Study of Chemical Additives for Optimization of Binary Systems Used for Downhole Thermochemical Treatment of Heavy Oil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
- Ammonium nitrate and sodium nitrite were stirred in fresh water to complete solubility.
- Potassium nitrite was added to the aqueous solution of the two salts and brought to complete solubility without exposure to elevated temperatures
- When surfactants were used in the experiments, surfactants of a given concentration were added to the binary mixture solution with stirring until complete dissolution.
2.2. Petrophysical Measurement
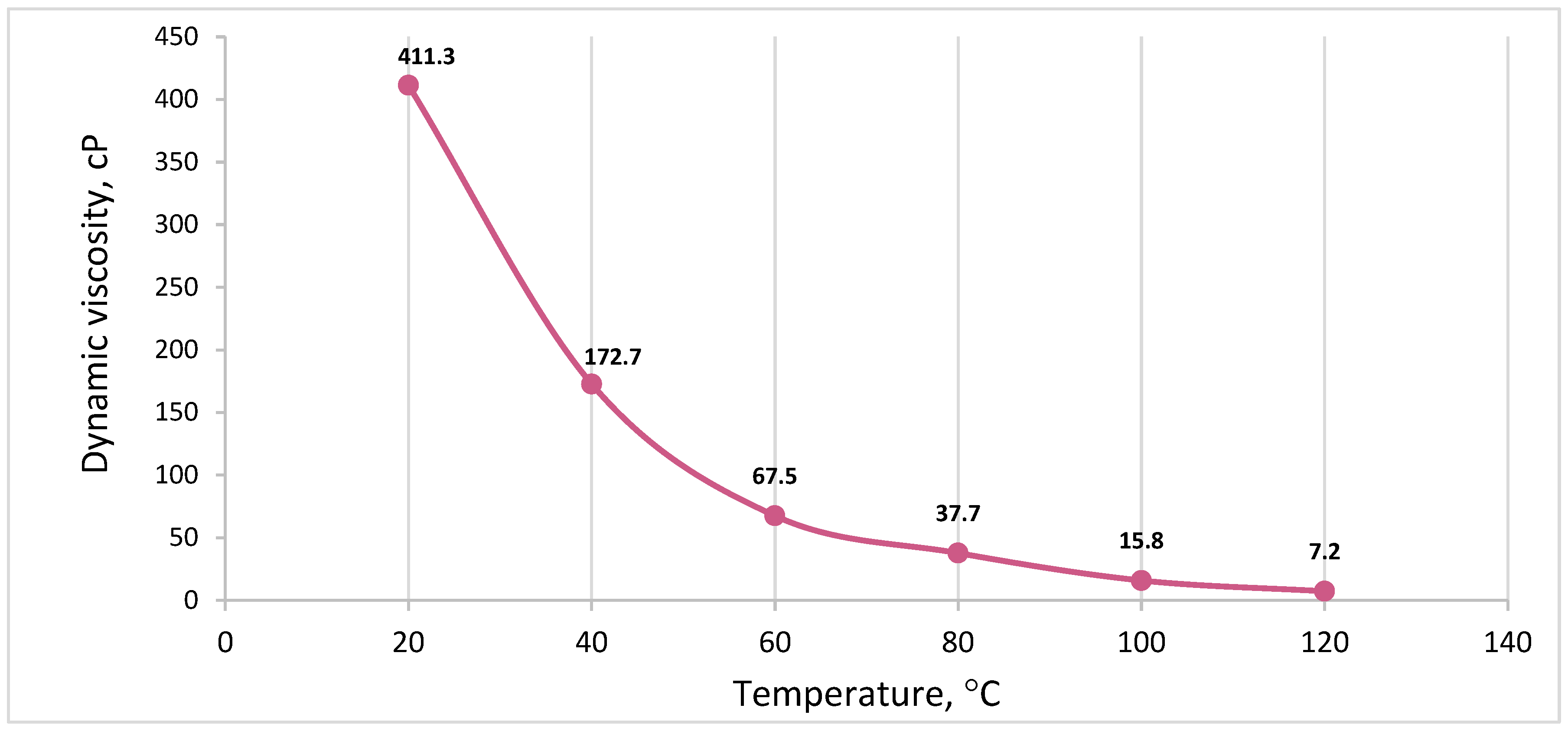
2.3. Gas Chromatography Analysis, Viscosity
2.4. Filtration Experiments
2.5. Hydrodynamic Modeling
3. Results and Discussion
4. Conclusions
- We have studied the effect on heat generation of the composition with increasing concentrations of salts in aqueous solution (72 %wt) of a binary mixture that includes ammonium nitrate, sodium nitrite, and potassium nitrite with a chemical initiation additive of delayed action HMBA. It has been shown that the reaction proceeds with a high exo effect in the solution and porous medium, and when EG (1:1 mass ratio H2O:EG,) is introduced into this mixture, the onset time increases by 1.5 times. However, as shown by the filtration experiment, with a simultaneous injection of this HMBA and binary mixture under reservoir conditions in porous medium, the treatment efficiency is very low.
- We also studied the foam (surfactant—alkylpolyglucosides) salt heat and gas-generating composition with sequential injection into oil-saturated cores at initiation with formaldehyde solution without lowering reaction initiation. However, a powerful reaction allowed the coverage due to foam flow formation and heat and gas spread to increase from the first to the third zone of the composite sandstone core model in the core holder at initial formation conditions of P = 5 MPa and T = 25 °C.
- We have also studied the application as an initiating additive on the basis of the binary mixture of formaldehyde solution and hydrogen peroxide solution in mass ratio 2:1 with sequential injection into carbonate cores under reservoir conditions without the introduction of surfactants into the salt mixture. It has been shown that it is possible to distribute thermal front in porous medium of oil-saturated carbonate cores more favourably and uniformly.
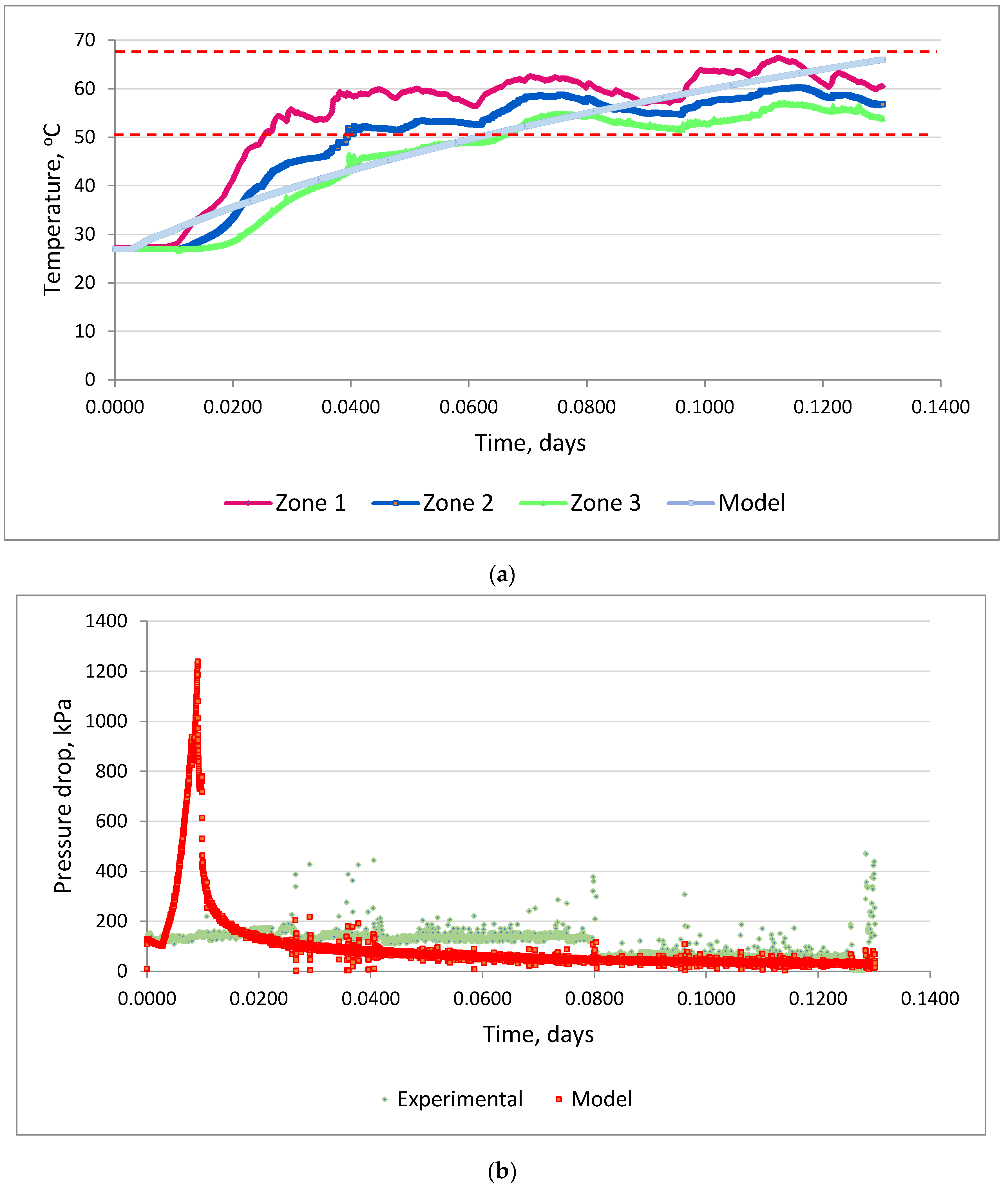
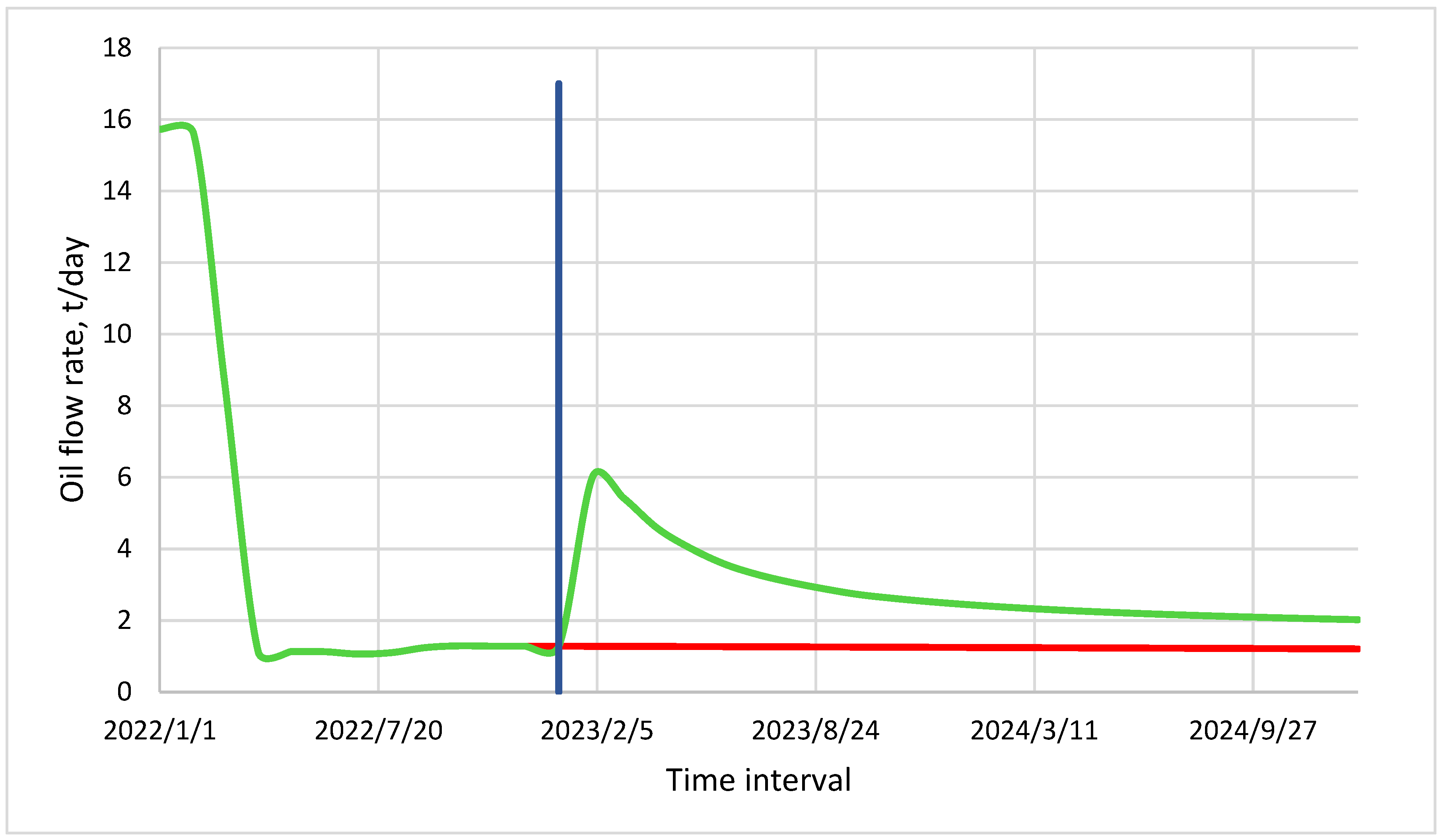
- Calculations were performed using a non-isothermal composite model in order to study the kinetics of chemical reactions and to find the activation energy for calculations on targeted field models to justify pilot tests. As a result of the injection of a chemical additive of a binary type on the basis of hydrogen peroxide and formaldehyde in a volume of 3 m3 and the salt composition (i.e., the one used in this study) nitrite in a volume of 20 m3 at well #8 of the Ilmenevskoye field, according to the results of the hydrodynamic modelling, an increase of 716 tons of additional oil production for 1 year was achieved. The results of this prediction demonstrate the efficiency of thermochemical technology. Oil mobility after the injection of the heat-generating system increased fourfold to fivefold on average, and, as a result of the dissolution of the colmatants in the bottomhole zone, the skin factor decreased to an average of “−2”.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Green, D.W.; Willhite, G.P. Enhanced Oil Recovery; Society of Petroleum Engineers Richardson: Richardson, TX, USA, 2018; 896p. [Google Scholar]
- Lake, L.; Johns, R.T.; Rossen, W.R.; Pope, G.A. Fundamentals of Enhanced Oil Recovery; Society of Petroleum Engineers: Richardson, TX, USA, 2014; 496p. [Google Scholar]
- Alfarge, D.; Wei, M.; Bai, B. Fundamentals of Enhanced Oil Recovery Methods for Unconventional Oil Reservoirs; Elsevier: Cambridge, MA, USA, 2020; 286p. [Google Scholar]
- Minkhanov, I.F.; Chalin, V.V.; Tazeev, A.R.; Bolotov, A.V.; Mukhamatdinov, I.I.; Sitnov, S.A.; Vakhin, A.V.; Varfolomeev, M.A.; Kudryashov, S.I.; Afanasiev, I.S.; et al. Integrated Modeling of the Catalytic Aquathermolysis Process to Evaluate the Efficiency in a Porous Medium by the Example of a Carbonate Extra-Viscous Oil Field. Catalysts 2023, 13, 283. [Google Scholar] [CrossRef]
- Xue, L.; Liu, P.; Zhang, Y. Development and Research Status of Heavy Oil Enhanced Oil Recovery. Geofluids 2022, 2022, 5015045. [Google Scholar] [CrossRef]
- Ushakova, A.S.; Zatsepin, V.Z.; KhelkhaL, M.A.; Sitnov, S.A.; Vakhin, A.V. In situ combustion of heavy, medium, and light crude oils: Low-temperature oxidation in terms of a chain reaction approach. Energy Fuels 2022, 36, 7710–7721. [Google Scholar] [CrossRef]
- Vakhin, A.V.; Khelkhal, M.A.; Mukhamatdinov, I.I.; Mukhamatdinova, R.E.; Tajik, A.; Slavkina, O.V.; Malaniy, S.Y.; Gafurov, M.R.; Nasybullin, A.R.; Morozov, O.G. Changes in Heavy Oil Saturates and Aromatics in the Presence of Microwave Radiation and Iron-Based Nanoparticles. Catalysts 2022, 12, 514. [Google Scholar] [CrossRef]
- Pituganova, A.; Nassan, T.; Amro, M.; Minkhanov, I.; Varfolomeev, M.; Bolotov, A. Experimental and Numerical Analysis of Thermal EOR Recovery Schemes for Extra-Heavy Oil of the Oykino-Altuninsky Uplift of the Romashkinskoye Oilfield. In Proceedings of the International Petroleum Technology Conference, Riyadh, Saudi Arabia, 21–23 February 2022; OnePetro: Richardson, TX, USA, 2022. [Google Scholar] [CrossRef]
- Pituganova, A.; Minkhanov, I.; Bolotov, A.; Varfolomeev, M. Screening of waterflooding, hot waterflooding and steam injection for extra heavy crude oil production from Tatarstan oilfield. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2021; Volume 931, p. 12002. [Google Scholar] [CrossRef]
- Alade, O.S.; Mahmoud, M.; Hassan, A.; Al-Shehri, D.; Al-Nakhl, A.; Bataweel, M. Evaluation of kinetics and energetics of thermochemical fluids for enhanced recovery of heavy oil and liquid condensate. Energy Fuels 2019, 33, 5538–5543. [Google Scholar] [CrossRef]
- Anikin, O.V.; Bolotov, A.V.; Minkhanov, I.F.; Varfolomeev, M.A.; Tazeev, A.R.; Chalin, V.V.; Lutfullin, A.A.; Abusalimov, E.M. Factors influencing hydrogen peroxide decomposition dynamics for thermochemical treatment of bottomhole zone. J. Pet. Explor. Prod. Technol. 2022, 12, 2587–2598. [Google Scholar] [CrossRef]
- Mahmoud, M. Well clean-up using a combined thermochemical/chelating agent fluids. J. Energy Resour. Technol. 2019, 141, 102905. [Google Scholar] [CrossRef]
- Al-Taq, A.A.; Aljawad, M.S.; Alade, O.S.; Mahmoud, M.; Alrustum, A. Thermally Activated Nitrogen/Heat Generating Reaction: A Kinetic Study. ACS Omega 2023, 8, 10139–10147. [Google Scholar] [CrossRef] [PubMed]
- Alade, O.S.; Hassan, A.; Mahmoud, M.; Al-Shehri, D.; Al-Majed, A. Novel Approach for Improving the Flow of Waxy Crude Oil Using Thermochemical Fluids: Experimental and Simulation Study. ACS Omega 2020, 5, 4313–4321. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsov, N.M.; Aleksandrov, E.N. Safety in applying binary mix-tures for oil production stimulation. Georesursy 2017, 19, 379–382. [Google Scholar] [CrossRef]
- Al-Taq, A.A.; Al-Haji, H.H.; Saleem, J.A. First Successful Filtercake Damage Removal Treatment Utilizing In-situ Nitrogen/Heat Generating system for Relatively Heavy Oil Wells. In Proceedings of the SPE Annual Technical Conference and Exhibition, Amsterdam, The Netherlands, 27–29 October 2014. [Google Scholar] [CrossRef]
- Moussa, T.; Patil, S.; Mahmoud, M. Feasibility Study of Heavy oil Recovery Using In-situ Steam Generated by Thermochemicals. In Proceedings of the SPE Western Regional Meeting, San Jose, CA, USA, 23–26 April 2019. [Google Scholar] [CrossRef]
- Moussa, T.; Mahmoud, M.; Mokheimer, E.M.A.; Habib, M.A.; Elkatatny, S. Well-Placement Optimization in Heavy Oil Reservoirs Using a Novel Method of In Situ Steam Generation. J. Energy Resour. Technol. 2018, 141, 032906. [Google Scholar] [CrossRef]
- Mahmoud, M.; Alade, O.S.; Hamdy, M.; Patil, S.; Mokheimer, E.M.A. In situ steam and nitrogen gas generation by thermochemical fluid Injection: A new approach for heavy oil recovery. Energy Convers. Manag. 2019, 202, 112203. [Google Scholar] [CrossRef]
- Babadagli, T. Technology Focus: Heavy Oil. J. Pet. Technol. 2019, 71, 68. [Google Scholar] [CrossRef]
- Hassan, A.; Mahmoud, M.; Al-Majed, A.; Al-Nakhli, A.; BaTaweel, M.; Elktatany, S. Permanent removal of condensate banking in tight gas reservoirs using thermochemicals. In Proceedings of the SPE International Conference on Oilfield Chemistry, Galveston, TX, USA, 8–9 April 2019. [Google Scholar] [CrossRef]
- Wang, F.; Chen, H.; Alzobaidi, S.; Li, Z. Application and mechanisms of self-generated heat foam for enhanced oil recovery. Energy Fuels 2018, 32, 9093–9105. [Google Scholar] [CrossRef]
- Varfolomeev, M.A.; Yuan, C.; Bolotov, A.V.; Minkhanov, I.F.; Mehrabi-Kalajahi, S.; Saifullin, E.R.; Marvanov, M.M.; Emil, R.; Baygildin, E.R.; Sabiryanov, R.M.; et al. Effect of copper stearate as catalysts on the performance of in-situ combustion process for heavy oil recovery and upgrading. J. Pet. Sci. Eng. 2021, 207, 109125. [Google Scholar] [CrossRef]
- Minkhanov, I.F.; Bolotov, A.V.; Al-Muntaser, A.A.; Mukhamatdinov, I.I.; Vakhin, A.V.; Varfolomeev, M.A.; Slavkina, O.V.; Shchekoldin, K.A.; Darishchev, V.I. Experimental study on the improving the efficiency of oil displacement by co-using of the steam-solvent catalyst. Oil Ind. 2021, 6, 54–57. [Google Scholar]
- Kitchens, J.F.; Casner, R.E.; Edwards, G.S.; William, I.; Harvard, E.; Macri, B.J. Investigation of Selected Potential Environmental Contaminants: Formaldehyde; US Environmental Protection Agency: Washington, DC, USA, 1976; 217p.
- Leal, J.F.; Neves, M.G.P.; Santos, E.B.; Esteves, V.I. Use of formalin in intensive aquaculture: Properties, application and effects on fish and water quality. Rev. Aquac. 2016, 10, 281–295. [Google Scholar] [CrossRef]
- Monzani, E.; Roncone, R.; Galliano, M.; Koppenol, W.H.; Casella, L. Mechanistic insight into the peroxidase catalyzed nitration of tyrosine derivatives by nitrite and hydrogen peroxide. Eur. J. Biochem. 2004, 271, 895–906. [Google Scholar] [CrossRef] [PubMed]
- Qi, Q.; Cai, C.F.; Sun, P.; Wang, D.W.; Zhu, H.J. Crude oil cracking in deep reservoirs: A review of the controlling factors and estimation methods. Pet. Sci. 2023, in press. [Google Scholar] [CrossRef]


| Length, cm | Diameter, cm | Gas Porosity, % | Gas Permeability, mD |
|---|---|---|---|
| Column #1 (sandstone) | |||
| 4.85 | 2.93 | 24.19 | 1262.68 |
| 5.17 | 2.92 | 23.98 | 1092.54 |
| 4.99 | 2.92 | 24.15 | 1016.92 |
| Column #2 (carbonate) | |||
| 4.99 | 2.95 | 18.81 | 1562.95 |
| 4.86 | 2.93 | 16.50 | 1102.31 |
| 5.01 | 2.95 | 20.45 | 749.25 |
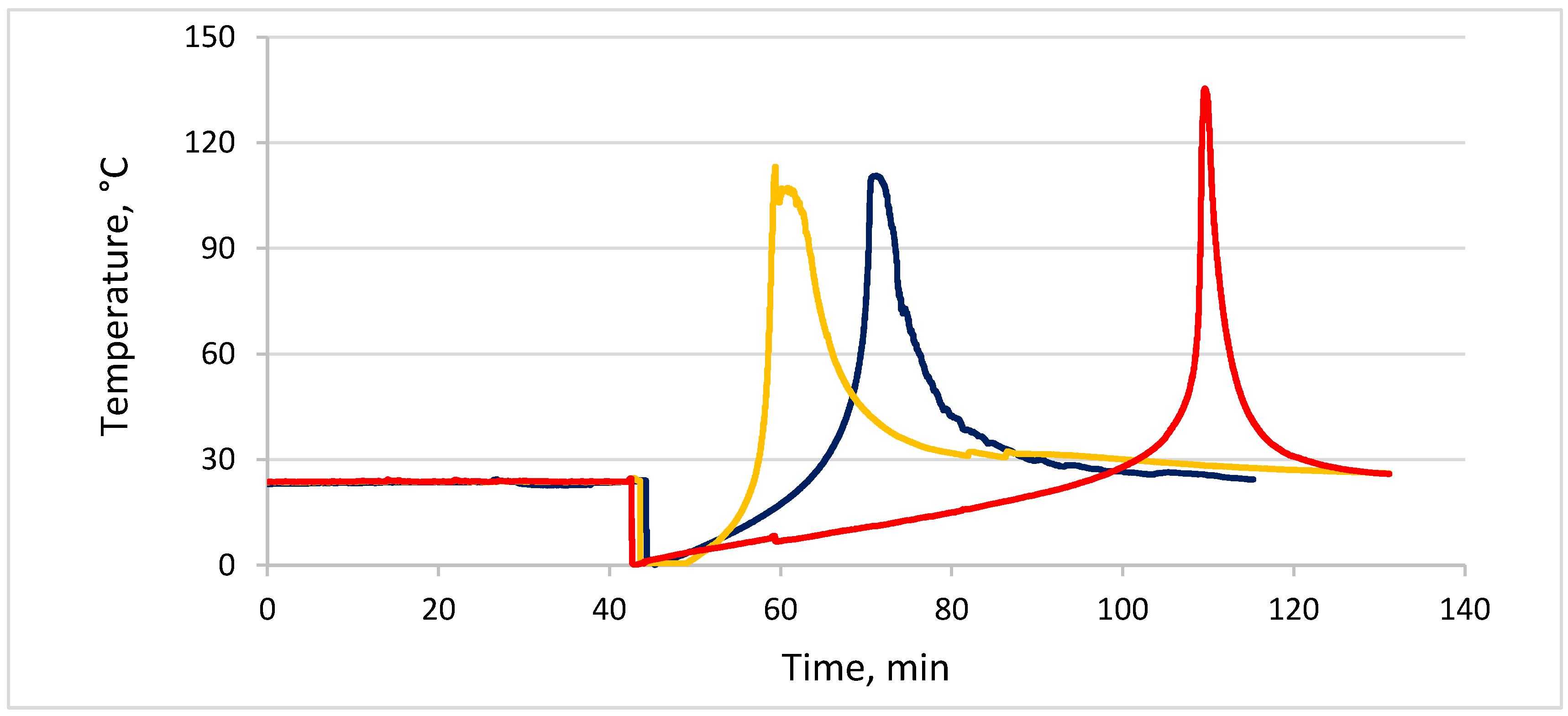
| # | Concentration of HMBA, %wt | Delay Time, min | Tmax, °C |
|---|---|---|---|
| 1 | 0.08 | 72 min | >120 |
| 2 | 0.1 | 30 min | >100 |
| 3 | 0.2 | 4 min | >100 |
| 4 | 0.3 | 3 min | >100 |
| 5 | 0.5 | 1min | >110 |
| Time, min | PV | ΔP, MPa | Zone 1, °C | Zone 2, °C | Zone 3, °C |
|---|---|---|---|---|---|
| 9 | 0.3 | 0.45 | 48.6 | 35.5 | 27.0 |
| 22 | 0.8 | 0.19 | 115.7 | 71.1 | 46.5 |
| 40 | 1.1 | 0.18 | 92.7 | 73.8 | 58.5 |
| Component | Concentration, %mol | ||
|---|---|---|---|
| Sampling Time | |||
| 15 min | 25 min | 45 min | |
| Carbon dioxide | 0.74 | 0.65 | 0.53 |
| Nitrogen | 99.11 | 98.73 | 99.44 |
| Ethane | 0.00 | 0.00 | 0.00 |
| Methane | 0.00 | 0.32 | 0.02 |
| Propane | 0.13 | 0.28 | 0.00 |
| Iso-butane | 0.02 | 0.00 | 0.00 |
| Butane | 0.00 | 0.02 | 0.00 |
| ∑ | 100.00 | 100.00 | 100.00 |
| Component | H2O | NH4NO2 | Oil | N2 |
|---|---|---|---|---|
| Molecular weight, g/mol | 18.015 | 64.04 | 426 | 28.013 |
| Critical pressure, kPa | 21,800 | 40,530 | 936 | 3394 |
| Critical temperature, °C | 374 | 69.85 | 620.9 | −146.95 |
| Density, kg/m3 | 1168.43 | 2390.18 | 933 | 0.65 |
| Parameter | Units of Measure | Value |
|---|---|---|
| Reaction frequency factor | day−1 | 1,995,840 |
| Enthalpy | J/mol | 334,000 |
| Activation energy | J/mol | 22,500 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anikin, O.V.; Bolotov, A.V.; Minkhanov, I.F.; Tazeev, A.R.; Varfolomeev, M.A.; Demin, S.V.; Pchela, K.V.; Dyrkin, S.M.; Amirov, A.A.; Kozlov, S.A.; et al. Study of Chemical Additives for Optimization of Binary Systems Used for Downhole Thermochemical Treatment of Heavy Oil. Processes 2023, 11, 2465. https://doi.org/10.3390/pr11082465
Anikin OV, Bolotov AV, Minkhanov IF, Tazeev AR, Varfolomeev MA, Demin SV, Pchela KV, Dyrkin SM, Amirov AA, Kozlov SA, et al. Study of Chemical Additives for Optimization of Binary Systems Used for Downhole Thermochemical Treatment of Heavy Oil. Processes. 2023; 11(8):2465. https://doi.org/10.3390/pr11082465
Chicago/Turabian StyleAnikin, Oleg V., Alexander V. Bolotov, Ilgiz F. Minkhanov, Aidar R. Tazeev, Mikhail A. Varfolomeev, Sergey V. Demin, Konstantin V. Pchela, Sergey M. Dyrkin, Albert A. Amirov, Sergey A. Kozlov, and et al. 2023. "Study of Chemical Additives for Optimization of Binary Systems Used for Downhole Thermochemical Treatment of Heavy Oil" Processes 11, no. 8: 2465. https://doi.org/10.3390/pr11082465
APA StyleAnikin, O. V., Bolotov, A. V., Minkhanov, I. F., Tazeev, A. R., Varfolomeev, M. A., Demin, S. V., Pchela, K. V., Dyrkin, S. M., Amirov, A. A., Kozlov, S. A., Frolov, D. A., Smirnov, E. A., & Abramov, V. V. (2023). Study of Chemical Additives for Optimization of Binary Systems Used for Downhole Thermochemical Treatment of Heavy Oil. Processes, 11(8), 2465. https://doi.org/10.3390/pr11082465







