Performance of Regenerated Activated Carbons on Pesticides Removal from the Aqueous Phase
Abstract
:1. Introduction
2. Materials and Methods
2.1. ACs Structural and Chemical Characterization
2.2. Pesticide Adsorption from Liquid-Phase
2.3. ACs Regeneration
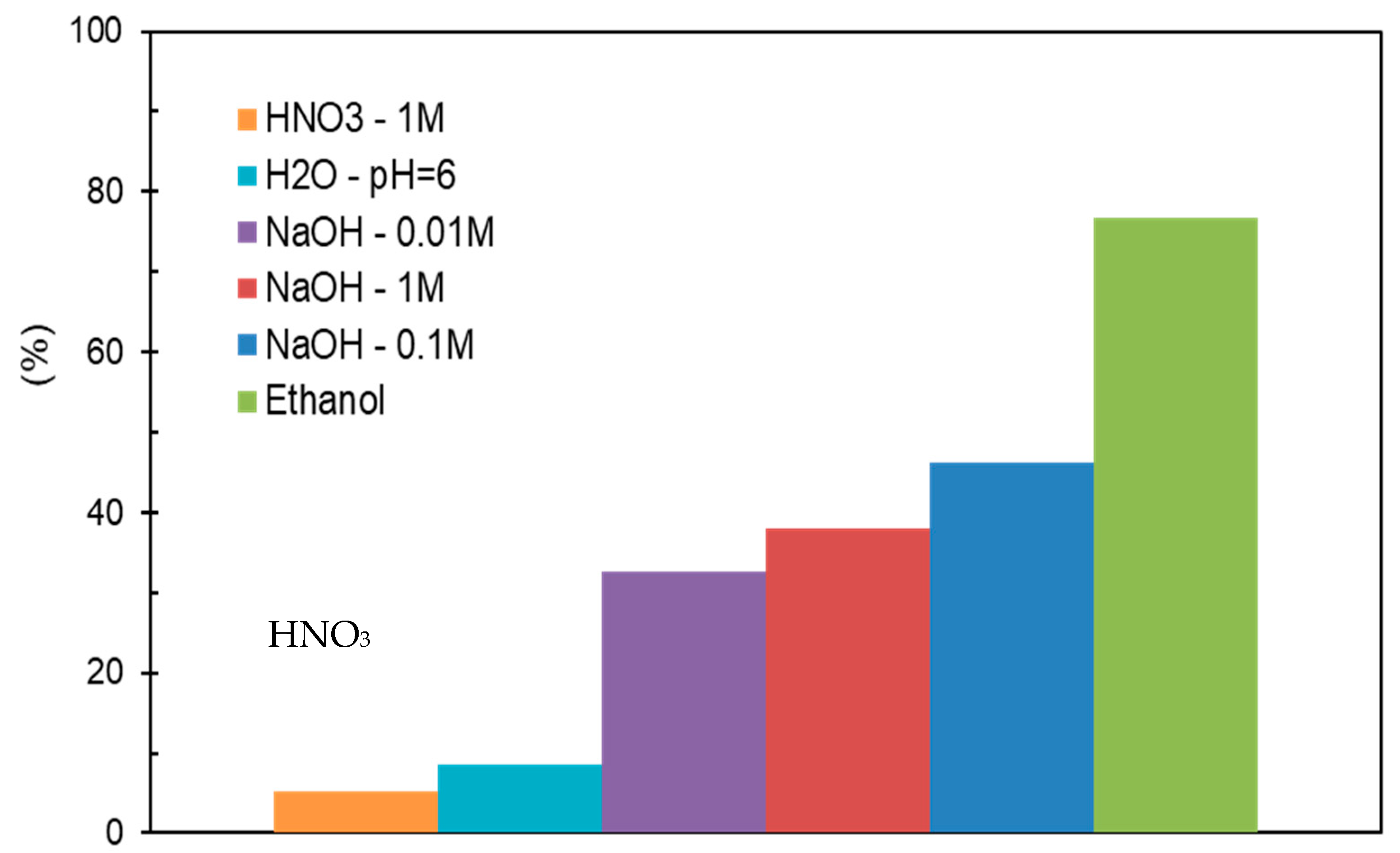
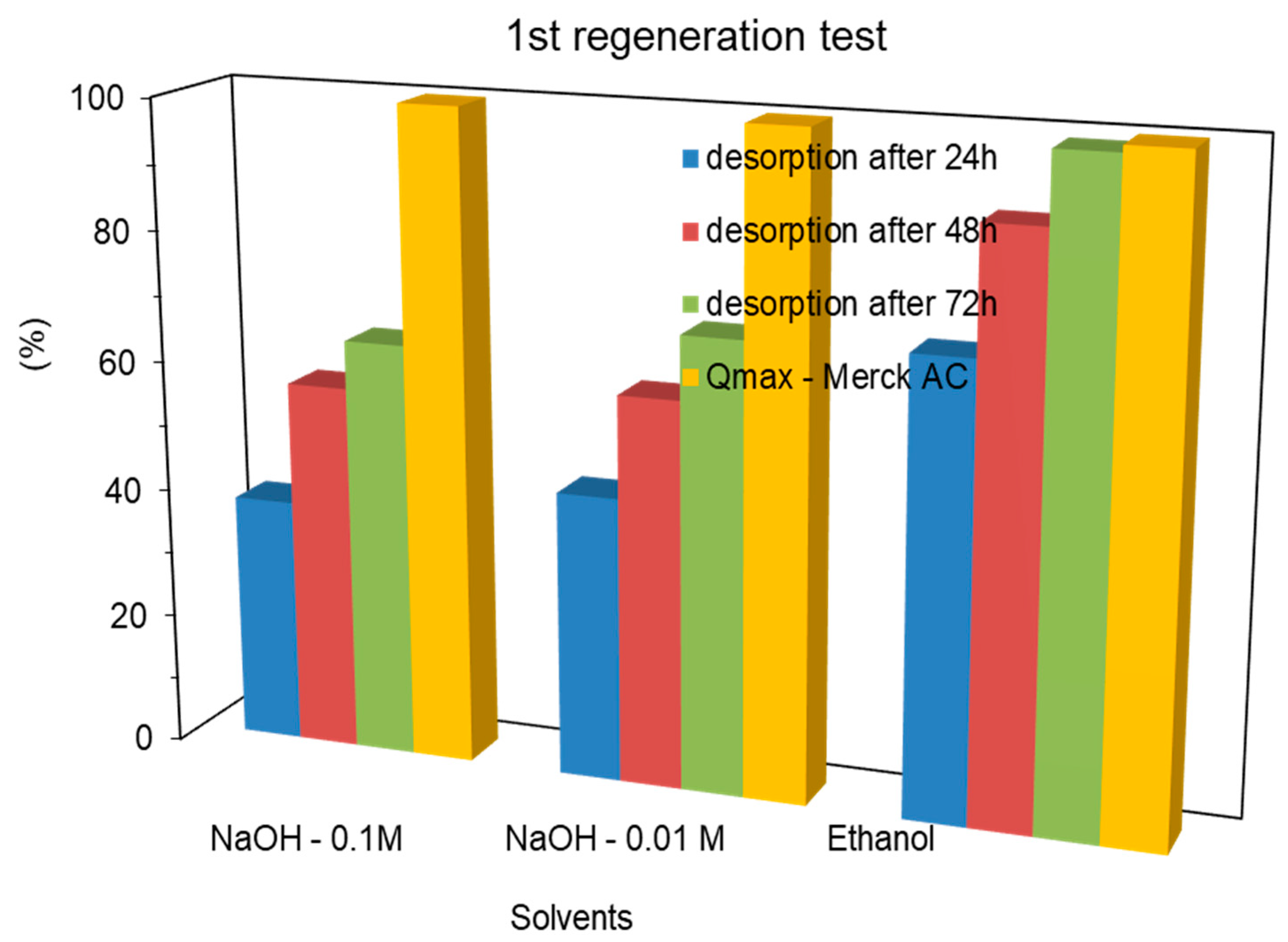
2.3.1. ACs Regeneration by Washing with Solvents
2.3.2. AC Regeneration by Basic Aqueous Washing Followed by Thermal Treatment
3. Results and Discussion
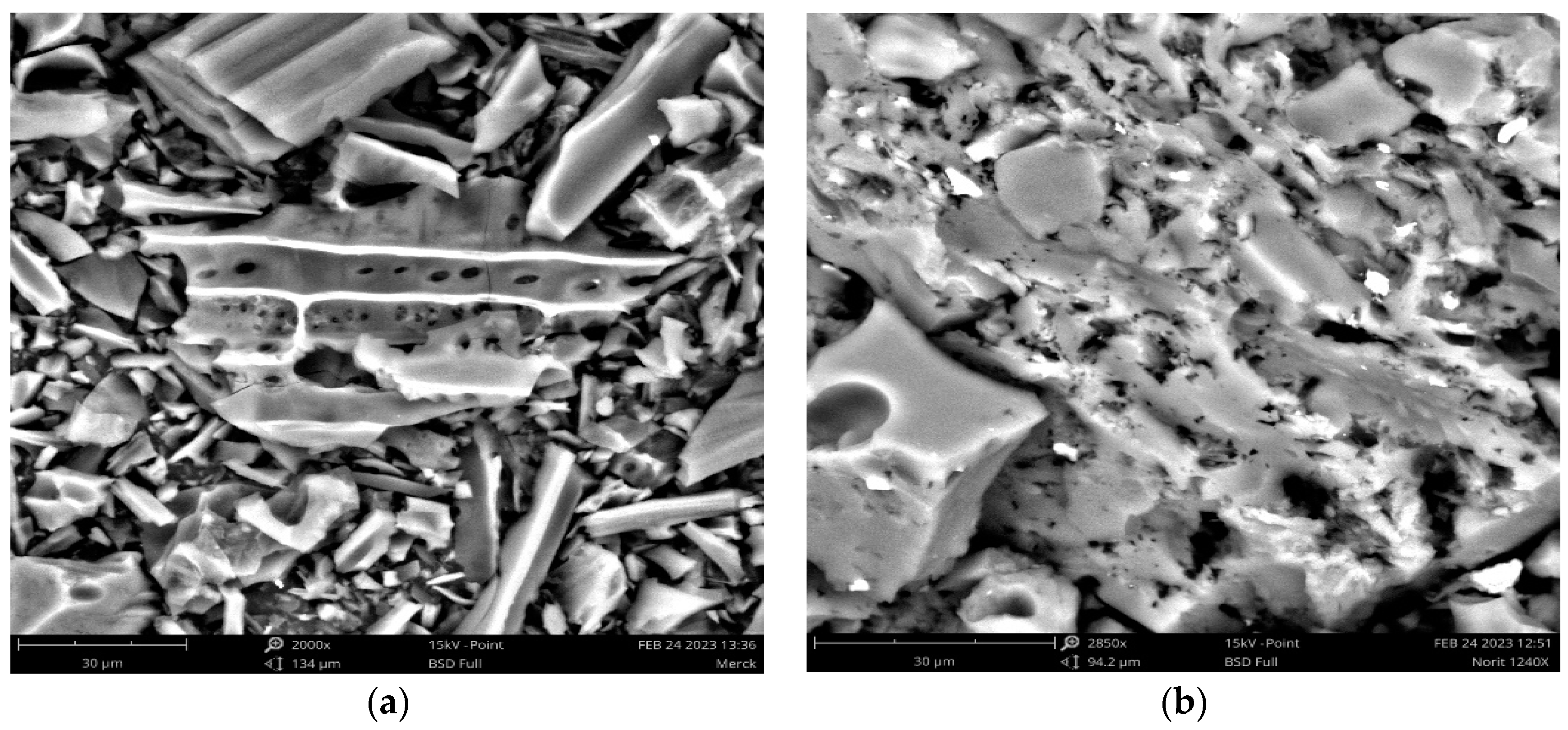
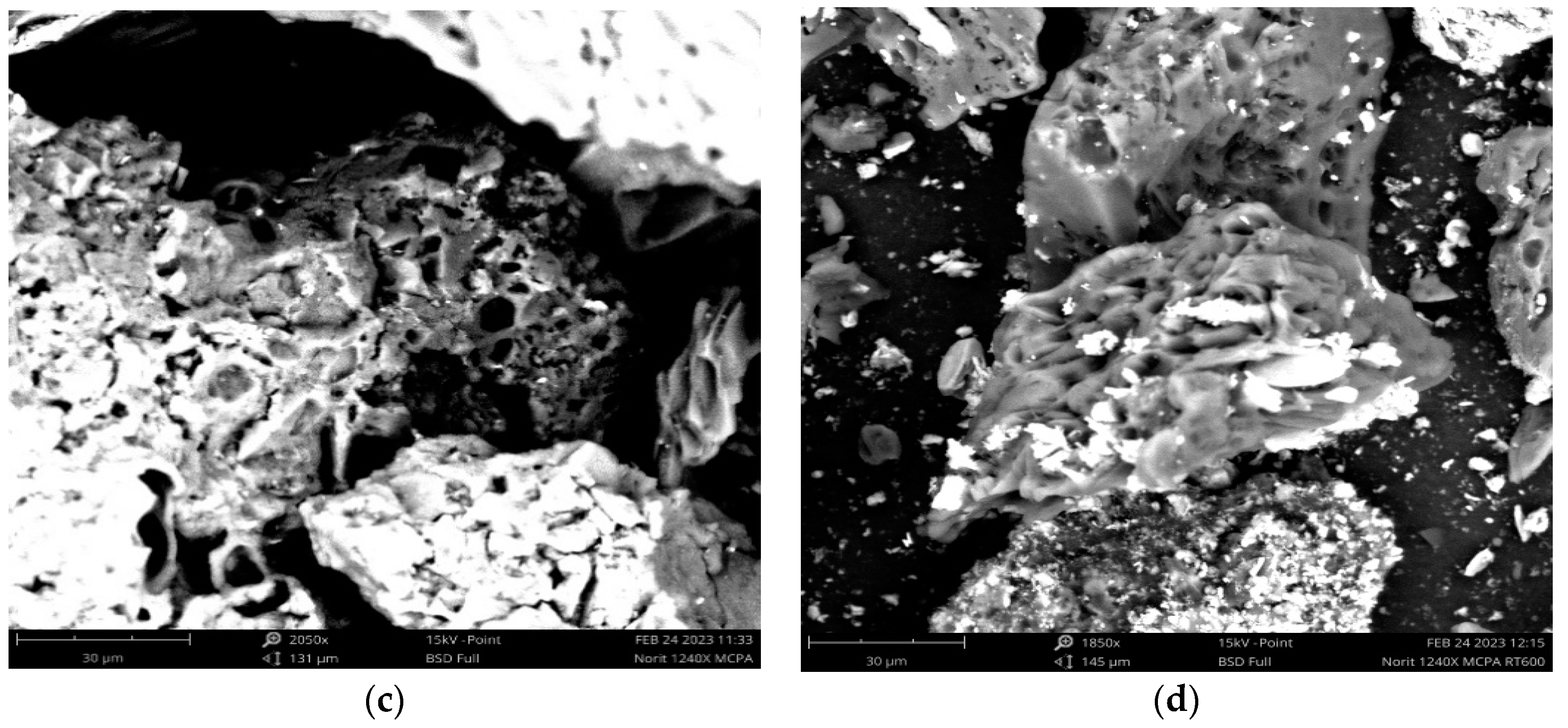
3.1. Characterization of the Adsorbent Materials
3.2. Chemical Characterization of the Adsorbent Materials
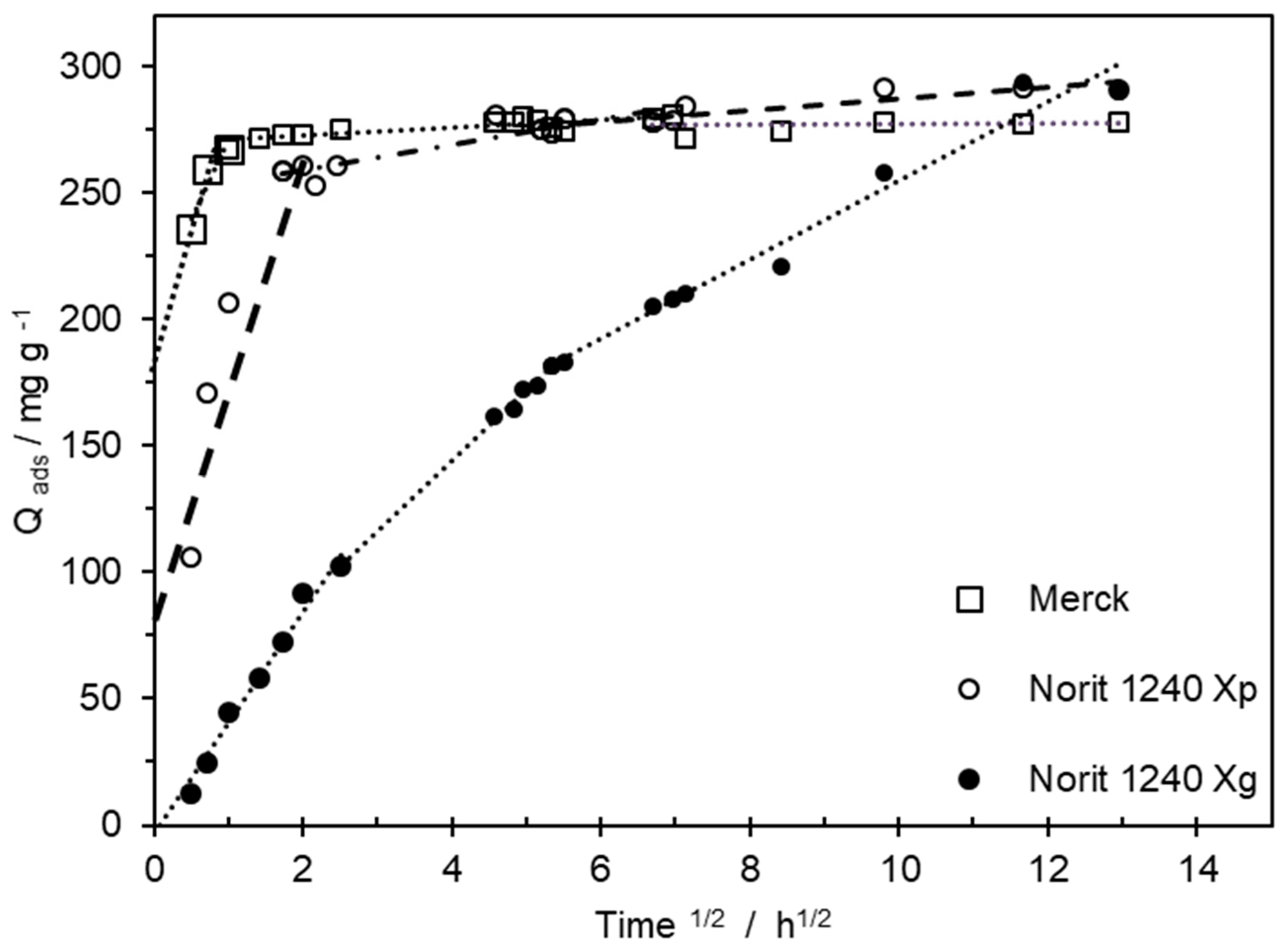
3.3. MCPA Kinetic Studies
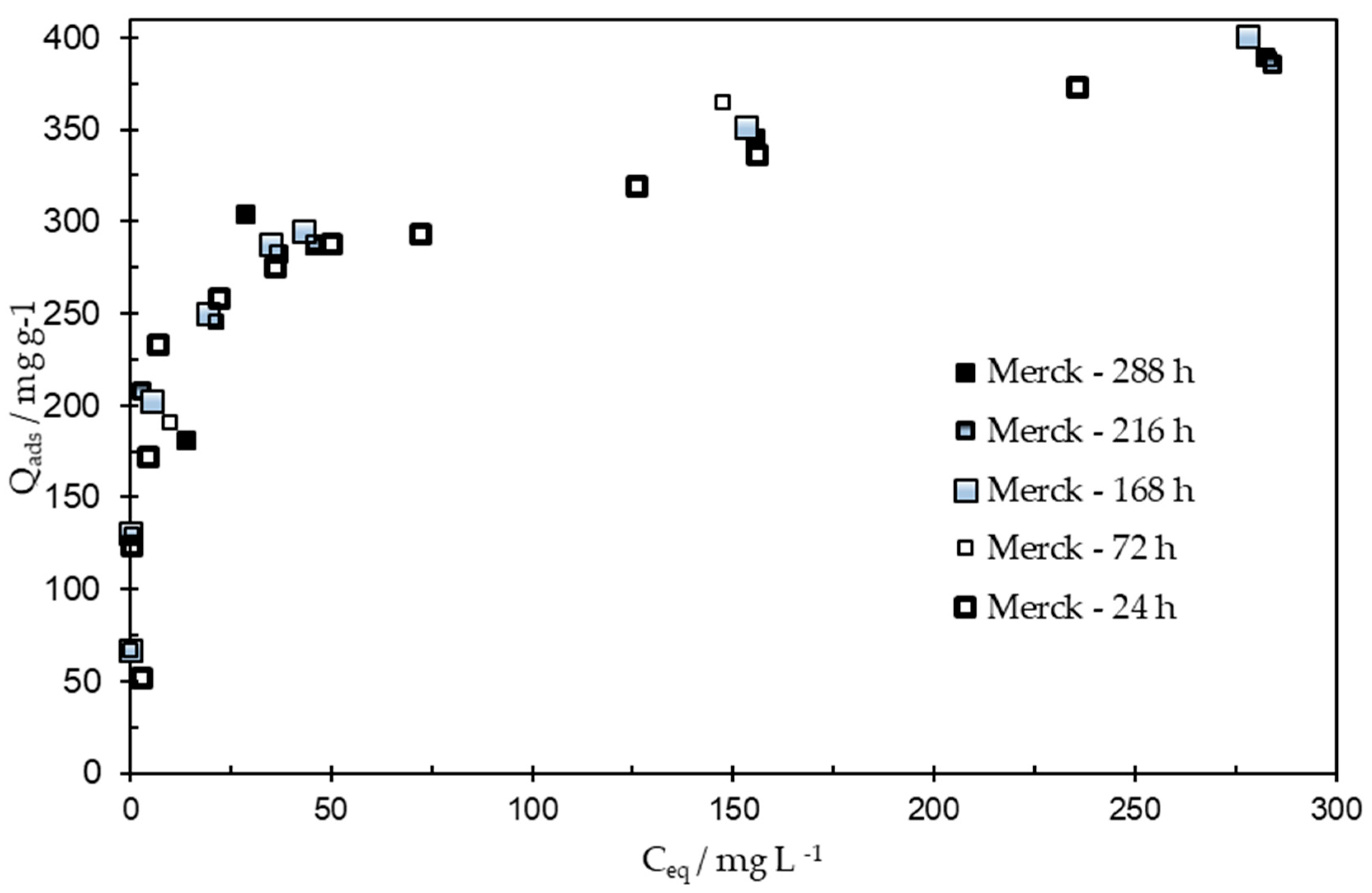
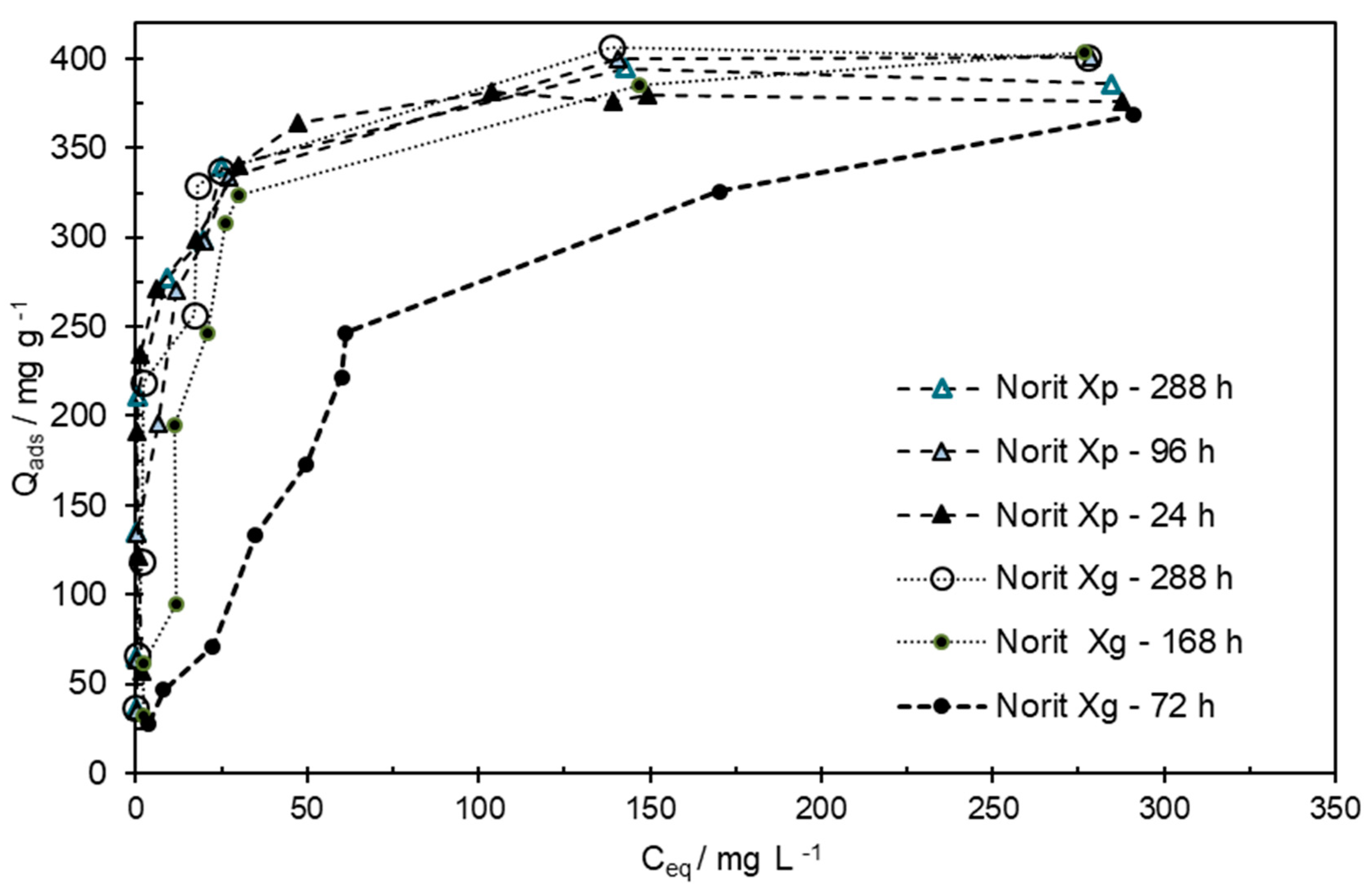
3.4. Pesticide Removal from the Aqueous Phase
3.5. Regeneration of ACs
3.6. Regeneration by Basic Aqueous Washing Followed by Thermal Treatment
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- He, H.; Liu, Y.; You, S.; Liu, J.; Xiao, H.; Tu, Z. A Review on Recent Treatment Technology for Herbicide Atrazine in Contaminated Environment. Int. J. Environ. Res. Public Health 2019, 16, 5129. [Google Scholar] [CrossRef] [PubMed]
- Kulaishin, S.A.; Vedenyapina, M.D.; Kurmysheva, A.Y. Influence of the Surface Characteristics of Activated Carbon on the Adsorption of Herbicides (A Review). Solid Fuel Chem. 2022, 56, 181–198. [Google Scholar] [CrossRef]
- Baskar, A.V.; Bolan, N.; Hoang, S.A.; Sooriyakumar, P.; Kumar, M.; Singh, L.; Jasemizad, T.; Padhye, L.P.; Singh, G.; Vinu, A.; et al. Recovery, regeneration and sustainable management of spent adsorbents from wastewater treatment streams: A review. Sci. Total. Environ. 2022, 822, 153555–153579. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, B.; Zheng, T.; Wang, P. Regeneration of activated carbon saturated with chloramphenicol by microwave and ultraviolet irradiation. Chem. Eng. J. 2017, 320, 264–270. [Google Scholar] [CrossRef]
- Zhang, L.-Q.; Jiang, H.-T.; Ma, C.-Y.; Yong, D. Microwave regeneration characteristics of activated carbon for flue gas desulfurization. J. Fuel Chem. Technol. 2012, 40, 1366–1371. [Google Scholar] [CrossRef]
- González-Poggini, S.; Rosenkranz, A.; Colet-Lagrille, M. Two-Dimensional Nanomaterials for the Removal of Pharmaceuticals from Wastewater: A Critical Review. Processes 2021, 9, 2160. [Google Scholar] [CrossRef]
- Cansado, I.P.d.P.; Belo, C.R.; Mourão, P.A.M. Valorisation of Tectona Grandis tree sawdust through the production of high activated carbon for environment applications. Bioresour. Technol. 2018, 249, 328–333. [Google Scholar] [CrossRef]
- Cansado, I.P.d.P.; Mourão, P.A.M.; Belo, C.R. Using Tectona Grandis Biomass to Produce Valuable Adsorbents for Pesticide Removal from Liquid Effluent. Materials 2022, 15, 5842. [Google Scholar] [CrossRef]
- Husien, S.; El-Taweel, R.M.; Salim, A.I.; Fahim, I.S.; Said, L.A.; Radwan, A.G. Review of activated carbon adsorbent material for textile dyes removal: Preparation, and modelling. Curr. Res. Green Sustain. Chem. 2022, 5, 100325–100340. [Google Scholar] [CrossRef]
- Cazetta, A.L.; Junior, O.P.; Vargas, A.M.; da Silva, A.P.; Zou, X.; Asefa, T.; Almeida, V.C. Thermal regeneration study of high surface area activated carbon obtained from coconut shell: Characterization and application of response surface methodology. J. Anal. Appl. Pyrolysis 2013, 101, 53–60. [Google Scholar] [CrossRef]
- Rao, M.M.; Ramana, D.; Seshaiah, K.; Wang, M.; Chien, S.C. Removal of some metal ions by activated carbon prepared from Phaseolus aureus hulls. J. Hazard. Mater. 2009, 166, 1006–1013. [Google Scholar] [CrossRef] [PubMed]
- Mourão, P.A.M.; Di Caprio, F.; Cansado, I.P.P.; Castanheiro, J.; Falcone, I.; Astolfi, M.L.; Pagnanelli, F. Granulation and ac-tivation of an arsenic adsorbent made of iron oxide doped hydrochar. Chem. Eng. Trans. 2022, 93, 91–96. [Google Scholar] [CrossRef]
- Ouyang, J.; Zhou, L.; Liu, Z.; Heng, J.Y.; Chen, W. Biomass-derived activated carbons for the removal of pharmaceutical mircopollutants from wastewater: A review. Sep. Purif. Technol. 2020, 253, 117536–117553. [Google Scholar] [CrossRef]
- Ania, C.; Parra, J.; Menéndez, J.; Pis, J. Microwave-assisted regeneration of activated carbons loaded with pharmaceuticals. Water Res. 2007, 41, 3299–3306. [Google Scholar] [CrossRef] [PubMed]
- Omorogie, M.O.; Babalola, J.O.; Unuabonah, E.I. Regeneration strategies for spent solid matrices used in adsorption of organic pollutants from surface water: A critical review. Desalinat. Water Treat. 2016, 57, 518–544. [Google Scholar] [CrossRef]
- Nahm, S.W.; Shim, W.G.; Park, Y.-K.; Kim, S.C. Thermal and chemical regeneration of spent activated carbon and its adsorption property for toluene. Chem. Eng. J. 2012, 210, 500–509. [Google Scholar] [CrossRef]
- Hwang, S.Y.; Lee, G.B.; Kim, J.H.; Hong, B.U.; Park, J.E. Pre-Treatment Methods for Regeneration of Spent Activated Carbon. Molecules 2020, 25, 4561. [Google Scholar] [CrossRef]
- Román, S.; Ledesma, B.; González, J.; Al-Kassir, A.; Engo, G.; Álvarez-Murillo, A. Two stage thermal regeneration of exhausted activated carbons. Steam gasification of effluents. J. Anal. Appl. Pyrolysis 2013, 103, 201–206. [Google Scholar] [CrossRef]
- Salvador, F.; Martin-Sanchez, N.; Sanchez-Hernandez, R.; Sanchez-Montero, M.J.; Izquierdo, C. Regeneration of carbonaceous adsorbents. Part I: Thermal Regeneration. Microporous Mesoporous Mater. 2015, 202, 259–276. [Google Scholar] [CrossRef]
- Salvador, F.; Martin-Sanchez, N.; Sanchez-Hernandez, R.; Sanchez-Montero, M.J.; Izquierdo, C. Regeneration of carbonaceous adsorbents. Part II: Chemical, Microbiological and Vacuum Regeneration. Microporous Mesoporous Mater. 2015, 202, 277–296. [Google Scholar] [CrossRef]
- Soto, M.L.; Moure, A.; Domínguez, H.; Parajó, J.C. Recovery, concentration and purification of phenolic compounds by adsorption: A review. J. Food Eng. 2011, 105, 1–27. [Google Scholar] [CrossRef]
- Álvarez, P.; Beltrán, F.; Gómez-Serrano, V.; Jaramillo, J.; Rodríguez, E. Comparison between thermal and ozone regenerations of spent activated carbon exhausted with phenol. Water Res. 2004, 38, 2155–2165. [Google Scholar] [CrossRef] [PubMed]
- Pak, S.-H.; Jeon, Y.-W. Effect of vacuum regeneration of activated carbon on volatile organic compound adsorption. Environ. Eng. Res. 2017, 22, 169–174. [Google Scholar] [CrossRef]
- Larasati, A.; Fowler, G.D.; Graham, N.J. Extending granular activated carbon (GAC) bed life: A column study of in-situ chemical regeneration of pesticide loaded activated carbon for water treatment. Chemosphere 2022, 286, 131888–131998. [Google Scholar] [CrossRef] [PubMed]
- Mourão, P.A.; Carrott, P.J.; Carrott, M.M.R.; Marques, L. Different Ways to Regenerate an Activated Carbon: Comparison between an Activated Carbon from Cork and a Commercial Carbon. Mater. Sci. Forum 2008, 587–588, 844–848. [Google Scholar] [CrossRef]
- Liao, P.; Yuan, S.; Xie, W.; Zhang, W.; Tong, M.; Wang, K. Adsorption of nitrogen-heterocyclic compounds on bamboo charcoal: Kinetics, thermodynamics, and microwave regeneration. J. Colloid Interface Sci. 2013, 390, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Cansado, I.P.d.P.; Mourão, P.A.M.; Morais, I.D.; Peniche, V.; Janeirinho, J. Removal of 4-Ethylphenol and 4-Ethylguaiacol, from Wine-like Model Solutions, by Commercial Modified Activated Carbons Produced from Coconut Shell. Appl. Sci. 2022, 12, 11754. [Google Scholar] [CrossRef]
- Belo, C.R.; Cansado, I.P.d.P.; Mourão, P.A.M. Synthetic polymers blend used in the production of high activated carbon for pesticides removals from the liquid phase. Environ. Technol. 2017, 38, 285–296. [Google Scholar] [CrossRef]
- Spaltro, A.; Pila, M.; Simonetti, S.; Álvarez-Torrellas, S.; Rodríguez, J.G.; Ruiz, D.; Compañy, A.D.; Juan, A.; Allegretti, P. Adsorption and removal of phenoxy acetic herbicides from water by using commercial activated carbons: Experimental and computational studies. J. Contam. Hydrol. 2018, 218, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization for the United Nation. FAO Specifications and Evaluations for Agricultural Pesticides. 2022. Available online: https://www.fao.org/3/cc4226en/cc4226en.pdf (accessed on 22 July 2023).
- Simonin, J.P. On the comparison of pseudo-first order and pseudo-second order rate laws in the modeling of adsorption kinetics. Chem. Eng. J. 2016, 300, 254–263. [Google Scholar] [CrossRef]
- Tochetto, G.A.; Brandler, D.; Pigatto, J.; Pasquali, G.D.L.; Alves, A.A.d.A.; Kempka, A.P.; da Luz, C.; Dervanoski, A. Kinetic modeling of the adsorption and desorption of metallic ions present in effluents using the biosorbent obtained from Syagrus romanzoffiana. Environ. Monit. Assess. 2023, 195, 844–902. [Google Scholar] [CrossRef] [PubMed]
- Revellame, E.D.; Fortela, D.L.; Sharp, W.; Hernandez, R.; Zappi, M.E. Adsorption kinetic modeling using pseudo-first order and pseudo-second order rate laws: A review. Clean. Eng. Technol. 2020, 1, 100032–100045. [Google Scholar] [CrossRef]
- An, F.-Q.; Wu, R.-Y.; Li, M.; Hu, T.-P.; Gao, J.-F.; Yuan, Z.-G. Adsorption of heavy metal ions by iminodiacetic acid functionalized D301 resin: Kinetics, isotherms and thermodynamics. React. Funct. Polym. 2017, 118, 42–50. [Google Scholar] [CrossRef]


| Activated Carbon | ABET/m2 g−1 | As/m2 g−1 | Vs/cm3 g−1 | V0/cm3 g−1 | L0/nm | C/% | H/% | S/% | pHpzc |
|---|---|---|---|---|---|---|---|---|---|
| Norit 1240 X | 975 | 60 | 0.43 | 0.23 | 1.04 | 84.4 | 0.19 | 0.49 | 9.73 |
| Merck | 927 | 170 | 0.36 | 0.23 | 1.02 | 85.2 | 0.22 | 0.32 | 7.27 |
| ACs | Qmax, exp /mg g−1 | Pseudo-First-Order Model | Pseudo-Second-Order Model | |||||
|---|---|---|---|---|---|---|---|---|
| Qmax1, cal1 /mg g−1 | K1 /h−1 | R2 | Qmax, cal2 /mg g−1 | V0/ mg g−1 h−1 | K2 /h−1 | R2 | ||
| Merck | 288.1 | 10.2 | 0.0086 | 0.95 | 277.8 | 5000 | 0.13 | 0.98 |
| Norit 1240 Xp | 290.7 | 3.1 | 0.019 | 0.75 | 285.7 | 833 | 0.020 | 0.99 |
| Norit 1240 Xg | 289.6 | 10.3 | 0.011 | 0.99 | 188.7 | 52.6 | 0.0015 | 0.96 |
| ACs | Qads (max) mg g−1 | Kip1 mg g−1 h1/2 | C | Kip2 mg g−1 h1/2 | C | Kip3 mg g−1 h1/2 | C |
|---|---|---|---|---|---|---|---|
| Merck | 288.1 | 114.0 | 178.8 | 0.8 | 271.3 | 0.2 | 75.2 |
| Norit 1240 Xp | 290.7 | 112.2 | 74.8 | 4.7 | 249.8 | 3.2 | 264.0 |
| Norit 1240 Xg | 289.6 | 44.0 | -- | 27.8 | 32.4 | 15.6 | 98.6 |
| Activated Carbon | Regeneration Cycles | Qads/ mg g−1 | Qads/ mg g−1 | Qads/ mg g−1 |
|---|---|---|---|---|
| NaOH—0.1 mol dm−3 | NaOH—0.01 mol dm−3 | Etanol | ||
| Merck | R1 | 229.7 | 230.7 | 231.6 |
| R2 | 169.9 | 176.4 | 197.3 | |
| R3 | 76.3 | 55.7 | 96.5 | |
| R4 − (R3 + T = 573 K) | 124.6 | 147.4 | 182.7 | |
| Norit 1240 Xp (powder) | R1 | 226.9 | 232.2 | 231.9 |
| R2 | 207.4 | 178.0 | 212.1 | |
| R3 | 79.2 | 67.4 | 88.8 | |
| R4 − (R3 + T = 573 K) | 230.4 | 221.9 | 239.4 | |
| Norit 1240 Xg (granules) | R1 | 157.9 | 148.6 | 155.8 |
| R2 | 84.5 | 103.3 | 102.8 | |
| R2 + 96 h * | 141.3 | 167.8 | 180.4 | |
| R3 | 77.5 | 74.3 | 142.7 | |
| R4 (R3 + T = 573 K) | 126.1 | 154.3 | 181.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cansado, I.P.d.P.; Mourão, P.A.M.; Castanheiro, J.E.d.S.F. Performance of Regenerated Activated Carbons on Pesticides Removal from the Aqueous Phase. Processes 2023, 11, 2496. https://doi.org/10.3390/pr11082496
Cansado IPdP, Mourão PAM, Castanheiro JEdSF. Performance of Regenerated Activated Carbons on Pesticides Removal from the Aqueous Phase. Processes. 2023; 11(8):2496. https://doi.org/10.3390/pr11082496
Chicago/Turabian StyleCansado, Isabel Pestana da Paixão, Paulo Alexandre Mira Mourão, and José Eduardo dos Santos Félix Castanheiro. 2023. "Performance of Regenerated Activated Carbons on Pesticides Removal from the Aqueous Phase" Processes 11, no. 8: 2496. https://doi.org/10.3390/pr11082496
APA StyleCansado, I. P. d. P., Mourão, P. A. M., & Castanheiro, J. E. d. S. F. (2023). Performance of Regenerated Activated Carbons on Pesticides Removal from the Aqueous Phase. Processes, 11(8), 2496. https://doi.org/10.3390/pr11082496








