potential strategies for improving the product’s stability.
Therefore, this study aims to evaluate the combined effects of surfactant solution and EFOP addition on the in vitro antioxidant activity of sausage using HSI and multivariate analysis. Following this, the key wavelengths associated with the in vitro antioxidant activity of sausages treated with different combinations will be identified through multivariate data analysis. Ultimately, multivariate calibration models will be developed using both full and significant wavelengths to establish the relationship between spectral data and in vitro antioxidant activity.
2. Materials and Methods
2.1. Orange Crude Extraction and Casing Modification Solution Preparation
Oranges were harvested in Katsuragi Town, Wakayama Prefecture, in March and were transported using a cold chain system. The orange peels (Citrus sinensis) were dried in an oven at 40 °C for 7 days. Forty grams of finely ground orange powder, obtained from an electric powder mill machine, was extracted using 100% ethanol via Soxhlet extraction [42]. The extracts were left overnight in a fume hood to allow for natural solvent evaporation. The extracts were washed with distilled water, transferred to filter paper, and dried in a desiccator to obtain crude precipitation. The dried precipitation was added to a casing modification solution composed of soy lecithin and soy oil. A length of 30 cm natural hog casing was put in this modification, stirred by a magnetic stirrer at 500 rpm at room temperature for a specific treatment time. The casing section without rinsing was then put in slush salt supplemented with lactic acid (LA) for the same treatment duration. A central composite design was employed to determine the concentrations of casing modification solution, the addition of EFOP, the addition of LA, and treatment time (Table 1). This results in 32 group combinations with 6 replications at the center points. Minitab software (version 21.1, Kozo Keikaku Engineering Inc., Tokyo, Japan) was employed for further data analysis.

Table 1.
Combinations of casing modification added with EFOP.
2.2. Preparation of Pork Sausages Using Modified Casing and Controlled Natural Casing
The sausage filling composition and concentration are shown in Table 2. The meat and fat were sterilely cut into small pieces and thoroughly mixed with the spices and wine. The resulting mixture was then stuffed into modified casings along with untreated natural hog casings (used as a control) using a stuffing machine (STX-4000-TB2-PD-BL, Electric Meat Grinder & Sausage Stuffer, STX International, Lincoln, NE, USA).

Table 2.
Materials for sausage production.
The stuffed sausage sections were immediately dried in a sterilized oven at 45 °C for 24 h and then aged at 20 °C for an additional 48 h. Consequently, the dry-cured sausage sections were cut in a sterilized clean bench, vacuum-packaged, and stored in a refrigerator at 4 °C for one year for further analysis.
2.3. In Vitro Antioxidant Activity of Pork Sausages with Different Types of Casings
To obtain sausage extract, three grams of one-year stored sausage (with casing) was weighed, minced, and homogenized with 10 mL of methanol for 2 min and then subjected to centrifugation (MX-301, Tomy, Tokyo, Japan) at 10,000× g for 10 min at 4 °C. Following this, the methanolic sample solution (100 μL) was added with 3.9 mL of 60 μM 1,1-diphenyl-2-picrylhydrazyl (DPPH) solution, vortexed well, and left for 10 min in an incubator (20 °C). The resulting mixture’s absorbance at 515 nm, with a blank solution consisting of 3.9 mL of 60 μM DPPH and 100 μL of methanol, was measured by a UV spectrophotometer (U5100, Hitachi High-Tech Science Corporation, Tokyo, Japan) to determine the antioxidant activity. The percent inhibition, representing the absorbance at 515 nm, was calculated using the formula:
where A1 and A2 denote the absorbance of the blank and the sample, respectively.
This method was according to the methods of Manzoor et al. (2023) [18] and Manzoor et al. (2022) [17] with a slight modification. The measurement was carried out in quadruplicate.
2.4. Hyperspectral Data Extraction and Processing
The sausage sample (both front and back side) after 1 year of cold room storage was placed perpendicularly under the hyperspectral camera, rendering the sample’s imaging within the measuring range as much as possible. Images were captured using a hyperspectral camera (EBA Japan, Tokyo, Japan) of model NH-4-KIT. The camera employed a push-broom line scanning method, capturing 151 contiguous spectra with an exposure time of 12.47 ms. To ensure uniform lighting and prevent shadows, a white sheet was utilized, and three halogen lamp lights were positioned around it. Ice bags were placed beneath a black sheet and two fans were set beside the halogen lamp, to avoid the sample temperature increasing greatly due to the halogen lamp heat. The black sheet served to create a clear distinction between the background and the sample. Imaging calibration was conducted using the following equation:
Ri is the raw reflectance image. Rii and Riii are the reflectance images of white (obtained using a 100% white reference) and dark (captured by covering the camera lens in a dark room), respectively. HSIs spectra were extracted and processed using HSAnalyzer software (EBA Japan, Tokyo, Japan). To select the region of interest (ROI), a manual separation process is performed to separate the sample from the background or any undesired elements. The average spectra of the ROIs selected from each sausage are utilized to develop the model.
2.5. Data Pretreatment & Partial Least Square Regression Model Development and Estimation
Prior to fitting the partial least square regression model (PLSRM), several spectral data pre-treatments, including first and second derivations, normalization, standard normal variate treatment (SNVT), and multiplicative scatter correction treatment (MSCT) were employed to enhance the performance of the model. One-third of the samples were designated for the prediction set, while the remaining two-thirds were assigned to the calibration set. The relationship between antioxidant activity and spectra was investigated using a partial least square regression model using the full range of wavelengths. To assess the predictive capabilities, the developed model was compared using metrics such as determination coefficients of validation (Rv2) and calibration (Rc2), as well as root mean square error of calibration (RMSEC) and validation (RMSEV). The Vektor Direktor (v1.1, KAX group, Sydney, Australia) was employed to conduct the multivariate analyses, including PLSRM, and principal component analysis (PCA), and perform all necessary computations.
2.6. Statistical Analysis
The effects of different casing treatments on antioxidant activity were analyzed by the Tukey method via Minitab (version 21.1, Kozo Keikaku Engineering Inc., Tokyo, Japan; one-way ANOVA).
3. Results and Discussion
3.1. Simultaneous Effects on In Vitro Antioxidant Activity of Sausages with Different Casings
Response surface methodology (RSM) was employed to analyze the five concurrent effects on the antioxidant activity of sausages with different casings. The developed regression model for antioxidant activity yields an R2 value of 65.28%, and the lack of fit was found to be not statistically significant at a 5% significance level. The polynomial equations for predicting the antioxidant activity of sausages in uncoded units are as follows:
where Xa, Xb, Xc, Xd, and Xe are the soy lecithin concentration, soy oil concentration, treated time, lactic acid addition, and EFOP addition. Although statistically insignificant at a 5% level, the addition of orange extract had the most significant impact on the in vitro antioxidant activity of the sausage after one year of storage at 4 °C, as indicated by the corresponding coefficient (97) in Equation (3). The interactive effects of soy lecithin concentration (Xa) and lactic acid (Xd) on antioxidant activity were found at a 10% significant level (Table 3). The 95% Confidence Interval (CI) refers to a range of values that provides an estimate of the uncertainty associated with a parameter or a result obtained from a statistical analysis. Generally, it is expected that true parameters or results should fall within the calculated interval of about 95% during the experimental repetitions. The Variance Inflation Factor (VIF) in RSM is a statistical measure used to assess multicollinearity among the predictor variables in a regression model. The VIF is a metric that quantifies the extent to which the variance of the estimated regression coefficient for a predictor variable is increased due to multicollinearity. A VIF value of 1 indicates no multicollinearity. VIF values greater than 1 but less than 5 generally suggest moderate multicollinearity. VIF values greater than 5 are often considered high, indicating strong multicollinearity that might be problematic for the interpretation and stability of regression coefficients.

Table 3.
Summary of regression for response variables: coefficients presented in relation to coded factors, along with goodness-of-fit statistics.
According to the surface (Figure 1a) and contour plot (Figure 1b), the values of the DPPH assay increase (>35%) when the SLC was over 1:33.33 along with the lactic acid addition of over 20%. A biodegradable film made from chitosan with tea tree essential oil added with soy lecithin and lactic acid solution was comprehensively studied [43]. The radical scavenging activity (indicated by DPPH) of the film with soy lecithin added to a lactic acid solution (0.75 ± 0.40%) was significantly higher than that of the film without soy lecithin (0.57 ± 0.41%) (p < 0.05) [43]. The authors also stated that lactic acid will positively influence scavenging activity [44]. It is known that soy lecithin is a complex mixture of phospholipids and other compounds found in soybeans. The impact of lecithin as a single additive was weak and provided around 7–17% inhibition of DPPH· radicals [45]. DPPH is a free radical molecule with an unpaired electron. When it comes into contact with an antioxidant compound, the unpaired electron in DPPH is donated to the antioxidant, neutralizing the radical and causing the purple DPPH solution to lose its color. The reduction in DPPH from purple to yellow indicates the scavenging of the free radical and is used to quantify the antioxidant activity. The decrease in the color intensity (absorbance) of the solution is measured spectrophotometrically at a specific wavelength (often around 517 nm). As depicted in Equation (1), a higher inhibition percentage indicates stronger antioxidant activity. Although lecithin alone may show less powerful antioxidants, other factors such as oxygen concentration, metal contaminants, lipid hydroxy compounds, enzymes, light and so on may influence the oxidative stability of lecithin. The higher the unsaturation of lecithin, the more susceptible to oxidative deterioration [45].
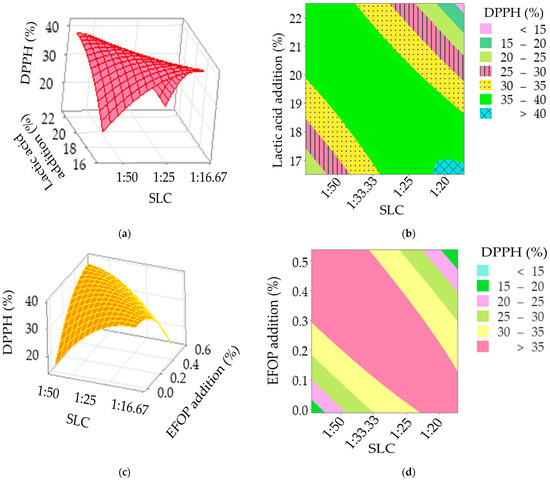
Figure 1.
The simultaneous effects [Soy lecithin concentration (SLC) and lactic acid addition (a,b); SLC and extracts from orange peel (EFOP) (c,d)] on DPPH radical scavenging activity of sausages.
With regard to the EFOP addition, the values of the DPPH assay can achieve over 35% when a high SLC (>1:25) is combined with a high EFOP addition (>0.3%). The average antioxidant activity for the control sausage was 32.64 ± 0.86%. The average DPPH radical scavenging activity of treatment 26 (SLC: 1:30, SOC: 1.78%, Treated time: 75 min, lactic acid addition: 19.5 mL/kg NaCl, EFOP addition: 0.26%) was the highest than the other 31 treatments (Table 4). The increasing trend of DPPH radical scavenging activity was also found when the extracts from betel leaf added to sausage increased [18]. The authors attribute this to the existence of bioactive compounds such as chavicol, carvacrol, eugenol, isoeugenol, and hydroxychavicol in the betel leaf extracts [18]. Those polyphenols possess redox properties that exert a useful impact on decomposing peroxide, quenching singlet oxygen, and neutralizing or adsorbing free radicals [18]. Similar observations were reported in the studies of Tran et al. (2020) [46], Manzoor et al. (2022) [17], and Bellucci et al. (2021) [47] for fresh pork sausages added with guava leaves extract, chicken sausages incorporated with mango peel extract, and pork patties added with acai extract, respectively. As for the current study, an increase in DPPH radical scavenging activity may probably be due to the presence of hesperidin, which has antioxidative, anti-inflammatory, and anti-cancer attributes [48]. The content of hesperidin in orange extracts was determined to be 46.82 ± 5.23 μg/40 g of dried orange [49].

Table 4.
Antioxidant activity of sausage with different modified casing treatments added with orange extracts.
The DPPH assay provides a general measure of the ability of compounds to scavenge free radicals, which are known to cause oxidative damage to cells and tissues. In the context of evaluating the preservative effects of compounds or extracts, the assay can be used to indicate their potential to prevent or mitigate oxidative deterioration of products. For example, in the field of food preservation, compounds with strong antioxidant activity as determined by the DPPH assay might be effective in extending the shelf life of food products by preventing lipid oxidation, color changes, and other forms of oxidative degradation. Similarly, in cosmetics or pharmaceuticals, substances with high antioxidant activity could help maintain the stability and effectiveness of formulations by protecting them from oxidation-induced degradation.
However, it’s important to note that while the DPPH assay provides valuable insights into the antioxidant potential of compounds, it represents a simplified in vitro model and does not necessarily replicate the complex interactions that occur within a biological system. Therefore, the results from the DPPH assay should be considered alongside other assays and studies to comprehensively evaluate the preservative effects of compounds or extracts.
3.2. Overview of Extracted Spectra
Figure 2a illustrates the average reflectance of sausages with modified casing under treatment 26 (SLC: 1:30, SOC: 1.78%, Treated time: 75 min, lactic acid addition: 19.5 mL/kg NaCl, EFOP addition: 0.26%) was higher than that of the control. Further investigation shows that the measured average DPPH radical scavenging activity of the treatment 26 sample (49.68 ± 0.65%) was significantly higher than that of the control (32.64 ± 0.86%) (p < 0.05, Table 4), indicating distinct spectral characteristics in the near-infrared (NIR) range for sausages stuffed with different casing treatments. The variations in absorbance are ascribed to the intrinsic vibrations of functional groups, indicating internal structural changes or fluctuations in intermolecular forces [50,51]. For example, the consistent slope observed in the 600–700 nm range signifies the presence of oxymyoglobin, while the transmittance absorption band at 790 nm corresponds to the third overtone of N-H stretching combined with protein [52]. Additionally, subtle absorption of 780 nm corresponds to the third overtones of O-H stretching, and that of 980 nm is linked to its second overtones, which are associated with water [53]. Finally, the absorption peak of 940 nm is related to fat and represents the third overtone of C-H stretching [53].
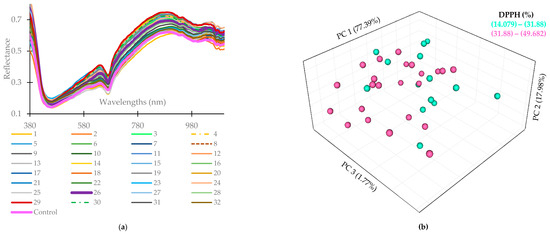
Figure 2.
Average samples with different modified casing treatments (denoted as the number) in the spectral range of 380 to 1100 nm (a); PCA score of DPPH radical scavenging activity of sausage with different casing treatment after 1-year storage (b). PC 1: the first principal component, PC2: the second principal component, PC3: the third principal component.
Figure 2b depicts that the first principal component (PC 1) can elucidate 77.39% of the whole attributes and can comparably divide the sausages’ antioxidant activity from 31.88%.
3.3. PLSRM Model Performance
The development of multivariate calibration models using spectral data indicates an intention to establish a relationship between the measured in vitro antioxidant activity and the spectral characteristics of the sausages. Both the full and average spectra were utilized for multivariate analysis. According to Table 5, the use of SNVT for the full wavelengths resulted in a higher Rc2 value (0.319) compared to the raw data (0.218), indicating a slight amelioration in the model. SNVT is used to minimize variability in reflectance spectra caused by light scattering [52]. Similarly, normalization is known to enhance spectral characteristics by producing spectra with consistent areas under the curve, facilitating feature comparison within the same plot [54]. Furthermore, it is more effective than conventional spectrophotometry in achieving a higher signal-to-noise ratio. The application of first and second derivations helps eliminate background noise and baseline drift as well as enhances the clarity of subtle spectral features. As for multiplicative scatter correction treatment, it compensates for additive and multiplicative effects [55]. The combination was employed as both SNVT and normalization achieved better R2 values than raw data (untreated). However, it did not show improvement compared to using SNVT alone. Using the full spectra in the calibration model enables the detection and correction of instrument drift or non-linearity. Incorporating all spectral features in the calibration improves the model’s accuracy and ensures that relevant information is considered. This is crucial for applications where even subtle differences between samples are significant. Furthermore, utilizing the full spectra lessens the risk of overfitting, especially when the model becomes overly complex and fails to generalize well to new data.

Table 5.
Hyperspectral data analyzed by chemometrics.
In order to enhance the model’s performance, the average spectra were also employed to fit the PLSRM model. The R2 values for both calibration and prediction sets were surprisingly improved in most cases, which may be credited to removing the noise and pixel outliers.
4. Conclusions
The objective of this study was to evaluate the potential of a hyperspectral imaging system for in vitro antioxidant activity of sausage surface after 1 year of storing in a cold room (4 °C). It reveals how antioxidant activity responds to different casing modifications with the addition of orange extracts during long-term storage. The study likely explores the technological aspect of utilizing natural extracts as a potential strategy for enhancing the oxidative stability and overall quality of the sausages during storage. The main findings can be summarized as follows:
- (1)
- Response surface methodology was employed to study the impact of different modification processes with varying concentrations of extracts addition from orange peel on the in vitro antioxidant activity of sausages. The model achieved an R2 value of 65.28% with no significant lack of fit. The values of the DPPH assay can achieve over 35% when a high soy lecithin concentration (>1:25) was associated with a high extract addition from orange peel (>0.3%).
- (2)
- The average reflectance of sausages with treatment 26 (central point) was higher than that of the control group using natural hog casing.
- (3)
- PLSRM developed from the average spectra achieved comparably higher than that using full spectra.
The current study exhibits both a technological interest in evaluating the effects of orange peel extract addition on sausages’ antioxidant capacity, as well as an inspection and quality control interest through the development of multivariate calibration models based on spectral data. The current study tried to explore the potential benefits of natural extracts and establish tools for assessing the sausages’ antioxidant activity or quality in a more efficient and cost-effective manner. However, further studies on lipid peroxidation, microbial, textural, sensorial analysis, physicochemical properties, and so on need to be conducted before this type of sausage comes to the consumers’ table.
Funding
This research was funded by the FY 2022 Mishima Kaiun Memorial Foundation, Iijima Tojuro Memorial Foundation for the Promotion of Food Science, Leading Initiative for Excellent Young Researchers (LEADER) from the Government of Japan Ministry of Education, Culture, Sports, Science and Technology (MEXT) (2020L0277), the Japan Society for the Promotion of Science Grant-in-Aid for Early Career Scientists (20K15477), Grants-in-Aid for Regional R&D Proposal-Based Program from Northern Advancement Center for Science & Technology of Hokkaido Japan (T-2-4), Sasakawa Scientific Research Grant from The Japan Science Society (2022-3005), FY 2022 and FY2021 President’s Discretionary Grants, funded by the Kitami Institute of Technology.
Data Availability Statement
Data are contained within this article.
Acknowledgments
The authors would also like to thank the anonymous reviewers for their constructive comments.
Conflicts of Interest
The author declares no conflict of interest.
References
- Feng, C.H. Quality evaluation and mathematical modelling approach to estimate the growth parameters of total viable count in sausages with different casings. Foods 2022, 11, 634. [Google Scholar] [PubMed]
- Feng, C.H.; Drummond, L.; Zhang, Z.H. Evaluation of innovative immersion vacuum cooling with different pressure reduction rates and agitation for cooked sausages stuffed in natural or artificial casing. LWT Food Sci. Technol. 2014, 59, 77–85. [Google Scholar] [CrossRef]
- Feng, C.H.; Drummond, L.; Sun, D.W.; Zhang, Z.-H. Evaluation of natural hog casings modified by surfactant solutions combined with lactic acid by response surface methodology. LWT Food Sci. Technol. 2014, 58, 427–438. [Google Scholar]
- Zamuz, S.; López-Pedrouso, M.; Barba, F.J.; Lorenzo, J.M.; Domínguez, H.; Franco, D. Application of hull, bur and leaf chestnut extracts on the shelf-life of beef patties stored under MAP: Evaluation of their impact on physicochemical properties, lipid oxidation, antioxidant, and antimicrobial potential. Food Res. Int. 2018, 112, 263–273. [Google Scholar]
- Serdaroğlu, M.; Can, H.; Sari, B.; Kavuşan, H.S.; Yılmaz, F.M. Effects of natural nitrite sources from arugula and barberry extract on quality characteristic of heat-treated fermented sausages. Meat Sci. 2023, 198, 109090. [Google Scholar]
- Cenci-Goga, B.T.; Rossitto, P.V.; Sechi, P.; Parmegiani, S.; Cambiotti, V.; Cullor, J.S. Effect of selected dairy starter cultures on microbiological, chemical and sensory characteristics of swine and venison (Dama dama) nitrite-free dry-cured sausages. Meat Sci. 2012, 90, 599–606. [Google Scholar] [PubMed]
- García-Martín, J.F.; Olmo, M.; García, J.M. Effect of ozone treatment on postharvest disease and quality of different citrus varieties at laboratory and at industrial facility. Postharvest. Biol. Technol. 2018, 137, 77–85. [Google Scholar] [CrossRef]
- García, J.F.; Olmo, M.; García, J.M. Decay incidence and quality of different citrus varieties after postharvest heat treatment at laboratory and industrial scale. Postharvest. Biol. Technol. 2016, 118, 96–102. [Google Scholar] [CrossRef]
- Xu, M.L.; Ran, L.; Chen, N.; Fan, X.W.; Ren, D.B.; Yi, L.Z. Polarity-dependent extraction of flavonoids from citrus peel waste using a tailor-made deep eutectic solvent. Food Chem. 2019, 297, 124970. [Google Scholar]
- Satari, B.; Karimi, K. Citrus processing wastes: Environmental impacts, recent advances, and future perspectives in total valorization. Resour. Conserv. Recycl. 2018, 129, 153–167. [Google Scholar]
- Ishiwata, N.; Feng, L.; Yian, N.; Yoshida, M.; Palihakkara, I.R.; Silva, N.; Tanaka, H.; Nga, N.T. Reduction of Waste from Beverage Industry in Shizuoka Prefecture. Available online: http://www.iai.ga.a.u-tokyo.ac.jp/mizo/lecture/noukoku-1/group-work/2012/G2_e.pdf (accessed on 31 January 2013).
- Feng, C.-H.; Otani, C.; Ogawa, Y. Innovatively identifying naringin and hesperidin by using terahertz spectroscopy and evaluating flavonoids extracts from waste orange peels by coupling with multivariate analysis. Food Control 2022, 137, 108897. [Google Scholar]
- Sammani, M.S.; Cerdà, V. Sample pre-treatment and flavonoids analytical methodologies for the quality control of foods and pharmaceutical matrices. In The Book of Flavonoids, 1st ed.; Feng, C.-H., García Martín, J.F., Eds.; Nova Science Publishers, Inc.: New York, NY, USA, 2021; Chapter 1; pp. 1–130. [Google Scholar]
- Bellavite, P.; Donzelli, A. Hesperidin and SARS-CoV-2: New light on the healthy function of citrus fruits. Antioxidants 2020, 9, 742. [Google Scholar] [CrossRef] [PubMed]
- Sawalha, S.M.S.; Arráez-Román, D.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Quantification of main phenolic compounds in sweet and bitter orange peel using CE-MS/MS. Food Chem. 2009, 116, 567–574. [Google Scholar] [CrossRef]
- Li, X.W.; Geng, M.M.; Peng, Y.Z.; Meng, L.S.; Lu, S.M. Molecular immune pathogenesis and diagnosis of COVID-19. J. Pharm. Anal. 2020, 10, 102–108. [Google Scholar] [PubMed]
- Manzoor, A.; Ahmad, S.; Yousuf, B. Effect of bioactive-rich mango peel extract on physicochemical, antioxidant and functional characteristics of chicken sausage. Appl. Food Biotechnol. 2022, 2, 100183. [Google Scholar]
- Manzoor, A.; Haque, A.; Ahmad, S.; Hopkins, D.L. Incorporation of betel leaf extract provides oxidative stability and improves phytochemical, textural, sensory and antimicrobial activities of buffalo meat sausages. Meat Sci. 2023, 200, 109157. [Google Scholar]
- Mokhtar, S.M.; Youssef, K.M. Antioxidant effect of some plant extracts as compared with BHA/BHTon lipidoxidation andsomequality properties of fresh beef burgers stored at 4 °C. Suez Canal Univ. J. Food Sci. 2014, 2, 19–29. [Google Scholar]
- Rodríguez-Pulido, F.J.; Gordillo, B.; Heredia, F.J.; González-Miret, M.L. CIELAB—Spectral image matching: An app for merging colorimetric and spectral images for grapes and derivatives. Food Control 2021, 125, 108038. [Google Scholar] [CrossRef]
- Rodríguez-Pulido, F.J.; Hernández-Hierro, J.M.; Nogales-Bueno, J.; Gordillo, B.; González-Miret, M.L.; Heredia, F.J. A novel method for evaluating flavanols in grape seeds by near infrared hyperspectral imaging. Talanta 2014, 122, 145–150. [Google Scholar] [PubMed]
- Rodríguez-Pulido, F.J.; Mora-Garrido, A.B.; González-Miret, M.L.; Heredia, F.J. Research progress in imaging technology for assessing quality in wine grapes and seeds. Foods 2022, 11, 254. [Google Scholar] [PubMed]
- Hu, M.-H.; Dong, Q.-L.; Liu, B.-L. Classification and characterization of blueberry mechanical damage with time evolution using reflectance, transmittance and interactance imaging spectroscopy. Comput. Electron. Agric. 2016, 122, 19–28. [Google Scholar]
- Hu, M.-H.; Dong, Q.-L.; Liu, B.-L.; Opara, U.L. Prediction of mechanical properties of blueberry using hyperspectral interactance imaging. Postharvest Biol. Technol. 2016, 115, 122–131. [Google Scholar]
- Cheng, J.H.; Sun, J.; Yao, K.S.; Xu, M.; Tian, Y.; Dai, C.X. A decision fusion method based on hyperspectral imaging and electronic nose techniques for moisture content prediction in frozen-thawed pork. LWT Food Sci. Technol. 2022, 165, 113778. [Google Scholar] [CrossRef]
- Zhuang, Q.B.; Peng, Y.K.; Yang, D.Y.; Wang, Y.L.; Zhao, R.H.; Chao, K.L.; Guo, Q.H. Detection of frozen pork freshness by fluorescence hyperspectral image. J. Food Eng. 2022, 316, 110840. [Google Scholar] [CrossRef]
- Cheng, J.H.; Sun, J.; Yao, K.S.; Xu, M.; Zhou, X. Nondestructive detection and visualization of protein oxidation degree of frozen-thawed pork using fluorescence hyperspectral imaging. Meat Sci. 2022, 194, 108975. [Google Scholar]
- Song, K.; Wang, S.-H.; Yang, D.; Shi, T.-Y. Combination of spectral and image information from hyperspectral imaging for the prediction and visualization of the total volatile basic nitrogen content in cooked beef. J. Food Meas. Charact. 2021, 15, 4006–4020. [Google Scholar] [CrossRef]
- Xie, A.G.; Sun, J.; Wang, T.M.; Liu, Y.H. Visualized detection of quality change of cooked beef with condiments by hyperspectral imaging technique. Food Sci. Biotechnol. 2022, 31, 1257–1266. [Google Scholar] [CrossRef]
- Ahmed, M.; Reed, D.D.; Young, J.M.; Eshkabilov, S.; Berg, E.P.; Sun, X. Beef quality grade classification based on intramuscular fat content using hyperspectral imaging technology. Appl. Sci. 2021, 11, 4588. [Google Scholar] [CrossRef]
- Wan, G.-L.; Fan, S.-X.; Liu, G.S.; He, J.G.; Wang, W.; Li, Y.; Cheng, L.J.; Ma, C.; Guo, M. Fusion of spectra and texture data of hyperspectral imaging for prediction of myoglobin content in nitrite-cured mutton. Food Control 2023, 144, 109332. [Google Scholar]
- Zhang, J.J.; Ma, Y.H.; Liu, G.S.; Fan, N.Y.; Li, Y.; Sun, Y.R. Rapid evaluation of texture parameters of Tan mutton using hyperspectral imaging with optimization algorithms. Food Control 2022, 135, 108815. [Google Scholar] [CrossRef]
- Zhang, J.J.; Liu, G.S.; Li, Y.; Guo, M.; Pu, F.N.; Wang, H. Rapid identification of lamb freshness grades using visible and near-infrared spectroscopy (Vis-NIR). J. Food Compos. Anal. 2022, 111, 104590. [Google Scholar]
- Ma, F.; Zhang, B.; Wang, W.; Li, P.J.; Niu, X.L.; Chen, C.; Zheng, L. Potential use of multispectral imaging technology to identify moisture content and water-holding capacity in cooked pork sausages. J. Sci. Food Agric. 2018, 98, 1832–1838. [Google Scholar] [PubMed]
- Aheto, J.H.; Huang, X.Y.; Tian, X.Y.; Ren, Y.; Bonah, E.; Alenyorege, E.A.; Lv, R.Q.; Dai, C.X. Combination of spectra and image information of hyperspectral imaging data for fast prediction of lipid oxidation attributes in pork meat. J. Food Process Eng. 2019, 42, e13225. [Google Scholar]
- Liu, S.J.; Dong, F.J.; Hao, J.; Qiao, L.; Guo, J.H.; Wang, S.L.; Luo, R.M.; Lv, Y.; Cui, J.R. Combination of hyperspectral imaging and entropy weight method for the comprehensive assessment of antioxidant enzyme activity in Tan mutton. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2023, 291, 122342. [Google Scholar]
- Feng, C.H.; Makino, Y.; García-Martín, J.F. Hyperspectral imaging coupled with multivariate analysis and image processing for detection and visualisation of colour in cooked sausages stuffed with different modified casings. Foods 2020, 9, 1089. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.H.; Makino, Y.; Yoshimura, M.; Rodríguez-Pulido, F.J. Real-time prediction of pre-cooked Japanese sausages color with different storage days using hyperspectral imaging. J. Sci. Food Agric. 2018, 98, 2564–2572. [Google Scholar]
- Feng, C.H.; Makino, Y.; Yoshimura, M.; Thuyet, D.C.; García-Martín, J.F. Hyperspectral imaging in tandem with R statistics and image processing for detection and visualisation of pH in Japanese big sausages under different storage conditions. J. Food Sci. 2018, 83, 358–366. [Google Scholar]
- Feng, C.H.; Arai, H.; Rodríguez-Pulido, F.J. Evaluation of pH in sausages stuffed in a modified casing with orange extracts by hyperspectral imaging coupled with response surface methodology. Foods 2022, 11, 2797. [Google Scholar] [CrossRef]
- Feng, C.H.; Makino, Y.; Yoshimura, M.; Rodríguez-Pulido, F.J. Estimation of adenosine triphosphate content in ready-to-eat sausages with different storage days, using hyperspectral imaging coupled with R statistics. Food Chem. 2018, 264, 419–426. [Google Scholar]
- Feng, C.H.; García-Martín, J.F.; Lavado, M.B.; López-Barrera, M.C.; Álvarez-Mateos, P. Evaluation of different solvents on flavonoids extraction efficiency from sweet oranges and ripe and immature Seville oranges. Int. J. Food Sci. 2020, 55, 3123–3134. [Google Scholar] [CrossRef]
- Cazón, P.; Antoniewska, A.; Rutkowska, J.; Vázquez, M. Evaluation of easy-removing antioxidant films of chitosan with Melaleuca alternifolia essential oil. Int. J. Biol. Macromol. 2021, 186, 365–376. [Google Scholar] [PubMed]
- Groussard, C.; Morel, I.; Chevanne, M.; Monnier, M.; Cillard, J.; Delamarche, A. Free radical scavenging and antioxidant effects of lactate ion: An in vitro study. J. Appl. Physiol. 2000, 89, 169–175. [Google Scholar]
- Ramadan, M.F. Antioxidant characteristics of phenolipids (quercetin-enriched lecithin) in lipid matrices. Ind. Crops. Prod. 2012, 36, 363–369. [Google Scholar]
- Tran, T.T.T.; Ton, N.M.N.; Nguyen, T.T.; Sajeev, D.; Schilling, M.W.; Dinh, T.T. Application of natural antioxidant extract from guava leaves (Psidium guajava L.) in fresh pork sausage. Meat Sci. 2020, 165, 108106. [Google Scholar] [PubMed]
- Bellucci, E.R.B.; Munekata, P.E.; Pateiro, M.; Lorenzo, J.M.; da Silva Barretto, A.C. Red pitaya extract as natural antioxidant in pork patties with total replacement of animal fat. Meat Sci. 2021, 171, 108284. [Google Scholar] [PubMed]
- Feng, C.H.; Arai, H. Evaluation of hesperidin on sausages stuffed in a new modified casing during long-term storage—A preliminary study. Sustainability 2022, 14, 9071. [Google Scholar] [CrossRef]
- Feng, C.H.; Arai, H. Estimating moisture content of sausages with different types of casings via hyperspectral imaging in tandem with multivariate. Appl. Sci. 2023, 13, 5300. [Google Scholar]
- Sanz, J.A.; Fernandes, A.M.; Barrenechea, E.; Silva, S.; Santos, V.; Gonçalves, N.; Paternain, D.; Jurio, A.; Melo-Pinto, P. Lamb muscle discrimination using hyperspectral imaging: Comparison of various machine learning algorithms. J. Food Eng. 2016, 174, 92–100. [Google Scholar]
- Jamil, Z.; Mohite, A.; Sharma, N. Selected engineering properties and drying behavior of tendu (diospyros melanoxylon roxb.) fruit. Curr. Res. Nutr. Food Sci. 2020, 8, 622–629. [Google Scholar]
- Jia, B.B.; Yoon, S.-C.; Zhuang, H.; Wang, W.; Li, C.Y. Prediction of pH of fresh chicken breast fillets by VNIR hyperspectral imaging. J. Food Eng. 2017, 08, 57–65. [Google Scholar]
- Feng, C.H. Optimizing procedures of ultrasound-assisted extraction of waste orange peels by response surface methodology. Molecules 2022, 27, 2268. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.L.; Riccioli, C.; Sun, D.-W. Development of an alternative technique for rapid and accurate determination of fish caloric density based hyperspectral imaging. J. Food Eng. 2016, 190, 185–194. [Google Scholar]
- Helland, I.S.; Næs, T.; Isaksson, T. Related versions of the multiplicative scatter correction method for preprocessing spectroscopic data. Chemom. Intell. Lab. Syst. 1995, 29, 233–241. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).