Synthesis and Properties of Polyhydroxyalkanoates on Waste Fish Oil from the Production of Canned Sprats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Obtaining and Characterization of Sprat Oil
2.2. Analysis of the Chemical Composition of Sprat Oil
2.3. PHA Producer Strain and Cultivation Technique
2.4. Analysis and Properties of PHA
2.5. Statistics
3. Results and Discussion
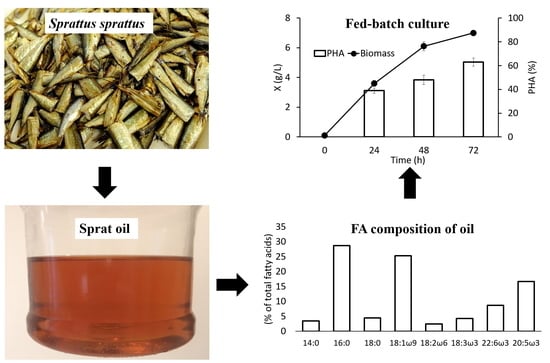
3.1. Characteristics of Sprat Oil
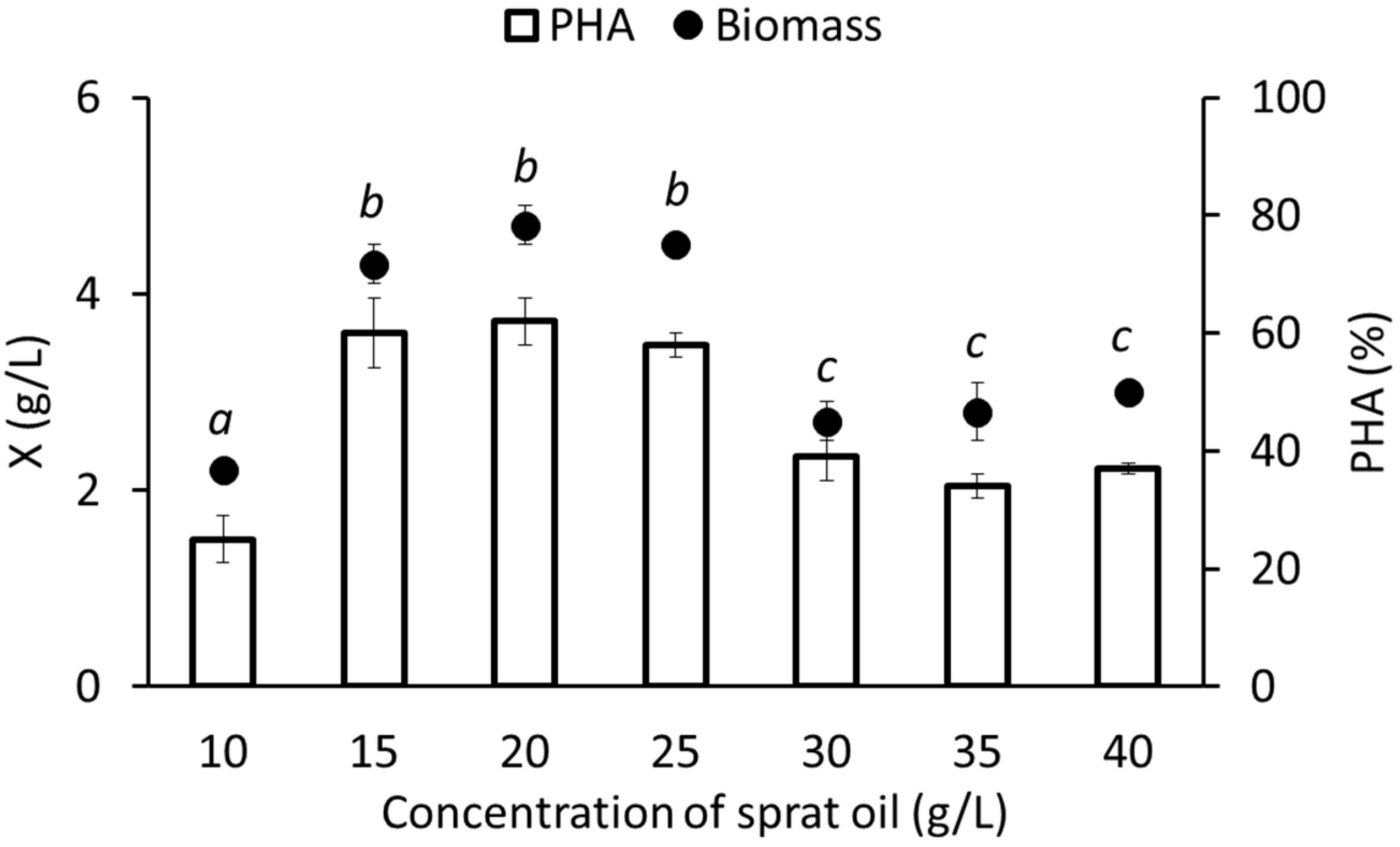
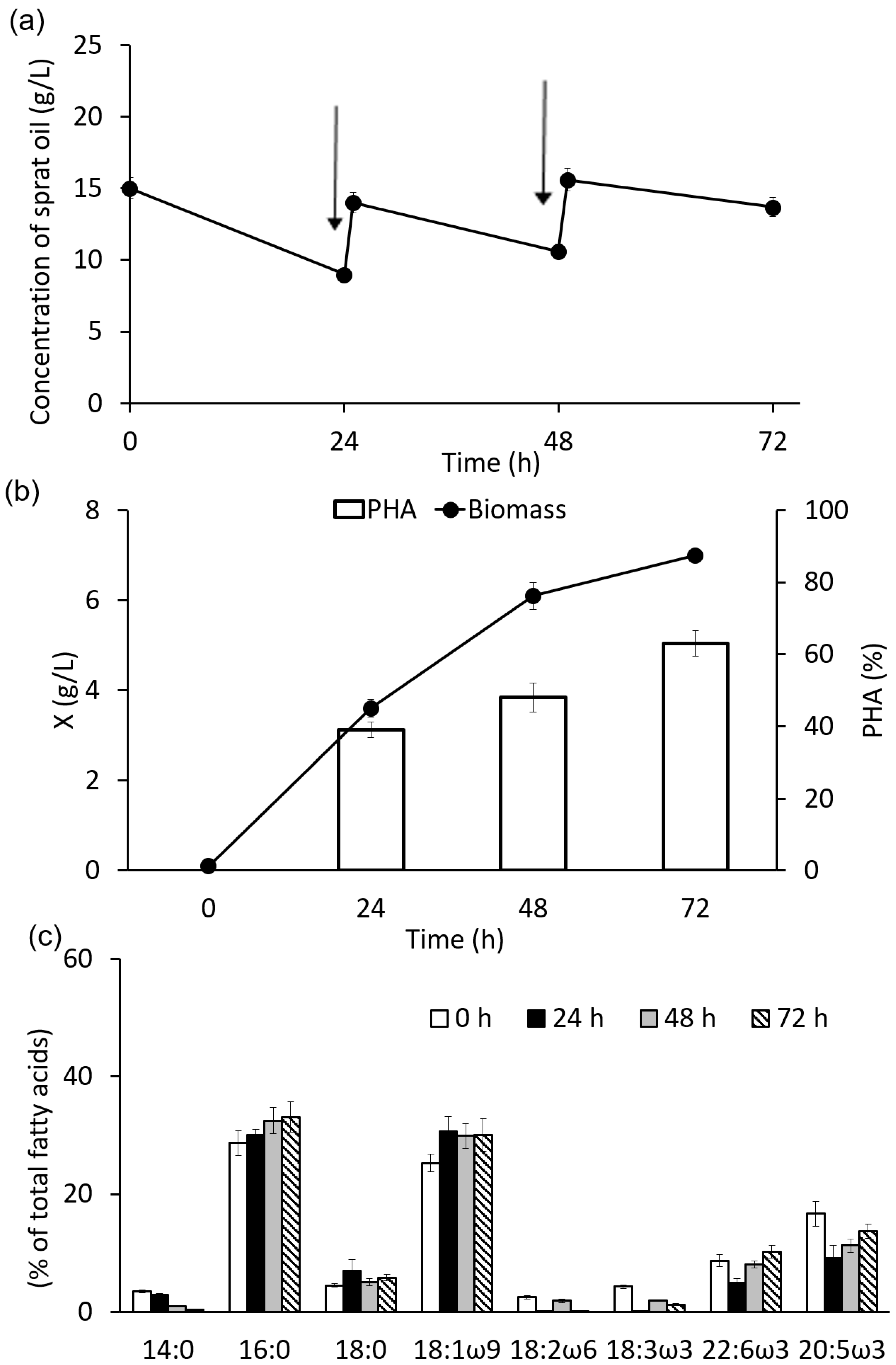
3.2. Growth and Synthesis of PHA by C. necator B-10646 from of Sprat Oil
3.3. Composition and Properties of PHA Synthesized on Sprat Oil
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mitra, R.; Xu, T.; Chen, G.Q.; Xiang, H.; Han, J. An updated overview on the regulatory circuits of polyhydroxyalkanoates synthesis. Microb. Biotechnol. 2022, 15, 1446–1470. [Google Scholar] [CrossRef] [PubMed]
- Dalton, B.; Bhagabati, P.; De Micco, J.; Padamat, R.B.; O’Connor, K. A review on biological synthesis of the biodegradable polymers polyhydroxyalkanoates and the development of multiple applications. Catalysts 2022, 12, 319. [Google Scholar] [CrossRef]
- Gutschmann, B.; Huang, B.; Santolin, L.; Thiele, I.; Neubauer, P.; Riedel, S.L. Native feedstock options for the polyhydroxyalkanoate industry in Europe: A review. Microbiol. Res. 2022, 264, 127177. [Google Scholar] [CrossRef] [PubMed]
- Koller, M.; Obruča, S. Biotechnological production of polyhydroxyalkanoates from glycerol: A review. Biocatal. Agric. Biotechnol. 2022, 42, 102333. [Google Scholar] [CrossRef]
- Mahato, R.P.; Kumar, S.; Singh, P. Production of polyhydroxyalkanoates from renewable resources: A review on prospects, challenges and applications. Arch. Microbiol. 2023, 205, 172. [Google Scholar] [CrossRef]
- Koller, M.; Mukherjee, A. A new wave of industrialization of PHA biopolyesters. Bioengineering 2022, 9, 74. [Google Scholar] [CrossRef]
- Maddikeri, G.L.; Pandit, A.B.; Gogate, P.R. Adsorptive removal of saturated and unsaturated fatty acids using ion-exchange resins. Ind. Eng. Chem. Res. 2012, 51, 6869–6876. [Google Scholar] [CrossRef]
- Riedel, S.L.; Jahns, S.; Koenig, S.; Bock, M.C.; Brigham, C.J.; Bader, J.; Stahl, U. Polyhydroxyalkanoates production with Ralstonia eutropha from low quality waste animal fats. J. Biotechnol. 2015, 214, 119–127. [Google Scholar] [CrossRef]
- Saad, V.; Gutschmann, B.; Grimm, T.; Widmer, T.; Neubauer, P.; Riedel, S.L. Low-quality animal by-product streams for the production of PHA-biopolymers: Fats, fat/protein-emulsions and materials with high ash content as low-cost feedstocks. Biotechnol. Lett. 2021, 43, 579–587. [Google Scholar] [CrossRef]
- Bong, C.P.C.; Alam, M.N.H.Z.; Samsudin, S.A.; Jamaluddin, J.; Adrus, N.; Yusof, A.H.M.; Muis, Z.A.; Hashim, H.; Salleh, M.M.; Abdullah, A.R.; et al. A review on the potential of polyhydroxyalkanoates production from oil-based substrates. J. Environ. Manag. 2021, 298, 113461. [Google Scholar] [CrossRef]
- Ghaly, A.E.; Ramakrishnan, V.V.; Brooks, M.S.; Budge, S.M.; Dave, D. Fish Processing Wastes as a Potential Source of Proteins. Amino Acids and Oils: A Critical Review. J. Microb. Biochem. Technol. 2013, 2, 107–129. [Google Scholar] [CrossRef] [Green Version]
- Caruso, G. Fishery wastes and by-products: A resource to be valorised. J. Fish. Sci. 2015, 9, 080–083. [Google Scholar]
- Ashby, R.D.; Solaiman, D.K.Y. Poly(hydroxyalkanoate) biosynthesis from crude alaskan pollock (Theragra chalcogramma) oil. J. Polym. Environ. 2008, 16, 221–229. [Google Scholar] [CrossRef]
- Mohapatra, S.; Sarkar, B.; Samantaray, D.P.; Daware, A.; Maity, S.; Pattnaik, S.; Bhattacharjee, S. Bioconversion of fish solid waste into PHB using Bacillus subtilis based submerged fermentation process. Env. Technol. 2017, 38, 3201–3208. [Google Scholar] [CrossRef]
- Argiz, L.; Gonzalez-Cabaleiro, R.; Correa-Galeote, D.; del Rio, A.V.; Mosquera-Corral, A. Open-culture biotechnological process for triacylglycerides and polyhydroxyalkanoates recovery from industrial waste fish oil under saline conditions. Sep. Purif. Technol. 2021, 270, 118805. [Google Scholar] [CrossRef]
- Sangkharak, K.; Paichid, N.; Yunu, T.; Klomklao, S.; Prasertsan, P. Utilisation of tuna condensate waste from the canning industry as a novel substrate for polyhydroxyalkanoate production. Biomass Convers. Biorefinery 2021, 11, 2053–2064. [Google Scholar] [CrossRef]
- Correa-Galeote, D.; Argiz, L.; Val del Rio, A.; Mosquera-Corral, A.; Juarez-Jimenez, B.; Gonzalez-Lopez, J.; Rodelas, B. Dynamics of PHA-Accumulating Bacterial Communities Fed with Lipid-Rich Liquid Effluents from Fish-Canning Industries. Polymers 2022, 14, 1396. [Google Scholar] [CrossRef] [PubMed]
- Thuoc, D.V.; My, D.N.; Loan, T.T.; Sudesh, K. Utilization of waste fish oil and glycerol as carbon sources for polyhydroxyalkanoate production by Salinivibrio sp. M318. Int. J. Biol. Macromol. 2019, 141, 885–892. [Google Scholar] [CrossRef]
- Thuoc, D.V.; Anh, V.T.M. Bioconversion of Crude Fish Oil into Poly-3-hydroxybutyrate by Ralstonia sp. M91. Appl. Biochem. Microbiol. 2021, 57, 219–225. [Google Scholar] [CrossRef]
- Loan, T.T.; Trang, D.T.Q.; Huy, P.Q.; Ninh, P.X.; Thuoc, V.D. A fermentation process for the production of poly(3-hydroxybutyrate) using waste cooking oil or waste fish oil as inexpensive carbon substrate. Biotechnol. Rep. 2022, 33, e00700. [Google Scholar] [CrossRef]
- Jiang, G.; Hill, D.J.; Kowalczuk, M.; Johnston, B.; Adamus, G.; Irorere, V.; Radecka, I. Carbon sources for polyhydroxyalkanoates and an integrated biorefinery. Int. J. Mol. Sci. 2016, 17, 1157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- European Market Observatory for Fisheries and Aquaculture Products. Canned Sprat in the EU. Available online: https://www.eumofa.eu/documents/20178/355514/PTAT_Canned+sprat.pdf/ (accessed on 4 June 2023).
- State Standard 280-2021; Canned Fish. “Sprats in Oil”. Specifications. Russian Institute of Standardization: Moscow, Russia, 2021. (In Russian)
- Ermakov, A.I.; Arasimovich, V.V.; Smirnova-Ikonnikova, M.I.; Yarosh, N.P.; Lukovnikova, G.A. Metody Biokhimicheskogo Issledovaniya Rastenii (Methods of Biochemical Plant Research); Kolos: Leningrad, Russia, 1972. (In Russian) [Google Scholar]
- State Standard 7636-85; Fish, Marine Mammals, Marine Invertebrates and Their Processed Products. Analysis Methods. Standartinform Publishing: Moscow, Russia, 2010. (In Russian)
- Volova, T.G.; Shishatskaya, E.I. Shtamm Bakterii Cupriavidus eutrophus VKPM B-10646—Producent Polyhidroxyalkanoatov i Sposob Ich Poluchenia (Bacterial Strain Cupriavidus eutrophus VKPM B-10646—Producer of Polyhydroxyalkanoates and Method for Their Production). RF Patent 2439143, 10 January 2012. (In Russian). [Google Scholar]
- Schlegel, H.G.; Kaltwasser, H.; Gottschalk, G. A submersion method for culture of hydrogen-oxidizing bacteria: Growth physiological studies. Arch. Microbiol. 1961, 38, 209–222. [Google Scholar]
- Budde, C.F.; Riedel, S.L.; Hübner, F.; Risch, S.; Popović, M.K.; Rha, C.; Sinskey, A.J. Growth and polyhydroxybutyrate production by Ralstonia eutropha in emulsified plant oil medium. Appl. Microbiol. Biotechnol. 2011, 89, 1611–1619. [Google Scholar] [CrossRef] [PubMed]
- Takaç, S.; Marul, B. Effects of lipidic carbon sources on the extracellular lipolytic activity of a newly isolated strain of Bacillus subtilis. Ind. Microbiol. Biotechnol. 2008, 35, 1019–1025. [Google Scholar] [CrossRef] [PubMed]
- Volova, T.; Kiselev, E.; Nemtsev, I.; Lukyanenko, A.; Sukovatyi, A.; Kuzmin, A.; Ryltseva, G.; Shishatskaya, E. Properties of degradable polyhydroxyalkanoates with different monomer compositions. Int. J. Biol. Macromol. 2021, 182, 98–114. [Google Scholar] [CrossRef]
- Mezenova, O.Y.; Heling, A.; Mersel, J.T.; Volkov, V.V.; Mezenova, N.Y.; Agafonova, S.V.; Sauskan, V.I.; Altshul, B.A.; Rosenstein, M.M.; Andreev, M.P. Analis sostoyaniya ekonomiki I perspektiv primeneniya biotechnologii v rybnoi otrasli Kalliningradskoi oblasti (Analysis of the state of the economy and prospects for the use of biotechnology in the fishing industry of the Kaliningrad region). Rybn. Chozyaistvo 2020, 5, 38–50. [Google Scholar] [CrossRef]
- Mezenova, O.Y.; Pyanov, D.S.; Agafonova, S.V.; Mezenova, N.Y.; Volkov, V.V. Proektirovanie sbalansirovannyh kormov dlya industrialnoyi akvakultury s primeneniem proteinovyx hydrolysatov pobochnogo rubnogo syria (Design of balanced feed for industrial aquaculture using protein hydrolysates of by-pay fish raw materials). Rybn. Chozyaistvo 2021, 4, 81–88. [Google Scholar] [CrossRef]
- Srivastava, S.K.; Tripathi, A.D. Effect of saturated and unsaturated fatty acid supplementation on bio-plastic production under submerged fermentation. 3 Biotech. 2013, 3, 389–397. [Google Scholar] [CrossRef] [Green Version]
- Volova, T.G.; Kiselev, E.G.; Shishatskaya, E.I.; Zhila, N.O.; Boyandin, A.N.; Syrvacheva, D.A.; Vinogradova, O.N.; Kalacheva, G.S.; Vasiliev, A.D.; Peterson, I.V. Cell growth and accumulation of polyhydroxyalkanoates from CO2 and H2 of a hydrogen-oxidizing bacterium, Cupriavidus eutrophus B-10646. Bioresour. Technol. 2013, 146, 215–222. [Google Scholar] [CrossRef]
- Volova, T.; Demidenko, A.; Kiselev, E.; Baranovskiy, S.; Shishatskaya, E.; Zhila, N. Polyhydroxyalkanoate synthesis based on glycerol and implementation of the process under conditions of pilot production. Appl. Microbiol. Biotechnol. 2019, 103, 225–237. [Google Scholar] [CrossRef] [Green Version]
- Volova, T.G.; Kiselev, E.G.; Zhila, N.O.; Shishatskaya, E.I. Synthesis of PHAs by Hydrogen Bacteria in a Pilot Production Process. Biomacromolecules 2019, 20, 3261–3270. [Google Scholar] [CrossRef] [PubMed]
- Volova, T.; Sapozhnikova, K.; Zhila, N. Cupriavidus necator B-10646 growth and polyhydroxyalkanoates production on different plant oils. Int. J. Biol. Macromol. 2020, 164, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Kiselev, E.G.; Demidenko, A.V.; Zhila, N.O.; Shishatskaya, E.I.; Volova, T.G. Sugar beet molasses as a potential C-substrate for PHA production by Cupriavidus necator. Bioengineering 2022, 9, 154. [Google Scholar] [CrossRef] [PubMed]
- Volova, T.G.; Kiselev, E.G.; Demidenko, A.V.; Zhila, N.O.; Nemtsev, I.V.; Lukyanenko, A.V. Production and properties of microbial polyhydroxyalkanoates synthesized from hydrolysates of Jerusalem artichoke tubers and vegetative biomass. Polymers 2022, 14, 132. [Google Scholar] [CrossRef] [PubMed]
- Salmond, C.V.; Kroll, R.G.; Booth, I.R. The effect of food preservatives on pH homeostasis in Escherichia coli. J. Gen. Microbiol. 1984, 130, 2845–2850. [Google Scholar] [CrossRef] [Green Version]
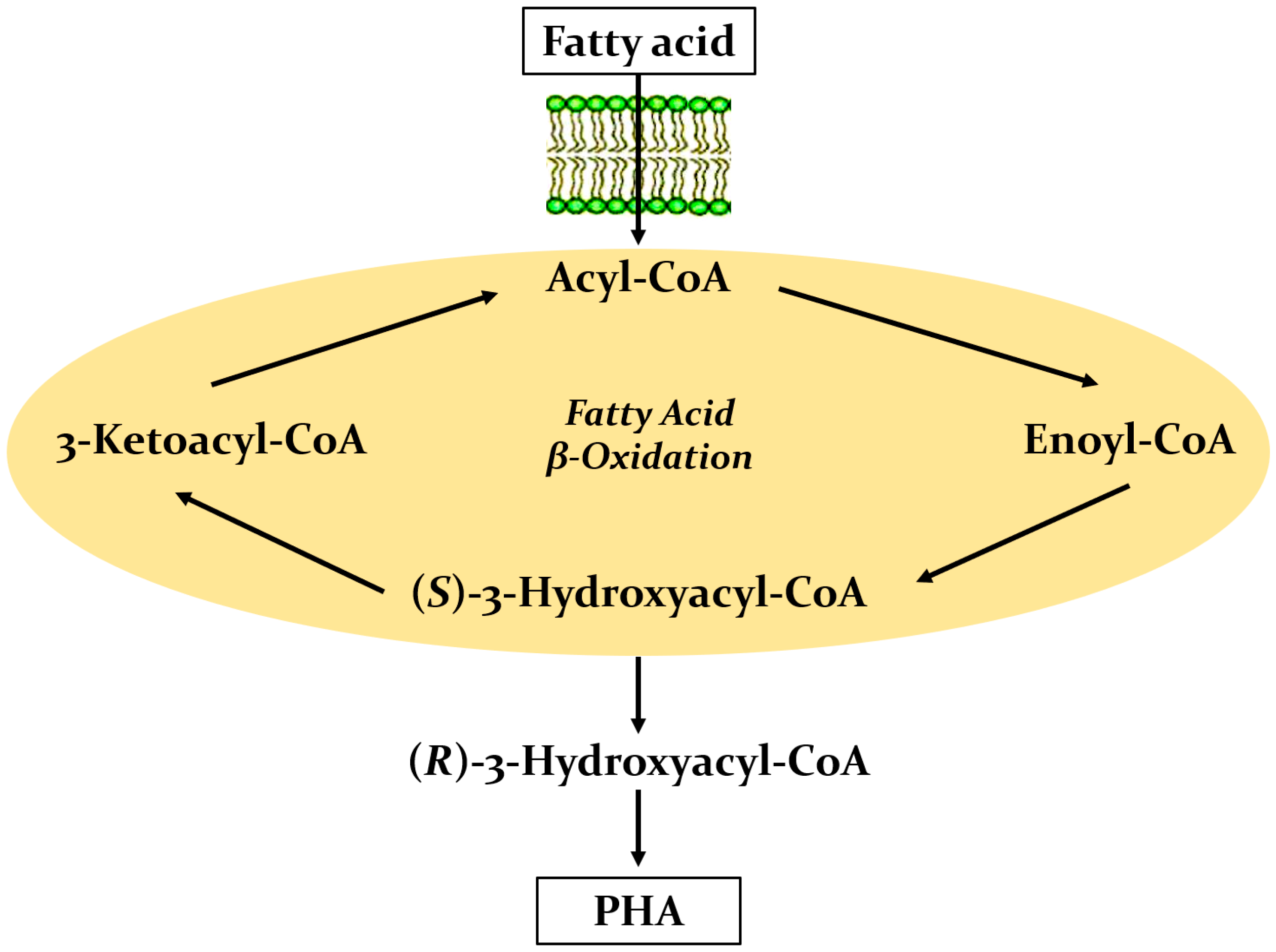
- Riedel, S.L.; Lu, J.; Stahl, U.; Brigham, C.J. Lipid and fatty acid metabolism in Ralstonia eutropha: Relevance for the biotechnological production of value-added products. Appl. Microbiol. Biotechnol. 2014, 98, 1469–1483. [Google Scholar] [CrossRef] [Green Version]
- Fukui, T.; Doi, Y. Efficient production of polyhydroxyalkanoates from plant oils by Alcaligenes eutrophus and its recombinant strain. Appl. Microbiol. Biotechnol. 1998, 49, 333–336. [Google Scholar] [CrossRef]
- Green, P.R.; Kemper, J.; Schechtman, L.; Guo, L.; Satkowski, M.; Fiedler, S.; Steinbüchel, A.; Rehm, B.H. Formation of short chain length/medium chain length polyhydroxyalkanoate copolymers by fatty acid β-oxidation inhibited Ralstonia eutropha. Biomacromolecules 2002, 3, 208–213. [Google Scholar] [CrossRef]
- Riedel, S.L.; Bader, J.; Brigham, C.J.; Budde, C.F.; Yusof, Z.A.M.; Rha, C.; Sinskey, A.J. Production of poly (3-hydroxybutyrate-co-3-hydroxyhexanoate) by Ralstonia eutropha in high cell density palm oil fermentations. Biotechnol. Bioeng. 2012, 109, 74–83. [Google Scholar] [CrossRef] [Green Version]
- Zhila, N.; Kalacheva, G.; Volova, T. Fatty acid composition and polyhydroxyalkanoates production by Cupriavidus eutrophus B-10646 cells grown on different carbon sources. Process Biochem. 2015, 50, 69–78. [Google Scholar] [CrossRef]
- Jambunathan, P.; Zhang, K. Engineered biosynthesis of biodegradable polymers. J. Ind. Microbiol. Biotechnol. 2016, 43, 1037–1058. [Google Scholar] [CrossRef] [PubMed]
- Tropea, A.; Ferracane, A.; Albergamo, A.; Potortì, A.G.; Lo Turco, V.; Di Bella, G. Single cell protein production through multi food-waste substrate fermentation. Fermentation 2022, 8, 91. [Google Scholar] [CrossRef]
- Richard, N.; Mourente, G.; Kaushik, S.; Corraze, G. Replacement of a large portion of fish oil by vegetable oils does not affect lipogenesis, lipid transport and tissue lipid uptake in European seabass (Dicentrarchus labrax L.). Aquaculture 2006, 261, 1077–1087. [Google Scholar] [CrossRef]
- Babalola, T.O.O.; Apata, D.F. Chemical and quality evaluation of some alternative lipid sources for aqua feed production. Agric. Biol. J. N. Am. 2011, 2, 935–943. [Google Scholar] [CrossRef]
- Oseni, O.A.; Akindahunsi, A.A. Some phytochemical properties and effect of fermentation on the seed of Jatropha curcas L. Am. J. Food Technol. 2011, 6, 158–165. [Google Scholar] [CrossRef] [Green Version]
- Yano, Y.; Oikawa, H.; Satomi, M. Reduction of lipids in fish meal prepared from fish waste by a yeast Yarrowia lipolytica. Int. J. Food Microbiol. 2008, 121, 302–307. [Google Scholar] [CrossRef]
- Volova, T.; Kiselev, E.; Vinogradova, O.; Nikolaeva, E.; Chistyakov, A.; Sukovatiy, A.; Shishatskaya, E. A glucose-utilizing strain, Cupriavidus euthrophus B-10646: Growth kinetics, characterization and synthesis of multicomponent PHAs. PLoS ONE 2014, 9, e87551. [Google Scholar] [CrossRef]
- Bhubalan, K.; Rathi, D.N.; Abe, H.; Iwata, T.; Sudesh, K. Improved synthesis of P (3HB-co-3HV-co-3HHx) terpolymers by mutant Cupriavidus necator using the PHA synthase gene of Chromobacterium sp. USM2 with high affinity towards 3HV. Polym. Degrad. Stab. 2010, 95, 1436–1442. [Google Scholar] [CrossRef]
- Zhang, H.F.; Ma, L.; Wang, Z.H.; Chen, G.Q. Biosynthesis and characterization of 3-hydroxyalkanoate terpolyesters with adjustable properties by Aeromonas hydrophila. Biotechnol. Bioeng. 2009, 104, 582–589. [Google Scholar] [CrossRef]
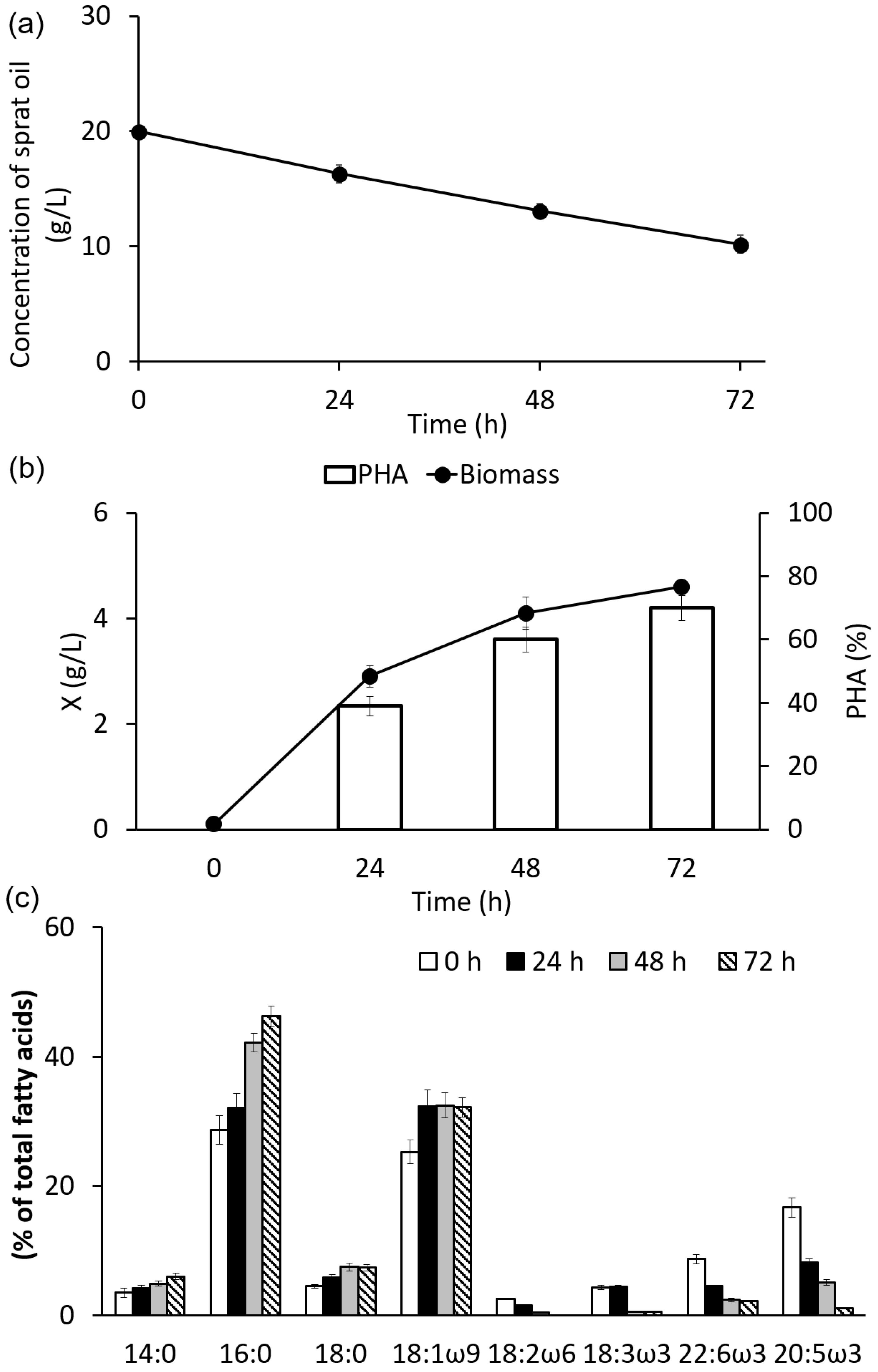
| Fatty Acid Index | Fatty Acid Type | Content, % of Sum of Fatty Acids |
|---|---|---|
| 14:0 | Myristic | 3.5 ± 0.1 |
| 15:0 | Pentadecanoic | 0.5 ± 0.0 |
| 16:0 | Palmitic | 28.0 ± 1.2 |
| 16:1ω7 | Palmitoleic | 0.3 ± 0.0 |
| 18:0 | Stearic | 4.5 ± 0.2 |
| 18:1ω9 | Oleic | 25.3 ± 1.3 |
| 18:1ω6 | Linoleic | 2.5 ± 0.1 |
| 18:1ω3 | Linolenic | 4.3 ± 0.1 |
| 20:0 | Arachidic | 0.3 ± 0.0 |
| 20:1 | Eicosenoic | 1.1 ± 0.0 |
| 20:2 | Eicosadienoic | 0.4 ± 0.0 |
| 20:5ω3 | Eicosapentaenoic | 8.7 ± 0.3 |
| 22:0 | Docosanoic | 0.5 ± 0.0 |
| 22:6ω3 | Docosahexaenoic | 16.7 ± 0.2 |
| 24:1 | Tetracosenoic | 1.5 ± 0.1 |
| Other * | 1.3 ± 0.1 | |
| ∑saturated FAs/∑unsaturated FAs | 0.63 |
| Waste Fish Oil Concentration, g/L | Specific Growth Rate, h−1 | PHA Specific Synthesis Rate, h−1 | Biomass Productivity, g/L·h | PHA Productivity, g/L·h |
|---|---|---|---|---|
| 10.0 | 0.116 | 0.020 | 0.046 | 0.011 |
| 15.0 | 0.139 | 0.056 | 0.090 | 0.054 |
| 20.0 | 0.140 | 0.059 | 0.098 | 0.061 |
| 25.0 | 0.139 | 0.054 | 0.094 | 0.054 |
| 30.0 | 0.123 | 0.052 | 0.056 | 0.022 |
| 35.0 | 0.118 | 0.047 | 0.058 | 0.020 |
| 40.0 | 0.120 | 0.051 | 0.063 | 0.023 |
| Concentration of Sprat Oil in the Medium, g/L | Composition of PHA, mol.% | Mn, kDa | Mw, kDa | Đ | Cx, % | Tmelt, °C | Tdegr., °C | ||
|---|---|---|---|---|---|---|---|---|---|
| 3HB | 3HV | 3HHx | |||||||
| 15 | 98.0 | 1.3 | 0.7 | 116 | 540 | 4.6 | 67 | 158/163 | 287 |
| 20 | 97.8 | 1.7 | 0.5 | 117 | 669 | 5.7 | 66 | 159/165 | 292 |
| 25 | 98.1 | 1.6 | 0.3 | 133 | 762 | 5.7 | 72 | 160/165 | 293 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhila, N.O.; Sapozhnikova, K.Y.; Kiselev, E.G.; Shishatskaya, E.I.; Volova, T.G. Synthesis and Properties of Polyhydroxyalkanoates on Waste Fish Oil from the Production of Canned Sprats. Processes 2023, 11, 2113. https://doi.org/10.3390/pr11072113
Zhila NO, Sapozhnikova KY, Kiselev EG, Shishatskaya EI, Volova TG. Synthesis and Properties of Polyhydroxyalkanoates on Waste Fish Oil from the Production of Canned Sprats. Processes. 2023; 11(7):2113. https://doi.org/10.3390/pr11072113
Chicago/Turabian StyleZhila, Natalia O., Kristina Yu. Sapozhnikova, Evgeniy G. Kiselev, Ekaterina I. Shishatskaya, and Tatiana G. Volova. 2023. "Synthesis and Properties of Polyhydroxyalkanoates on Waste Fish Oil from the Production of Canned Sprats" Processes 11, no. 7: 2113. https://doi.org/10.3390/pr11072113
APA StyleZhila, N. O., Sapozhnikova, K. Y., Kiselev, E. G., Shishatskaya, E. I., & Volova, T. G. (2023). Synthesis and Properties of Polyhydroxyalkanoates on Waste Fish Oil from the Production of Canned Sprats. Processes, 11(7), 2113. https://doi.org/10.3390/pr11072113