1. Introduction
Currently, crude oils are getting scarcer and more expensive, and the need for light oil is continually rising. Oil deep processing, especially under cost-effective energy management, is essential. The FCC unit is a significant deep processing procedure for crude oil, a high-energy-consuming component of a fuel-based refinery, and is undergoing extraordinary development [
1]. The FCC is the modern refinery’s mainstay on a global scale. Its purpose was to transform heavy hydrocarbon petroleum compounds into a variety of more usable products, including petrol, intermediate distillates, and light olefins [
2]. The product from the FCC reaction is then separated into rich gas, unsaturated gasoline, light diesel oil, re-refined oil, and oil slurry based on the various boiling point ranges of the distillates, while also making sure that the dry point, freezing point, and flash point of light diesel oil are acceptable [
3]. Compressed-rich gas (H
2, CO, CO
2, C
1–6 components) and crude gasoline are distillated from the top of the main fractionation tower are commonly co-processed in ASS. Prior to ASS, a two-stage turbine was used to compress the rich gas to the required pressure for the subsequent operation. In particular, the dry gas (C
2− components) and liquefied petroleum gas (LPG, C
3–4 components) are separated from the compressed rich gas, while the stabilized gasoline is upgraded from crude gasoline in this process [
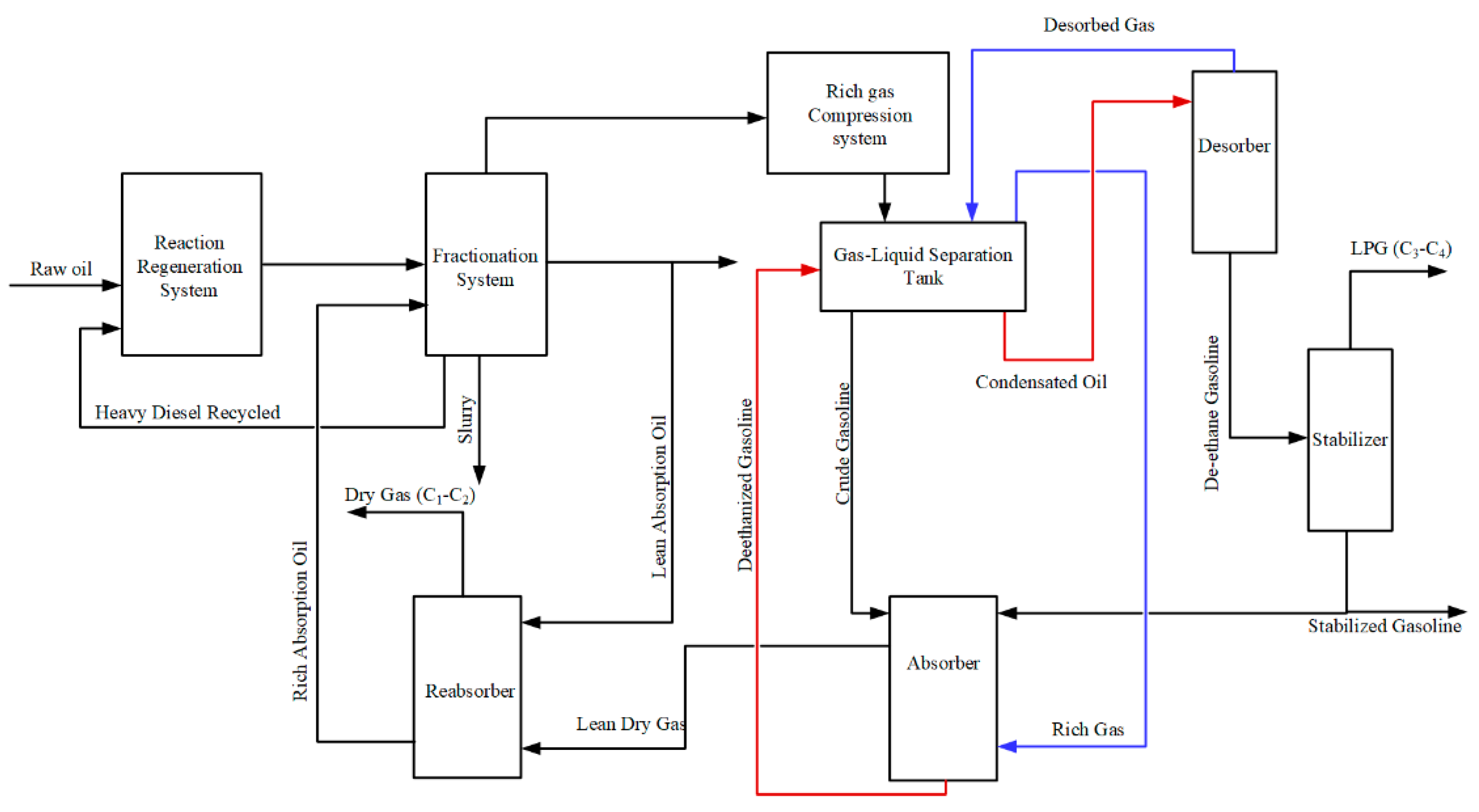
4]. The ASS comprises four columns: absorber, desorber, reabsorber, and stabilizer [
5,
6]. As the ASS consists of four recycles with substantial flow rates, the interaction between the various process parameters is intense. As an illustration, the absorber and desorber interact closely because they feed each other. Specifically, the rich absorption oil from the absorption tower is the liquid feed for the desorption tower. Likewise, the desorbed gas from the desorption tower is recycled as the gas feed to the absorption tower, as shown in
Figure 1. The system’s temperature and pressure influence the absorption and desorption effects. There is a tradeoff between desorbed gas quantity, dry gas quality, and LPG quality [
7]. Chen et al. [
8] introduced a novel GASP to enhance the separation process and greatly simplify the existing GASP flowsheet. They analyzed the solubility and volatility of the C
5–C
11 hydrocarbons in their study and introduced a new indicator to reflect the effectiveness of additional absorbent streams. Pan et al. [
9] presented a rigorous dynamic simulation of the fluid catalytic cracking unit’s (FCCU) absorption-stabilization system.
The issues with the GCS and ASS have been investigated by other researchers in terms of process modeling, simulation, and operational optimization. Liu et al. [
10] used process simulation and pinch analysis to conduct a thorough investigation of the process retrofit along with heat integration for an existing ASS with feed splitting. They introduced a new ASS with a two-stage condensation section. Zhang et al. [
11] employed HYSYS software to model the mechanism of the FCC unit and proposed an optimization method to improve the product yield and processing capacity through the model research. Liu et al. [
12] simulated the FCC flowsheet and proposed case studies to quantify the effects of key FCC operating variables. Sui et al. [
13] employed an organic rankine cycle (ORC) to convert low-grade process heat to electricity by integrating it into the fluid catalytic cracking (FCC) absorption-stabilization system. Yang et al. [
14] have proposed a brand-new flowsheet that uses less energy by adding a side extraction stream to the absorber. Pinheiro et al. [
15] explored novel developments in the modeling, monitoring, control, and optimization of the fluid catalytic cracking (FCC) process. He et al. [
16] proposed a modeling strategy that combines molecular mechanisms and data models to explain the maximizing iso-paraffins (MIP) technology of the FCC process.
Therefore, much research is required to gain a thorough grasp of the two systems. Only in this manner can an optimal design be achieved. The impact of the GCS outlet pressure on the ASS absorption efficiency, however, was not taken into account by the aforementioned literature as a crucial decision variable to be optimized. Given the above literature, we can suggest that changing the outlet pressure of GCS and the inlet flowrate of absorbent to the absorption tower in ASS would effectively intensify the mass transfer and absorption efficiency of the absorber and further improve the utility-use performance of the existing GCS and ASS.
The flow rate and inlet temperature of unsaturated gasoline and diesel from the main fractionator and the supplementary absorbent from the stabilizer plays a vital role in the absorption efficiency of absorbers, reducing C3+ light components in lean gas and dry gas from the top of the absorber and reabsorber, respectively. The operational pressure, flow rate, and temperature of the absorbent are key parameters to the absorption process. Higher pressure and flow rate and lower inlet temperature of the absorbent are beneficial for absorption. The absorbent temperature cannot be reduced below 40 °C due to the limitations of the temperature of fresh cooling water. Thus, the energy-saving effects are relatively limited by optimizing the inlet temperature of the absorbent. However, the electricity consumption of rich gas compressors and pumps will be increased correspondingly along with the increment of the outlet pressure of the compressor, tower pressure of absorber, and quantity of the absorbents. Hence, there is a tradeoff between the compression system and absorption-stabilization system. In the past, few researchers optimized these two key parameters simultaneously.
The objective of this paper is to conduct a systematic study on process retrofit through process simulation and optimization on the upstream GCS and downstream ASS. The key novelty of this work is the simultaneous consideration of tower operational pressure and absorbent flow rate for the energy-use efficiency of the two systems mentioned above.
3. Simulation and Optimization of Existing GCS and ASS
3.1. Case Study One
We take a 725 kt/a rich gas processing flowsheet of an FCC refinery in China as the research object.
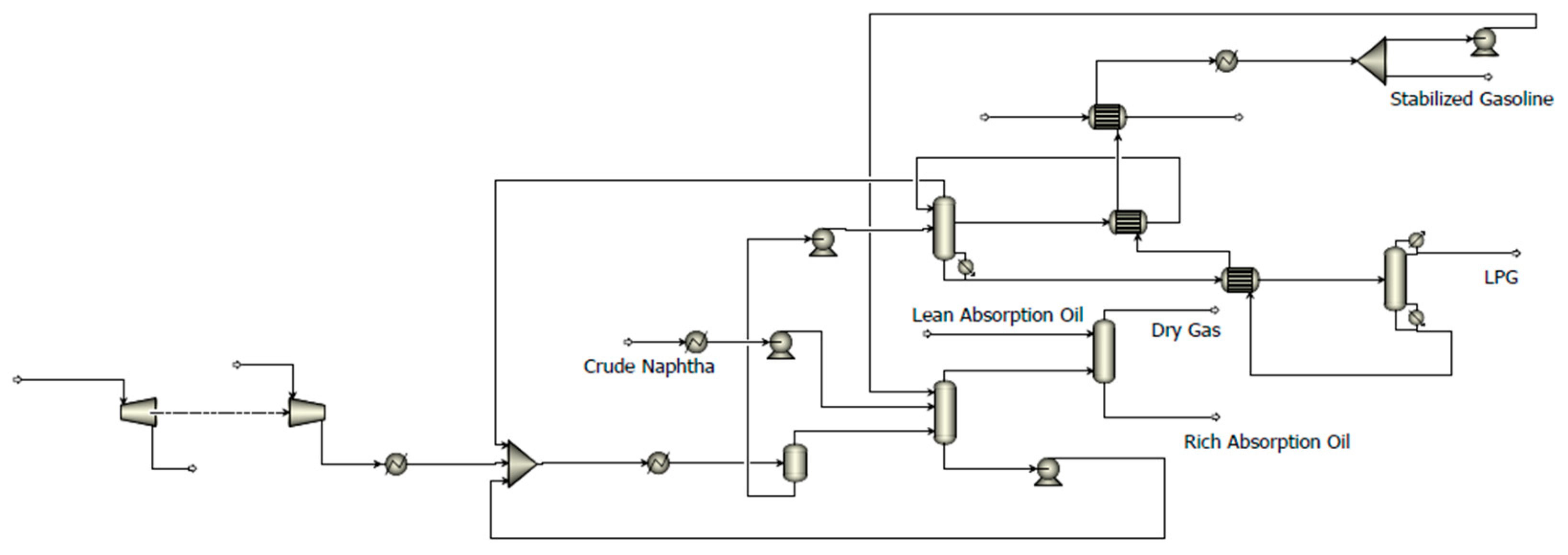
Figure 3 illustrates the overall Aspen Plus model of GCS and ASS. In this work, the Aspen Plus V12.0 simulator is used to represent this integrated system. For predicting the thermodynamic properties of all fluids, the Peng–Robinson state equation is chosen.
Table A1 lists Data of streams on the rich gas compression system and absorption stabilization system in
Appendix A. The optimization module is utilized for optimization purposes.
A “RadFrac” module is used to simulate each of the four distillation columns. In this study, there are five recycle streams in the ASS: two pumparounds as coolers for absorber, intermediate heater recycle for desorber, recycled desorbed gas, rich absorption oil, and supplemental absorbent. To avoid the convergence issue, we divide the recycle streams in the simulation model and connect them until the difference between the values of the divided streams becomes negligible. This simulation technique has been extensively studied in the literature [
18].
Table 1 and
Table 2 list the property parameters of all feed streams (S1, S7, S27 in
Figure 2) including molar percentage (mol%), pressure (P), temperature (T), mass flow rate (F), density (ρ, tested at 20 °C), and the true boiling point (TBP).
The specifications of the absorber, desorber, stabilizer, reabsorber, and compressor are listed in
Table 3. Thus, to guarantee the LPG quality, this model specifies that the concentrations of the total C
1–2 components in the desorbed oil should be lower than 7.0 mol%. Here, RVP is Reid Vapor Pressure, which represents gasoline vapor pressure with a quarter volumetric ratio of vapor to oil at 37.8 °C. The molar concentrations of the total C
1–4 components at the bottom stream of the stabilized tower are specified to be less than 1.0 mol% to meet the product specifications of the stabilized gasoline.
Olefins in the rich gas, especially propylene, are valuable chemical raw materials. The LPG in the ASS should contain as much propylene as possible, and the amount of propylene and butene entrained in the dry gas should be limited as much as possible. Specifically, C3–6 molar concentrations of the dry gas product in S25 should be lower than 3 mol%. The light diesel oil is used only to absorb C5+ components in the reabsorption tower.
Table 4 compares simulation results with industrial data, indicating a high level of agreement. It also demonstrates that the thermodynamic method of Peng-Robinson and the simulation strategy utilized in this model are capable of producing accurate and reliable simulations.
3.2. Gas-Liquid Concentration Distribution Analysis of the Towers
Both the absorption and reabsorption towers are used to absorb C
3+ components from compressed rich gas and recover C
2− components (i.e., dry gas). Additionally, the stabilization tower is used to simultaneously separate C
4− (LPG) and C
5+ (stable gasoline) components. Therefore, C
2 and C
3 component concentrations are considered indicators of the separation abilities of the absorption and reabsorption towers. Similar to this, the concentrations of C
3–4 and C
5–6 components can be used to gauge how well the stabilization towers work during separation. Here,
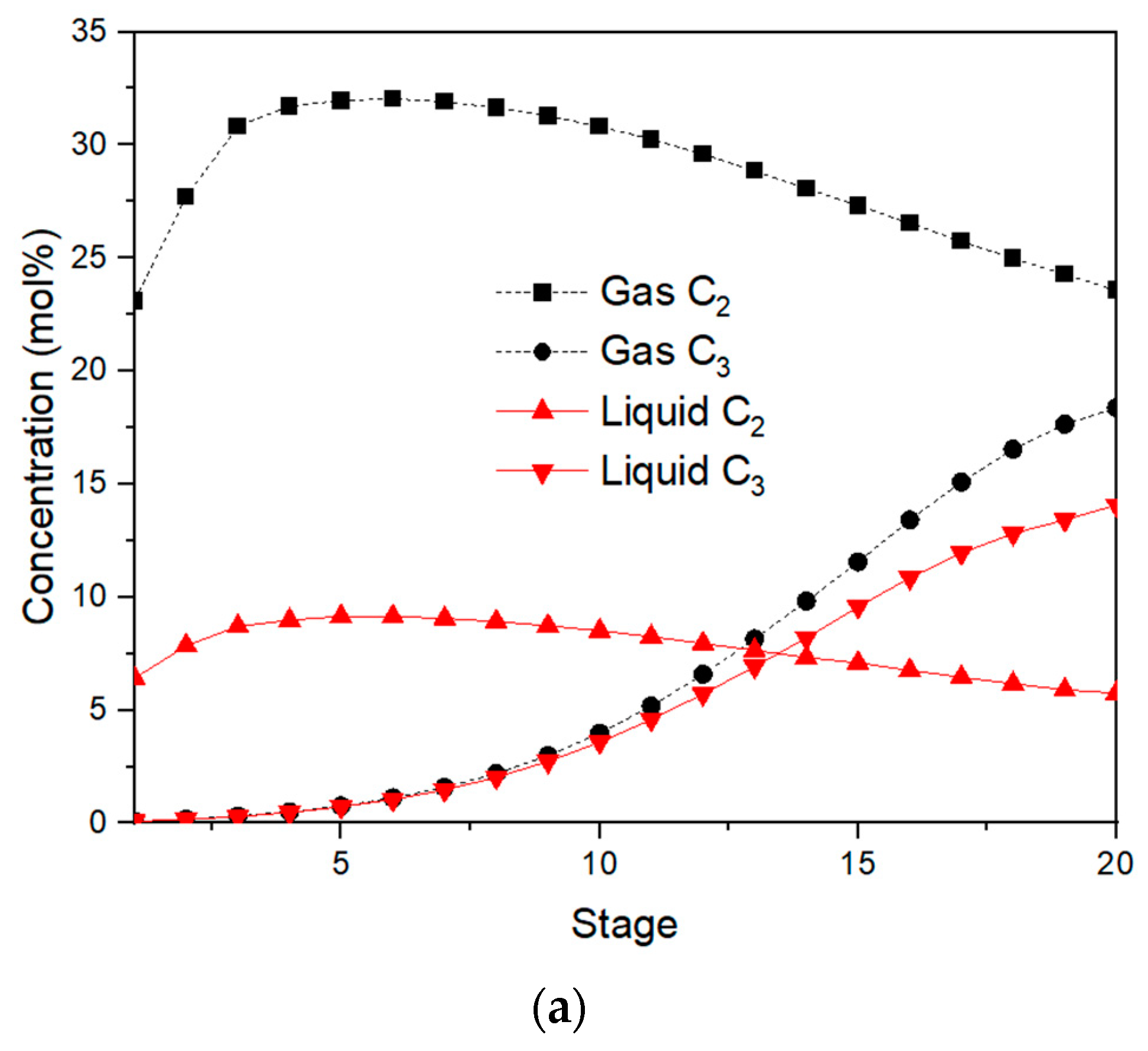
Figure 4a,b displays the concentration distributions of the C
2 and C
3 components, as well as the C
3–4 and C
5–6 components. Since absorbent naphtha is fed from the absorption tower’s top, it is simple to transport some petrol components there. This portion of the petrol carryover must be recovered in the reabsorber using diesel as an absorbent.
Ethane and ethylene concentrations for the absorption tower are depicted in
Figure 4a as steadily increasing from bottom to top, but propane and propylene concentrations are the opposite. The lean dry gas (S9) from the top of the absorption tower will carry part of the absorbent. In order to recover this part of the absorbent, lean absorption oil with light ends of components similar to naphtha is used as a re-absorbent to recover naphtha. It can be seen from
Table 2 that the boiling point ranges of naphtha and lean absorption oil overlap, which means that their components are similar. Part of the C
2- components can be dissolved in the absorbent, resulting in a decrease in the C
2- concentration in the early stages of the absorber, as shown in
Figure 4a.
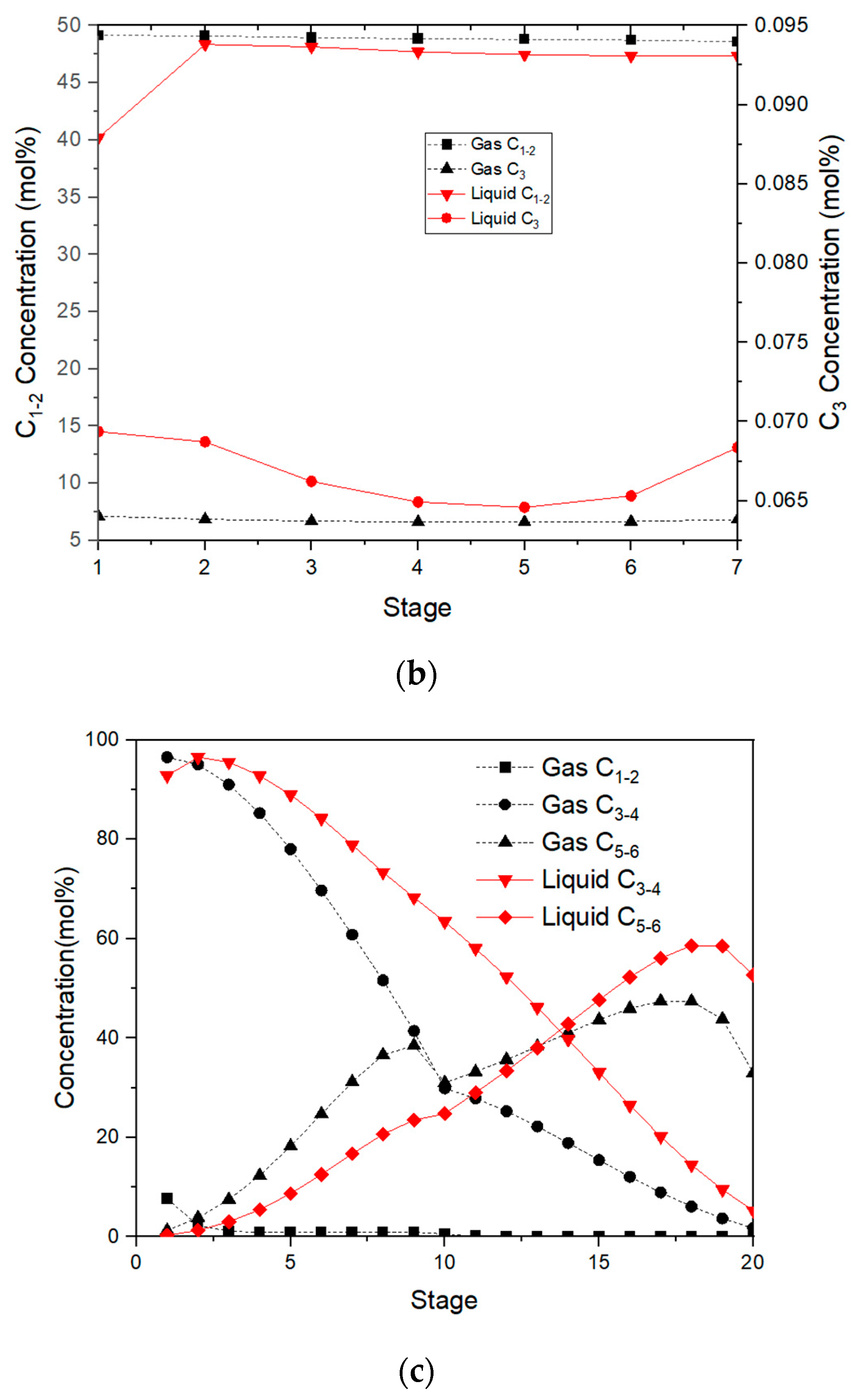
The mole fraction of C
3 components in the gas phase on the first stage for the desorption tower is just 0.087%, as illustrated in
Figure 4b. As depicted in
Figure 4b, the mole fractions of C
2 and lighter components and C
5 and heavier components for the stabilization tower are 2.9% and 0.3%, respectively, and both are within LPG standards.
The Aspen Plus program defaults the reboiler as a theoretical plate, and the last plate can only be written as N-1. The 20th plate in the stabilizer is the reboiler. The concentration of C
5–6 (gas) decreases in the reboiler of the stabilization tower since it evaporates back to the bottom of the tower, as shown in
Figure 4c.
The detailed specification for dry gas, LPG, and stabilized gasoline are given in
Table 5.
3.3. System Optimization Parameters
The compression of rich gas depends on the expansion of a medium-pressure steam turbine to do its work. The larger the compression ratio, the more steam will be consumed for doing work, which determines the operating pressure of the absorption tower and constitutes the main energy-consuming part of the GCS. In addition to operating pressure, the absorption effect of the absorber is also closely related to the temperature and flow rate of the absorbent and supplementary absorbent in the absorption tower. The higher the absorbent’s circulation volume, the less pressure the rich gas needs to be compressed to have the same absorption effect. In this instance, while the compressor requires less steam, the pump consumes more power. In contrast, the rich gas needs to be compressed to a higher pressure for the same absorption effect since the absorbent’s circulation volume is smaller. In this instance, the compressor consumes more energy than the pump. The absorption effect influences the separation energy consumption of other towers in a direct manner. This study focuses on the optimization of the GCS and ASS’s operating parameters.
3.3.1. Objective Function
The rich gas compressor is powered by medium-pressure steam in the process flow. Electricity is utilized to power the pumps that deliver crude gasoline and stabilized gasoline, respectively, as an absorbent and supplementary absorbent to the absorption tower. Therefore, it is important to take into account both medium-pressure steam and electricity simultaneously. Since this study focuses on the operational optimization of the existing process, our primary goal is to reduce the system’s operational costs.
where C
s and C
e represent the price of medium-pressure steam, electricity, which are
$50.15/t and
$0.13/kWh, respectively [
19]; F
s: medium-pressure steam usage, t/h; P
e: pump’s power consumption, kWh/h.
3.3.2. Manipulate Variables
Absorption, reabsorption, desorption, and stabilization towers are interconnected and influence one another within the FCC ASS. In addition to modifying the output pressure of the compressor in GCS, it is necessary to adjust the parameters of each tower to achieve optimal operation in ASS. Manipulated variables include flowrate of the absorbent (
), flowrate of the Supplementary absorbent (
), medium-pressure steam consumption (
), reflux ratio and reboiler load of stabilizer (
), inlet temperature of absorbent (
) and Desorber Reboiler Load(
).
3.3.3. Constraint Conditions
To ensure that the products (dry gas, liquefied petroleum gas, and stabilized gasoline) obtained by the simulation of the absorption stabilization system meet the process indicators while simultaneously optimizing the compressor outlet pressure, absorbent, and supplementary absorbent dosage, it is necessary to impose constraints to ensure that the optimization also satisfies the constraints as follows:
3.4. Optimization and Analysis
The objective function is defined within the optimization model in Aspen Plus Analysis Tools, minimizing the operational cost of medium-pressure steam and electricity for the system. The upper and lower bounds of manipulated variables are also defined in the optimization block. Flowrate of the absorbent, the flow rate of the Supplementary absorbent, medium-pressure steam consumption, reflux ratio, reboiler load of stabilizer, inlet temperature of absorbent, and Desorber Reboiler Load were considered as decision variables in the optimization. The Constraints are defined within the constraint model in Aspen Plus Analysis Tools, including quality specifications for dry gas, liquefied petroleum gas, and stabilized gasoline. The SQP optimization algorithm is used in Aspen Optimizer. The SQP algorithm is designed to handle both equality and inequality constraints. It incorporates the constraints into the optimization problem, ensuring that the solution satisfies the specified constraints. This makes it useful for problems where constraints on process variables or system limitations need to be considered during optimization.
The Base case is from the FCC Rich Gas Compression System and Absorption-stabilization System in fuel-based refineries. The entire system is simulated using the commercial software environment Aspen Plus V12.0, and it is optimized using the optimization module of model analysis software. The stream parameters of the Steam Turbine and rich gas compressor in GCS are illustrated in
Table 6.
The feed temperature of the absorbent has both positive and negative effects on the total energy consumption of the system. In particular, the low feed temperature of the absorbent helps decrease the concentrations of C3+ components, and flow rate of the dry gas, thus maximizing the recovery of propylene and butene. However, a low feed temperature of absorbent increases the content of C2+ and flow rate of the feed stream from the gas-liquid separation tank, which inevitably raises the cold load of absorption tower and the heat load of desorption tower to meet the product specifications. On the contrary, the higher feed temperature of the absorbent is harmful to improving the absorbing efficiency of the absorption tower while increasing the energy requirement of the pumps and compressors, as well as the reboiler duty of the desorber and stabilizer. Therefore, we need to appropriately adjust the feed temperature of the absorbent to determine the trade-off between product quality and energy requirements. The feed temperature of the absorbent becomes a key design parameter in the new process design and will be investigated later.
Based on the assumption that chilled water is available as a cold utility, the temperature range of the absorbent can be determined by replacing the cooling water with chilled water. By using chilled water instead of cooling water, the absorbent temperature can be maintained within a specific range. For example, let us consider a scenario where the previous cooling water temperature ranged from 25 °C to 35 °C and the absorbent temperature cannot be reduced below 40 °C. By replacing it with chilled water, the absorbent temperature range could be narrowed down to 10 °C to 30 °C. This narrower range can help optimize the absorbent’s performance and improve the overall efficiency of the system.
Table 7 shows the bounds on these manipulated variables and the results obtained by adjusting the decision variables through the optimization module in Aspen Plus V12.0. The objective function converged at the optimal operational parameters.
The higher the compressor outlet pressure, the smaller the amount of stabilized gasoline required as supplemental absorbent to meet the same separation requirements, but at a higher total utility cost. This is because compressors work far less efficiently than pumps and therefore consume more energy.
As stated previously, the utility cost is based on the minimum operating cost of the steam turbine and pump as the objective function, which is calculated by manipulating the decision variables using the optimization module. The operating costs for the base case and after optimization are compared in
Table 8.
According to
Table 8, the operating cost prior to optimization was
$1985.667/h, whereas it was
$1937.624/h after optimization. After calculation, the total savings are
$48.043/h, and the annual cost savings are
$413,1698 (Based on the annual operation hour of 8600 h). Under optimal conditions of operation, a refinery can save 2.42% of utility expenses when compared to the base scenario of a 725 kt/a rich gas FCC unit.
4. Results and Discussion
Since the absorption tower is the core equipment connecting the rich gas compression system and the system stabilization system, it is necessary to optimize the decision variables, such as the amount of absorbent (crude gasoline) and supplementary absorbent (stabilized gasoline) to the absorption tower. The pressure of the absorption tower is determined by the rich gas compressor, so it is necessary to optimize the outlet pressure of the rich gas compressor as well. To achieve the expected goal of minimizing the operating costs of steam turbines and pumps as the objective function, the compressor outlet pressure was reduced from 1300 kPa (prior to optimization) to 1260 kPa (after optimization). Under optimal operation, a refinery can save 2.42% of utility expenses, when compared to the base scenario of a 725 kt/a rich gas FCC unit.
To compensate for the effect of pressure reduction, the circulation volume of absorbent and supplementary absorbent is increased, thereby enhancing the absorption effect of light components C3+ in the absorption tower as a whole. The C2− light components are supposed to separate from the top of the absorption tower, thereby reducing the amount of C2− light components fed to the stabilization tower. However, the absorption effect of the absorption tower should not be too high; otherwise, the operating cost of the desorption tower will increase.
It is important to note that the absorbent’s inlet temperature is 16.56 °C when operating expenses are at their lowest. Since the temperature of the absorbent is limited by the temperature of the cooling water supply, it is difficult to achieve the best absorption effect of the absorption tower, and future research will employ the lithium bromide absorption refrigeration system to recover the heat of the high-temperature stable gasoline in the stabilization system to supply refrigerant water, replacing the traditional circulating cooling water system as the cold source of the absorbent. This strategy can be considered to cool the absorbent temperature of the absorption tower to a lower temperature, thereby improving the absorption effect and the economic efficiency of the whole system.