Cost-Effective Processes for Denim Production Wastewater: Dual Criterial Optimization of Techno-Economical Parameters by RSM and Minimization of Energy Consumption of Photo Assisted Fenton Processes via Direct Photovoltaic Solar Panel Integration
Abstract
1. Introduction
2. Materials and Methods
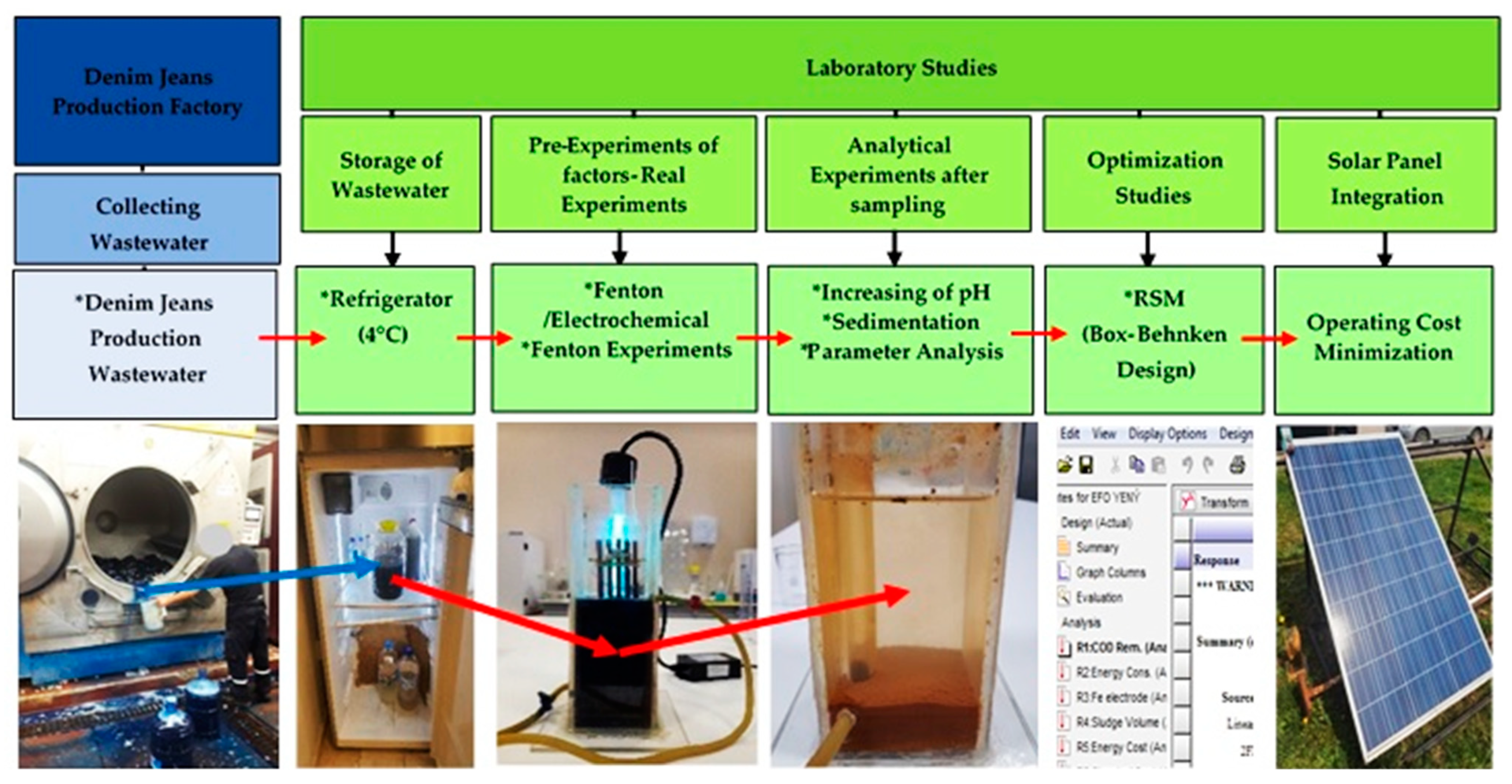
2.1. Experimental Flow-Chart and Characterization of Denim Jean Production Wastewater
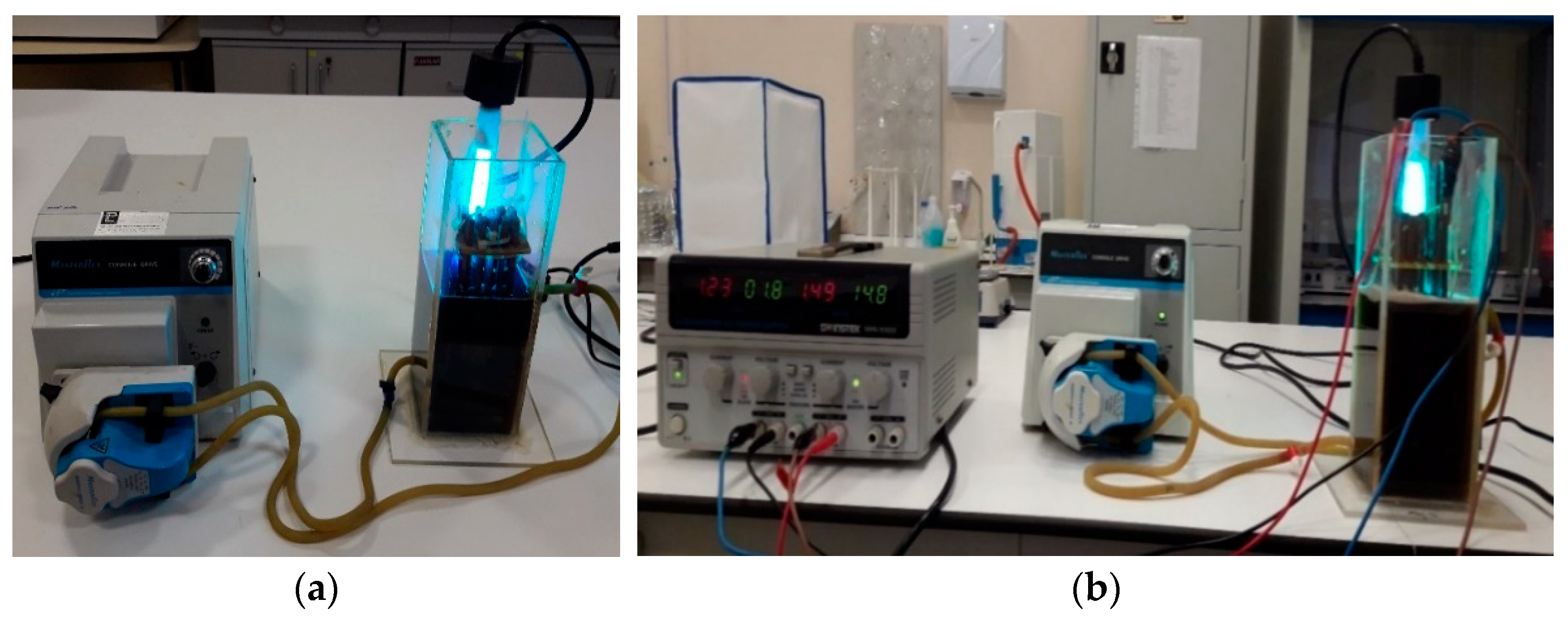
2.2. PFP and PEFP Experimental Procedure
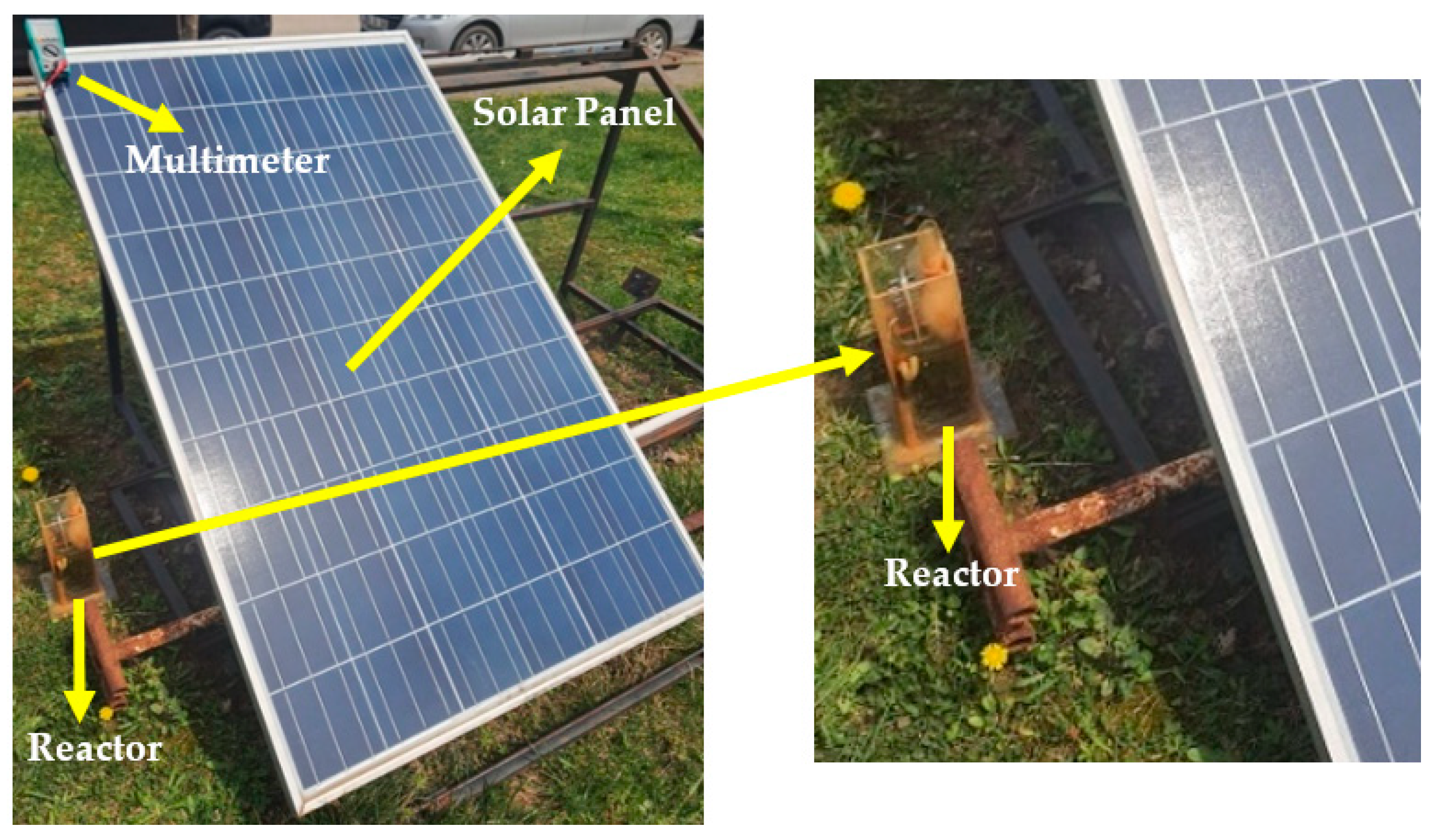
2.3. Photovoltaic Solar Panel Integration to the Fenton Processes
2.4. Analytic Methods
2.5. Response Surface Methodology (RSM)
2.6. Equations
| OCPFP | Operating cost of PFP (USD/m3); |
| OCPEFP | Operating cost of PEFP (USD/m3); |
| EUV | Energy consumption of UV lamp (kWh/m3); |
| EDC | Energy consumption of DC power supply (kWh/m3); |
| EPP | Energy consumption of peristaltic pump (kWh/m3); |
| CFeSO4 | Consumption of FeSO4 (kg/m3); |
| CH2O2 | Consumption of H2O2 (m3/m3); |
| CNaOH | Consumption of NaOH (kg/m3); |
| CH2SO4 | Consumption of H2SO4 (kg/m3); |
| CFe el. | Consumption of Fe electrode (kg/m3); |
| Vsludge | Sludge volume (kg/m3). |
3. Results and Discussion
3.1. Statistical Analysis of COD Removal Efficiency of PFP and PEFP
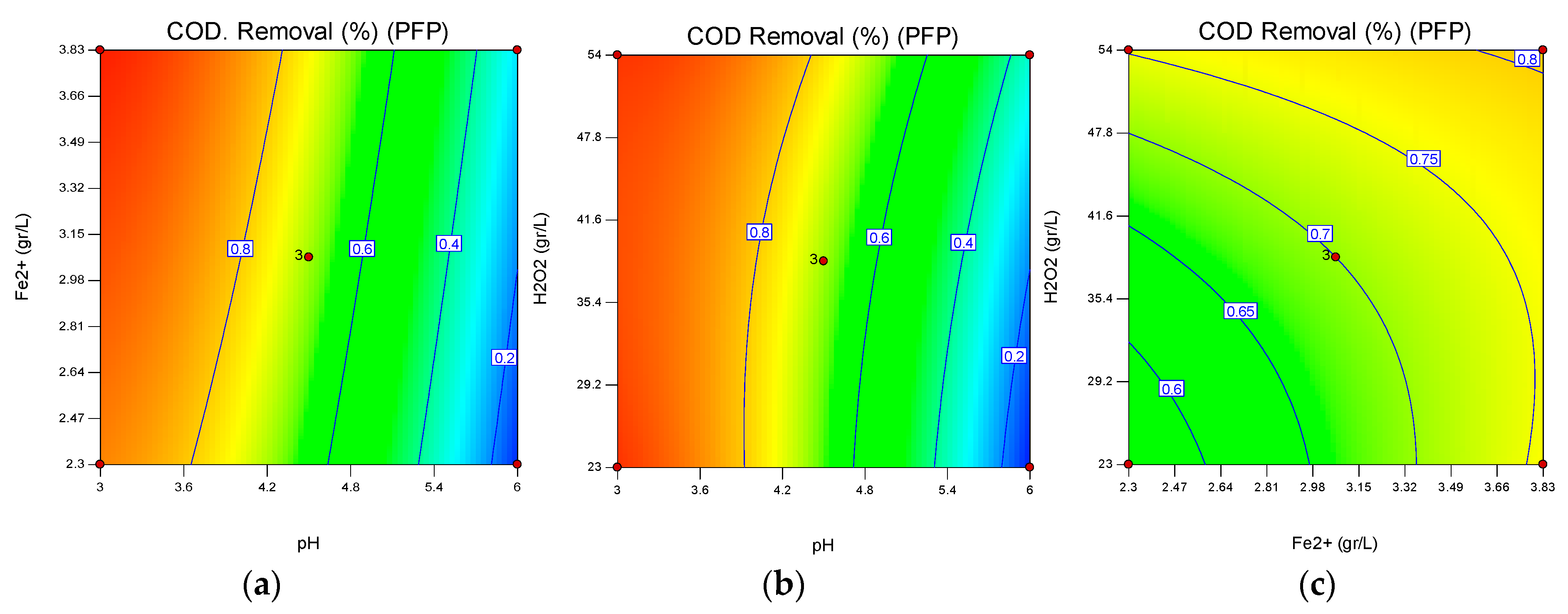
3.2. Interactive Effects of pH, Fe2+ Concentration/Current Density, H2O2 Concentration on PFP and PEFP for COD Degradation
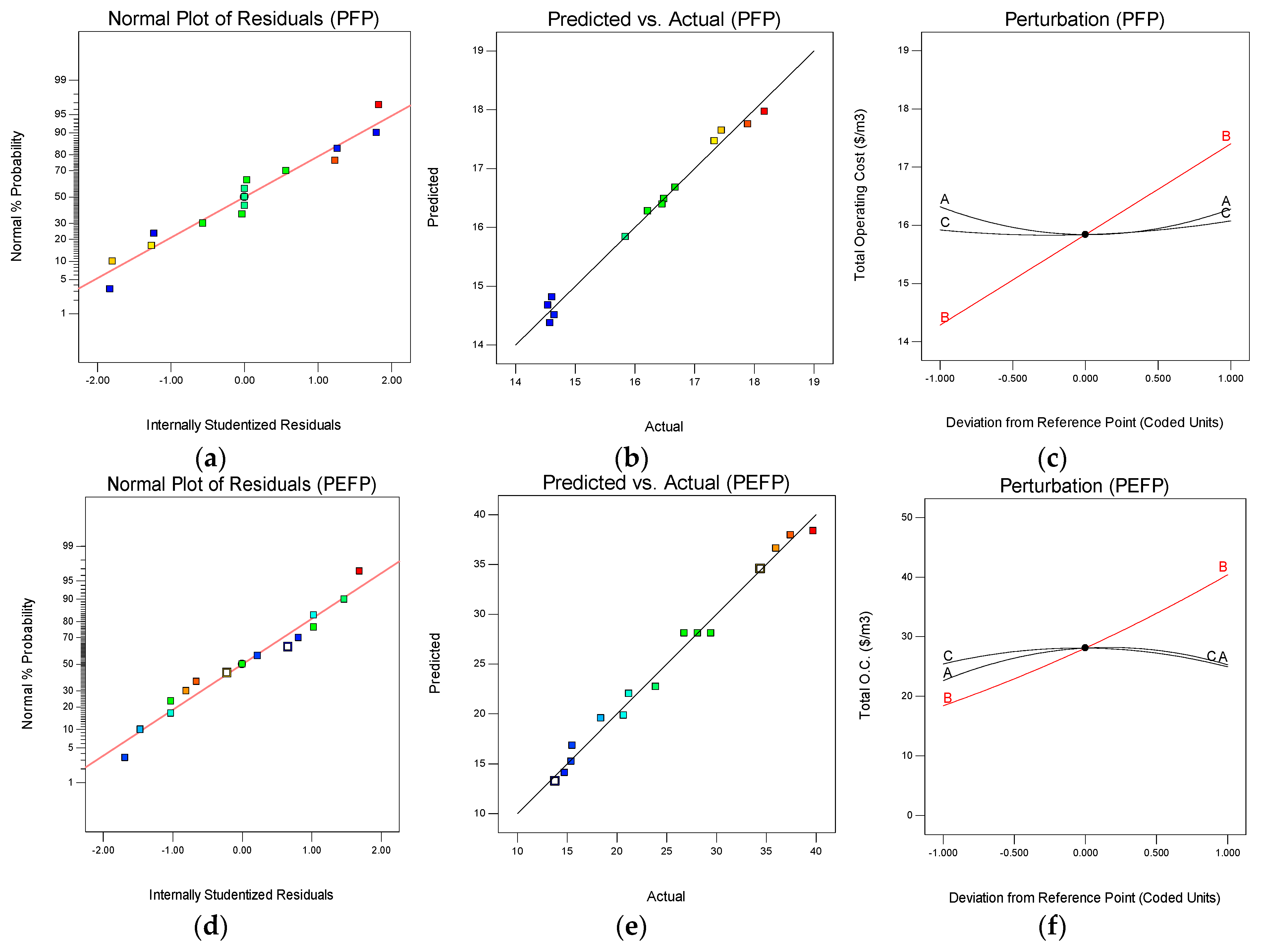
3.3. Statistical Analysis of Total Operating Cost of PFP and PEFP
3.4. Optimization of Other Economical Parameters
3.5. Effect of Solar Panel Integration on Processes
3.6. Biodegradability of Wastewater
4. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Moreno-Casillas, H.A.; Cocke, D.L.; Gomes, J.A.G.; Morkovsky, P.; Parga, J.R.; Peterson, E. Electrocoagulation mechanism for COD removal. Sep. Purif. Technol. 2007, 56, 204–211. [Google Scholar] [CrossRef]
- Mousavi, S.; Nazari, S. Applying Response Surface Methodology to Optimize the Fenton Oxidation Process in the Removal of Reactive Red 2. Pol. J. Environ. Stud. 2017, 26, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Dotto, J.; Fagundes-Klen, M.R.; Veit, M.T.; Palacio, S.M.; Bergamasco, R. Performance of different coagulants in the coagulation/flocculation. J. Clean. Prod. 2019, 208, 656–665. [Google Scholar] [CrossRef]
- Bener, S.; Bulca, Ö.; Palas, B.; Tekin, G.; Atalay, S.; Ersöz, G. Electrocoagulation process for the treatment of real textilewastewater: Effect of operative conditions on the organic carbon removal and kinetic study. Process Saf. Environ. Prot. 2019, 129, 47–54. [Google Scholar] [CrossRef]
- Shoukat, R.; Khan, S.J.; Jamal, Y. Hybrid anaerobic-aerobic biological treatment for real textile wastewater. J. Water Process Eng. 2019, 29, 100804. [Google Scholar] [CrossRef]
- Aydıner, C.; Kiril Mert, B.; Can Dogan, E.; Yatmaz, H.C.; Dagli, S.; Aksu, S.; Tilki, Y.M.; Esin Balci, A.Y.G. Novel hybrid treatments of textile wastewater by membrane oxidation reactor: Performance investigations, optimizations and efficiency comparisons. Sci. Total Environ. 2019, 683, 411–426. [Google Scholar] [CrossRef]
- Kuleyin, A.; Gök, A.; Akbal, F. Treatment of textile industry wastewater by electro-Fenton process using graphite electrodes in batch and continuous mode. J. Environ. Chem. Eng. 2021, 9, 104782. [Google Scholar] [CrossRef]
- Deniz, Ç.Ç.; Çifçi, İ. Comparison of kinetics and costs of Fenton and photo-Fenton processes used for the treatment of a textile industry wastewater. J. Environ. Manag. 2022, 304, 114234. [Google Scholar]
- Khan, N.A.; Khan, A.H.; Tiwari, P.; Zubair, M.; Naushad, M. New insights into the integrated application of Fenton-based oxidation processes for the treatment of pharmaceutical wastewater. J. Water Process Eng. 2021, 44, 102440. [Google Scholar] [CrossRef]
- Rezgui, S.; Ghazouani, M.; Bousselmi, L.; Akrout, H. Efficient treatment for tannery wastewater through sequential electro-Fenton and electrocoagulation processes. J. Environ. Chem. Eng. 2022, 10, 107424. [Google Scholar] [CrossRef]
- Can-Güven, E. Advanced treatment of dye manufacturing wastewater by electrocoagulation and electro-Fenton processes: Effect on COD fractions, energy consumption, and sludge analysis. J. Environ. Manag. 2021, 300, 113784. [Google Scholar] [CrossRef]
- Asaithambi, P.; Govindarajan, R.; Yesuf, M.B.; Alemayehun, E. Removal of color, COD and determination of power consumption from landfill leachate wastewater using an electrochemical advanced oxidation processes. Sep. Purif. Technol. 2020, 233, 115935. [Google Scholar] [CrossRef]
- Meng, G.; Jiang, N.; Wang, Y.; Zhang, H.Y.; Tang, Y.; Bai, L.J. Treatment of coking wastewater in a heterogeneous electro-Fenton system: Optimization of treatment parameters, characterization, and removal mechanism. J. Water Process Eng. 2022, 45, 102482. [Google Scholar] [CrossRef]
- Ribeiro, J.P.; Marques, C.C.; Portugal, I.; Nunes, M.I. AOX removal from pulp and paper wastewater by Fenton and photo-Fenton processes: A real case-study. Energy Rep. 2020, 6, 770–775. [Google Scholar] [CrossRef]
- Mirzaei, A.; Chen, Z.; Haghighat, F.; Yerushalmi, L. Removal of pharmaceuticals from water by homo/heterogonous Fenton-type processes—A review. Chemosphere 2017, 174, 665–688. [Google Scholar] [CrossRef]
- Casado, J. Towards industrial implementation of electro-Fenton and derived technologies for wastewater treatment: A review. J. Environ. Chem. Eng. 2019, 7, 102823. [Google Scholar] [CrossRef]
- Exposito, A.J.; Monteagudo, J.M.; Díaz, I.; Dur´an, A. Photo-fenton degradation of a beverage industrial effluent: Intensification with persulfate and the study of radicals. Chem. Eng. J. 2016, 306, 1203–1211. [Google Scholar] [CrossRef]
- Gamarra-Güere, C.D.; Dionisio, D.; Santos, G.O.S.; Lanza, M.R.V.; Motheo, A.J. Application of Fenton, photo-Fenton and electro-Fenton processes for the methylparaben degradation: A comparative study. J. Environ. Chem. Eng. 2022, 10, 106992. [Google Scholar] [CrossRef]
- Moradi, M.; Elahinia, A.; Vasseghian, Y.; Dragoi, E.N.; Omidi, F.; Khaneghah, A.M. A review on pollutants removal by Sono-photo -Fenton processes. J. Environ. Chem. Eng. 2020, 8, 104330. [Google Scholar] [CrossRef]
- Muruganandham, M.; Swaminathan, M. Decolourisation of Reactive Orange 4 by Fenton and photo-Fenton oxidation technology. Dye. Pigment. 2004, 63, 315–321. [Google Scholar] [CrossRef]
- Ting, W.P.; Lu, M.C.; Huang, Y.H. The reactor design and comparison of fenton, electro-fenton and photoelectron-fenton processes for mineralization of benzene sulfonic acid (BSA). J. Hazard. Mater. 2008, 156, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Babuponnusami, A.; Muthukumar, K. Advanced oxidation of phenol: A comparison between Fenton, electro-Fenton, sono-electro-Fenton and photo-electro-Fenton processes. Chem. Eng. J. 2012, 183, 1–9. [Google Scholar] [CrossRef]
- GilPavas, E.; Dobrosz-Gómez, I.; Ángel Gómez-García, M. Optimization of solar-driven photo-electro-Fenton process for the treatment of textile industrial wastewater. J. Water Process Eng. 2018, 24, 49–55. [Google Scholar] [CrossRef]
- APHA (American Public Health Association). Standard Methods for the Examination of Waste and Wastewater, 19th ed.; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- Aravind, P.; Subramanyan, V.; Ferro, S.; Gopalakrishnan, R. Eco-friendly and facile integrated biological-cum-photo assisted electrooxidation process for degradation of textile wastewater. Water Res. 2016, 93, 230–241. [Google Scholar] [CrossRef] [PubMed]
- Tak, B.; Tak, B.; Kim, Y.; Park, Y.; Yoon, Y.; Min, G. Optimization of color and COD removal from livestock wastewater by electrocoagulation process: Application of Box–Behnken design (BBD). J. Ind. Eng. Chem. 2015, 28, 307–315. [Google Scholar] [CrossRef]
- Jing, L.; Chen, B.; Wen, D.; Zheng, J.; Zhang, B. Pilot-scale treatment of atrazine production wastewater by UV/O3/ultrasound: Factor effects and system optimization. J. Environ. Manag. 2017, 203, 182–190. [Google Scholar] [CrossRef]
- Ghanbari, F.; Moradi, M. A comparative study of electrocoagulation, electrochemical Fenton, electro-Fenton and peroxi-coagulation for decolorization of real textile wastewater: Electrical energy consumption and biodegradability improvement. J. Environ. Chem. Eng. 2015, 3, 499–506. [Google Scholar] [CrossRef]
- Trigueros, D.E.G.; Braun, L.; Hinterholz, C.L. Environmental and economic feasibility of the treatment of dairy industry wastewater by photo-Fenton and electrocoagulation process: Multicriteria optimization by desirability function. J. Photochem. Photobiol. A Chem. 2022, 427, 113820. [Google Scholar] [CrossRef]
- Wang, Z.; Qiao, H.; Yu, Z.; Yang, X.; Tang, Z.; Zhou, W.; Zhao, H. Source analysis of benzene degradability in floating cathode electro-fenton system based on COD removal ratio. J. Water Process Eng. 2022, 46, 102568. [Google Scholar] [CrossRef]
- Wang, Q.; Lemley, A.T. Kinetic model and optimization of 2,4-D degradation by anodic Fenton treatment. Environ. Sci. Technol. 2001, 35, 4509–4514. [Google Scholar] [CrossRef]
- Shemer, H.; Linden, K.G. Degradation and by-product formation of diazinon in water during UV and UV/H2O2 treatment. J. Hazard. Mater. 2006, 136, 553–559. [Google Scholar] [CrossRef]
- Nidheesh, P.N.; Gandhimathi, R. Trends in electro-Fenton process for water and wastewater treatment: An overview. Desalination 2012, 299, 1–15. [Google Scholar] [CrossRef]
- Mohajeri, S.; Aziz, H.A.; Isa, M.H.; Zahed, M.A.; Adlan, M.N. Statistical optimization of process parameters for landfill leachate treatment using electro-Fenton technique. J. Hazard Mater. 2010, 176, 749–758. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Luan, Z.; Yu, L.; Ji, Z. Pretreatment of acrylic fiber manufacturing wastewater by the Fenton process. Desalination 2012, 284, 62–65. [Google Scholar]
- Babuponnusami, A.; Muthukumar, K. A review on Fenton and improvements to the Fenton process for wastewater treatment. J. Environ. Chem. Eng. 2014, 2, 557–572. [Google Scholar] [CrossRef]
- Ting, W.P.; Lu, M.C.; Huang, Y.H. Kinetics of 2,6-dimethylaniline degradation by electro-Fenton process. J. Hazard. Mater. 2009, 161, 1484–1490. [Google Scholar] [CrossRef]
- Zhang, H.; Fei, C.; Zhang, D.; Tang, F. Degradation of 4-nitrophenol in aqueous medium by electro-fenton method. J. Hazard. Mater. 2007, 145, 227–232. [Google Scholar] [CrossRef]
- Welling, C. Fenton’s reagent revisited. Acc. Chem. Res. 1975, 8, 125–131. [Google Scholar] [CrossRef]
- Hu, Y.; Lu, Y.; Liu, G.; Luo, H.; Zhang, R.; Cai, X. Effect of the structure of stacked electro-Fenton reactor on treating nanofiltration concentrate of landfill Leachate. Chemosphere 2008, 202, 191–197. [Google Scholar] [CrossRef]
- El Kateb, M.; Trellu, C.; Darwich, A.; Rivallin, M.; Bechelany; Nagarajan, M.S. Electrochemical advanced oxidation processes using novel electrode materials for mineralization and biodegradability enhancement of nanofiltration concentrate of landfill leachates. Water Res. 2019, 162, 446–455. [Google Scholar] [CrossRef]
- Kaur, P.; Sangal, V.; Kushwaha, J.P.K. Parametric study of electro-Fenton treatment for real textile wastewater, disposal study and its cost analysis. Int. J. Environ. Sci. Technol. 2019, 16, 801–810. [Google Scholar] [CrossRef]
- Ismail, S.A.; Ang, W.L.; Mohammad, A.W. Electro-Fenton technology for wastewater treatment: A bibliometric analysis of current research trends, future perspectives and energy consumption analysis. J. Water Process Eng. 2021, 40, 101952. [Google Scholar] [CrossRef]
- Gaied, F.; Louhichi, B.; Jeday, M.R. Tertiary treatment of waste water by electro-fenton process: Economical study. In Proceedings of the 2017 International Conference on Green Energy Conversion Systems (GECS), Hammamet, Tunisia, 23–25 March 2017; pp. 2–5. [Google Scholar]
- Li, M.; Zhou, M.; Qin, X. A feasible electro-Fenton treatment of landfill leachate diluted by electro-Fenton effluent: Evaluation of operational parameters, effect of dilution ratio and assessment of treatment cost. J. Water Process Eng. 2022, 47, 102754. [Google Scholar] [CrossRef]
- Chai, Y.; Qin, P.; Zhang, J.; Li, T.; Dai, Z.; Wu, Z. Simultaneous removal of Fe(II) and Mn(II) from acid mine wastewater by electro-Fenton process. Process Saf. Environ. Prot. 2020, 143, 76–90. [Google Scholar] [CrossRef]
- Varank, G.; Yazıcı, S.; Güvenç, K.; Dinçer, D.A. Concentrated leachate treatment by electro-Fenton and electro-persulfate processes using central composite design. Int. J. Environ. Res. 2020, 14, 439–461. [Google Scholar] [CrossRef]
- Tripathy, B.K.; Kumar, M. Sequential coagulation/flocculation and microwavepersulfate processes for landfill leachate treatment: Assessment of bio-toxicity, effect of pretreatment and cost-analysis. Waste Manag. (Oxford) 2019, 85, 18–29. [Google Scholar] [CrossRef]
- Zhang, Z.; Meng, H.; Wang, Y.; Shi, L.M.; Wang, X.; Chai, S. Fabrication of graphene@ graphite-based gas diffusion electrode for improving H2O2 generation in Electro- Fenton process. Electrochim. Acta 2018, 260, 112–120. [Google Scholar] [CrossRef]
- GilPavas, E.; Correa-Sanchez, S. Optimization of the heterogeneous electro-Fenton process assisted by scrap zero-valent iron for treating textile wastewater: Assessment of toxicity and biodegradability. J. Water Process. Eng. 2019, 32, 100924. [Google Scholar] [CrossRef]
- Ahmadi, M.; Ghanbari, F.; Madihi-Bidgoli, S. Photoperoxi-coagulation using activated carbon fiber cathode as an efficient method for benzotriazole removal from aqueous solutions: Modelling, optimization and mechanism. J. Photochem. Photobiol. A 2016, 322–323, 85–94. [Google Scholar] [CrossRef]




| Parameter | Value/Concentration | Parameter | Value/Concentration |
|---|---|---|---|
| pH | 6.82 ± 0.32 | TDS | 4.89 ± 0.40 g/L |
| Conductivity | 5.16 ± 0.21 mS/cm | Color 460 nm | 16 m−1 |
| COD | 4100 ± 2000 mg/L | Color 525 nm | 35 m−1 |
| TOC | 2780 ± 200 mg/L | Color 620 nm | 47 m−1 |
| SS | 3200 ± 190 mg/L |
| Parameter | Technical Value | Parameter | Technical Value |
|---|---|---|---|
| Solar module type | SPE250 | Max system voltage | 1000 VDC |
| Max power (Pmax) | 250 W | Dimensions | 1001 × 1665 × 42 mm |
| Max power voltage (Vmp) | 30.50 V | Application class | Class A |
| Max power current (Ipmax) | 8.2 A | Weight | 18.5 kg |
| Open circuit voltage (Voc) | 37.8 V | Power tolerance up to | +4.9 W |
| Short circuit voltage (Isc) | 8.7 A | Measurement tolerance | ±3% |
| Parameter | Method | Method No. | Instrument |
|---|---|---|---|
| COD | Photometric | SM 5220 | Hach DR5000 UV-VIS |
| TOC | High temperature combustion method | SM 5310-B | TOC analyzer with NDIR detector |
| Suspended Solid | Gravimetric | SM 2540-D | Vacuum filtration unit |
| Turbidity | Photometric | SM 2130-B | Hach DR5000 UV-VIS |
| pH | Electrometric | SM 4500-B | Hanna Ins. |
| Conductivity | Electrometric | SM 2510-B | Hach 7100e |
| Color | Photometric | EN ISO 7887 | Hach DR5000 UV-VIS |
| PFP | PEFP | |||||||
|---|---|---|---|---|---|---|---|---|
| Levels | Levels | |||||||
| Coded Variables (Xi) | Factors | Unit | Low (−1) | Center (0) | High (+1) | Low (−1) | Center (0) | High (+1) |
| (X1) | pH | - | 3 | 4.5 | 6 | 3 | 4.5 | 6 |
| (X2) | Fe2+/C.D. | g/L-A/m2 | 2.3 | 3.06 | 3.83 | 24 | 48 | 72 |
| (X3) | H2O2 | g/L | 23 | 38.5 | 54 | 23 | 38.5 | 54 |
| Parameter | Unit Price | Parameter | Unit Price |
|---|---|---|---|
| a-Energy | USD 0.22/m3 | e-NaOH | USD 1.74/m3 |
| b-Fe Electrode | USD 1.00/kg | f-H2SO4 | USD 1.53/m3 |
| c-FeSO4·7H2O | USD 1.18/kg | g-Sludge Disposal | USD 0.19/kg |
| d-H2O2 | USD 1.76/m3 |
| PFP | PEFP | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Source | SS | DF | MS | F Value | p Value | SS | DF | MS | F Value | p Value |
| Model | 1.15 | 9 | 0.13 | 116.59 | <0.0001 | 0.94 | 9 | 0.10 | 225.77 | <0.0001 |
| X1-pH | 0.99 | 1 | 0.99 | 903.68 | <0.0001 | 0.83 | 1 | 0.83 | 1775.51 | <0.0001 |
| X2-Fe2+/i | 0.031 | 1 | 0.031 | 28.41 | 0.0031 | 0.063 | 1 | 0.063 | 135.51 | <0.0001 |
| X3-H2O2 | 0.029 | 1 | 0.029 | 26.18 | 0.0037 | 4.5 × 10−4 | 1 | 4.5 × 10−4 | 0.97 | 0.3704 |
| X1X2 | 1.6 × 10−3 | 1 | 1.6 × 10−3 | 1.45 | 0.2818 | 3.6 × 10−3 | 1 | 3.6 × 10−3 | 7.74 | 0.0388 |
| X1X3 | 0.014 | 1 | 0.014 | 13.09 | 0.0152 | 2.25 × 10−4 | 1 | 2.25 × 10−4 | 0.48 | 0.5177 |
| X2X3 | 4.9 × 10−3 | 1 | 4.9 × 10−3 | 4.45 | 0.0886 | 0.011 | 1 | 0.011 | 23.71 | 0.0046 |
| X12 | 0.075 | 1 | 0.075 | 68.16 | 0.0004 | 2.792 × 10−3 | 1 | 2.792 × 10−3 | 6.00 | 0.0579 |
| X22 | 2.3 × 10−5 | 1 | 2.308 × 10−5 | 0.021 | 0.8905 | 0.022 | 1 | 0.022 | 47.69 | 0.0010 |
| X32 | 1.869 × 10−3 | 1 | 1.869 × 10−3 | 1.70 | 0.2492 | 0.021 | 1 | 0.021 | 44.67 | 0.0011 |
| Residual | 5.5 × 10−3 | 5 | 1.1 × 10−3 | 2.325 × 10−3 | 5 | 4.650 × 10−4 | ||||
| Lack of fit | 5.3 × 10−3 | 3 | 1.767 × 10−3 | 17.67 | 0.0540 | 9.25 × 10−4 | 3 | 3.083 × 10−4 | 0.44 | 0.7491 |
| Pure error | 2 × 10−4 | 2 | 1 × 10−4 | 1.4 × 10−3 | 2 | 7 × 10−4 | ||||
| Cor total | 1.16 | 14 | 0.95 | 14 | ||||||
| PFP | PEFP | |||||||||
| R2 | 0.99 | Std. Dev. | 0.033 | R2 | 0.99 | Std. Dev. | 0.022 | |||
| Adj R2 | 0.97 | Mean | 0.63 | Adj R2 | 0.99 | Mean | 0.62 | |||
| Pred R2 | 0.93 | C.V. (%) | 5.23 | Pred R2 | 0.98 | C.V. (%) | 3.46 | |||
| A.P. | 31.11 | PRESS | 0.085 | A.P. | 46.57 | PRESS | 0.018 | |||
| PFP | PEFP | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Source | SS | DF | MS | F Value | p Value | SS | DF | MS | F Value | p Value |
| Model | 20.41 | 9 | 2.27 | 46.18 | 0.0003 | 1090.12 | 9 | 121.12 | 47.94 | 0.0003 |
| X1-pH | 2.946 × 10−3 | 1 | 2.946 × 10−3 | 0.060 | 0.8163 | 14.32 | 1 | 14.32 | 5.67 | 0.0631 |
| X2-Fe2+/i | 19.43 | 1 | 19.43 | 395.50 | <0.0001 | 969.03 | 1 | 969.03 | 383.58 | <0.0001 |
| X3-H2O2 | 0.048 | 1 | 0.048 | 0.99 | 0.3662 | 0.50 | 1 | 0.50 | 0.20 | 0.6764 |
| X1X2 | 0.030 | 1 | 0.030 | 0.62 | 0.4670 | 0.50 | 1 | 0.50 | 0.20 | 0.6749 |
| X1X3 | 0.061 | 1 | 0.061 | 1.23 | 0.3170 | 0.043 | 1 | 0.043 | 0.017 | 0.9018 |
| X2X3 | 5.565 × 10−4 | 1 | 5.565 × 10−4 | 0.011 | 0.9194 | 5.05 | 1 | 5.05 | 2.00 | 0.2166 |
| X12 | 0.78 | 1 | 0.78 | 15.92 | 0.0104 | 63.38 | 1 | 63.38 | 25.09 | 0.0041 |
| X22 | 3.5 × 10−5 | 1 | 3.5 × 10−5 | 7.127 × 10−4 | 0.9797 | 6.30 | 1 | 6.30 | 2.49 | 0.1753 |
| X32 | 0.092 | 1 | 0.092 | 1.88 | 0.2286 | 31.41 | 1 | 31.41 | 12.43 | 0.0168 |
| Residual | 0.25 | 5 | 0.049 | 12.63 | 5 | 2.53 | ||||
| Lack of Fit | 0.25 | 3 | 0.082 | 9.08 | 3 | 3.03 | 1.70 | 0.3909 | ||
| Pure Error | 0.000 | 2 | 0.000 | 3.56 | 2 | 1.78 | ||||
| Cor Total | 20.66 | 14 | 1102.75 | 14 | ||||||
| PFP | PEFP | |||||||||
| R2 | 0.98 | Std. Dev. | 0.22 | R2 | 0.98 | Std. Dev. | 1.59 | |||
| Adj R2 | 0.97 | Mean | 16.17 | Adj R2 | 0.97 | Mean | 25.03 | |||
| Pred R2 | 0.80 | C.V. (%) | 1.37 | Pred R2 | 0.87 | C.V. (%) | 6.35 | |||
| A.P. | 19.84 | PRESS | 3.93 | A.P. | 19.34 | PRESS | 153.22 | |||
| PFP | PEFP | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameter | Unit | Value | Eq. | ANOVA Results/Equations | Parameter | Unit | Value | Eq. | ANOVA Results/Equations |
| pH | - | 3.00 | - | - | pH | - | 3.00 | - | - |
| CFe2+ | g/L | 2.30 | - | - | C.D. | A/m2 | 27.06 | - | - |
| CH2O2 | g/L | 27.00 | - | - | CH2O2 | g/L | 28.16 | - | - |
| ECOD | % | 84 | Q | It is given in Equation (11) | ECOD | % | 90 | Q | It is given in Equation (12) |
| Vsludge | kg/m3 | 5.97 | Q | R2 = 0.99, R2Ad j= 0.96, R2Pred = 0.80 Ysludge = 6.2 − 0.25 × X1 + 2.82 × X2 − 0.035 × X3 − 0.26 × X1X2 − 0.23 × X1X3 + 0.076 × X2X3+ 2.26 × X12 0.12 × X22 + 0.56 × X32 | Vsludge | kg/m3 | 3.21 | Q | R2 = 0.93, R2Adj = 0.81 Ysludge = 7.2 − 0.06 × X1 + 0.74 × X2 + 0.24 × X3 + 0.22 × X1X2 +0.06 × X1X3 + 0.053 × X2X3 − 2.3 × X12 − 1.08 × X22 − 0.84 × X32 |
| CFeSO4 | kg/m3 | 3.61 | L | R2 = 1 R2Adj = 1, R2Pred = 1 YFeso4 = 4.82 − 7.85046e − 016 × X1 + 1.204 × X2 + 0 × X3 | CFe electrode | kg/m3 | 4.12 | Q | R2 = 0.98 R2Adj = 0.94, R2Pred = 0.75 YFe = 16.39 + 1.3 × X1 + 8.20 × X2 − 0.27 × X3 − 0.057 × X1X2 − 0.36 × X1X3 + 0.89 × X2X3 − 3.9 × X12 + 1.4 × X22 − 3.15 × X32 |
| - | ECons. | kWh/m3 kWh/kgCOD | 26.90 7.29 | L | R2 = 0.96, R2Adj = 0.94, R2Pred = 0.91 Yenergy = 39.73 + 0.27 × X1+ 14.85 × X2 − 0.24 × X3 | ||||
| - | Celectrode | USD/m3 USD/kgCOD | 4.08 1.106 | Q | R2 = 0.98 R2Adj = 0.94, R2Pred = 0.75 YFe = 16.39 + 1.3 × X1 + 8.20 × X2 − 0.27 × X3 − 0.057 × X1X2 − 0.36 × X1X3 + 0.89 × X2X3 − 3.9 × X12 + 1.4 × X22 − 3.15 × X32 | ||||
| CChemical | USD/m3 USD/kgCOD | 3.35 0.97 | L | R2 = 0.97, R2Ad j= 0.97, R2Pred = 0.95 Ychemical = 4.3 + 0.045 × X1+ 0.85 × X2 + 0.087 × X3 | CChemical | USD/m3 USD/kgCOD | 0.105 0.028 | L | R2 = 0.99 R2Adj = 0.99, R2Pred = 0.99 Ychemical = 0.13 – 0.005 × X1 – 0.0014 × X2 + 0.048 × X3 |
| CEnergy | USD/m3 USD/kgCOD | 9.52 2.76 | L | - | CEnergy | USD/m3 USD/kgCOD | 10.91 2.96 | L | R2 = 0.94 R2Adj = 0.92, R2Pred = 0.87 Ychemical = 13.23 + 0.061 × X1+ 2.68 × X2 − 0.053 × X3 |
| Csludge | USD/m3 USD/kgCOD | 1.51 0.43 | Q | R2 = 0.99 R2Adj = 0.96, R2Pred = 0.80 Yslduge = 1.57 − 0.06 × X1 + 0.71 × X2 − 0.009 × X3 − 0.07 × X1X2 − 0.058 × X1X3 + 0.019 × X2X3+ 0.57 × X12 + 0.030 × X22 + 0.14 × X32 | Csludge | USD/m3 USD/kgCOD | 0.61 0.16 | Q | R2 = 0.93, Adj R2 = 0.81 Ysludge = 7.2 − 0.06 × X1 + 0.74 × X2 + 0.24 × X3 + 0.22 × X1X2 + 0.06 × X1X3 + 0.053 × X2X3 − 2.3 × X12 − 1.08 × X22 − 0.84 × X32 |
| Total O.C. | USD/m3 USD/kgCOD | 14.62 4.25 | Q | It is given in Equation (19) | Total O.C. | USD/m3 USD/kgCOD | 13.79 3.73 | Q | It is given in Equation (20) |
| Total Operating Cost | |||||||
|---|---|---|---|---|---|---|---|
| Method | Type of Wastewater | Removal Efficiency (%) | Optimum Conditions | Sludge Volume Energy/Chemical Consumption | Before a Photovoltaic Solar Panel Integration | After a Photovoltaic Solar Panel Integration | Literature |
| Photo/Fenton | Denim jean production wastewater | COD: 84% (CODi: 4100 mg/L) TOC: 61% | pH 3, CFe2+: 2.3 g/L, H2O2: 27 gr/L, R.T.: 30 min | S.V.: 5.97 kg/m3 C FeSO4: 3.61 kg/m3 | USD 4.25/kgCOD USD 14.62/m3 | USD 1.61/kgCOD USD 5.7/m3 | Present Study |
| Photo/Electrochemical Fenton | COD: 90% (CODi: 4100 mg/L) TOC: 73% | pH 3, CFe2+: 27 A/m2, H2O2: 28 g/L, R.T.: 30 min | S.V.: 3.21 kg/m3 E.C.:7.29 kWh/kgCOD (26.90 kWh/m3) | USD 3.73/kgCOD USD 13.79/m3 | USD 1.34/kg COD USD 4.96/m3 | ||
| Solar-Photo-electro-Fenton | Textile wastewater | COD: 83% (CODi: 545 mg/L) | pH: 4, C.D.: 40 mA/cm2, C[FeSO4]: 0.3 mM | - | USD 3.45/kgCOD USD 1.56/m3 | [23] | |
| Photo/Fenton | Dairy industry wastewater | COD: 60% (CODi: 2136 mg/L) | pH 3.5, C[FeSO4]: 198 mg/L, H2O2 14,000 mg/L, R.T.: 180 min | - | USD 40.24/kg COD | [29] | |
| Electrochemical Fenton | Nanofiltration concentrate wastewater | COD: 71% (CODi: 3100 mg/L) | pH: 3, C.D.: 15 mA/cm2, C[FeSO4]: 560 mg/L, R.T.: 360 min | 207 kWh/kgCOD | USD 15.93/kgCOD | [40] | |
| Electro-Fenton | Landfill leachate wastewater | COD: 96% (CODi: 1827 mg/L) | pH: 3, C.D.: 1A, C[FeSO4]: 0.2 mM, R.T.: 480 min | 110–350 kWh/kgCOD | USD 8.61–26.72/kgCOD | [41] | |
| Electro-Fenton | Textile wastewater | COD: 96% (CODi: 544 mg/L) | pH: 3, C.D.: 0.32 A, C[FeSO4]: 0.53 mM, R.T.: 90 min | 1.31 kWh/kgCOD | USD 5.76/kgCOD | [42] | |
| Electro-Fenton | Landfill leachate wastewater | COD: 92.82% (CODi: 825 mg/L) | pH: 4, U: 5.5 V, H2O2/Fe2+: 2.5, R.T.: 50 min, (E:L = 1:2) | 3.32 kWh/kgCOD | USD 1.719/kgCOD USD 1.41/m3 | [45] | |
| COD: 93.35% (CODi: 792 mg/L) | pH: 4, U: 5.5 V, H2O2/Fe2+: 2.5, R.T.: 50 min, (E:L = 1:1) | 3.44 kWh/kgCOD | USD 1.722/kgCOD USD 1.36/m3 | ||||
| COD: 91.90% (CODi: 444 mg/L) | pH: 4, U: 5.5 V, H2O2/Fe2+: 2.5, R.T.: 50 min, (E:L = 2:1) | 6.24 kWh/kgCOD | USD 1.92/kgCOD USD 0.85/m3 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Solak, M. Cost-Effective Processes for Denim Production Wastewater: Dual Criterial Optimization of Techno-Economical Parameters by RSM and Minimization of Energy Consumption of Photo Assisted Fenton Processes via Direct Photovoltaic Solar Panel Integration. Processes 2023, 11, 1903. https://doi.org/10.3390/pr11071903
Solak M. Cost-Effective Processes for Denim Production Wastewater: Dual Criterial Optimization of Techno-Economical Parameters by RSM and Minimization of Energy Consumption of Photo Assisted Fenton Processes via Direct Photovoltaic Solar Panel Integration. Processes. 2023; 11(7):1903. https://doi.org/10.3390/pr11071903
Chicago/Turabian StyleSolak, Murat. 2023. "Cost-Effective Processes for Denim Production Wastewater: Dual Criterial Optimization of Techno-Economical Parameters by RSM and Minimization of Energy Consumption of Photo Assisted Fenton Processes via Direct Photovoltaic Solar Panel Integration" Processes 11, no. 7: 1903. https://doi.org/10.3390/pr11071903
APA StyleSolak, M. (2023). Cost-Effective Processes for Denim Production Wastewater: Dual Criterial Optimization of Techno-Economical Parameters by RSM and Minimization of Energy Consumption of Photo Assisted Fenton Processes via Direct Photovoltaic Solar Panel Integration. Processes, 11(7), 1903. https://doi.org/10.3390/pr11071903







