1. Introduction
Climate changes observed in recent years are the reason for the search for new environmentally friendly techniques and processes. One is the fluidization process, used in many industrial processes, including drying, mixing, granulation and chemical reactions [
1,
2,
3]. The wide application of fluidization processes is due to its numerous advantages [
4,
5]. One of them is increased gas–solid contact and the mixing of particles, which improves the bed’s thermal conductivity [
6,
7,
8]. In addition, the fluidization process ensures that the bed material is in constant motion and the particles are in close contact, which can help improve adsorption/desorption processes [
9]. Many studies concern applying fluidized bed units under atmospheric and elevated pressure [
10,
11].
There are several research works on the adsorption and desorption of silica gel in a circulating fluidized bed for an air-conditioning system. Chen et al. examined silica gel in circulating fluidized beds used for dehumidification in air-conditioning systems [
12]. The fluidization process is forced by fans that lift the silica gel particles upward. In the study, the air velocity ranged from 4.0 m/s to 6.0 m/s, and the regeneration temperature, which simulates low-temperature waste heat, varied from 40 °C to 60 °C. In another similar study, Ahmed M. Hamed [
13] presented research on adsorption and desorption processes in an inclined fluidized bed using silica gel. The regeneration temperature was maintained at approx. 90 °C, showing the possibility of applying solar energy for desiccant reactivation when using an inclined fluidized bed. The investigations were carried out under atmospheric pressure conditions.
Initially, the low-pressure fluidization seemed to have few practical applications. However, over time, researchers found a use for low-pressure fluidization in fine chemicals solids and the food and pharmaceutical industry [
14]. In the work of Grabowska et al. [
15], the authors presented research on heat transfer in a fluidized adsorption bed at low pressures. Two types of silica gel were tested. In addition, the possibility of obtaining fluidization was assessed using the pressure difference between the evaporator and the adsorption chamber. It has been shown that a pressure difference of 10 mbar allowed one to obtain the fluidized state.
In recent years, the energy demand for cooling has also increased, in addition to the increase in water demand. The International Institute of Refrigeration (IIR) reported that 15% of the world’s electricity production is used for refrigeration and air-conditioning systems [
16].
This type of fluidization can also be important in water desalination [
16] and adsorption chillers [
17]. Water scarcity is now a serious global problem, and it is estimated that by 2050, around 6 billion people will suffer from it. Saltwater is the most significant amount of water available on Earth. Freshwater is a scarce resource, accounting for only 0.75% of the total amount available for human consumption on Earth, of which 7.75% is stored in glaciers and ice caps. The current civilization development causes an increased demand for water, so seawater desalination is used to meet these demands [
18].
Reverse osmosis (RO) is the most widely used among the commonly known water desalination methods. However, this system has disadvantages related to bromide, chloride and boron residues, and above all, it requires high operating costs for mechanical equipment [
9,
19]. Moreover, the traditional powering of desalination plants and cooling systems with energy from fossil fuels will cause further environmental problems. Therefore, renewable energy or waste heat is a good solution to drive these systems [
20]. Currently, adsorption technology that uses waste heat is a promising alternative to conventional desalination and cooling technologies [
21,
22]. This type of technology is used in the industry, where the adsorption process is used in the first phase (evaporation process) to produce cooling energy. In the second phase, fresh water is obtained during desorption and condensation [
23,
24,
25].
A relatively significant disadvantage of using conventional adsorption cooling and desalination systems with packed adsorption beds is their low coefficient of performance (COP). This is due to the low thermal conductivity of the adsorption fixed material employed in the beds. Fluidization can improve heat and mass transfer processes, while the fluidization process itself may be influenced by the composition of the adsorption bed. Therefore, the purpose of the paper is to study the influence of adsorption bed composition on the fluidization process of an adsorption bed [
26,
27].
This study analyzes the effect of adding Al powder and nanotubes to the adsorption bed mixture on its performance at low-pressure fluidization.
The particular practical significance of this work is to carry out the process under low-pressure conditions and to induce fluidization with a pressure difference. These two features distinguish the research presented in this paper and constitute its main novelty. Furthermore, in the literature, there is no data on the effect of the adsorption bed mixture on fluidization under low-pressure conditions; therefore, this is an essential novel scientific aspect and, simultaneously, a new scientific contribution resulting from this work.The practical significance of the paper follows from the fact that the obtained results allow for an increase in the performance of the adsorption chillers and, therefore, an increase in the energy efficiency of the adsorption cooling and desalination systems. Moreover, since adsorption chillers accept low-grade thermal energy sources, including renewable and waste thermal energy, the presented paper contributes to works on sustainable development. Therefore, the presented results contribute to the zero-emissions concept of energy processes.
2. Materials and Methods
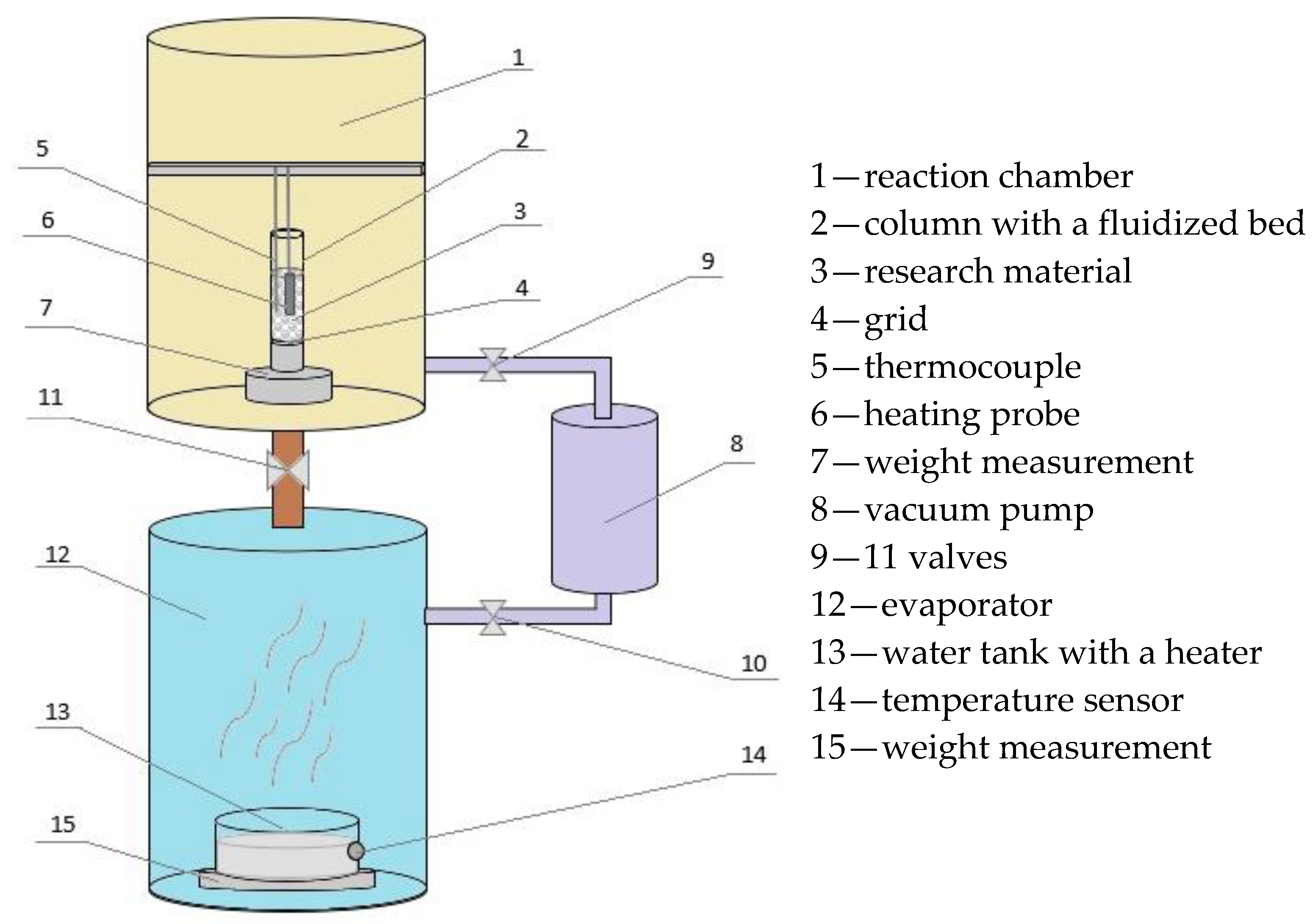
An intensified heat transfer adsorption bed (IHTAB) was used in the study (
Figure 1) [
15]. The facility comprises two primary chambers: the evaporator (12) and the reaction chamber (1). The evaporator and reaction chamber are interconnected with a vacuum pump (8) via respective control valves (9) and (10). The valve (11) regulates the connection between the evaporator and the reaction chamber. The evaporator is equipped with a heater, facilitating water heating to generate water vapor under low-pressure conditions. The pressure difference between the evaporator and the reaction chamber causes fluidization.
The picture of the unit is shown in
Figure 2. The main components of the research unit, shown in
Figure 2, are: I—steel construction of the test unit, II—reaction chamber equipped with a viewfinder, III—evaporator, IV—vacuum pump, V—control system and VI—computer.
An evaporator chamber at the bottom of the unit has a water tank, electric heater, temperature sensors and a weight sensor.
The gas temperature was 25 °C, while the velocity of the gas fluidizing the bed (in this case, it was water vapor) was caused by the pressure difference in both chambers.
The minimum fluidization velocity of the solid particles is in the range of 0.43–0.68 m/s. The system operated in transient regimes corresponding to bubbling and circulating fluidized beds (superficial gas velocity: 6.6–14 m/s), but the bed did not circulate and was held inside the chamber by the net.
A more detailed description of the research unit can be found in the paper [
15].
It should be noted that in this study, the fluidized bed was not reheated. Additionally, the heater was not inserted into the bed to not disturb the fluidization caused by the steam flow. A detailed description of the facility can be found elsewhere [
15].
The adsorption bed was composed of silica gel (a particle sphericity of 0.65, a density of 2200 kg/m
3 and a bulk density of 850 kg/m
3) supplied by Sigma-Aldrich. An aluminum powder of 5 and 15% of the weight was added to the adsorption bed. Powder of aluminum was supplied from KAMB Import-Export. Mixtures containing 5 and 15% of carbon nanotubes were also prepared. The ingredients were measured in the appropriate proportion to prepare the SG-Al and SG-CNT mixtures, then combined and mechanically mixed in three directions. The mixture prepared in this way was poured into the bed column. Carbon nanotubes were made by the 3D-nano company. The properties of the bed composition are shown in
Table 1.
Figure 3 shows the microscopic photograph of silica gel, aluminum powder, and carbon nanotubes. The carbon nanotubes form the agglomerates seen in
Figure 3c.
The theoretical basis of the experiment was to induce a fluidization process by differential pressure with an appropriate choice of materials to support the fluidization of adsorption parent material (in this case, silica gel) under low-pressure conditions. Materials used in the tests were selected by taking into account their good thermal properties to enhance heat transfer in the fluidized bed.
The pressure losses generated by the fluidized bed in the reactors are calculated using the following parameters [
5]:
- –
Sauter mean diameter of the particles in the bed (,
- –
the height of the fluidized bed (ZD),
- –
superficial gas velocity (U),
- –
temperatures (T),
- –
pressure of gas fluidizing the bed (P0),
- –
- –
average gas velocity in the fluidized bed (Uav)—gas (steam) velocity caused by the pressure difference between the evaporator and the chamber,
- –
gas density ρ𝐺,
- –
gas viscosity μ𝐺,
- –
Pressure losses (pressure difference) in the bed are determined from the following formula [
5]:
where the dimensionless parameters a
1, a
2, a
3, a
4 are determined as follows:
where 𝑃 is the average bed pressure.
3. Results and Discussion
Studies of adsorption/desorption cycles under fluidization conditions have been conducted. To maintain fluidization before the adsorption stage (the moment the valve was opened), the pressure was about 1000 mbar. This pressure drop allows fluidization to be achieved in each test cycle, and the assumed active flow time (open valve) is 30 s, while the desorption time (closed valve) is 360 s. The opening and closing times of the valve have been chosen experimentally. An opening time of 30 s is sufficient for the fluidization process, extinguishing it before the valve closes. On the other hand, the valve closing time (360 s) is necessary to obtain the correct pressure difference between the evaporator and the chamber. The pressure difference between the evaporator and adsorption chamber is necessary to obtain the fluidization state [
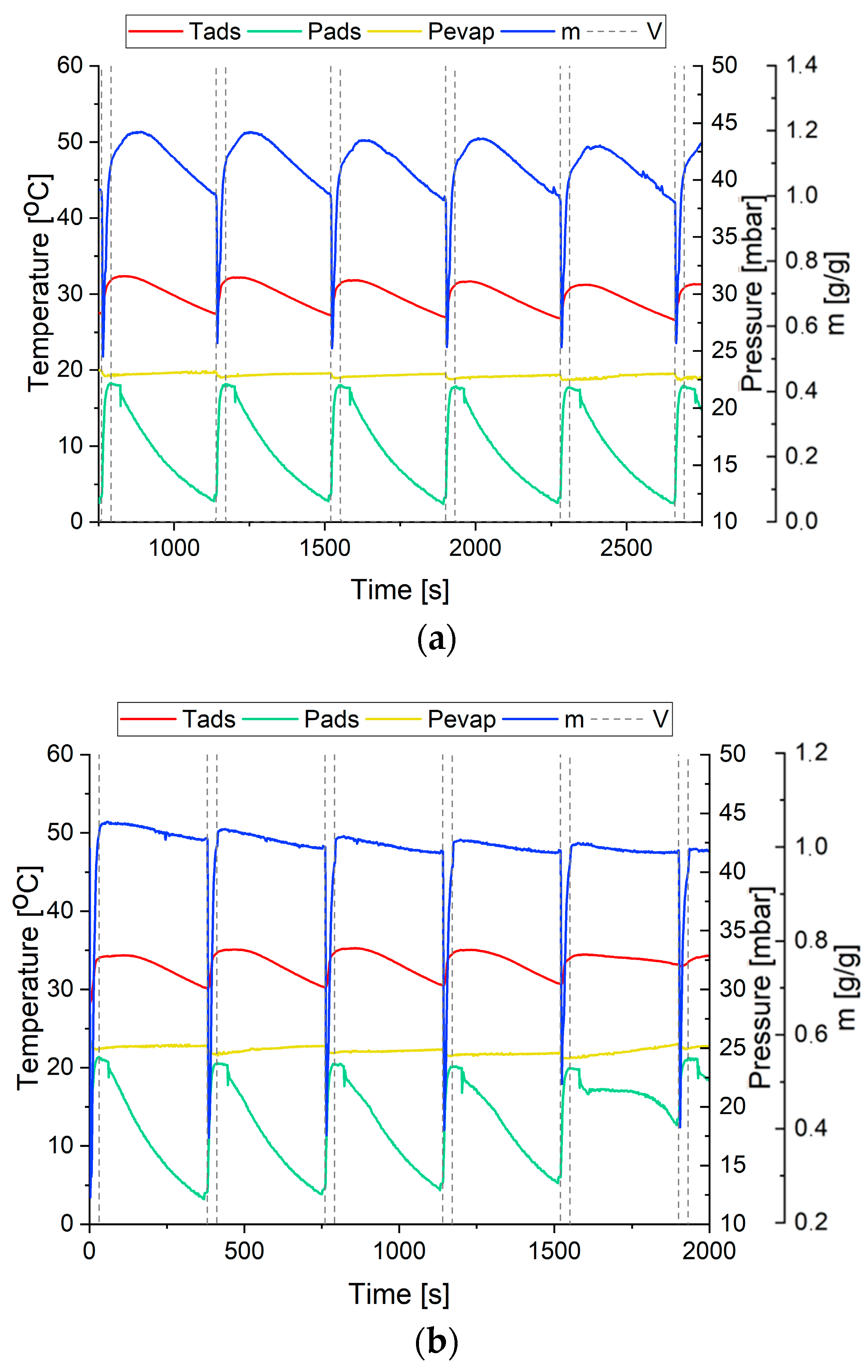
28]. The characteristic cycles for each mixture are shown in
Figure 4.
The purpose of creating mixtures with aluminum powder and nanotubes was to improve heat transfer coefficients, enhancing the intensity of sorption processes involving SG, the parent sorbent material in the adsorption bed. As a metal, aluminum does not have a sorption capacity, but it was selected for the tests due to its high thermal conductivity coefficient. However, good thermal properties did not compensate for its lack of sorption capacities.
In the case of CNT, the situation is different. The material itself does not show a sorption capacity in the water-CNT pair, but it has a shape that leads to the creation of a potential difference at the opposite ends of the nanotube, which accelerates the transport of water or steam through the nanotube, thus facilitating the access of water vapor to the sorbent. This phenomenon probably improved the efficiency of sorption processes in the deposit.
During the study, parameters, such as pressure in the chamber (P
ads) and pressure in the evaporator (P
evap), the temperature of adsorption (T
ads) and the mass of the bed with adsorbed water vapor in relation to the initial mass of the bed (m), were registered. In addition, the diagrams (
Figure 4) show the moments of the open-close cycle of the valve (dashed line). Using the example of a bed containing 100% SG (
Figure 4a), it can be seen that at the moment just before the valve was opened, the pressure difference between the chamber and the evaporator was at a maximum of 1000 mbar. This difference allowed for a fluidized state, as evidenced by a significant decrease in mass when the valve was open. When the valve was closed, an increase in the weight of the bed was observed, with an increase in the weight of the bed up to 1.2 g/g during adsorption up to a certain point in each cycle and a decrease in the weight up to 1.0 g/g due to desorption of water vapor. Such behavior of the bed is the result of the continuation of the process of water vapor adsorption, which begins while the valve is still open and which is well shown by the increase in the temperature of the bed while the valve is still open. Then, the cycle shows a moment of reduction in the mass of the bed as well as its temperature, which indicates the desorption of water vapor. The observed desorption process results from the decreasing pressure, which, in addition to a higher temperature [
29], favors desorption processes. Temperature changes in the bed containing 100% SG were observed in the range of 10 °C, and mass changes of about 0.2 g/g were observed for all cycles. The addition of 5% Al to the bed had a negative effect on the sorption processes.
Figure 4b shows that after fluidization, the mass increase is insignificant, the increase in the bed temperature is about 5 °C, and the mass decrease is imperceptible. The increased aluminum content in the adsorption bed to 15% increases the water vapor uptake up to 1.2 g/g compared to the 5% Al content (
Figure 4c). In this case, the temperature variation in the cycle is within 5 °C.
The most significant changes in mass during the process were observed for a bed with a composition of 5% CNTs (
Figure 4d). The maximum value of the mass is 1.3 g/g, while the decrease in mass during the cycle occurs at a value of 1.0 g/g; in addition, an increase in the maximum mass during the process was observed with each successive cycle, indicating an increasingly better adsorption. The heat effect at this point is also within 5 °C. Increasing the nanotube content to 15% in the bed no longer has the same effect as for 5% carbon nanotubes (
Figure 4e). There is a mass change in the range of 0.9–1.1 g/g and a temperature change in the range of 5 °C.
The best addition to the bed to intensify the adsorption/desorption process is 5% CNTs, as can be seen from the weight and temperature of the bed.
Figure 5 shows the bed pressure difference at heights of 3 and 4 cm.
Heights of 3 and 4 cm turned out to be the most appropriate heights, as these heights constituted the top of the bed in the stationary state for all the materials tested. Hence, pressure changes at these heights were beneficial to compare and assess the intensity of the fluidization process of all tested materials.
The data presented in
Figure 5 show that the pressure difference is the smallest at 3 cm of the bed (
Figure 5a) and at 4 cm (
Figure 5b) when the valve opens. It is worth noting that the minimum pressure difference at a height of 3 cm is 50 mbar, while at a height of 4 cm it is 75 mbar.
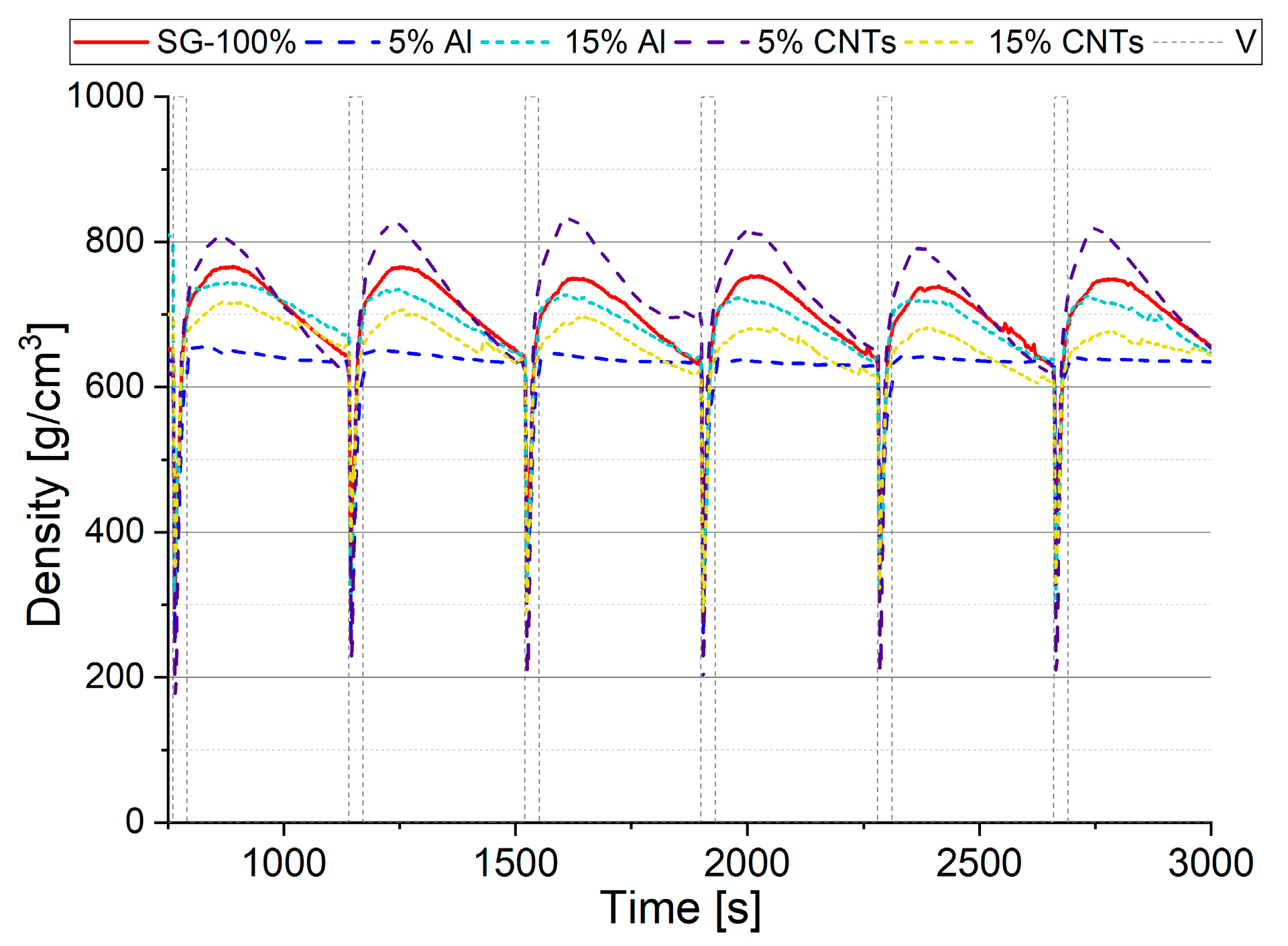
Changes in the bed density at a height of 4 cm during the test are shown in
Figure 6. These results showed that a minimum value of bed density equal to 175 g/cm
3 is reported for a bed containing 5% CNTs. This indicates that the mixture allows the best conditions for fluidization. In addition, when the valve is closed, the maximum density is observed as reaching 800 g/cm
3. A smaller range of density change at 4 cm was observed in the adsorption bed SG, equal to 325–780 g/cm
3, while the minimum extent of change was observed for a mixture with a content of 5 % Al (180–440 g/cm
3).
4. Conclusions
This paper deals with time subjects of a high practical significance related to net-zero emission, sustainability, and energy efficiency. Based on the obtained results, the following conclusions can be formulated.
In a bed of any composition, fluidization was achieved, as evidenced by the rapid decrease in mass during the opening of the valve. In addition, in the bed of each composition, during valve closure, an increase in bed weight was observed due to adsorption, while a decrease in bed weight was observed due to desorption. Even though mixtures with aluminum powder showed the best coefficient of thermal diffusivity and the best heat and mass transfer conditions, in a fluidized bed, aluminum powder as a component of the mixture interferes with sorption processes as well as fluidization itself, compared to a bed containing 100% SG.
The most extensive range of bed mass change due to adsorption/desorption processes was observed in an adsorption bed with a content of 5% CNTs. Thus, the most significant changes in bed density at 4 cm were observed for an adsorption bed with a content of 5% CNTs. The minimum bulk density values were 175 g/cm3, while the maximum was equal to 800 g/cm3, thus, the analysis shows that the silica gel mixture containing 5% CNTs was the best in terms of mass transfer in the fluidized adsorption bed.
As can be concluded based on the presented results, the adsorption bed composition significantly affects its performance. The study proved that 5% and 15% Al powder introduced to the bed negatively affect its sorption properties, while the addition of 15% CNTs improves the sorption properties of the bed. This finding is essential regarding guidelines for researchers and engineers aiming to develop adsorption technology dedicated to cooling and desalination applications. The findings presented in the paper allow for an increase in the performance of adsorption chillers and, therefore, an increase in the energy efficiency of adsorption cooling and desalination systems, and they contribute to works on sustainable development working towards a zero-emissions concept of energy processes.
Author Contributions
Conceptualization, A.K., A.Z. and J.K.; methodology, A.K. and A.Z.; software, J.K.; validation, J.K., K.G. and M.S.; formal analysis, W.N., J.K. and M.S.; investigation, A.K., A.Z., K.G. and D.S.; resources, M.S.; data curation, J.K.; writing—original draft preparation, A.K. and A.Z.; writing—review and editing, J.K. and M.S.; visualization, K.G. and D.S.; supervision, J.K. and W.N.; project administration, K.G.; funding acquisition, J.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Science Center, Poland, project number 018/29/B/ST8/00442, “Research on sorption process intensification methods in modified construction of adsorbent beds”.
Acknowledgments
This work was performed within project No. 2018/29/B/ST8/00442, “Research on sorption process intensification methods in modified construction of adsorbent beds”, supported by the National Science Center, Poland.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| a1–a4 | dimensionless parameters |
| Sauter mean diameter of the particles in the bed |
| Al | aluminum particles |
| CNTs | carbon nanotubes |
| m | water vapor uptake, g/g |
| P | the average bed pressure, mbar |
| Pads | absolute pressure in the adsorption chamber, mbar |
| Pevap | absolute pressure in the evaporator, mbar |
| P0 | pressure of gas fluidizing the bed |
| apparent density of the material, kg/m3 |
| bulk density of the material, g/cm3 |
| 𝜌𝐺 | gas density, g/cm3 |
| Reynolds numbers |
| SG | silica gel |
| Tads | temperature of the adsorption bed, °C |
| Uav | average gas velocity in the fluidized bed, m/s |
| V | valve between the evaporator and adsorption chamber |
| ΔP | pressure difference (pressure drop) in the bed, mbar |
| ZD | height of the fluidized bed, mm |
| 𝜇𝐺 | gas viscosity, m/s |
| ε | material porosity |
References
- Kunii, D.; Levenspiel, O. Fluidization Engineering II; Elsevier: Amsterdam, The Netherlands, 2013; ISBN 0-409-90233-0. [Google Scholar]
- Yang, W.-C. Handbook of Fluidization and Fluid-Particle Systems; CRC Press: Boca Raton, FL, USA, 2003; ISBN 978-0-8247-4836-4. [Google Scholar]
- Mawatari, Y.; Koide, T.; Ikegami, T.; Tatemoto, Y.; Noda, K. Characteristics of Vibro-Fluidization for Fine Powder under Reduced Pressure. Adv. Powder Technol. 2003, 14, 559–570. [Google Scholar] [CrossRef]
- Idziak, K.; Czakiert, T.; Krzywanski, J.; Zylka, A.; Kozlowska, M.; Nowak, W. Safety and Environmental Reasons for the Use of Ni-, Co-, Cu-, Mn- and Fe-Based Oxygen Carriers in CLC/CLOU Applications: An Overview. Fuel 2020, 268, 117245. [Google Scholar] [CrossRef]
- Muskała, W.; Krzywański, J.; Sekret, R.; Nowak, W. Model research of coal combustion in circulating fluidized bed boilers. Chem. Process Eng.-Inz. Chem. Proces. 2008, 29, 473–492. Available online: https://www.researchgate.net/publication/261712888_Model_research_of_coal_combustion_in_circulating_fluidized_bed_boilers (accessed on 1 January 2023).
- Krzywanski, J.; Grabowska, K.; Sosnowski, M.; Zylka, A.; Kulakowska, A.; Czakiert, T.; Sztekler, K.; Wesolowska, M.; Nowak, W. Heat Transfer in Adsorption Chillers with Fluidized Beds of Silica Gel, Zeolite, and Carbon Nanotubes. Heat Transf. Eng. 2022, 43, 172–182. [Google Scholar] [CrossRef]
- Krzywanski, J.; Grabowska, K.; Sosnowski, M.; Zylka, A.; Kulakowska, A.; Czakiert, T.; Sztekler, K.; Wesolowska, M.; Nowak, W. Heat Transfer in Fluidized and Fixed Beds of Adsorption Chillers. E3S Web Conf. 2019, 128, 01003. [Google Scholar] [CrossRef]
- Krzywanski, J.; Blaszczuk, A.; Czakiert, T.; Rajczyk, R.; Nowak, W. Artificial intelligence treatment of NOx emissions from CFBC in air and oxy-fuel conditions. In Proceedings of the 11th International Conference on Fluidized Bed Technology, Beijing, China, 14–17 May 2014; pp. 619–624. [Google Scholar] [CrossRef]
- Khawaji, A.D.; Kutubkhanah, I.K.; Wie, J.-M. Advances in Seawater Desalination Technologies. Desalination 2008, 221, 47–69. [Google Scholar] [CrossRef]
- Vershinina, K.; Nyashina, G.; Strizhak, P. Combustion, Pyrolysis, and Gasification of Waste-Derived Fuel Slurries, Low-Grade Liquids, and High-Moisture Waste: Review. Appl. Sci. 2022, 12, 1039. [Google Scholar] [CrossRef]
- Muskala, W.; Krzywański, J.; Czakiert, T.; Nowak, W. The research of CFB boiler operation for oxygen-enhanced dried lignite combustion. Rynek Energii 2011, 92, 172–176. Available online: https://dc.engconfintl.org/cfb10/52/ (accessed on 1 January 2023).
- Chen, C.-H.; Ma, S.-S.; Wu, P.-H.; Chiang, Y.-C.; Chen, S.-L. Adsorption and Desorption of Silica Gel Circulating Fluidized Beds for Air Conditioning Systems. Appl. Energy 2015, 155, 708–718. [Google Scholar] [CrossRef]
- Hamed, A.M. Experimental Investigation on the Adsorption/Desorption Processes Using Solid Desiccant in an Inclined-Fluidized Bed. Renew. Energy 2005, 30, 1913–1921. [Google Scholar] [CrossRef]
- Majid, I.; Abdul Wahab, K.; Khalid, H. Rheima Drying of Solid Materials by Vacuum Fluidized Bed Dryer. Iraqi J. Chem. Pet. Eng. 2009, 10, 1–11. [Google Scholar]
- Grabowska, K.; Zylka, A.; Kulakowska, A.; Skrobek, D.; Krzywanski, J.; Sosnowski, M.; Ciesielska, K.; Nowak, W. Experimental Investigation of an Intensified Heat Transfer Adsorption Bed (IHTAB) Reactor Prototype. Materials 2021, 14, 3520. [Google Scholar] [CrossRef]
- Ali, S.M.; Chakraborty, A. Adsorption Assisted Double Stage Cooling and Desalination Employing Silica Gel+water and AQSOA-Z02+water Systems. Energy Convers. Manag. 2016, 117, 193–205. [Google Scholar] [CrossRef]
- Krzywanski, J.; Grabowska, K.; Sosnowski, M.; Zyłka, A.; Sztekler, K.; Kalawa, W.; Wojcik, T.; Nowak, W. Modeling of a re-heat two-stage adsorption chiller by AI approach. In Proceedings of the MATEC Web of Conferences, Bandung, Indonesia, 18 April 2018; p. 240. [Google Scholar] [CrossRef]
- Riaz, N.; Sultan, M.; Miyazaki, T.; Shahzad, M.W.; Farooq, M.; Sajjad, U.; Niaz, Y. A Review of Recent Advances in Adsorption Desalination Technologies. Int. Commun. Heat Mass Transf. 2021, 128, 105594. [Google Scholar] [CrossRef]
- Ghaffour, N.; Lattemann, S.; Missimer, T.; Ng, K.C.; Sinha, S.; Amy, G. Renewable Energy-Driven Innovative Energy-Efficient Desalination Technologies. Appl. Energy 2014, 136, 1155–1165. [Google Scholar] [CrossRef]
- Chakraborty, A.; Thu, K.; Saha, B.B.; Ng, K.C. Adsorption-Desalination Cycle. In Advances in Water Desalination; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2012; pp. 377–451. ISBN 978-1-118-34773-7. [Google Scholar]
- Sztekler, K.; Kalawa, W.; Nowak, W.; Mika, L.; Gradziel, S.; Krzywanski, J.; Radomska, E. Experimental study of three-bed adsorption chiller with desalination function. Energies 2020, 13, 5827. [Google Scholar] [CrossRef]
- Sztekler, K.; Kalawa, W.; Nowak, W.; Mika, Ł.; Krzywański, J.; Grabowska, K.; Sosnowski, M.; Alharbi, A.A. Performance evaluation of a single-stage two-bed adsorption chiller with desalination function. J. Energy Resour. Technol. 2021, 143, 082101. [Google Scholar] [CrossRef]
- Kim, Y.-D.; Thu, K.; Ng, K.C. Adsorption Characteristics of Water Vapor on Ferroaluminophosphate for Desalination Cycle. Desalination 2014, 344, 350–356. [Google Scholar] [CrossRef]
- Thu, K.; Ng, K.C.; Saha, B.B.; Chakraborty, A.; Koyama, S. Operational Strategy of Adsorption Desalination Systems. Int. J. Heat Mass Transf. 2009, 52, 1811–1816. [Google Scholar] [CrossRef]
- Wu, J.W.; Hu, E.J.; Biggs, M.J. Thermodynamic Cycles of Adsorption Desalination System. Appl. Energy 2012, 90, 316–322. [Google Scholar] [CrossRef]
- Kulakowska, A.; Pajdak, A.; Krzywanski, J.; Grabowska, K.; Zylka, A.; Sosnowski, M.; Wesolowska, M.; Sztekler, K.; Nowak, W. Effect of Metal and Carbon Nanotube Additives on the Thermal Diffusivity of a Silica Gel-Based Adsorption Bed. Energies 2020, 13, 1391. [Google Scholar] [CrossRef]
- Krzywanski, J.; Skrobek, D.; Zylka, A.; Grabowska, K.; Kulakowska, A.; Sosnowski, M.; Nowak, W.; Blanco-Marigorta, A.M. Heat and Mass Transfer Prediction in Fluidized Beds of Cooling and Desalination Systems by AI Approach. Appl. Therm. Eng. 2023, 225, 120200. [Google Scholar] [CrossRef]
- Win, K.K.; Nowak, W.; Hitoki, H.; Matsuda, M.; Hasatani, M.; Bis, Z.; Krzywanski, J.; Gajewski, W. Transport velocity of coarse particles in multi-solid fluidized bed. J. Chem. Eng. Jpn. 1995, 28, 535–540. [Google Scholar] [CrossRef]
- Krzywanski, J.; Sztekler, K.; Bugaj, M.; Kalawa, W.; Grabowska, K.; Chaja, P.R.; Bykuc, S. Adsorption chiller in a combined heating and cooling system: Simulation and optimization by neural networks. Bull. Pol. Acad. Sci. Technol. Sci. 2021, 69, e137054. [Google Scholar] [CrossRef]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).