Development of a Continuous Process Chain for Selective Recovery and Purification of Rare Metals
Abstract
1. Introduction
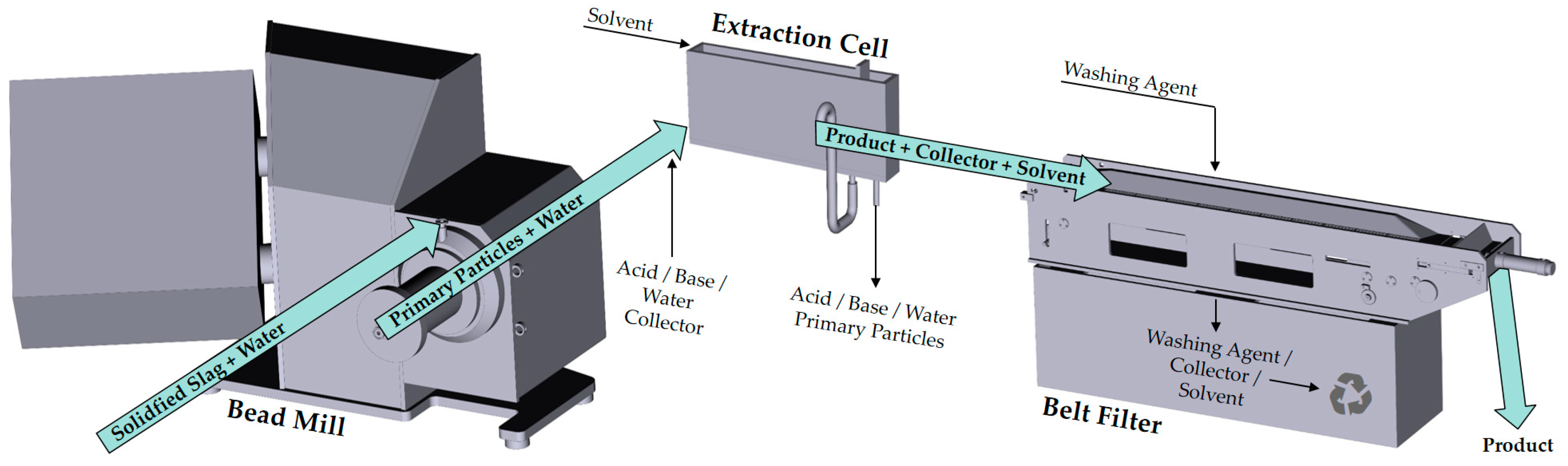
2. Materials and Methods
2.1. Particle System
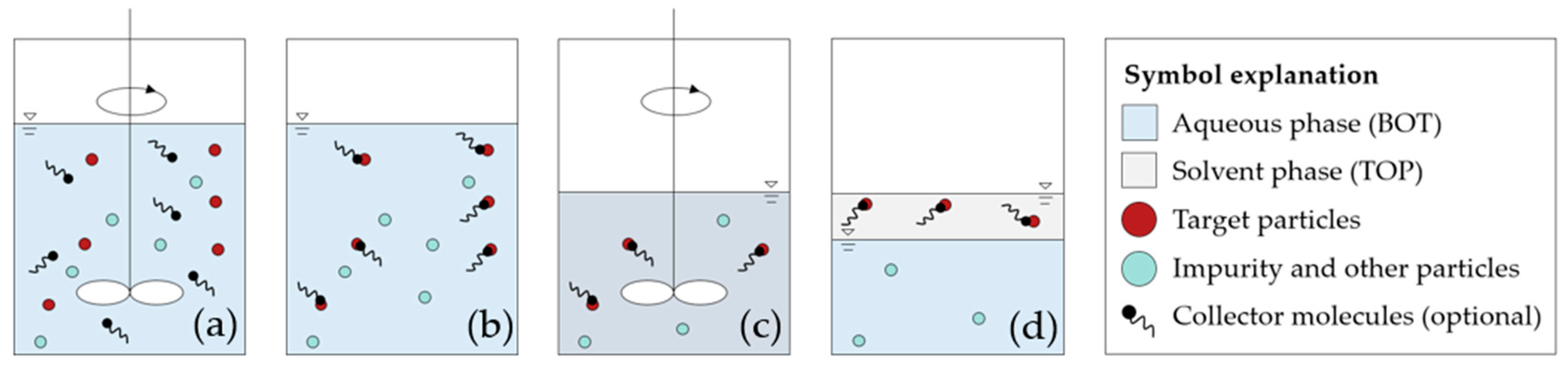
2.2. Liquid–Liquid Extraction
2.2.1. Experimental Procedure
2.2.2. Test Evaluation
2.3. Cake Washing
- (1)
- Removal of the collector molecules adsorbed on the particle surface;
- (2)
- Recovery of the applied solvent.
2.3.1. Experimental Procedure
2.3.2. Test Evaluation
3. Results and Discussion
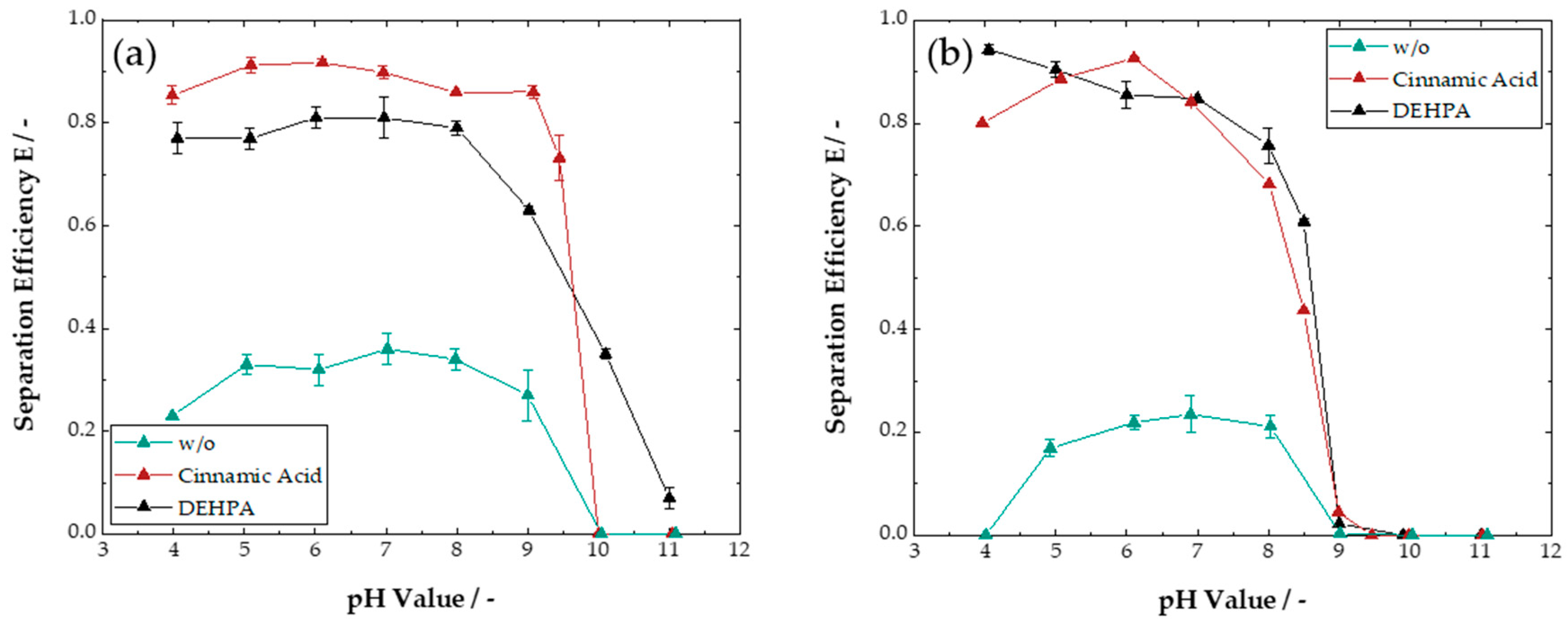
3.1. Liquid–Liquid Extraction
3.1.1. Single-System Extraction
3.1.2. Multi-System Extraction
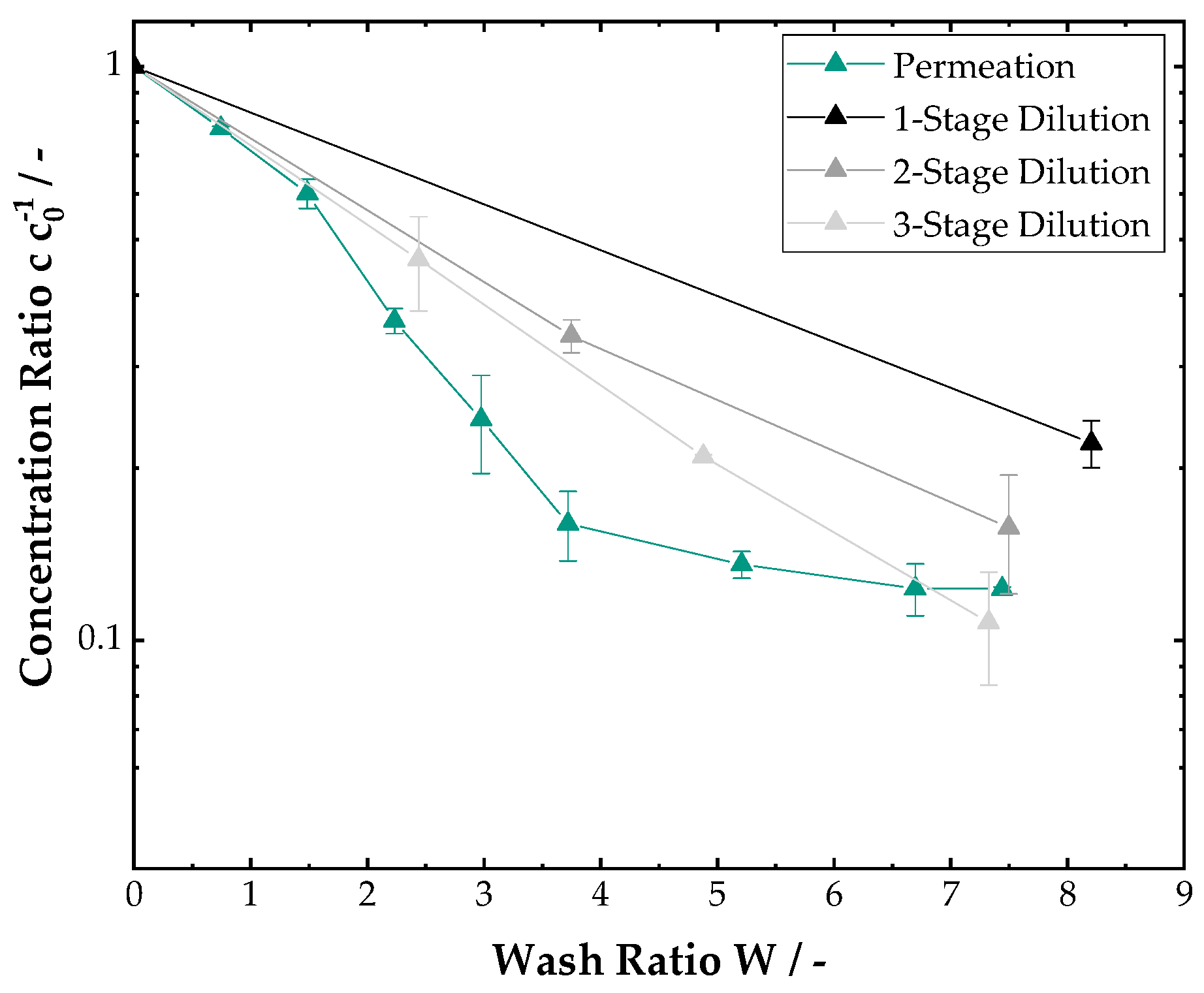
3.2. Filter Cake Washing
3.3. Continuous Process Chain
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Andersson, M.; Ljunggren Söderman, M.; Sandén, B.A. Challenges of recycling multiple scarce metals: The case of Swedish ELV and WEEE recycling. Resour. Policy 2019, 63, 101403. [Google Scholar] [CrossRef]
- Sinclar, W.D. Electronic Metals (In, Ge and Ga): Present and Future Resources. Acta Geol. Sin.-Engl. Ed. 2014, 88, 463–465. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Ding, Y.; Liu, B.; Chang, C.-C. Supply and demand of some critical metals and present status of their recycling in WEEE. Waste Manag. 2017, 65, 113–127. [Google Scholar] [CrossRef] [PubMed]
- U.S. Geological Survey. Mineral Commodity Summaries 2001. Available online: https://www.usgs.gov/publications/mineral-commodity-summaries-2001 (accessed on 15 May 2023).
- U.S. Geological Survey. Mineral Commodity Summaries 2023. Available online: https://www.usgs.gov/publications/mineral-commodity-summaries-2023 (accessed on 15 May 2023).
- Eggert, R.G. Minerals go critical. Nat. Chem. 2011, 3, 688–691. [Google Scholar] [CrossRef]
- Redlinger, M.; Eggert, R.; Woodhouse, M. Evaluating the availability of gallium, indium, and tellurium from recycled photovoltaic modules. Sol. Energy Mater. Sol. Cells 2015, 138, 58–71. [Google Scholar] [CrossRef]
- Licht, C.; Peiró, L.T.; Villalba, G. Global Substance Flow Analysis of Gallium, Germanium, and Indium: Quantification of Extraction, Uses, and Dissipative Losses within their Anthropogenic Cycles. J. Ind. Ecol. 2015, 19, 890–903. [Google Scholar] [CrossRef]
- Nagy, S.; Bokányi, L.; Gombkötő, I.; Magyar, T. Recycling of Gallium from End-of-Life Light Emitting Diodes. Arch. Metall. Mater. 2017, 62, 1161–1166. [Google Scholar] [CrossRef]
- Swain, B.; Mishra, C.; Kang, L.; Park, K.-S.; Lee, C.G.; Hong, H.S. Recycling process for recovery of gallium from GaN an e-waste of LED industry through ball milling, annealing and leaching. Environ. Res. 2015, 138, 401–408. [Google Scholar] [CrossRef]
- Zhan, L.; Xia, F.; Ye, Q.; Xiang, X.; Xie, B. Novel recycle technology for recovering rare metals (Ga, In) from waste light-emitting diodes. J. Hazard. Mater. 2015, 299, 388–394. [Google Scholar] [CrossRef]
- Virolainen, S.; Ibana, D.; Paatero, E. Recovery of indium from indium tin oxide by solvent extraction. Hydrometallurgy 2011, 107, 56–61. [Google Scholar] [CrossRef]
- de La Torre, E.; Vargas, E.; Ron, C.; Gámez, S. Europium, Yttrium, and Indium Recovery from Electronic Wastes. Metals 2018, 8, 777. [Google Scholar] [CrossRef]
- Fontana, D.; Forte, F.; de Carolis, R.; Grosso, M. Materials recovery from waste liquid crystal displays: A focus on indium. Waste Manag. 2015, 45, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Retegan, T.; Steenari, B.-M.; Ekberg, C. Recovery of indium and yttrium from Flat Panel Display waste using solvent extraction. Sep. Purif. Technol. 2016, 166, 117–124. [Google Scholar] [CrossRef]
- Pereira, E.B.; Suliman, A.L.; Tanabe, E.H.; Bertuol, D.A. Recovery of indium from liquid crystal displays of discarded mobile phones using solvent extraction. Miner. Eng. 2018, 119, 67–72. [Google Scholar] [CrossRef]
- Yang, J.; Retegan, T.; Ekberg, C. Indium recovery from discarded LCD panel glass by solvent extraction. Hydrometallurgy 2013, 137, 68–77. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, Z. A review of current progress of recycling technologies for metals from waste electrical and electronic equipment. J. Clean. Prod. 2016, 127, 19–36. [Google Scholar] [CrossRef]
- Ebin, B.; Isik, M.I. Pyrometallurgical Processes for the Recovery of Metals from WEEE. In WEEE Recycling; Elsevier: Amsterdam, The Netherlands, 2016; pp. 107–137. [Google Scholar]
- Sawistowski, H. Physical Aspects of Liquid-Liquid Extraction. In Mass Transfer with Chemical Reaction in Multiphase Systems. Alper, E., Ed.; Springer Netherlands: Dordrecht, The Netherlands, 1983; pp. 613–635. [Google Scholar]
- Kumar, A.; Negi, S.; Choudhury, T.; Mutreja, V.; Sunaina, S.; Sahoo, S.C.; Singh, A.; Mehta, S.K.; Kataria, R.; Saini, V. A highly sensitive and specific luminescent MOF determines nitric oxide production and quantifies hydrogen sulfide-mediated inhibition of nitric oxide in living cells. Mikrochim. Acta 2023, 190, 127. [Google Scholar] [CrossRef]
- Kirandeep; Kaur, J.; Sharma, I.; Zangrando, E.; Pal, K.; Mehta, S.K.; Kataria, R. Fabrication of novel copper MOF nanoparticles for nanozymatic detection of mercury ions. J. Mater. Res. Technol. 2023, 22, 278–291. [Google Scholar] [CrossRef]
- Kumar, A.; Mutreja, V.; Anand, V.; Mehta, S.K.; Zangrando, E.; Kataria, R. Solvent templated luminescent metal-organic frameworks for specific detection of vitamin C in aqueous media. J. Mol. Struct. 2023, 1284, 135365. [Google Scholar] [CrossRef]
- Buchheiser, S.; Deutschmann, M.P.; Rhein, F.; Allmang, A.; Fedoryk, M.; Stelzner, B.; Harth, S.; Trimis, D.; Nirschl, H. Particle and Phase Analysis of Combusted Iron Particles for Energy Storage and Release. Materials 2023, 16, 2009. [Google Scholar] [CrossRef]
- Hubbard, C.R.; Snyder, R.L. RIR-Measurement and Use in Quantitative XRD. Powder Diffr. 1988, 3, 74–77. [Google Scholar] [CrossRef]
- Doebelin, N.; Kleeberg, R. Profex: A graphical user interface for the Rietveld refinement program BGMN. J. Appl. Crystallogr. 2015, 48, 1573–1580. [Google Scholar] [CrossRef] [PubMed]
- Sigma-Aldrich. Bis-(2-ethylhexyl)-phosphorsäure. Available online: https://www.sigmaaldrich.com/US/en/product/aldrich/237825 (accessed on 15 May 2023).
- Sigma-Aldrich. Cinnamic Acid. Available online: https://www.sigmaaldrich.com/US/en/substance/cinnamicacid14816140103 (accessed on 15 May 2023).
- Rhein, F.; Schmid, E.; Esquivel, F.B.; Nirschl, H. Opportunities and Challenges of Magnetic Seeded Filtration in Multidimensional Fractionation. Chem. Ing. Tech. 2020, 92, 266–274. [Google Scholar] [CrossRef]
- Cerbelaud, M.; Videcoq, A.; Abélard, P.; Ferrando, R. Simulation of the heteroagglomeration between highly size-asymmetric ceramic particles. J. Colloid Interface Sci. 2009, 332, 360–365. [Google Scholar] [CrossRef]
- Anlauf, H. Wet Cake Filtration: Fundamentals, Equipment, Strategies; WILEY VCH: Weinheim, Germany, 2020. [Google Scholar]
- Wilkens, M.; Peuker, U.A. Grundlagen und aktuelle Entwicklungen der Filterkuchenwaschung. Chem. Ing. Tech. 2012, 84, 1873–1884. [Google Scholar] [CrossRef]
- Dobler, T.; Buchheiser, S.; Gleiß, M.; Nirschl, H. Development and Commissioning of a Small-Scale, Modular and Integrated Plant for the Quasi-Continuous Production of Crystalline Particles. Processes 2021, 9, 663. [Google Scholar] [CrossRef]

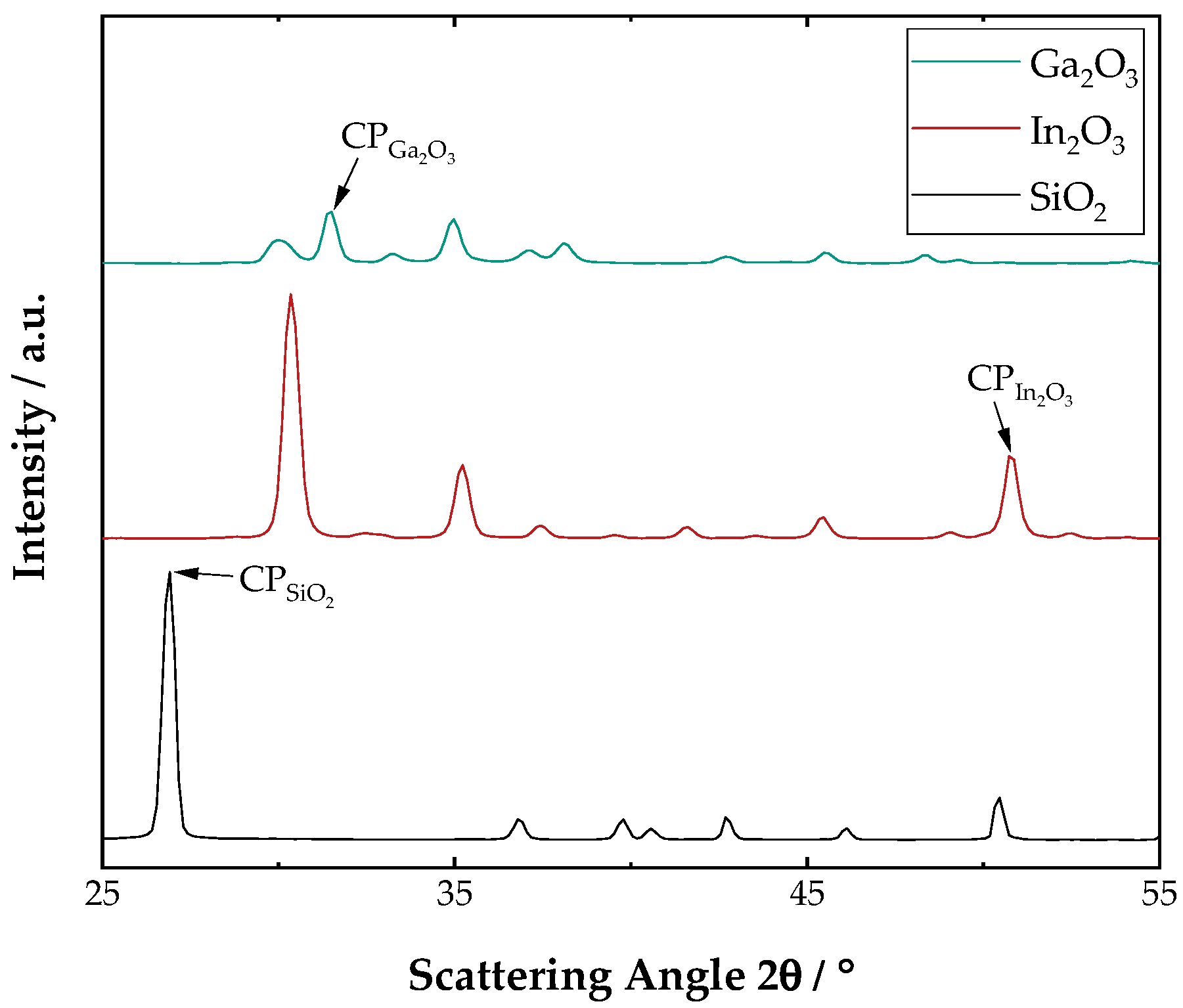



| Mixture () | Proportionality Constant /- | R-Square/- |
| In2O3/SiO2 | 0.333 | 0.995 |
| In2O3/Ga2O3 | 1.082 | 0.997 |
| Collector | Mixture | pH Value | Composition BOT (wt %) |
|---|---|---|---|
| Cinnamic Acid | In2O3SiO2 | 6 | 50|50 |
| In2O3|Ga2O3 | 9.5 | 50|50 | |
| In2O3|Ga2O3|SiO2 | 9.5 | 33.3|33.3|33.3 | |
| DEHPA | In2O3|SiO2 | 6 | 50|50 |
| In2O3|Ga2O3 | 10 | 50|50 | |
| In2O3|Ga2O3|SiO2 | 10 | 33.3|33.3|33.3 |
| Collector | Mixture | Composition TOP (wt %) | |
|---|---|---|---|
| Theoretical | Experimental | ||
| Cinnamic Acid | In2O3|SiO2 | 100|0 | 98.3|1.7 |
| In2O3|Ga2O3 | 100|0 | 76.7|23.3 | |
| In2O3|Ga2O3|SiO2 | 100|0|0 | 74.2|13.9|11.9 | |
| DEHPA | In2O3|SiO2 | 100|0 | 98.9|1.1 |
| In2O3|Ga2O3 | 100|0 | 93.1|6.9 | |
| In2O3|Ga2O3|SiO2 | 100|0|0 | 94.1|5.0|0.9 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dobler, T.; Buchheiser, S.; Gaschler, T.; Platzk, S.; Kruggel-Emden, H.; Nirschl, H.; Gleiß, M. Development of a Continuous Process Chain for Selective Recovery and Purification of Rare Metals. Processes 2023, 11, 1847. https://doi.org/10.3390/pr11061847
Dobler T, Buchheiser S, Gaschler T, Platzk S, Kruggel-Emden H, Nirschl H, Gleiß M. Development of a Continuous Process Chain for Selective Recovery and Purification of Rare Metals. Processes. 2023; 11(6):1847. https://doi.org/10.3390/pr11061847
Chicago/Turabian StyleDobler, Timo, Simon Buchheiser, Thomas Gaschler, Stefan Platzk, Harald Kruggel-Emden, Hermann Nirschl, and Marco Gleiß. 2023. "Development of a Continuous Process Chain for Selective Recovery and Purification of Rare Metals" Processes 11, no. 6: 1847. https://doi.org/10.3390/pr11061847
APA StyleDobler, T., Buchheiser, S., Gaschler, T., Platzk, S., Kruggel-Emden, H., Nirschl, H., & Gleiß, M. (2023). Development of a Continuous Process Chain for Selective Recovery and Purification of Rare Metals. Processes, 11(6), 1847. https://doi.org/10.3390/pr11061847







