1. Introduction
Oil shale is a solid underground resource with low maturity; it is difficult to exploit compared with traditional energy sources such as oil and coal [
1]. Pyrolysis is a popular method of converting oil shale into high-quality liquid fuel. The addition of appropriate catalysts can significantly enhance the thermal conversion efficiency of kerogen in oil shale, thereby improving the quality of the oil and gas products.
Recently, metal-based catalysts, especially metal oxides, have attracted significant attention owing to their excellent catalytic performances. They are believed to lead to high desulfurization rates of shale oil or low-sulfur diesel [
2,
3]. Chen et al. [
4] demonstrated the effectiveness of CuO nanoparticles as hydrothermal cracking catalysts in reducing the viscosity of heavy oil from the Shengli Oilfield by 94.6% and transforming 22.4% of asphaltene into lighter components. Zhong et al. [
5] further investigated the use of CuO nanoparticles in the catalytic pyrolysis of heavy oil and observed that the addition of a THF donor in the catalytic reaction further reduced the viscosity. Furthermore, a recent density functional theory (DFT) study [
6] reported the catalytic activity of several transition metal ions in the hydrothermal degradation of heavy oil; they found the order of catalytic activity as Cu
2+ > Co
2+ ≈ Ni
2+ > Fe
2+, with Cu
2+ exhibiting the highest catalytic activity. In our recent theoretical study on the catalytic activity of Cu-doped SBA-15 molecular sieves, we found that the decomposition of organic sulfur compounds can be promoted by doping with Cu [
7]. Moreover, research [
8] has shown that Fe, Ca, Zn, Ni, and other metal oxides and chloride expedite the pyrolysis of oil shale, facilitate the generation of hydrogen-free radicals, and cause the shale oil to become lighter. Lu et al. [
9] used MgO as a catalyst to investigate the catalytic pyrolysis of Changji oil shale in Xinjiang and found that the addition of MgO increased the yields of olefins and shale gas CH
4 in shale oil. Wang et al. [
8] reported that Fe
2O
3 has a better catalytic effect on oil shale pyrolysis as it increases the yield of shale oil. Esen et al. [
10] studied the catalytic pyrolysis of Turkish oil shale and found that the liquefaction rate was 23.4% at a concentration of 3% MoO
3, and that the catalytic effect of MoO
3 was better than that of Fe
2O
3. Given the effectiveness of metal oxides in oil shale cracking and the favorable catalytic properties of Cu-based catalysts in heavy oil cracking, CuO could possibly serve as an effective catalyst for oil shale cracking. Nevertheless, research on the specific catalytic effect of CuO on oil shale cracking is limited and the interaction mechanism between CuO and oil shale remains unclear.
The pyrolysis of kerogen in oil shale is highly complex because of its macromolecular organic structure. Therefore, elucidating the catalytic pyrolysis mechanism for oil shale is challenging. The application of molecular simulation technology can offer insights into the intricate changes that occur during catalytic transformation of complex molecular systems and provide a novel understanding of the underlying reaction mechanism. Guo et al. [
11] utilized DFT and found that the decomposition process of H
2O
2 on CuO and Ag-doped CuO surfaces follows the sequence of H
2O
2 → 2OH → H
2O + O, with the decomposition of H
2O
2 being comparatively easier on the Ag-doped CuO surface than on the pure CuO surface. Ahmad et al. [
12] employed dispersive-corrected Hubbard DFT to demonstrate that the decomposition of H
2O into OH and H occurs on the surface of CuO(111), with the oxygen-preadsorbed surface exhibiting the lowest reaction energy. Ye et al. [
13] employed ab initio quantum chemistry to elucidate the mechanism of the thermochemical reactions between organic carbon and H radicals during oil shale retorting. Tian et al. [
14] investigated the impact of electric fields on the macromolecular structure of kerogen at the M06-2X/6-311+G(d,p) level using DFT and established different reaction pathways. Chen et al. [
15] employed quantum chemical simulations and kinetic analysis to investigate the formation mechanism of nitrogen-containing substances during the pyrolysis of oil shale and obtained the energies of reactants, transition states, and intermediates involved in the reaction. Despite these studies, limited research has been conducted on the catalytic cracking mechanism of oil shale at the molecular level.
Kang et al. [
16] reported that the rupture of heteroatomic bonds is crucial for increasing the cracking rate of kerogen. They found that FeCl
3 promotes the rupture of heteroatomic bonds in kerogen and ring-opening reaction of aromatic structures, and that the rupture of heteroatomic bonds triggers a chain reaction network for the cracking of kerogen. To understand the catalytic pyrolysis mechanism of macromolecular aggregates, such as kerogen, the breaking of heteroatomic bonds in kerogen induced by catalysts must be investigated. The principal heteroatoms in kerogen are N and S, with the S atoms existing primarily as sulfone and sulfoxide. Dimethyl sulfoxide (C
2H
6OS) is a commonly used sulfoxide. In a recent study [
7], we selected C
2H
6OS as a model molecule for organic sulfur in oil shale and investigated its interaction with SBA-15 molecular sieves. To further investigate the interaction mechanisms between organic sulfur compounds in oil shale and different types of catalysts, the present study also employed C
2H
6OS as the model molecule. The interactions of C
2H
6OS with nano-CuO were examined using the DFT to determine the adsorption configuration, adsorption energy, and pyrolysis activation energy; additionally, the mechanism of the catalytic pyrolysis of C
2H
6OS by nano-CuO and activities of surface modifications of nano-CuO were studied. These theoretical findings are expected to provide valuable guidance for the design and development of catalysts for oil shale pyrolysis under specific conditions. Moreover, further comprehensive investigations are necessary to explore the thermal stability of C
2H
6OS, considering the potential risk of explosions associated with its thermal decomposition. Thus, the results of this study also contribute valuable evaluation data regarding the thermal stability of C
2H
6OS in the presence of incompatible substances.
2. Computational Method
The DMol
3 software package [
17] was utilized for all DFT calculations, with the electron exchange and correlation energy calculated using the Perdew–Wang (PW91) functional [
18] based on the generalized gradient approximation (GGA) [
19]. All electron double numerical (DND) [
20] basis sets that include a d-type polarized function on all non-hydrogen atoms were utilized. Numerical basis sets are well-known for their ability to minimize or eliminate basis set superposition errors (BSSE) [
21] and are thus highly valuable for the investigation of chemical reactions. Given that the CuO bulk possesses an antiferromagnetic ground state, the spin was not restricted in the calculations [
22,
23]. According to the literature [
24], the DFT semi-core pseudopot (DSPP) [
25] was utilized for the treatment of Cu atoms so that the electron behavior of Cu atoms can be described more accurately. Additionally, to ensure precise depiction of the van der Waals (VDW) interactions, the DFT-D
3 method developed by Grimme et al. [
26] was employed for correction of dispersion forces. The Broyden–Fletcher–Goldfarb–Shanno (BFGS) method [
27] was employed for structure relaxation. The transition states of each reaction were studied using the linear/quadratic synchronous transit (LST/QST) method [
28] and were verified through frequency analysis. The Brillouin region was integrated using a 3 × 3 × 1 k point grid sampled by the Monkhorst–Pack scheme. The cut-off value for real space was set to 4.2 Å. The convergence standard of optimization was that the total energy change of two adjacent steps in the optimization process was less than 2 × 10
−5 eV, the energy gradient was less than 0.004 eV/Å, and the atomic coordinate change was less than 0.005 Å.
Slab periodic models are commonly used to investigate surface reactions on catalysts or materials. A slab periodic model of a CuO(111) surface was adopted in view of its thermodynamically stability [
29] in this work. A 4 × 2 supercell was used on the side plane with a thickness of 9 layers. The model comprises a total of 64 atoms (Cu
32O
32), with a vacuum layer thickness of 15 Å. All atoms were relaxed in all of the geometry optimization calculations.
To determine the adsorption energy, the formula
Eads =
Emol +
Eslab −
E(mol+slab) was employed, where
Emol denotes the energy of the C
2H
6OS molecule in a cubic cell with dimensions of 15 Å × 15 Å × 15 Å,
Eslab represents the minimum energy of the CuO(111) surface, and
E(mol+slab) corresponds to the total energy of the CuO(111) surface in conjunction with the C
2H
6OS molecule. Based on this definition, the positive adsorption energy is associated with a stable adsorption phenomenon [
30].
3. Results and Discussion
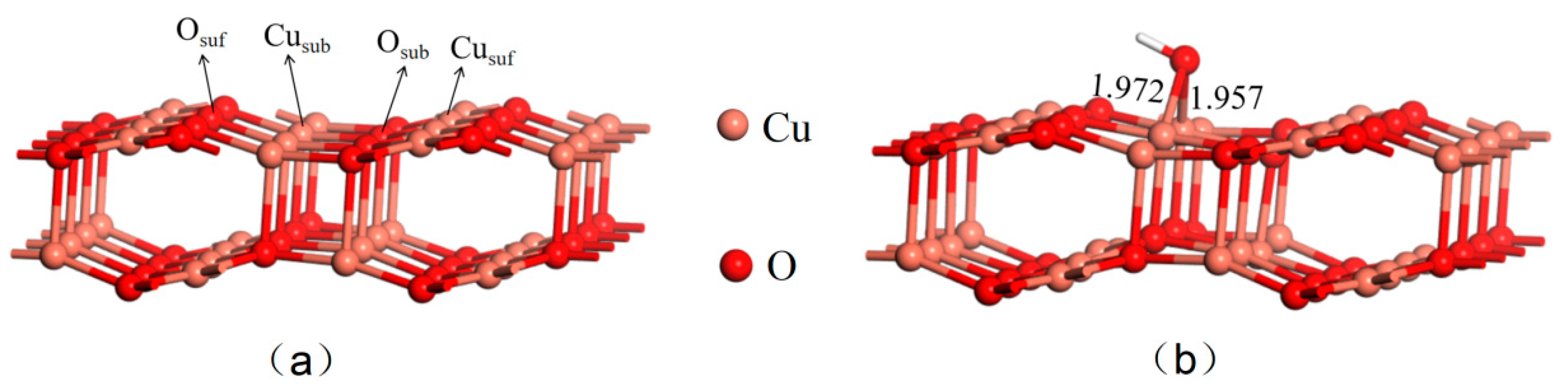
Two reaction environments were constructed in this work to account for the possible surface groups present on the CuO surface when preparing nano-CuO using Cu(OH)
2, as reported in the literature [
4], and the complex conditions encountered when CuO is used for underground in situ catalysis, specifically, the pure CuO(111) (a) and OH pre-adsorbed CuO(111) (b) surfaces, as shown in
Figure 1 which displays the optimized structures for both surfaces. The pure CuO(111) surface contains four unique adsorption sites known as “Cu
suf”, “Cu
sub”, “O
suf”, and “O
sub”. Among these, “Cu
suf” and “O
suf” are located in the outermost layer, whereas “Cu
sub” and “O
sub” are situated in the subouter layer. Moreover, the outermost layer’s Cu
sub atoms are tetra-coordinated, while the subouter layer’s Cu
sub atoms are tri-coordinated. The outermost layer’s O
suf atoms are tri-coordinated, and the subouter layer’s O
sub atoms are tetra-coordinated.
3.1. Adsorption Structures and Energies of C2H6OS on the Pure CuO(111) Surface
The C
2H
6OS molecule exhibited a strong affinity toward the Lewis acid site, i.e., the Cu ion on the CuO surface, wherein the O and S atoms in the molecule were responsible for anchoring the adsorbate onto the surface. Four types of adsorption on a pure CuO(111) surface are known, wherein the S and O atoms of the molecule are in proximity to the Cu
suf and Cu
sub atoms, as shown in
Figure 2. For ease of discussion, the notation “DS” is employed to denote the stable configuration for C
2H
6OS adsorbed on the pure CuO(111) surface.
In
Figure 2, DS-1 and DS-2 correspond to the stable structures of the O and S atoms adsorbed on Cu
suf, whereas DS-3 and DS-4 depict the stable structures of the O and S atoms adsorbed on Cu
sub, respectively. Notably, the adsorption energies presented in
Figure 2 suggest that the interaction of the S atom in C
2H
6OS molecule with the Cu atom on the surface is stronger than that of the O atom with the surface Cu atom. Moreover, the Cu atom in the subouter layer exhibited stronger adsorption affinity compared with the outermost Cu atom, thus suggesting that the subouter-layer Cu atom plays a crucial role in the adsorption process. The interaction between the S atom in the molecule and Cu atom on the subouter layer of the surface formed the most stable adsorption structure (DS-4). The bond length between S and Cu in DS-4 was 2.326 Å, and the corresponding adsorption energy was 31.3 kcal/mol, thus indicating a chemical adsorption behavior. The second most stable structure (DS-3) involved the adsorption of an O atom onto a Cu
sub atom, with an O–Cu bond length of 1.999 Å and adsorption energy of 28.3 kcal/mol. The adsorption structures formed by the S and O atoms with the Cu
suf atoms (i.e., DS-1 and DS-2) had longer S–Cu and O–Cu bond lengths (2.579 and 2.370 Å, respectively) than those formed by the Cu
sub atoms, thus indicating weaker interactions. The adsorption energies for DS-1 and DS-2 were 20.2 and 18.8 kcal/mol, respectively, which are lower than those for the structures formed with the subouter Cu atoms.
3.2. Adsorption Structures and Energies of C2H6OS on the OH Pre-Adsorbed CuO(111) Surface
The adsorption of C
2H
6OS on the OH pre-adsorbed CuO(111) surface occurred on the Lewis acid sites via the O and S atoms in the molecule. This study focused primarily on the C
2H
6OS molecules in the vicinity of OH, essentially resulting in four types of adsorption. These include the adsorption of the S and O atoms in the molecule close to the Cu
suf atoms, and those close to the Cu
sub atoms near the OH on the surface, as depicted in
Figure 3. To facilitate the description, “DS-OH” is used to represent the stable adsorption structure of C
2H
6OS on the OH preadsorbed CuO(111) surface.
In
Figure 3, DS-OH-1 and DS-OH-2 represent the stable adsorption configurations of S and O atoms in the C
2H
6OS molecules adsorbed with Cu
suf atoms, whereas DS-OH-3 and DS-OH-4 correspond to the stable adsorption configurations of O and S atoms adsorbed with Cu
sub atoms near OH. As illustrated in
Figure 3, little difference was noted in the adsorption energies for C
2H
6OS on the OH preadsorbed CuO(111) surface compared with the pure CuO(111) surface regardless of the type of adsorption, thus indicating that the preadsorption of OH does not significantly affect the adsorption of C
2H
6OS molecules on the CuO(111) surface. The most stable adsorption structure (DS-OH-4) was characterized by the adsorption of S atoms in the molecule and Cu
sub atoms near the surface OH. Notably, the S–Cu bond length in DS-OH-4 was 2.299 Å, which is shorter than that on the pure surface (2.326 Å). Moreover, the corresponding adsorption energy for DS-OH-4 was 32.9 kcal/mol, which is higher than that on the pure surface. The second most stable structure (DS-OH-3) corresponds to the adsorption of the O atom in the molecule and the Cu
sub atom near the surface OH. In particular, the O–Cu bond length in DS-OH-3 was 2.042 Å, and the corresponding adsorption energy was 28.5 kcal/mol, which is almost equal to that of the pure surface.
To further understand the interaction between C
2H
6OS and the pure or OH pre-adsorbed CuO(111) surface, the electronic structures of the most stable adsorption configurations, DS-4 and DS-OH-4, were analyzed. The partial densities of states (PDOS) of these configurations before and after C
2H
6OS adsorption are shown in
Figure 4. Evidently, both the s-PDOS and p-PDOS of S in C
2H
6OS exhibited a leftward shift, with the p-PDOS of S moving to a lower energy compared with the initial configuration. Moreover, after adsorption, the S p-PDOS interacted with the Cu p-PDOS at −2.09 and −4.99 eV on the pure CuO(111) surface, thus implying the presence of strong interactions between C
2H
6OS and the pure CuO(111) surface. In the case of OH preadsorbed CuO(111) surface, the S p-PDOS interacted with the Cu p-PDOS by state overlap at −3.16 and −5.03 eV, as shown in
Figure 4b. These energies correspond to the S–Cu bonds in DS-OH-4. The characteristic peaks of the p orbitals near the Fermi level became wider after adsorption, thus indicating a significant delocalization of the p orbitals of the S atom in C
2H
6OS.
3.3. Decomposition of C2H6OS on the Pure and OH Pre-Adsorbed CuO(111) Surfaces
3.3.1. Decomposition of C2H6OS on the Pure CuO(111) Surface
As illustrated in
Figure 2, DS-4 was the most stable configuration. Accordingly, DS-4 served as the initial state for subsequent analyses. C
2H
6OS contains three types of bonds, namely C–H, C–S, and S=O. Because double bonds possess a higher bond energy compared with single bonds, this study focused primarily on the breaking of C–H and C–S bonds. First, from the DS-4 configuration, numerous possible C–H and C–S bond breaks were explored to identify transition states. Subsequently, the activation energies associated with each reaction pathway were compared to determine the preferentially broken bonds, and three main reaction pathways were identified.
Table 1 lists the intermediate relative energies, reaction energy barriers, and reaction heats of the three main reaction pathways. The structures of the intermediates, transition states, and products of each pathways are shown in
Figure S1.
Evidently from
Table 1, on the pure CuO(111) surface, the transfer of an H atom from C
2H
6OS to the O
suf site had a lower energy barrier (33.9 kcal/mol) compared with the C–H bond energies (96–99 kcal/mol) reported in the literature [
31,
32]. Note that, if a -CH
3 group of C
2H
6OS transfers to the O
suf and Cu
sub sites, the energy barriers were 58.2 and 59.8 kcal/mol, respectively. Therefore, when C
2H
6OS molecules were adsorbed onto the pure CuO(111) surface, the H atom in C
2H
6OS was preferentially transferred to the O
suf site to generate C
2H
5OS and H. The C–H bond rupture occurred via a seven-center transition state (TS1). The S and C atoms in the intermediate C
2H
5OS (see IM2 in
Figure 5) formed stable chemical bonds with the surface Cu atoms, with bond lengths of 2.261 and 2.003 Å, respectively, and the H atoms formed a hydroxyl group with the surface O atoms. The intermediate state, transition state, and product structures of the process are shown in
Figure 5; the energy path of the reaction is shown in
Figure 6.
The intermediate C
2H
5OS (IM2 in
Figure 5) underwent further C–H and C–S bond cleavage on the pure CuO(111) surface. Various possibilities for C–H and C–S bond cleavage were considered; however, no stable configuration with C–H bond cleavage was identified. Instead, two dissociative structures resulting from the C–S bond cleavage were observed. These structures involve the -CH
2 group attacking either the O
suf of the surface OH or the Cu
sub atom near the OH, and thereby resulting in C–S bond cleavage (
Figure S2). The Gibbs free energy changes at room temperature for these two processes were calculated and are listed in
Table 2. The results reveal that the transfer of -CH
2 to the O
suf site represents a thermodynamically stable reaction with a −11.4 kcal/mol change in Gibbs free energy. By contrast, if -CH
2 is transferred to the Cu
sub site, then 26.4 kcal/mol energy must be overcome for the reaction to occur. Furthermore, the energy barriers for both processes were calculated and revealed that if -CH
2 is transferred to the O
suf site, then 38.1 kcal/mol through the transition state TS2 must be overcome, as shown in
Figure 6; this value is much lower than that reported (66 kcal/mol) [
31,
32] for a typical C−S bond breakage. Finally, if -CH
2 is instead transferred to the Cu
sub site, then 50.0 kcal/mol must be overcome, indicating that the transfer of -CH
2 from the IM2 intermediate state toward the O
suf site represents both a thermodynamically and kinetically favorable reaction path.
3.3.2. Decomposition Reaction of C2H6OS on the OH Pre-Adsorbed CuO(111) Surface
The most stable adsorption configuration of C
2H
6OS on the OH pre-adsorbed CuO(111) surface, denoted as DS-OH-4 in
Figure 3, served as the initial state for exploring the breaking of various C–H and C–S bonds in the molecule on the OH pre-adsorbed CuO(111) surface. Thus, five main reaction pathways were determined. The intermediate relative energies, reaction energy barriers, and reaction heats for each pathway are listed in
Table 3. The intermediates, transition states, and product structures involved in these pathways are shown in
Figure S3.
Figure 5 shows that transferring a H atom from C
2H
6OS to OH to form H
2O on the OH preadsorbed CuO(111) surface requires passing through a transition state, TS1′, and generates an intermediate species, IM2′. Evidently from
Table 3, the energy barrier for this process was only 20.0 kcal/mol, which is lower than the barriers for H transfers to the O
suf and O
sub sites to form C
2H
5OS, which were 27.2 and 40.5 kcal/mol, respectively. Moreover, transferring -CH
3 in C
2H
6OS to the Cu
suf or O
suf site incurs higher energy barriers (57.8 and 60.4 kcal/mol, respectively), as shown in
Table 3. Therefore, when a C
2H
6OS molecule is adsorbed onto the OH preadsorbed CuO(111) surface, the initial reaction is the cleavage of the C–H bond, thereby resulting in the generation of C
2H
5OS and H. The cleaved H then combined with the surface OH to form H
2O, whereas the S atom in the intermediate species C
2H
5OS formed a stable chemical bond with the Cu
sub atom on the surface, with a bond length of 2.281 Å (IM2′ in
Figure 5). Furthermore, the energy barrier of 20.0 kcal/mol for the C–H bond cleavage is approximately 15 kcal/mol lower than that on the pure CuO(111) surface. Therefore, the presence of preadsorbed OH significantly enhanced the dehydrogenation of C
2H
6OS compared with that on pure CuO(111) surfaces.
Furthermore, the C–H and C–S bond cleavage of the intermediate IM2′ on the OH preadsorbed CuO(111) surface was investigated. Three primary reaction pathways were identified: transfer of -CH2 from C2H5OS to Osuf atoms, and transfer of H atom to Osub or Osuf atoms. The calculations of Gibbs free energy changes revealed that transferring -CH2 to the Osuf site was thermodynamically stable and spontaneous, with a Gibbs free energy change of −17.0 kcal/mol, which is more negative than on the pure CuO(111) surface, thus indicating that the C–S bond cleavage trend on the OH-preadsorbed CuO(111) surface was greater. By contrast, transferring H to the Osub and Osuf sites resulted in positive Gibbs free energy changes of 10.3 and 16.7 kcal/mol, respectively, which must be overcome to achieve the reaction. The energy barrier for transferring -CH2 to the Osuf site was 19.3 kcal/mol through transition state TS2′, whereas transferring H had energy barriers of 28.0 and 43.9 kcal/mol to the Osub and Osuf sites, respectively. These results suggest that the transfer of -CH2 to the Osuf site is a favorable reaction pathway that is both thermodynamically and kinetically feasible. The energy barrier of this pathway is approximately 20 kcal/mol lower than that of a pure CuO(111) surface (38.1 kcal/mol). Thus, the presence of preadsorbed OH on the CuO(111) surface promotes the C–S bond cleavage of C2H6OS compared with the pure CuO(111) surface.
4. Conclusions
This study aimed to investigate the mechanism of CuO on oil shale and its surface modification from the atomic and molecular levels. Considering the complexity of the molecular structure and pyrolysis process of kerogen in oil shale, C2H6OS was used as a representative model molecule. Periodic models and first-principle calculations based on the DFT were used. Two distinct reaction environments were analyzed to compare the catalytic effects of different CuO(111) surfaces: pure and OH preadsorbed CuO(111) surfaces.
The findings herein indicate that C
2H
6OS interacts primarily with the Lewis acid site of both surfaces through O and S atoms, thus leading to strong chemical adsorption. On both surfaces, C
2H
6OS tended to break the C-H bond first, followed by the C-S bond, which is different from the order of bond energies for C-H and C-S bonds [
31,
32]. Notably, besides the stronger adsorption affinity of Cu
sub atoms towards molecules, as reported in the literature [
12], the O
suf atom demonstrated favorable reactivity, attracting both H and -CH
2 fragments formed by the decomposition of C
2H
6OS. On the pure CuO(111) surface, C
2H
6OS removed one H to O
suf first, followed by breakage of the C-S bond to remove one -CH
2 atom from O
suf. However, on the OH preadsorbed CuO(111) surface, C
2H
6OS initially removed one H to OH on the surface and then broke the C-S bond to remove one -CH
2 from O
suf. The activation energies for dehydrogenation and C-S bond cleavage were significantly lower, measuring 20.0 and 19.3 kcal/mol, which are 41% and 49% lower than those on the pure surface, respectively. These results suggest that the presence of OH greatly facilitates the surface decomposition of C
2H
6OS. They also indicate a potential risk of long-term storage of C
2H
6OS and CuO leading to hazardous conditions. These findings are helpful for understand the catalytic transformation mechanism of nano-CuO into oil shale and provide a theoretical basis for the design and synthesis of catalysts for the thermal transformation of oil shale. Additionally, they provide valuable data to evaluate the thermal hazards of C
2H
6OS when combined with incompatible substances. Future studies will explore alternative surface modification techniques, such as metal atom doping, to further enhance the catalytic activity of CuO in oil shale cracking.