Effects of Surfactant Characteristics on Fuel Properties of Emulsions of Alternative Engine Fuel through the Phase Inversion Method
Abstract
1. Introduction
2. Experimental Details
2.1. Emulsification Materials
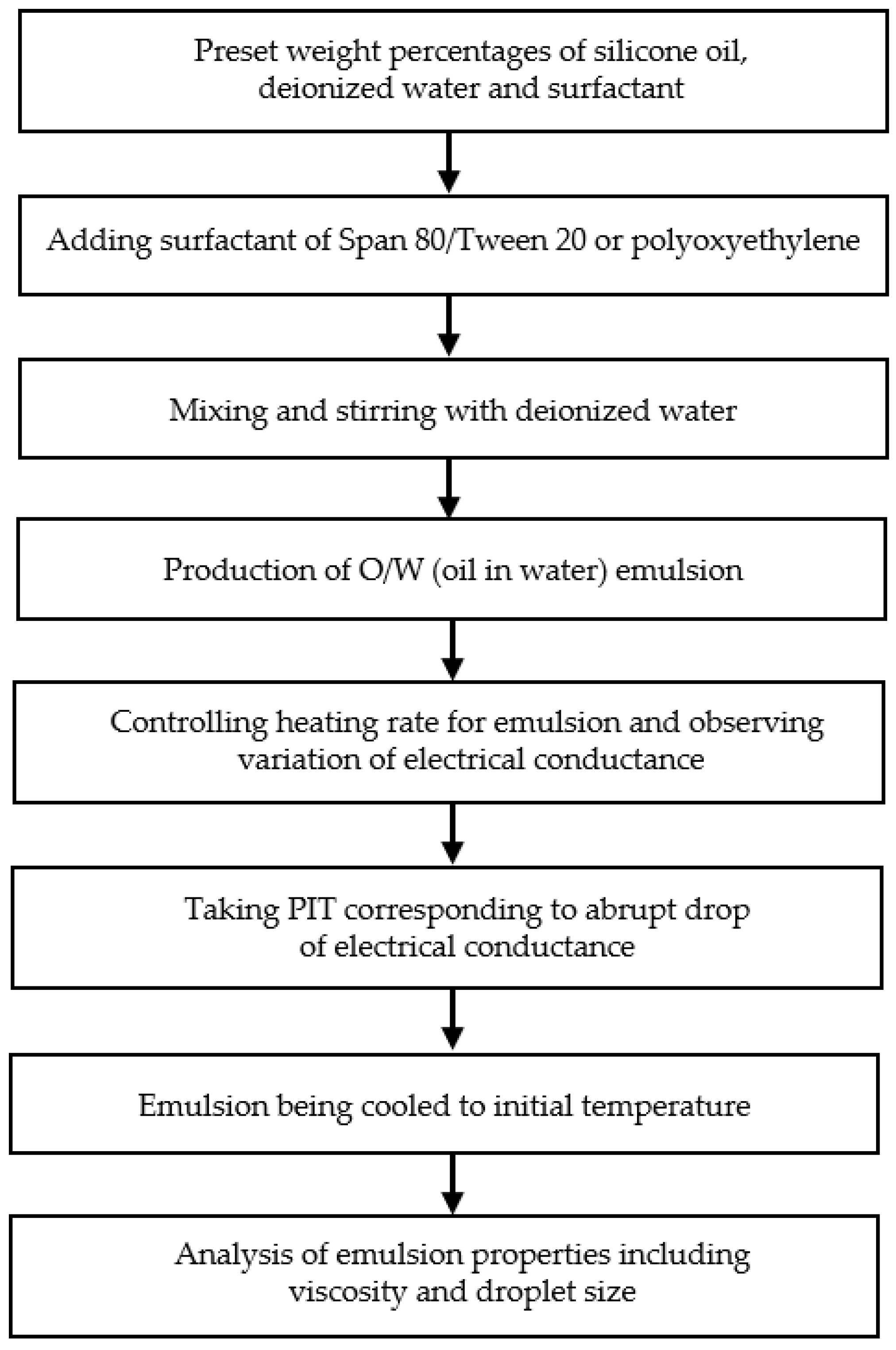
2.2. Emulsions Preparation Procedures
2.3. Measurement of Emulsion Properties
3. Results and Discussion
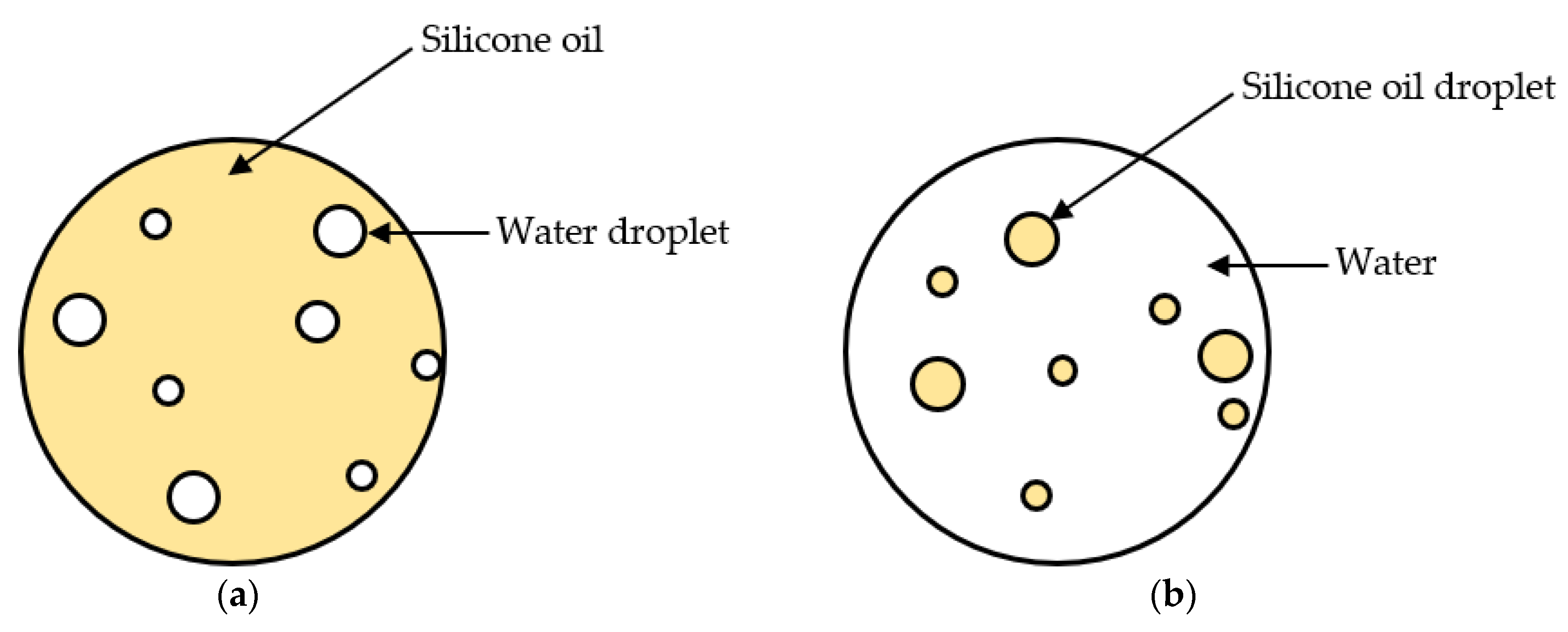
3.1. Effects of Surfactant Type on Mean Droplet Size
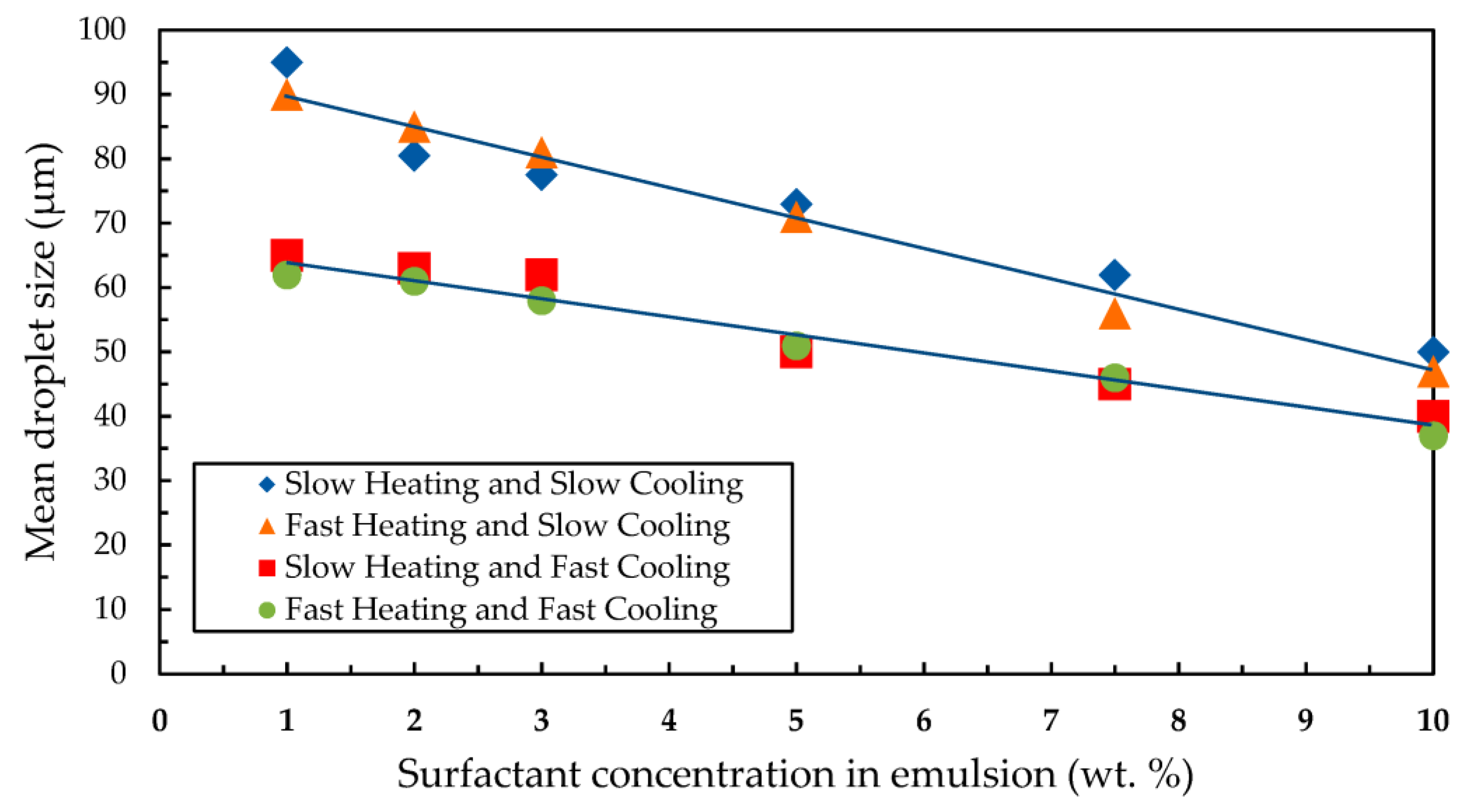
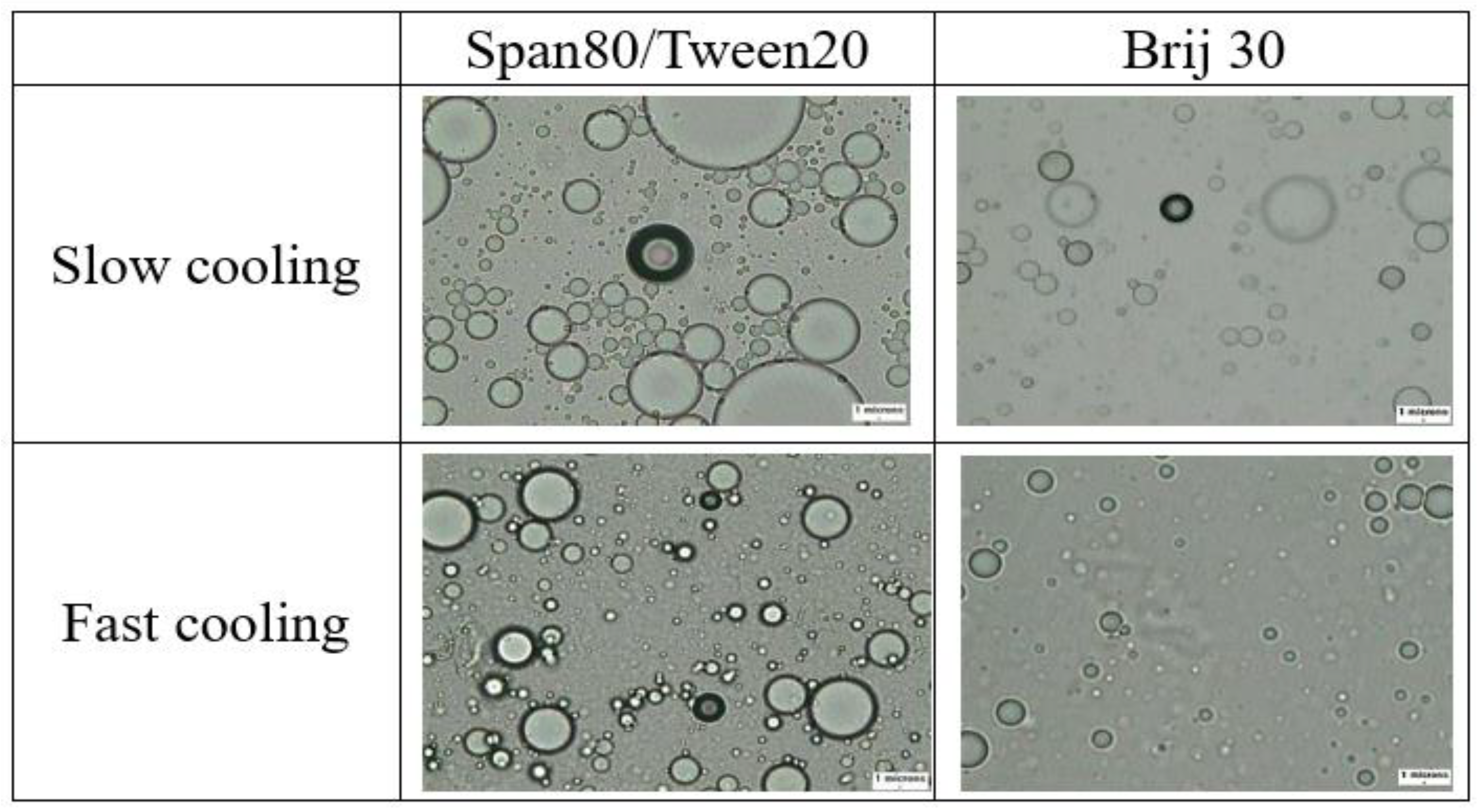
3.2. Effects of Heating and Cooling Processes on Mean Droplet Size
3.3. Effects of Surfactant Type and Concentration on Kinematic Viscosity
4. Conclusions
- The mean droplet sizes of the dispersed phase of the silicone oil emulsions were reduced with the increase of surfactant concentrations of Brij 30 or the mixture of Span 80 and Tween 20. Slow or fast heating followed by fast cooling significantly decreased the emulsions’ mean droplet sizes. However, the mean droplet sizes of the emulsions emulsified with Brij 30 were considerably lower than those prepared with a Tween 20 and Span 80 mixture.
- The emulsions either added with Brij 30 or Tween 20 and Span 80 mixture subjected to fast cooling were observed to have smaller droplets and a wider range of droplet sizes than those subjected to slow cooling.
- The emulsions added with Brij 30 surfactant appeared to have larger kinematic viscosity and emulsification stability. The increased surfactant concentrations of either Span 80/Tween 20 or Brij 30 also increased kinematic viscosity.
- Adding a Tween 20 and Span 80 mixture to the emulsion increased the number of dispersed droplets more than that with Brij 30. The increase of surfactant concentration of Brij 30 or a Span 80 and Tween mixture raised the number of scattered droplets.
- The silicone oil emulsions by the PIT method were observed to have adequate fuel properties and, thus, are promising alternatives to fossil fuel.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abdollahi, M.; Ghobadian, B.; Najafi, G.; Hoseini, S.S.; Mofijur, M.; Mazlan, M. Impact of water–biodiesel–diesel nano-emulsion fuel on performance parameters and diesel engine emission. Fuel 2020, 280, 118576. [Google Scholar] [CrossRef]
- Abrar, I.; Arora, T.; Khandelwal, R. Bioalcohols as an alternative fuel for transportation: Cradle to grave analysis. Fuel Process. Technol. 2023, 242, 107646. [Google Scholar] [CrossRef]
- Rostampour, A.; Shojaeefard, M.H.; Molaeimanesh, G.R. Role of water micro-explosion on fuel droplet size distribution, engine performance, and emissions in a water-diesel emulsified engine: A comprehensive numerical investigation. Int. J. Engine Res. 2023, 24, 1110–1120. [Google Scholar] [CrossRef]
- González-Castaño, M.; Dorneanu, B.; Arellano-García, H. The reverse water gas shift reaction: A process systems engineering perspective. React. Chem. Eng. 2021, 6, 954–976. [Google Scholar] [CrossRef]
- Baraj, E.; Ciahotný, K.; Hlinčík, T. The water gas shift reaction: Catalysts and reaction mechanism. Fuel 2021, 288, 119817. [Google Scholar] [CrossRef]
- Najjar, R.; Heidari, S. Modified diesel prepared by stabilization of water as nanodroplets in diesel/colza oil blend: Study of phase behavior and affecting parameters. Fuel 2018, 214, 497–504. [Google Scholar] [CrossRef]
- Goodarzi, F.; Zendehboudi, S. A Comprehensive Review on Emulsions and Emulsion Stability in Chemical and Energy Industries. Can. J. Chem. Eng. 2019, 97, 281–309. [Google Scholar] [CrossRef]
- Kundu, P.; Arora, K.; Gu, Y.; Kumar, V.; Mishra, I.M. Formation and stability of water-in-oil nano-emulsions with mixed surfactant using in-situ combined condensation-dispersion method. Can. J. Chem. Eng. 2019, 97, 2039–2049. [Google Scholar] [CrossRef]
- Meng, L.; Wang, Z.; Wang, L.; Guo, L.; Guo, Z. Novel and efficient purification of scrap Al-Mg alloys using supergravity technology. Waste Manag. 2021, 119, 22–29. [Google Scholar] [CrossRef]
- Hoo, D.Y.; Low, Z.L.; Low, D.Y.S.; Tang, S.Y.; Manickam, S.; Tan, K.W.; Ban, Z.H. Ultrasonic cavitation: An effective cleaner and greener intensification technology in the extraction and surface modification of nanocellulose. Ultrason. Sonochem. 2022, 90, 106176. [Google Scholar] [CrossRef]
- Liu, L.; Niu, J.; Wu, J.Y. Formulation of highly stable PCM nano-emulsions with reduced supercooling for thermal energy storage using surfactant mixtures. Sol. Energy Mater. Sol. Cells 2021, 223, 110983. [Google Scholar] [CrossRef]
- Cholakova, D.; Vinarov, Z.; Tcholakova, S.; Denkov, N.D. Self-emulsification in chemical and pharmaceutical technologies. Curr. Opin. Colloid Interface Sci. 2022, 59, 101576. [Google Scholar] [CrossRef]
- Chuesiang, P.; Siripatrawan, U.; Sanguandeekul, R.; Yang, J.S.; McClements, D.J.; McLandsborough, L. Antimicrobial activity and chemical stability of cinnamon oil in oil-in-water nanoemulsions fabricated using the phase inversion temperature method. LWT 2019, 110, 190–196. [Google Scholar] [CrossRef]
- Sneha, K.; Kumar, A. Nanoemulsions: Techniques for the preparation and the recent advances in their food applications. Innov. Food Sci. Emerg. Technol. 2022, 76, 102914. [Google Scholar] [CrossRef]
- Saffarionpour, S. Preparation of Food Flavor Nanoemulsions by High- and Low-Energy Emulsification Approaches. Food Eng. Rev. 2019, 11, 259–289. [Google Scholar] [CrossRef]
- Kumar, N.; Verma, A.; Mandal, A. Formation, characteristics and oil industry applications of nanoemulsions: A review. J. Pet. Sci. Eng. 2021, 206, 109042. [Google Scholar] [CrossRef]
- Wang, F.; Lin, W.; Ling, Z.; Fang, X. A comprehensive review on phase change material emulsions: Fabrication, characteristics, and heat transfer performance. Sol. Energy Mater. Sol. Cells 2019, 191, 218–234. [Google Scholar] [CrossRef]
- Dastbaz, Z.; Dana, S.N.; Ashrafizadeh, S.N. Preparation of a stabilized aqueous polystyrene suspension via phase inversion. RSC Adv. 2021, 11, 17547–17557. [Google Scholar] [CrossRef]
- Rodríguez-Abreu, C. On the Relationships between the Hydrophilic-Lipophilic Balance and the Nanoarchitecture of Nonionic Surfactant Systems. J. Surfactants Deterg. 2019, 22, 1001–1010. [Google Scholar] [CrossRef]
- Doost, A.S.; Sinnaeve, D.; De Neve, L.; Van der Meeren, P. Influence of non-ionic surfactant type on the salt sensitivity of oregano oil-in-water emulsions. Colloids Surf. A Physicochem. Eng. Asp. 2017, 525, 38–48. [Google Scholar] [CrossRef]
- Aubry, J.-M.; Ontiveros, J.F.; Salager, J.-L.; Nardello-Rataj, V. Use of the normalized hydrophilic-lipophilic-deviation (HLDN) equation for determining the equivalent alkane carbon number (EACN) of oils and the preferred alkane carbon number (PACN) of nonionic surfactants by the fish-tail method (FTM). Adv. Colloid Interface Sci. 2020, 276, 102099. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.; Li, J.; Wang, Y.; Jiang, Y.; Wang, Z.; Geng, T. Synergistic effects of Gemini cationic surfactants with multiple quaternary ammonium groups and anionic surfactants with long EO chains in the mixed systems. J. Mol. Liq. 2023, 376, 121427. [Google Scholar] [CrossRef]
- Han, X.; Wang, Z.; Wang, J. Preparation of highly selective reverse osmosis membranes by introducing a nonionic surfactant in the organic phase. J. Membr. Sci. 2022, 651, 120453. [Google Scholar] [CrossRef]
- Ren, G.; Sun, Z.; Wang, Z.; Zheng, X.; Xu, Z.; Sun, D. Nanoemulsion formation by the phase inversion temperature method using polyoxypropylene surfactants. J. Colloid Interface Sci. 2019, 540, 177–184. [Google Scholar] [CrossRef]
- Perazzo, A.; Preziosi, V.; Guido, S. Phase inversion emulsification: Current understanding and applications. Adv. Colloid Interface Sci. 2015, 222, 581–599. [Google Scholar] [CrossRef]
- Jiang, J.; Wang, Z.; Wang, C.; Shi, L.; Hou, J.; Zhang, L. Model Emulsions Stabilized with Nonionic Surfactants: Structure and Rheology Across Catastrophic Phase Inversion. ACS Omega 2022, 7, 44012–44020. [Google Scholar] [CrossRef]
- Singpanna, K.; Charnvanich, D.; Panapisal, V. Effect of the hydrophilic-lipophilic balance values of non-ionic surfactants on size and size distribution and stability of oil/water soybean oil nanoemulsions. Thai J. Pharm. Sci. 2021, 45, 487–491. [Google Scholar]
- Volpe, V.; Giacomodonato, M.N.; Sordelli, D.O.; Insausti, M.; Buzzola, F.R.; Grünhut, M. Ciprofloxacin loaded o/w microemulsion against Staphylococcus aureus. Analytical and biological studies for topical and intranasal administration. J. Drug Deliv. Sci. Technol. 2020, 57, 101705. [Google Scholar] [CrossRef]
- Zhang, X.; Niu, J.; Wu, J.-Y. Evaluation and manipulation of the key emulsification factors toward highly stable PCM-water nano-emulsions for thermal energy storage. Sol. Energy Mater. Sol. Cells 2021, 219, 110820. [Google Scholar] [CrossRef]
- Liu, M.; Yang, C.; Liu, E.; Zhang, F.; Meng, X.; Liu, B. Effect of environmental stresses on physicochemical properties of ALA oil-in-water nanoemulsion system prepared by emulsion phase inversion. Food Chem. 2021, 343, 128475. [Google Scholar] [CrossRef]
- Mir, M.; Ghasemirad, S. Phase inversion emulsification of paraffin oil/polyethylene wax blend in water: A comparison between mixed monomeric and monomeric/gemini surfactant systems. J. Mol. Liq. 2022, 359, 119315. [Google Scholar] [CrossRef]
- Liu, L.; Niu, J.; Wu, J.-Y. Development of highly stable paraffin wax/water phase change material nano-emulsions as potential coolants for thermal management. Sol. Energy Mater. Sol. Cells 2023, 252, 112184. [Google Scholar] [CrossRef]
- Al-Mohammedawi, A.; Mollenhauer, K. Current Research and Challenges in Bitumen Emulsion Manufacturing and Its Properties. Materials 2022, 15, 2026. [Google Scholar] [CrossRef]
- Ren, G.; Li, B.; Lu, D.; Di, W.; Ren, L.; Tian, L.; Zhang, P.; He, J.; Sun, D. Preparation of polyoxypropylene surfactant-based nanoemulsions using phase inversion composition method and their application in oil recovery. J. Mol. Liq. 2021, 342, 117469. [Google Scholar] [CrossRef]
- Wang, F.; Cao, J.; Ling, Z.; Zhang, Z.; Fang, X. Experimental and simulative investigations on a phase change material nano-emulsion-based liquid cooling thermal management system for a lithium-ion battery pack. Energy 2020, 207, 118215. [Google Scholar] [CrossRef]
- Engls, T.; Forster, T.; von Rybinski, W. The influence of coemulsifier type on the stability of oil-in-water emulsions. Colloids Surf. A Physicochem. Eng. Asp. 1995, 99, 141–149. [Google Scholar] [CrossRef]
- Usón, N.; Garcia, M.; Solans, C. Formation of water-in-oil (W/O) nano-emulsions in a water/mixed non-ionic surfactant/oil systems prepared by a low-energy emulsification method. Colloids Surf. A Physicochem. Eng. Asp. 2004, 250, 415–421. [Google Scholar] [CrossRef]
- Aziz, T.; Fan, H.; Khan, F.U.; Haroon, M.; Cheng, L. Modified silicone oil types, mechanical properties and applications. Polym. Bull. 2019, 76, 2129–2145. [Google Scholar] [CrossRef]
- Mendichi, R.; Schieroni, A.G.; Piovani, D.; Allegrini, D.; Ferrara, M.; Romano, M.R. Comparative Study of Chemical Composition, Molecular and Rheological Properties of Silicone Oil Medical Devices. Transl. Vis. Sci. Technol. 2019, 8, 9. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Lin, K.-H.; Yang, H. The Influences of Emulsification Variables on Emulsion Characteristics Prepared through the Phase Inversion Temperature Method as Engine Fuel. Processes 2023, 11, 1091. [Google Scholar] [CrossRef]
- Sarheed, O.; Shouqair, D.; Ramesh, K.; Khaleel, T.; Amin, M.; Boateng, J.; Drechsler, M. Formation of stable nanoemulsions by ultrasound-assisted two-step emulsification process for topical drug delivery: Effect of oil phase composition and surfactant concentration and loratadine as ripening inhibitor. Int. J. Pharm. 2020, 576, 118952. [Google Scholar] [CrossRef] [PubMed]
- Cabaleiro, D.; Agresti, F.; Fedele, L.; Barison, S.; Hermida-Merino, C.; Losada-Barreiro, S.; Bobbo, S.; Piñeiro, M. Review on phase change material emulsions for advanced thermal management: Design, characterization and thermal performance. Renew. Sustain. Energy Rev. 2022, 159, 112238. [Google Scholar] [CrossRef]
- Kumar, M.; Bishnoi, R.S.; Shukla, A.K.; Jain, C.P. Development and optimization of drug-loaded nanoemulsion system by phase inversion temperature (PIT) method using Box–Behnken design. Drug Dev. Ind. Pharm. 2021, 47, 977–989. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Chen, Q.; Wu, X.; Jafari, S.M.; McClements, D.J. Formulation of oil-in-water emulsions for pesticide applications: Impact of surfactant type and concentration on physical stability. Environ. Sci. Pollut. Res. 2018, 25, 21742–21751. [Google Scholar] [CrossRef] [PubMed]
- Kharazi, M.; Saien, J.; Yarie, M.; Zolfigol, M.A. The superior effects of a long chain gemini ionic liquid on the interfacial tension, emulsification and oil displacement of crude oil-water. J. Pet. Sci. Eng. 2020, 195, 107543. [Google Scholar] [CrossRef]
- Lemahieu, G.; Ontiveros, J.F.; Molinier, V.; Aubry, J.-M. SPI-slope/PIT-slope mapping as a guiding tool for the selection of technical grade surfactants for chemical enhanced oil recovery. Colloids Surf. A Physicochem. Eng. Asp. 2023, 656, 130362. [Google Scholar] [CrossRef]
- Chuacharoen, T.; Prasongsuk, S.; Sabliov, C.M. Effect of Surfactant Concentrations on Physicochemical Properties and Functionality of Curcumin Nanoemulsions Under Conditions Relevant to Commercial Utilization. Molecules 2019, 24, 2744. [Google Scholar] [CrossRef]
- Posocco, P.; Perazzo, A.; Preziosi, V.; Laurini, E.; Pricl, S.; Guido, S. Interfacial tension of oil/water emulsions with mixed non-ionic surfactants: Comparison between experiments and molecular simulations. RSC Adv. 2016, 6, 4723–4729. [Google Scholar] [CrossRef]
- Yekeen, N.; Padmanabhan, E.; Syed, A.H.; Sevoo, T.; Kanesen, K. Synergistic influence of nanoparticles and surfactants on interfacial tension reduction, wettability alteration and stabilization of oil-in-water emulsion. J. Pet. Sci. Eng. 2020, 186, 106779. [Google Scholar] [CrossRef]
- Akhlaghi, N.; Riahi, S.; Parvaneh, R. Interfacial tension behavior of a nonionic surfactant in oil/water system; salinity, pH, temperature, and ionic strength effects. J. Pet. Sci. Eng. 2021, 198, 108177. [Google Scholar] [CrossRef]
- Shibata, Y.; Hyde, A.; Asakuma, Y.; Phan, C. Thermal response of a non-ionic surfactant layer at the water/oil interface during microwave heating. Colloids Surfaces A Physicochem. Eng. Asp. 2018, 556, 127–133. [Google Scholar] [CrossRef]
- Eras-Muñoz, E.; Farré, A.; Sánchez, A.; Font, X.; Gea, T. Microbial biosurfactants: A review of recent environmental applications. Bioengineered 2022, 13, 12365–12391. [Google Scholar] [CrossRef]
- Feng, J.; Esquena, J.; Rodriguez-Abreu, C.; Solans, C. Key features of nano-emulsion formation by the phase inversion temperature method. J. Dispers. Sci. Technol. 2021, 42, 1073–1081. [Google Scholar] [CrossRef]
- Vasistha, V.; Bharj, R.S. Analyzing the storage stability of diesel emulsified fuels: A comparative standpoint. Energy Sources Part A Recover. Util. Environ. Eff. 2022, 44, 5527–5544. [Google Scholar] [CrossRef]
- Sagdeev, D.I.; Khairutdinov, V.F.; Farakhov, M.; Alyaev, V.A.; Gumerov, F.M.; Zaripov, Z.I.; Minkin, V.S.; Abdulagatov, I.M. Measurements of the Density and Viscosity of Heavy Oil and Water-in-Oil Emulsions Over a Wide Temperature Range. Int. J. Thermophys. 2023, 44, 7. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Lin, S.-A. Effects of emulsification variables on fuel properties of two- and three-phase biodiesel emulsions. Fuel 2007, 86, 210–217. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, C.-Y.; Lin, K.-H.; Yang, H. Effects of Surfactant Characteristics on Fuel Properties of Emulsions of Alternative Engine Fuel through the Phase Inversion Method. Processes 2023, 11, 1864. https://doi.org/10.3390/pr11071864
Lin C-Y, Lin K-H, Yang H. Effects of Surfactant Characteristics on Fuel Properties of Emulsions of Alternative Engine Fuel through the Phase Inversion Method. Processes. 2023; 11(7):1864. https://doi.org/10.3390/pr11071864
Chicago/Turabian StyleLin, Cherng-Yuan, Keng-Hung Lin, and Hsuan Yang. 2023. "Effects of Surfactant Characteristics on Fuel Properties of Emulsions of Alternative Engine Fuel through the Phase Inversion Method" Processes 11, no. 7: 1864. https://doi.org/10.3390/pr11071864
APA StyleLin, C.-Y., Lin, K.-H., & Yang, H. (2023). Effects of Surfactant Characteristics on Fuel Properties of Emulsions of Alternative Engine Fuel through the Phase Inversion Method. Processes, 11(7), 1864. https://doi.org/10.3390/pr11071864






