Design and Optimization of the Insulation Performance of a 4000 m3 Liquid Hydrogen Spherical Tank
Abstract
1. Introduction
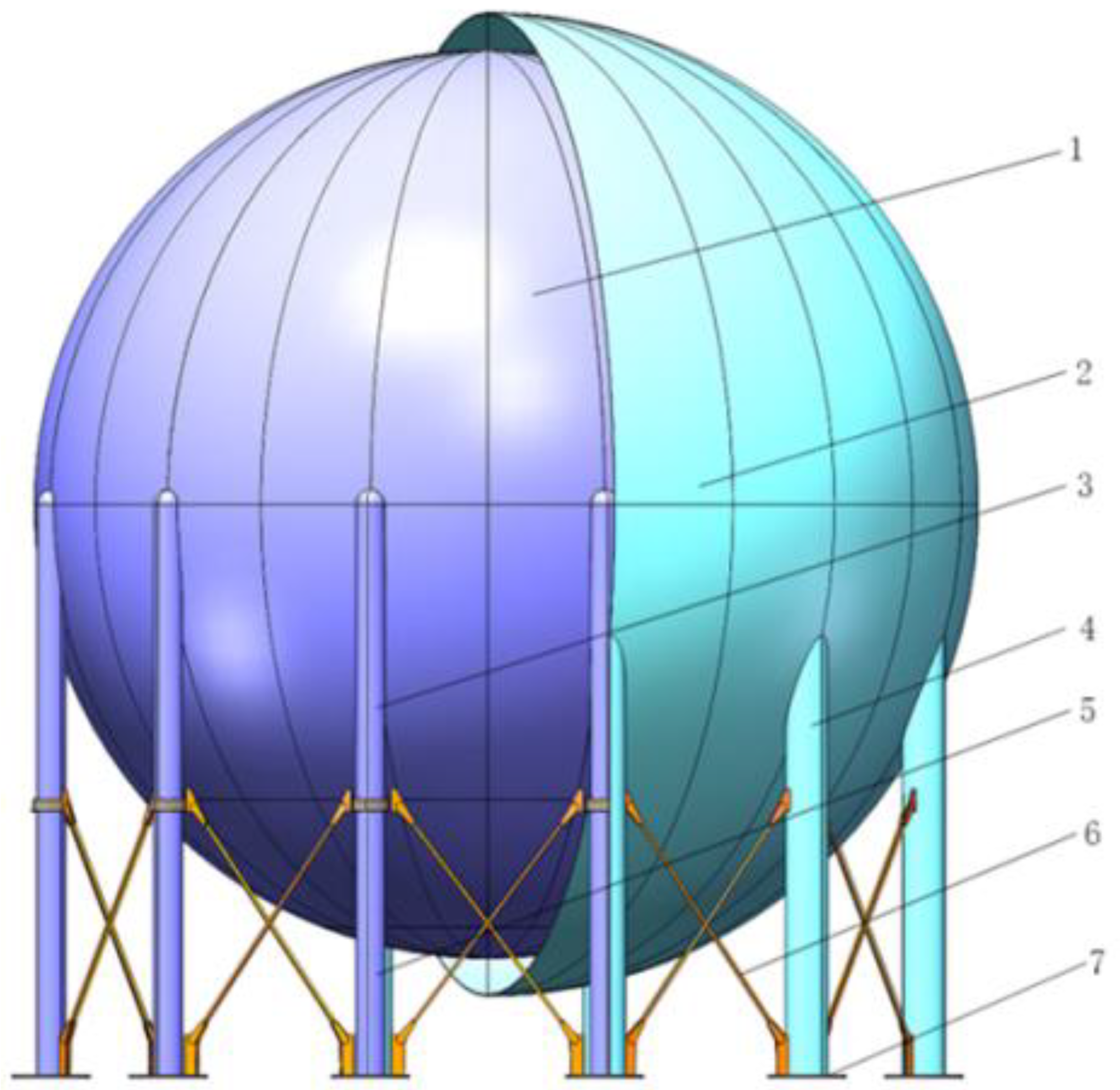
2. The 4000 m3 LH2 Tank Structure and Insulation Schemes
2.1. Physical Model of a 4000 m3 LH2 Tank
2.2. Design of Insulation Schemes
3. Development of Heat Transfer Model
3.1. Heat Transfer Model of MLI
- The spacer between two adjacent radiators has no effect on the radiation heat leakage;
- The spacer between two adjacent reflection shields is a finite space;
- The longitudinal conduction of the MLI is neglected, and overall conduction is assumed to be radial.
3.2. Heat Transfer Model of Single VCS
- (a)
- Between the VCS and MLI, there is no contact thermal resistance;
- (b)
- The cooling capacity of the VCS is totally used to absorb the heat;
- (c)
- The temperatures of both sides of the VCS are equivalent;
- (d)
- The heat leak into the inner tank through the support structure, pipeline, etc., is a constant value.
3.3. Heat Transfer Model of Double VCSs
3.4. Heat Transfer Model of LN2CS
3.5. Calculation of Daily Evaporation Rate
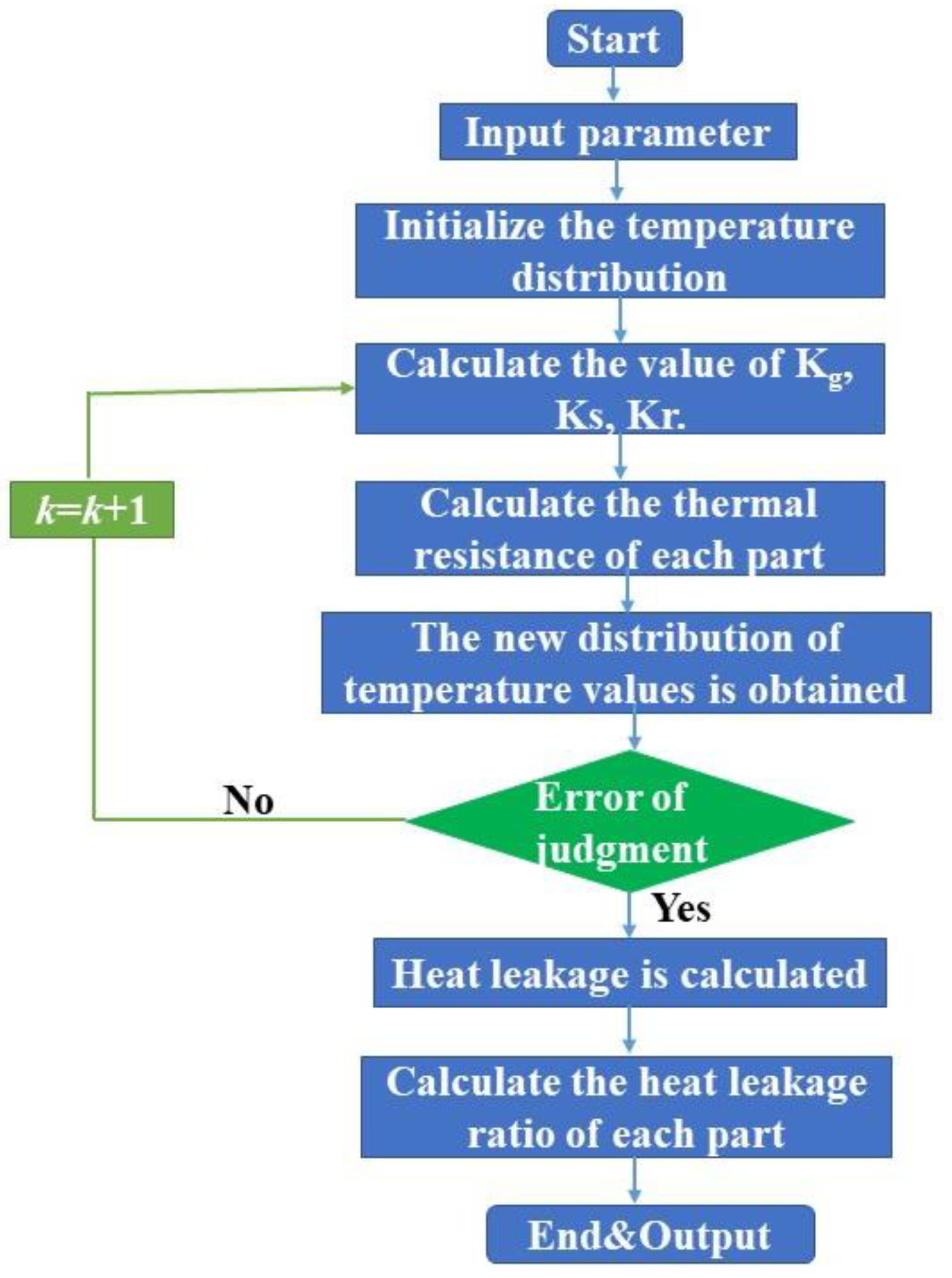
3.6. Solving Strategy of Heat Transfer Model
3.7. Verification of the Heat Transfer Model
4. Results and Discussion
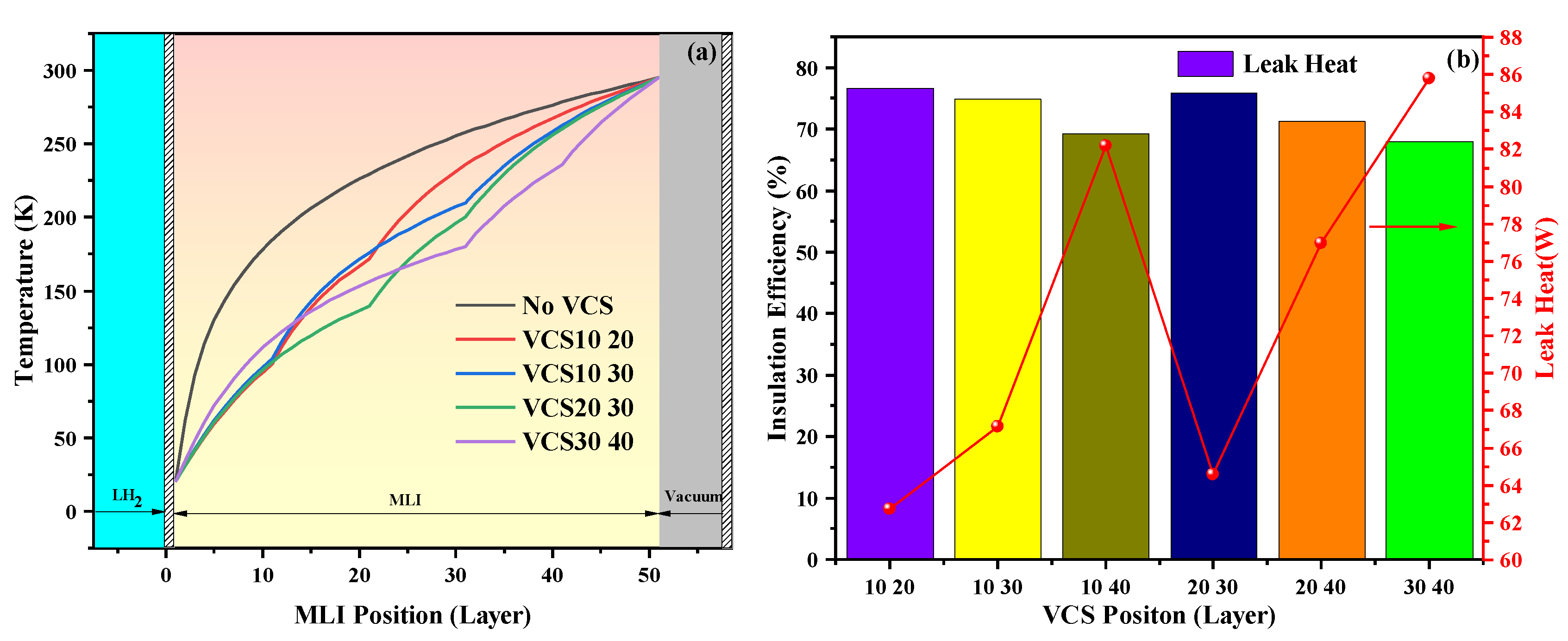
4.1. Comparison of Insulation Scheme
4.2. Percentage of Heat Leakage in MLI
4.3. Effect of Layer Number on MLI Performance
4.4. Effect of Ambient Temperature on MLI
4.5. Effect of Single VCS on the MLI
4.6. Effect of Double VCSs on MLI
4.7. Effect of LN2CS on MLI
5. Conclusions
- The insulation properties of HGMs and MLI is better than that of only vacuum insulation. When the pressure of the vacuum interlayer is less than 3.34 Pa, the insulation performance of MLI is better than that of HGMs. When the vacuum degree is in the range of 3.34 to 133.69 Pa, the insulation performance of HGMs is better than MLI.
- The daily evaporation rate of LH2 is increased by 1.82 times when the external environment temperature increases from 230K to 310K. Considering the cost, there is an optimal layer number of the MLI. As the number layer of MLI increases, the heat leakage first drops, then gradually slows. The optimal value is 50 layers for a 4000 m3 LH2 spherical tank.
- The minimum heat leakage of P-VCSs can be improved by 76.6% compared with that of No VCS. The heat leakage through the MLI will further decrease when S-VCSs is used. The minimum heat leakage of S-VCSs is 83.79%, 25.67% and 30.75% that of No VCS, VCS10 and P-VCS, respectively. The maximum insulation efficiency can be improved by 7.16% and 43.26% compared with that of VCS10 and P-VCS, respectively;
- Considering the comprehensive cost analysis, the optimal installation scheme is obtained when the LN2CS is installed at the 40th layer; consumption is decreased to less than 10W. The insulation efficiency is 96.05% higher than that of No VCS.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature
| LH2 | Liquid hydrogen |
| VCS | Vapor-cooled shield |
| LN2CS | Liquid-nitrogen-cooled shield |
| CS | Cooled shield |
| HV-MLI | High-vacuum multilayer insulation |
| VDMLI | Variable density multilayer insulation |
| HGMs | Hollow glass microspheres |
| H2 | Hydrogen |
| LO2 | Liquid oxygen |
| LN2 | Liquid nitrogen |
| No VCS | Without VCS |
| P-VCS | Parallel VCS |
| S-VCS | Series VCS |
| MLI | Multilayer insulation |
References
- Wu, Q.L.; Li, C.X. Economy-environment-energy benefit analysis for green hydrogen based integrated energy system operation under carbon trading with a robust optimization model. J. Energy Storage 2022, 55, 105560. [Google Scholar] [CrossRef]
- Buffi, M.; Prussi, M.; Scarlat, N. Energy and environmental assessment of hydrogen from biomass sources: Challenges and perspectives. Biomass Bioenergy 2022, 165, 106556. [Google Scholar] [CrossRef]
- Yang, W. Structural Statics and Dynamics Analysis of Large Vacuum Powder Insulation Cryogenic Spherical Tank; Lanzhou University of Technology: Lanzhou, China, 2021. [Google Scholar]
- Domashenko, A.; Golovchenko, A.; Gorbatsky, Y. Production, storage and transportation of liquid hydrogen. Experience of infrastructure development and operation. Int. J. Hydrogen Energy 2002, 27, 753–755. [Google Scholar] [CrossRef]
- Krenn, A.G. Diagnosis of a poorly performing liquid hydrogen bulk storage sphere. In Proceedings of the Transactions of the Cryogenic Engineering Conference (CEC), American Institute of Physics, Spokane, WA, USA, 13–17 June 2011; Volume 57. [Google Scholar]
- Chen, C.K. Development of LH2 Storage Tank for Liquid Hydrogen Transport Tank Wagon; Harbin Institute of Technology: Harbin, China, 2014; pp. 26–34. [Google Scholar]
- Wang, F. Structural Design of Large Liquid Hydrogen Storage Tank; Southeast University: Nan Jing, China, 2006; pp. 21–28. [Google Scholar]
- Liu, Z.; Li, Y.Z.; Zhou, G. Study on thermal stratification in liquid hydrogen tank under different gravity levels. Int. J. Hydrogen Energy 2018, 43, 9369–9378. [Google Scholar] [CrossRef]
- Rong, Y. Application of new thermal insulation materials in thick oil injection steam pipeline insulation. Mater Rep. 2020, 34, 5. [Google Scholar]
- Deng, B.C.; Yang, S.Q.; Xie, X.J.; Wang, Y.; Pan, W.; Li, Q.; Gong, L. Thermal performance assessment of cryogenic transfer line with support and multilayer insulation for cryogenic fluid. Appl. Energy 2019, 250, 895–903. [Google Scholar] [CrossRef]
- Monkam, L.K.; von Schweinitz, A.G.; Friedrichs, J.; Gao, X. Feasibility analysis of a new thermal insulation concept of cryogenic fuel tanks for hydrogen fuel cell powered commercial aircraft. Int. J. Hydrogen Energy 2022, 47, 31395–31408. [Google Scholar] [CrossRef]
- Joseph, J.; Agrawal, G.; Agarwal, D.K.; Pisharady, J.C.; Kumar, S.S. Effect of insulation thickness on pressure evolution and thermal stratification in a cryogenic tank. Appl. Therm. Eng. 2017, 111, 1629–1639. [Google Scholar] [CrossRef]
- Peterson, P. The Heat-Tight Vessel; University of Lund, Office of Naval Intelligence Translation: Lund, Sweden, 1953. [Google Scholar]
- Yu, Y.; Zhu, M.; Chen, S.P.; Gu, C.L.; Zhang, B.; Huang, Y.W. Study on the behavior of hydrogen removal materials to maintain high vacuum in high vacuum multilayer insulation equipment. Mater Rep. 2021, 35, 5. [Google Scholar]
- Martin, J.J.; Hastings, L. Large-Scale Liquid Hydrogen Testing of a Variable Density Multilayer Insulation with a Foam Substrate; Marshall Space Flight Center: Huntsville, AL, USA, 2001. [Google Scholar]
- Bapat, S.L.; Narayankhedkar, K.G.; Lukose, T.P. Experimental Investigation of Multilayer Insulation. Cryogenics 1990, 30, 711–719. [Google Scholar] [CrossRef]
- Sun, P.J.; Wu, J.Y.; Zhang, P.; Jiang, M.L.; Xu, L. Experimental study of the effect of gas heat transfer on the thermal performance of multilayer insulation. Cryog. Supercond. 2008, 11, 6–12. [Google Scholar]
- Li, F.J. Research on the Thermal Insulation Performance of Composite Multilayer Materials; Zhejiang University: Hangzhou, China, 2017. [Google Scholar]
- Ratnakar, R.R.; Sun, Z.; Balakotaiah, V. Effective Thermal Conductivity of Insulation Materials for Cryogenic LH2 Storage Tanks: A review. Int. J. Hydrogen Energy 2023, 48, 7770–7793. [Google Scholar] [CrossRef]
- Wang, B.; Luo, R.Y.; Chen, H.; Zheng, C.; Gao, Y.; Wang, H.; Hashmi, A.R.; Zhao, Q.; Gan, Z. Characterization and Monitoring of Vacuum Pressure of Tank Containers with Multilayer Insulation for Cryogenic Clean Fuels Storage and Transportation. Appl. Therm. Eng. 2021, 187, 116569. [Google Scholar] [CrossRef]
- Zheng, J.P.; Chen, L.B.; Wang, J.J.; Zhou, Y.; Wang, J. Thermodynamic modelling and optimization of self-evaporation vapor cooled shield for liquid hydrogen storage tank. Energy Convers. Manag. 2019, 184, 74–82. [Google Scholar] [CrossRef]
- Jiang, W.B.; Zuo, Z.Q.; Sun, P.J.; Li, P.; Huang, Y. Thermal analysis of coupled vapor-cooling-shield insulation for liquid hydrogen-oxygen pair storage. Int. J. Hydrogen Energy 2022, 12, 47. [Google Scholar] [CrossRef]
- Jiang, W.B.; Sun, P.J.; Li, P.; Zuo, Z.; Huang, Y. Transient thermal behavior of multi-layer insulation coupled with vapor cooled shield used for liquid hydrogen storage tank. Energy 2021, 231, 120859. [Google Scholar] [CrossRef]
- Jiang, W.B.; Zuo, Z.Q.; Huang, Y.H.; Wang, B.; Sun, P.; Li, P. Coupling optimization of composite insulation and vapor-cooled shield for on-orbit cryogenic storage tank. Cryogenics 2018, 96, 90–98. [Google Scholar] [CrossRef]
- Zheng, J.P.; Chen, L.B.; Xu, X.F.; Guo, L.; Zhou, Y.; Wang, J. A novel insulation system based on active cooling without power input for liquid hydrogen storage. Energy 2019, 182, 1–10. [Google Scholar] [CrossRef]
- Yang, Y.L.; Jiang, W.B.; Huang, Y.H. Experiment on transient thermodynamic behavior of a cryogenic storage tank protected by a composite insulation structure. Energy 2023, 270, 126929. [Google Scholar] [CrossRef]
- Zheng, J.P.; Chen, L.B.; Wang, J.J.; Xi, X.; Zhu, H.; Zhou, Y.; Wang, J. Thermodynamic analysis and comparison of four insulation schemes for liquid hydrogen storage tank. Energy Convers. Manag. 2019, 186, 526–534. [Google Scholar] [CrossRef]
- De Blasio, C. Preliminary Concepts. In Fundamentals of Biofuels Engineering and Technology; Green Energy and Technology; Springer: Cham, Switzerland, 2019; pp. 337–340. [Google Scholar]
- Niu, W.C.; Lin, Y.L.; Ju, Y.A. The daily evaporation rate test and conversion method for a new independent type B LNG mock-up tank. Cryogenics 2020, 111, 103168. [Google Scholar] [CrossRef]
- McIntosh, G.E. Layer by Layer MLI Calculation Using a Separated Mode Equation. Adv. Cryog. Eng. 1994, 39, 1683–1690. [Google Scholar]
- Tao, W.Q. Numerical Heat Transfer, 2nd ed.; Xi’an Jiaotong University Press: Xi’an, China, 2005. [Google Scholar]
- Zheng, J.P. Experimental Study on Ground Simulation of Composite Adiabatic System for Liquid Oxygen Storage; University of Chinese Academy of Sciences: Beijing, China, 2016. [Google Scholar]
- Fesmire, J.E. Standardization in Cryogenic Insulation Systems Testing and Performance Data. Phys. Procedia 2015, 67, 1089–1097. [Google Scholar] [CrossRef]
- Allen, M.S.; Baumgartner, R.G.; Fesmire, J.E.; Augustynowicz, S.D. Advances in microsphere insulation systems. Conf. Proc. 2004, 710, 619. [Google Scholar]
- Wang, P.; Liao, B.; An, Z.G.; Yan, K.; Zhang, J. Measurement and calculation of cryogenic thermal conductivity of HGMs. Int. J. Heat Mass Transf. 2018, 129, 591–598. [Google Scholar] [CrossRef]
















| LN2CS Position (Layer) | Heat Leakage (W) | Daily Evaporation Rate (%) | Absorbed Heat /W | LN2 Consumption (m3/day) |
|---|---|---|---|---|
| 1 | 368.73 | 2.79 × 10−3 | −105.70 | — |
| 5 | 74.21 | 5.62 × 10−4 | 212.28 | 0.114 |
| 8 | 46.64 | 3.53 × 10−4 | 260.37 | 0.140 |
| 10 | 37.45 | 2.84 × 10−4 | 284.95 | 0.154 |
| 15 | 25.20 | 1.91 × 10−4 | 343.35 | 0.185 |
| 20 | 19.07 | 1.44 × 10−4 | 410.0 | 0.221 |
| 25 | 15.40 | 1.17 × 10−4 | 500.78 | 0.270 |
| 30 | 13.31 | 1.01 × 10−4 | 168.42 | 0.091 |
| 35 | 11.77 | 8.91 × 10−5 | 848.77 | 0.457 |
| 40 | 10.57 | 8.01 × 10−5 | 1280.35 | 0.690 |
| 45 | 9.61 | 7.28 × 10−5 | 2572.42 | 1.39 |
| 49 | 8.97 | 6.79 × 10−5 | 12,902.91 | 6.95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Y.; Xie, F.; Zhu, M.; Yu, S.; Li, Y. Design and Optimization of the Insulation Performance of a 4000 m3 Liquid Hydrogen Spherical Tank. Processes 2023, 11, 1778. https://doi.org/10.3390/pr11061778
Yu Y, Xie F, Zhu M, Yu S, Li Y. Design and Optimization of the Insulation Performance of a 4000 m3 Liquid Hydrogen Spherical Tank. Processes. 2023; 11(6):1778. https://doi.org/10.3390/pr11061778
Chicago/Turabian StyleYu, Yang, Fushou Xie, Ming Zhu, Shuai Yu, and Yanzhong Li. 2023. "Design and Optimization of the Insulation Performance of a 4000 m3 Liquid Hydrogen Spherical Tank" Processes 11, no. 6: 1778. https://doi.org/10.3390/pr11061778
APA StyleYu, Y., Xie, F., Zhu, M., Yu, S., & Li, Y. (2023). Design and Optimization of the Insulation Performance of a 4000 m3 Liquid Hydrogen Spherical Tank. Processes, 11(6), 1778. https://doi.org/10.3390/pr11061778







