Preliminary Results of Innovative Two-Stage Torrefaction Technology Applied for Thermochemical Treatment of Sunflower Husk
Abstract
:1. Introduction
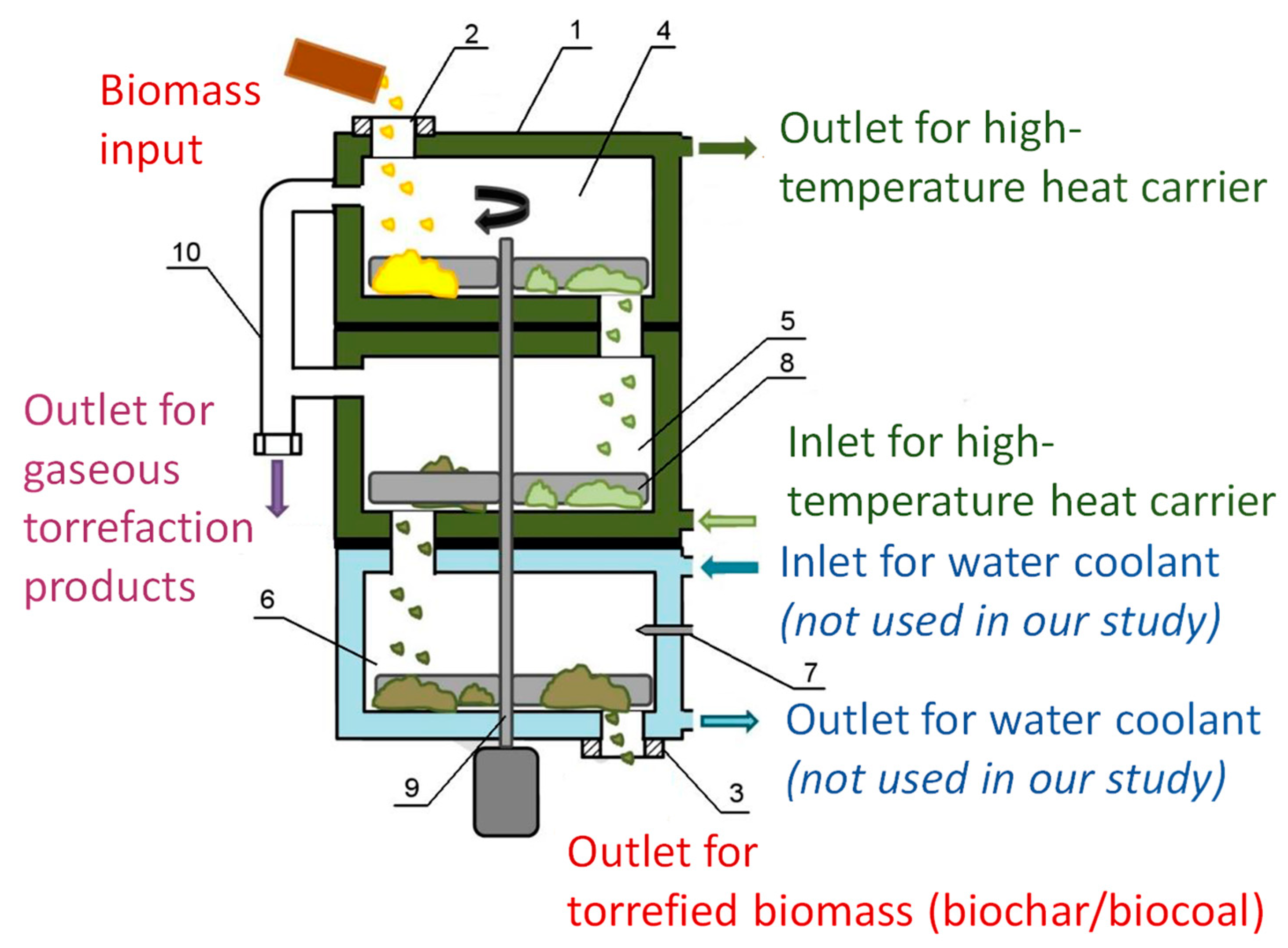
2. Materials and Methods
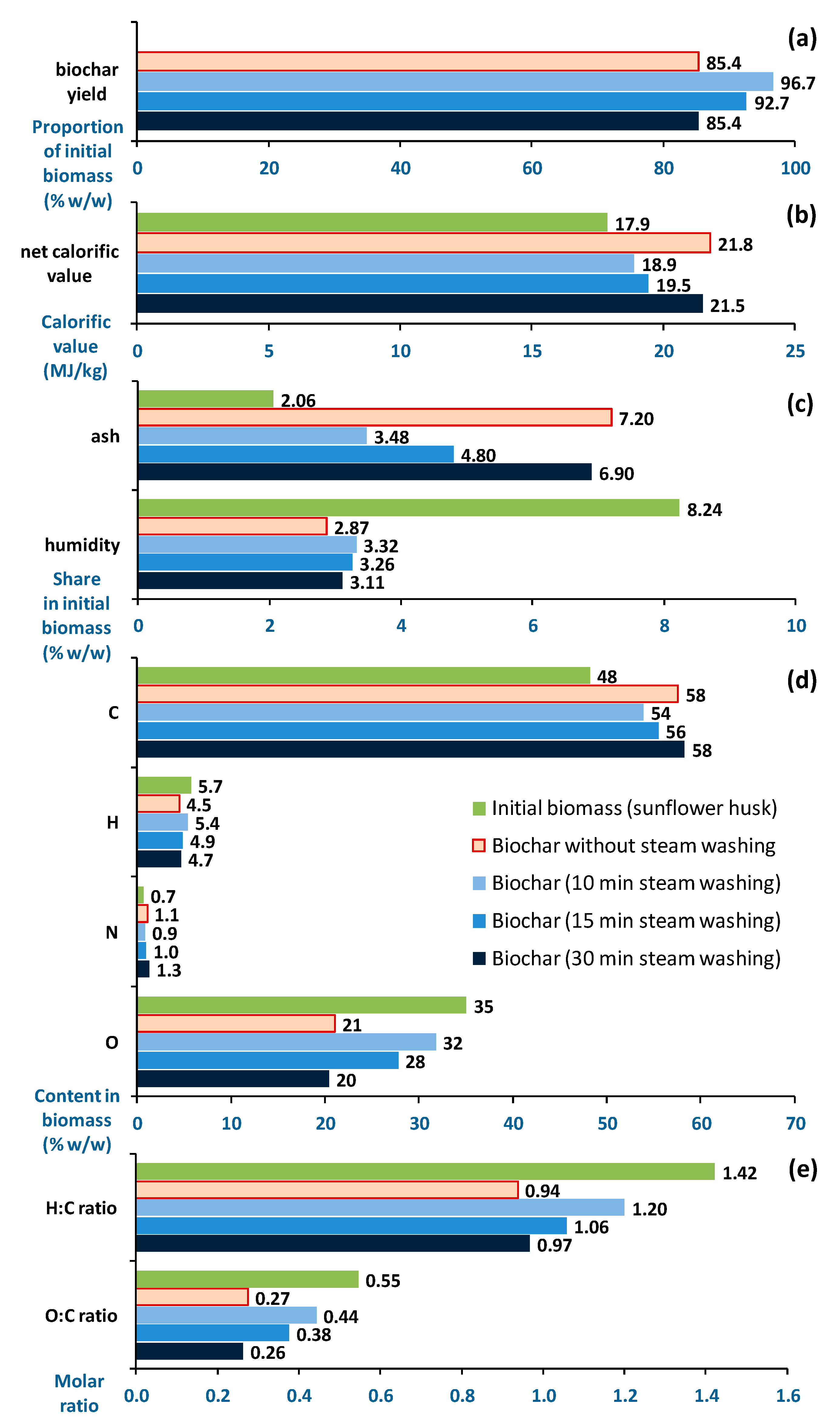
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- GEA. Global Energy Assessment—Toward a Sustainable Future; International Institute for Applied Systems Analysis: New York, NY, USA; Vienna, Austria; Cambridge University Press: Cambridge, UK, 2012. [Google Scholar]
- Perea-Moreno, M.-A.; Manzano-Agugliaro, F.; Perea-Moreno, A.-J. Sustainable Energy Based on Sunflower Seed Husk Boiler for Residential Buildings. Sustainability 2018, 10, 3407. [Google Scholar] [CrossRef]
- De Fusco, L.; Boucquey, A.; Blondeau, J.; Jeanmart, H.; Contino, F. Fouling propensity of high-phosphorus solid fuels: Predictive criteria and ash deposits characterization of sunflower hulls with P/Ca-additives in a drop tube furnace. Fuels 2016, 170, 16–26. [Google Scholar] [CrossRef]
- Singhal, A.; Konttinen, J.; Joronen, T. Effect of different washing parameters on the fuel properties and elemental composition of wheat straw in water-washing pre-treatment. Part 1: Effect of washing duration and biomass size. Fuel 2021, 292, 120206. [Google Scholar] [CrossRef]
- Singhal, A.; Konttinen, J.; Joronen, T. Effect of different washing parameters on the fuel properties and elemental composition of wheat straw in water-washing pre-treatment. Part 2: Effect of washing temperature and solid-to-liquid ratio. Fuel 2021, 292, 120209. [Google Scholar] [CrossRef]
- Deng, L.; Zhang, T.; Che, D. Effect of water washing on fuel properties, pyrolysis and combustion characteristics, and ash fusibility of biomass. Fuel Process. Technol. 2013, 106, 712–720. [Google Scholar] [CrossRef]
- Runge, T.; Wipperfurth, P.; Zhang, C. Improving biomass combustion quality using a liquid hot water treatment. Biofuels 2013, 4, 73–83. [Google Scholar] [CrossRef]
- Liu, T.; Wen, C.; Li, C.; Yan, K.; Li, R.; Jing, Z.; Zhang, B.; Ma, J. Integrated water washing and carbonization pretreatment of typical herbaceous and woody biomass: Fuel properties, combustion behaviors, and techno-economic assessments. Renew. Energy 2022, 200, 218–223. [Google Scholar] [CrossRef]
- Cen, K.; Chen, D.; Wang, J.; Cai, Y.; Wang, L. Effects of water washing and torrefaction pretreatments on corn stalk pyrolysis: Combined study using TG-FTIR and a fixed bed reactor. Energy Fuels 2016, 30, 10627–10634. [Google Scholar] [CrossRef]
- Chen, C.Y.; Chen, W.H.; Ilham, Z. Effects of torrefaction and water washing on the properties and combustion reactivity of various wastes. Int. J. Energy Res. 2021, 45, 8125–8139. [Google Scholar] [CrossRef]
- Chen, C.Y.; Chen, W.H.; Hung, C.H. Combustion performance and emissions from torrefied and water washed biomass using a kg-scale burner. J. Hazard. Mater. 2021, 402, 123468. [Google Scholar] [CrossRef]
- Zhang, S.; Zhu, S.; Zhang, H.; Liu, X.; Zhang, H. Evaluation of pyrolysis behavior and products properties of rice husk after combined pretreatment of washing and torrefaction. Biomass Bioenerg. 2019, 127, 105293. [Google Scholar] [CrossRef]
- Abelha, P.; Vilela, C.M.; Nanou, P.; Carbo, M.; Janssen, A.; Leiser, S. Combustion improvements of upgraded biomass by washing and torrefaction. Fuel 2019, 253, 1018–1033. [Google Scholar] [CrossRef]
- Saddawi, A.; Jones, J.M.; Williams, A.; Le Coeur, C. Commodity fuels from biomass through pretreatment and torrefaction: Effects of mineral content on torrefied fuel characteristics and quality. Energy Fuels 2012, 26, 6466–6474. [Google Scholar] [CrossRef]
- Zhang, S.; Dong, Q.; Zhang, L.; Xiong, Y. Effects of water washing and torrefaction on the pyrolysis behavior and kinetics of rice husk through TGA and Py-GC/MS. Bioresour. Technol. 2016, 199, 352–361. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Mei, J.; Li, H.; Li, Y.; Lu, M.; Ma, T.; Ma, Z. Combined pretreatment with torrefaction and washing using torrefaction liquid products to yield upgraded biomass and pyrolysis products. Bioresour. Technol. 2017, 228, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Mei, J.; Li, H.; Li, Y.; Lu, M.; Ma, T.; Ma, Z. An approach for upgrading biomass and pyrolysis product quality using a combination of aqueous phase bio-oil washing and torrefaction pretreatment. Bioresour. Technol. 2017, 233, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Teng, H.; Wei, Y.C. Thermogravimetric studies on the kinetics of rice hull pyrolysis and the influence of water treatment. Ind. Eng. Chem. Res. 1998, 37, 3806–3811. [Google Scholar] [CrossRef]
- Ross, A.B.; Anastasakis, K.; Kubacki, M.; Jones, J.M. Investigation of the pyrolysis behavior of brown algae before and after pre-treatment using PY-GC/MS and TGA. J. Anal. Appl. Pyrolysis 2009, 85, 3–10. [Google Scholar] [CrossRef]
- Zhang, L.; Li, S.; Ding, H.; Zhu, X. Two-step pyrolysis of corncob for value-added chemicals and high-quality bio-oil: Effects of alkali and alkaline earth metals. Waste Manag. 2019, 87, 709–718. [Google Scholar] [CrossRef]
- Zhang, L.; Huang, L.; Li, S.; Zhu, X. Study on two-step pyrolysis of walnut shell coupled with acid washing pretreatment. J. Anal. Appl. Pyrolysis 2018, 136, 1–7. [Google Scholar] [CrossRef]
- Chen, D.; Wang, Y.; Liu, Y.; Cen, K.; Cao, X.; Ma, Z.; Li, Y. Comparative study on the pyrolysis behaviors of rice straw under different washing pretreatments of water, acid solution, and aqueous phase bio-oil by using TG-FTIR and Py-GC/MS. Fuel 2019, 252, 1–9. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Singhal, A.; Tolvanen, H.; Valtonen, K.; Joronen, T.; Konttinen, J. Effect of pretreatment and biomass blending on bio-oil and biochar quality from two-step slow pyrolysis of rice straw. Waste Manag. 2022, 138, 298–3074. [Google Scholar] [CrossRef] [PubMed]
- Klasson, K.T.; Boihem Jr, L.L.; Uchimiya, M.; Lima, I.M. Influence of biochar pyrolysis temperature and post-treatment on the uptake of mercury from flue gas. Fuel Process. Technol. 2014, 123, 27–33. [Google Scholar] [CrossRef]
- Yan, K.; Wen, C.; Liu, T.; Ma, J.; Li, R.; Wu, J.; Jing, Z. Effects of torrefaction and water-washing on the mineral transformation behavior during co-combustion of straw and coal: A CCSEM analysis. J. Energy Inst. 2021, 98, 124–130. [Google Scholar] [CrossRef]
- Abelha, P.; Janssen, A.; Vilela, C.M.; Nanou, P.; Carbo, M. Low-grade biomass upgrading by washing and torrefaction: Lab and pilot-scale results. In Proceedings of the 26th European Biomass Conference and Exhibition, Copenhagen, Denmark, 14–18 May 2018; pp. 1209–1220. [Google Scholar]
- Yrjas, P.; Carbo, M.; Janssen, A.; Abelha, P.; Leino, T.; Hupa, L. Influence of fuel pre-treatments on ash-forming elements and implications on corrosion. In Proceedings of the 23rd International Conference on FBC, Grand Ambassador Hotel, Seoul, Republic of Korea, 13–17 May 2018. [Google Scholar]
- Bilgic, E.; Yama, S.; Haykiri-Acma, H.; Kucukbayrak, S. Limits of variations on the structure and the fuel characteristics of sunflower seed shell through torrefaction. Fuel Process. Technol. 2016, 144, 197–202. [Google Scholar] [CrossRef]
- EN 14774-3:2009; Solid Biofuels—Determination of Moisture Content—Oven Dry Method—Part 3: Moisture in General Analysis Sample. iTeh, Inc.: Newark, DE, USA, 2009.
- EN 14775:2009; Solid Biofuels—Determination of Ash Content. iTeh, Inc.: Newark, DE, USA, 2009.
- EN 15104:2011; Solid Biofuels—Determination of Total Content of Carbon, Hydrogen and Nitrogen—Instrumental Methods. iTeh, Inc.: Newark, DE, USA, 2011.
- EN 15289:2011; Solid Biofuels—Determination of Total Content of Sulfur and Chlorine. iTeh, Inc.: Newark, DE, USA, 2011.
- EN 14918:2009; Solid Biofuels—Determination of Calorific Value. iTeh, Inc.: Newark, DE, USA, 2009.
- EN 15148:2009; Solid Biofuels—Determination of the Content of Volatile Matter. iTeh, Inc.: Newark, DE, USA, 2009.
- GOST 10538-87; Solid Fuel. Methods for Determining the Chemical Composition of Ash. RussianGost: San Diego, CA, USA, 1987.
- GOST 32977-2014; Solid Mineral Fuel. Determination of Trace Elements in Ash by Atomic Absorption Method. RussianGost: San Diego, CA, USA, 2014.
- Van Krevelen, D.W. Coal: Typology-Physics-Chemistry-Constitution; Elsevier Science Publishers: Amsterdam, The Netherlands, 1993. [Google Scholar]
- Kew, W.; Blackburn, J.W.; Clarke, D.J.; Uhrín, D. Interactive van Krevelen diagrams—Advanced visualization of mass spectrometry data of complex mixtures. Rapid Comm. Mass Spectr. 2017, 31, 658. [Google Scholar] [CrossRef]
- Ma, S.; Chai, J.; Wu, K.; Wan, Z.; Xiang, Y.; Zhang, J.; Fan, Z. Experimental and mechanism research on volatilization characteristics of HCl in desulfurization wastewater evaporation process using high temperature flue gas. J. Ind. Eng. Chem. 2018, 66, 311–317. [Google Scholar] [CrossRef]
- Porter, R.F.; Schoonmaker, R.C. Gaseous species in the vaporization of sodium and potassium hydroxide. J. Phys. Chem. 1958, 62, 234–237. [Google Scholar] [CrossRef]
- Jones, J.M.; Darvell, L.I.; Bridgeman, T.G.; Pourkashanian, M.; Williams, A. An investigation of the thermal and catalytic behavior of potassium in biomass combustion. Proc. Combust. Inst. 2007, 31, 1955–1963. [Google Scholar] [CrossRef]
- Li, X.; He, F.; Behrendt, F.; Gao, Z.; Shi, J.; Li, C. Inhibition of K2SO4 on evaporation of KCl in combustion of herbaceous biomass. Fuel 2021, 289, 119754. [Google Scholar] [CrossRef]
- Li, X.; He, F.; Su, X.; Behrendt, F.; Gao, Z.; Wang, H. Evaporation rate of potassium chloride in combustion of herbaceous biomass and its calculation. Fuel 2019, 257, 116021. [Google Scholar] [CrossRef]
- Raask, E.; Wilkins, D.M. Volatilization of silica in gasification and combustion processes. J. Inst. Fuel 1965, 38, 255–262. [Google Scholar]
- Iribarne, J.V.; Thomson, B.A. On the evaporation of small ions from charged droplets. J. Chem. Phys. 1976, 64, 2287–2294. [Google Scholar] [CrossRef]
- Bach, Q.V.; Skreiberg, Ø. Upgrading biomass fuels via wet torrefaction: A review and comparison with dry torrefaction. Renew. Sust. Energy Rev. 2016, 54, 665–677. [Google Scholar] [CrossRef]
- Funke, A.; Reebs, F.; Kruse, A. Experimental comparison of hydrothermal and vapothermal carbonization. Fuel Process. Technol. 2013, 115, 261–269. [Google Scholar] [CrossRef]
- Niu, Y.; Zhu, Y.; Tan, H.; Hui, S.; Jing, Z.; Xu, W. Investigations on biomass slagging in utility boiler: Criterion numbers and slagging growth mechanisms. Fuel Process. Technol. 2014, 128, 499–508. [Google Scholar] [CrossRef]
- Abelha, P.; Kiel, J. Techno-economic assessment of biomass upgrading by washing and torrefaction. Biomass Bioenergy 2020, 142, 105751. [Google Scholar] [CrossRef]
- Meesters, K.; Elbersen, W.; van der Hoogt, P.; Hristov, H. Biomass Pre-Treatment for Bioenergy Case Study 5: Leaching as a Biomass Pre-Treatment Method for Herbaceous Biomass. Sugar Cane Trash and Palm Oil Mill Residues, IEA Bioenergy, InterTask Project on Fuel Pretreatment of Biomass Residues in the Supply Chain for Thermal Conversion. September 2018. Available online: https://www.ieabioenergy.com/wp-content/uploads/2018/11/CS5-Leaching-as-a-biomass-pre-treatment-method-for-herbaceous-biomass.pdf.1.08.2023 (accessed on 25 June 2023).
| Treatment, Biomasses, Reference | Treatment Steps and Conditions | Treatment Effects |
|---|---|---|
| pre-wash wheat straw Singhal et al. 2021 [4] | cuttings 3, 1 or 0.05–0.08 cm → water washing (0–180 min) | max. removal rate 87% Cl, 74% S, 68% K, 46% N, and 39% ash; efficiency increases with higher washing time and lower particle size, fuel: reduced fouling and corrosion |
| pre-wash wheat straw Singhal et al. 2021 [5] | cuttings (3 cm) → water washing (20–80 °C), Solid: Liquid ratio 1:15–1:50 → manual pressing → drying | optimal pre-wash conditions 10 min, 40 °C and 80 °C, fuel: reduction of fouling and slagging |
| pre-wash wheat straw, rice straw, corn stalk, cotton stalk, candlenut wood and rice hull Deng et al. 2013 [6] | water washing in deionized water for 3 h at 30–90 °C | removal of K, S and Cl; higher removal efficiencies of K and SiO2 at higher water temperatures; fuel: increased ash fusion temperatures for some biomasses |
| LHW treatment poplar wood, corn stover, switchgrass, Miscanthus Runge et al. 2013 [7] | debarking, chipping, screening 2–8 mm, drying (poplar); drying, chopping, screening 25 mm → liquid hot water (LHW) treatment (30–60 min, 150–170 °C) → cooling in ice water (10 min) → vacuum filtering → rinsing (deionized water), re-filtering (twice) | reduced ash content regardless of treatment severity; fuel: up to 25% increase in energy density from higher heating value and pellet density, improved durability of pellets |
| pre-wash → torrefaction straw, pine Liu et al. 2022 [8] | cuttings (3 cm, 1 cm, and 0.05–0.08 cm) → water washing (2–180 min) → filtration and drying → torrefaction or hydrothermal carbonization (HTC) | both washing-torrefaction treatment and HTC treatment have beneficial effects on fuel properties of biochar |
| pre-wash → torrefaction corn stalk Cen et al. 2016 [9] | drying, grinding → water washing (6 h, 60 °C) → torrefaction in tubular furnace (250 °C, 30 min) | removal of ash and metallic species, reduced acid and water contents, increased phenol content, fuel: increased HHV |
| pre-wash → torrefaction waste epoxy resin Chen et al. 2020 [10] | grinding → water washing (deionized water, 10–15 min) → torrefaction (300 °C, 20 min) | salt removal, fuel: improved properties, but torrefaction had little effect |
| pre-wash → torrefaction waste epoxy resin Chen et al. 2021 [11] | water washing (10–15 min, with shaking) → torrefaction (tubular furnace, 300 °C, 20 min) | removal of water-soluble ash (minerals), removal of Cl, fuel: risk of increased dioxin emissions from combustion of torrefied material due to de novo synthesis |
| pre-wash (acid) → torrefaction rice husk Zhang et al. 2019 [12] | sieving, drying → acid washing (2 h) → water washing (deionized water) → torrefaction in tubular furnace (210–270 °C, 1 h) | increased sugars and phenols contents in bio-oil, increased bio-oil quality |
| pre-wash → two-stage torrefaction (pilot) road side grass, miscanthus, wheat straw and spruce bark Abelha et al. 2019 [13] | water washing with hot water (50 °C) in 2 washing bins (15 min each) → drying → milling, pelletizing (except spruce bark) → two-stage torrefaction in moving bed reactor (drying stage 150–200 °C, torrefaction stage 240–320 °C) | removal of 90% of Cl and 60–80% of K; decreased NOx emissions, fuel: slagging only slightly reduced |
| pre-wash (water or chemicals) → two-stage torrefaction (pilot) short-rotation coppiced willow, eucalyptus, miscanthus, wheat straw Saddawi et al. 2012 [14] | cuttings (2 × 2–4 × 4 cm for SRC willow, eucalyptus; 2 × 1–3 × 1 cm for Miscanthus, wheat straw) → washing (deionized water with stirring for 20 h, or 1 M ammonium acetate solution for 60 h, then filtration and washing with deionized water, or 1 M HCl at 70 °C for 40 h, ratio ~1 L/ 60 g) → torrefaction in tube reactor (150 °C, 50 min and 290 °C, 60 min) | high removal of alkali metal ions and chloride, more efficient for herbaceous fuels (Miscanthus and wheat straw), low Ca removal rate, fuel: water washing pretreatment yielded best results in ash fusion tests of torrefied fuel; increased ash hemisphere temperature (ash melting) |
| pre-wash → torrefaction → pyrolysis rice husk Zhang et al. 2016 [15] | water washing (deionized water 60 °C, 6 h with stirring) → filtration, drying → torrefaction in vertical quartz reactor (250–280 °C) → pyrolysis in TGA analyzer (700 °C) | decreased contents of acids, ketones, aldehydes and furans, increased contents of sugars, including 9-fold increase in levoglucosan in bio-oil |
| pre-wash (liquid torrefaction products) → torrefaction → pyrolysis cotton stalk Chen et al. 2017a [16] | washing with liquid products of torrefaction → torrefaction (250 °C, 30 min) → pyrolysis in fixed-bed reactor (500 °C, 15 min) | reduced metallic species, decreased water and acids contents, increased phenols in bio-oil, decreased ash content in biochar, fuel: increased heating value of non-condensable gas |
| pre-wash (aqueous phase bio-oil) → torrefaction → pyrolysis cotton stalk Chen et al. 2017b [17] | drying, grinding →washing with aqueous phase bio-oil from pyrolysis (60 °C, 2 h) → filtration, rinsing (deionized water), drying → torrefaction (260 °C, 30 min), → pyrolysis in fixed-bed reactor (500 °C, 15 min) | removal of metallic species, improved yield, quality of bio-oil, fuel: improved heating value of bio-oil |
| pre-wash → pyrolysis rice hulls Teng and Wei 1998 [18] | water washing (80 °C) → vacuum-drying (50 °C, 24 h) → pyrolysis in TGA (900 °C) | increased volatile yield and decreased char yield due to removal of hydrocarbons which favor char formation |
| pre-wash → pyrolysis seaweed (brown algae) Ross et al. 2009 [19] | rinsing, drying, grinding (<90 μm) →water washing (6 h) or acid washing (2 M HCl, 6 h, 60 °C) → filtration, rinsing → pyrolysis (500 °C, 20 °C/ms, hold time 20 s) | 30–40% reduction of Mg, K and Na contents with water washing, >90% reduction of Mg, K, Na, Ca with acid washing |
| pre-wash (water or acid) → two-step pyrolysis corncob Zhang et al. 2019 [20] | crushing, sieving, drying → washing (deionized water or acid solution 5% HCl, room temperature, 3 h, stirring) → filtration, rinsing (deionized water), drying→ two-step pyrolysis in quartz tube (400 °C, 5–60 s and 650 °C, 20 s) | removal of alkaline earth metals, increase in bio-oil and decrease in biochar yield, increased yields of furfural, 4-vinylphenol and levoglucosan |
| pre wash (acid) → two-step pyrolysis walnut shell Zhang et al. 2018 [21] | milling, sieving, drying → acid washing (5% HCl, 25 °C, 3 h, stirring) → filtration, rinsing, drying → two-step pyrolysis in quarty tube (350–550 °C, 20 s and 650 °C, 40 s) | reduced acids, ketones, alcohols, aldehydes, phenols and increased N-compounds, sugars and furans in first step; decreased hydrocarbons and further increased sugars in second step, increased value-added chemicals including furfural, levoglucosan and 2-methoxy-4-methylphenol |
| pre-wash (warm water, dilute acid or aqueous phase bio-oil) → pyrolysis rice straw Chen et al. 2019 [22] | grinding, drying → washing with water, dilute HCl solution, or aqueous phase bio-oil (3 g/100 mL, 50 °C, 2 h)→ filtration, rinsing with distilled water → pyrolysis in TGA analyzer (50–650 °C at 10–40 °C/min) | washing with aqueous phase bio-oil yielded highest removal rate of alkaline earth metals and highest improvement of bio-oil quality, including increased content of anhydrosugars (mainly levoglucosan) |
| blending → pre-wash → two-step pyrolysis rice straw, groundnut shells, wheat straw Bhatnagar et al. 2022 [23] | grinding → blending of feedstocks → water washing (30–120 min, 40 °C, stirring 100 rpm) → pressing, drying (65 °C, 12 h) → two-step slow pyrolysis (20–340 and 340–600 °C at heating rate 5 °C/min, 15 min pause at 340 °C for 1st stage bio-oil recovery) | 1.6–2.1 fold increase in levoglucosan yield in bio-oil, ∼10% reduction in water content of bio-oil, fuel: reduced slagging and fouling, higher GCV |
| pyrolysis → post-wash (dilute acid) almond shells, cottonseed hulls, lignin, chicken manure Klasson et al. 2014 [24] | slow pyrolysis in box furnace(350–800 °C, 1–4 h) → dilute acid washing (0.1 M HCl, ratio 50:1 v/w, 1 h) → rinsing with deionized water (twice) | dilute acid reduces ash content in biochars, expose increased surface areas, increase biochar efficiency as adsorbent for Hg |
| torrefaction → post-wash → fuel blending straw Yan et al. 2021 [25] | drying, grinding → torrefaction (300 °C, 30 min) → water washing (ratio 1:40 w/w, 20 h, stirring) → filtration, drying, sieving (75–150 μm) → co-combustion with coal (ratios 1:1 and 1:4) in drop tube furnace (1400 °C) | reduced ash content reduced aluminosilicates content, fuel: ash slagging may be lower |
| pre-wash → two-stage torrefaction, or two-stage torrefaction → post-wash road side grass, miscanthus, wheat straw, spruce bark Abelha et al. 2018 [26] | drying (105 °C), milling (<4 mm) → water washing (liquid/solid ratios 5–40 on dry mass, shaking 1.25 Hz, 20–80 °C, 5 min–24 h) → two-stage torrefaction (drying stage 150–200 °C, torrefaction stage 240–280 °C), or water washing after torrefaction (post-washing) | removal of 90–95% Cl, 50–80% K, 30–60% S and 30% P: washing stage crucial for removal of K and P, which are not removed during torrefaction; post-washing (washing after torrefaction) yielded similar results with potential energy savings and easier dewatering of biomass |
| torrefaction → post-wash (water evaporating as water vapour >120 °C) sunflower husk Our study | torrefaction in hearth reactor (250 °C, 1 h) → water washing (thinly sprayed water) combined with biomass cooling, while maintaining biomass temperature >120 °C for efficient water evaporation and drying of treated biomass, along with evaporation of some minerals, not requiring additional treatment stages | biomass ash displays decreased Cl ratio (7.9-fold) and S ratio(10.7-fold), with increased CaO (3.1-fold), along with decreased K2O (2.1-fold), SO3 (up to 1.6-fold), and Cl (1.9-fold) hinting at reduced slagging |
| Content of Microelements, mg/kg of ash | Biomass Used for Combustion | ||||
|---|---|---|---|---|---|
| Original Sunflower Husk | Biochar without Water Washing | Biochar after Water Washing for 10 min | Biochar after Water Washing for 15 min | Biochar after Water Washing for 30 min | |
| V | <10 | <10 | <10 | <10 | 12 |
| Mn | 333 | 751 | 321 | 389 | 418 |
| Cu | 425 | 502 | 347 | 310 | 276 |
| Ni | 102 | 112 | 98 | 96 | 97 |
| Sr | 180 | 196 | 184 | 197 | 310 |
| Cr | 567 | 377 | 541 | 489 | 429 |
| Zn | 149 | 485 | 189 | 215 | 310 |
| Pb | 43 | 52 | 38 | 27 | 13 |
| As | <10 | <10 | <10 | <8 | <5 |
| Significance for the Modeling of Slagging Effects | Parameter | Biomass Used for Combustion | ||||
|---|---|---|---|---|---|---|
| Original Sunflower Husk | Biochar without Water Washing | Biochar after Water Washing for 10 min | Biochar after Water Washing for 15 min | Biochar after Water Washing for 30 min | ||
| Sulfur | S total in solid fuel (mg/kg) | 76.6 | 60.9 | 72.0 | 64.5 | 54.7 |
| SO3 in ash (mg/kg) | 6.1 | 6.25 | 8.9 | 5.02 | 3.75 | |
| S volatile (mg/kg) | 70.5 | 54.7 | 63.1 | 59.5 | 51.0 | |
| S volatilization rate (%) | 92.0 | 89.7 | 87.6 | 92.2 | 93.1 | |
| Chloride | Cl in ash (mg/kg) | 1.77 | 1.75 | 1.16 | 1.08 | 0.93 |
| Adverse elements | K2O in ash (mg/kg) | 38.99 | 34.05 | 39.16 | 23.03 | 18.14 |
| Na2O in ash (mg/kg) | 0.93 | 0.63 | 0.35 | 0.43 | 0.75 | |
| Protective elements | SiO2 in ash (mg/kg) | 1.24 | 2.91 | 2.42 | 4.18 | 8.6 |
| Al2O3 in ash (mg/kg) | 0.36 | 0.69 | 0.8 | 0.81 | 0.84 | |
| Ratios | Cl ratio | 26.1 | 10.1 | 12.6 | 4.9 | 2.1 |
| S ratio | 69.0 | 24.8 | 31.9 | 16.6 | 7.4 | |
| Other elements | TiO2 in ash (mg/kg) | 0.02 | 0.17 | 0.03 | 0.02 | 0.07 |
| Fe2O3 in ash (mg/kg) | 0.43 | 7.16 | 1.37 | 1.28 | 1.04 | |
| CaO in ash (mg/kg) | 6.39 | 6.36 | 11.88 | 13.02 | 19.93 | |
| MgO in ash (mg/kg) | 12.05 | 10.79 | 6.35 | 6.89 | 7.62 | |
| P2O5 in ash (mg/kg) | 11.72 | 9.24 | 10.13 | 11.6 | 13.93 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nebyvaev, A.; Klimov, D.; Ryzhenkov, A.; Brulé, M. Preliminary Results of Innovative Two-Stage Torrefaction Technology Applied for Thermochemical Treatment of Sunflower Husk. Processes 2023, 11, 2486. https://doi.org/10.3390/pr11082486
Nebyvaev A, Klimov D, Ryzhenkov A, Brulé M. Preliminary Results of Innovative Two-Stage Torrefaction Technology Applied for Thermochemical Treatment of Sunflower Husk. Processes. 2023; 11(8):2486. https://doi.org/10.3390/pr11082486
Chicago/Turabian StyleNebyvaev, Artemy, Dmitry Klimov, Artem Ryzhenkov, and Mathieu Brulé. 2023. "Preliminary Results of Innovative Two-Stage Torrefaction Technology Applied for Thermochemical Treatment of Sunflower Husk" Processes 11, no. 8: 2486. https://doi.org/10.3390/pr11082486
APA StyleNebyvaev, A., Klimov, D., Ryzhenkov, A., & Brulé, M. (2023). Preliminary Results of Innovative Two-Stage Torrefaction Technology Applied for Thermochemical Treatment of Sunflower Husk. Processes, 11(8), 2486. https://doi.org/10.3390/pr11082486









