Abstract
Formic acid is an intermediate of the steam methane reforming process for hydrogen production. According to International Standard ISO 14687, the amount fraction level of formic acid present in the hydrogen supplied to fuel cell electric vehicles must not exceed 200 nmol·mol−1. The development of formic acid standards in hydrogen is crucial to validate the analytical results and ensure measurement reliability for the fuel cell electric vehicles industry. NPL demonstrated that these standards can be gravimetrically prepared and validated at 4 to 100 µmol·mol−1, with a shelf-life of 1 year (stability uncertainty < 7%; k = 2). Stability was not affected over 1 year or by low temperature or pressure. At sub-µmol·mol−1 level, formic acid amount fraction was found to decrease due to adsorption on the gas cylinder surface; however, it is possible to certify the formic acid amount fraction after a period of 20 days and ensure the certified value validity for 1 year with an uncertainty below 7% (k = 1) confirmed by thermodynamic investigation. This study demonstrated that formic acid in hydrogen gas reference materials can be prepared with reasonable uncertainty (>7%, k = 1) and shelf life (>1 year). Potential applications include the calibration of analysers and for studying the impact of formic acid on future application with relevant traceability and accuracy.
1. Introduction
Hydrogen demand reached 94 million tonnes (Mt) in 2021, corresponding to approximately 2.5% of global final energy consumption [1]. Current projections share a relevant increase in hydrogen demand, resulting in a global hydrogen share of 4–11% of final energy consumption in 2050 [2]; However, most of the hydrogen demand in 2021 was met by hydrogen produced from unabated fossil fuels without the benefit towards mitigating climate change. The pipeline of projects to produce low emission hydrogen, however, is growing at an impressive rate, and by 2030, the production of low-emission hydrogen could reach 16–24 Mt per year, with 9–14 Mt based on electrolysis and 7–10 Mt on fossil fuels with CCUS [1]. Despite recent government incentives and support towards electrolyser deployment, a significant part of the low-emission hydrogen will still be from fossil fuels with CCUS in the foreseeable future.
Formic acid is an intermediate of the steam methane reforming process [3], which is currently the prevalent method for producing hydrogen. It may also be part of the hydrogen fuel impurities originating from biomass [4], which is one of the future low-emission hydrogen production methods. Moreover, recent studies suggested that formic acid could be a source of hydrogen [5] or used as a hydrogen carrier [6] or as a way to coproduce hydrogen and formic acid without CO2 emissions [7]. Depending on the future development in production and storage, formic acid may be present at low amount fraction in hydrogen. Without appropriate purification steps downstream of the process, formic acid may end up in the hydrogen product. The presence of trace amounts fraction of formic acid in hydrogen gas can be an issue as, for example, hydrogen fuel cell vehicles have strict requirements in terms of hydrogen purity [8]. These requirements include formic acid as one of the 14 gaseous impurities that should be monitored according to the international standards ISO 14687 [5], EN17124 [9], and SAE J2719 [10]. If present in hydrogen fuel, formic acid will have very weak but discernible poisoning effect [3,11], and it will reduce the lifetime of fuel cells [12]. It is therefore crucial for users and producers that formic acid in hydrogen is measured accurately. For example, in Europe, European Directive 2014/94/EU [13] requires the hydrogen supplier or system integrator to obtain the evidence proving that their hydrogen is of suitable purity (compliance with ISO 14786) for fuel cell electrical vehicle. The maximum limit for the amount fraction of formic acid is set at 200 nmol mol−1 in ISO 14687, which represents an analytical challenge.
Several literature reviews [14,15,16] compiled state-of-the-art gas analysis methods for performing purity analysis of fuel-cell hydrogen. According to these reviews, only Fourier-transform infrared (FT-IR) spectroscopy analysers have been reported for performing measurements of formic acid in hydrogen in ASTM standard [17]. There are several other candidate methods for performing this measurement, including gas chromatography coupled with mass spectrometry, chromatography coupled with flame ionisation detector, chromatography coupled pulse discharge helium ionisation detector, ion chromatography, selected ion flow tube mass spectrometry (SIFT-MS), or cavity ringdown spectroscopy, but there appears to be limited literature available indicating that these methods have been validated specifically for the purpose of hydrogen purity analysis [14]. The development and the validation of a new analytical methods requires the use of a calibrant or reference materials (RMs) in hydrogen to determine if the method is fit-for-purpose, especially regarding interferences or trueness, which can be affected by the matrix. To achieve this, the formic acid RMs need to be accurate and stable in the hydrogen matrix.
According to the literature, formic acid in hydrogen has never been prepared or studied with regard to its long-term stability (in the order of months) or under transport conditions (low temperature). Several approaches were investigated using diffusion or permeation tubes [18]; however, the use of gravimetric methods can provide transportable calibrants for online monitoring or reduce the infrastructure requirements to perform traceable and reliable formic acid measurements. There is currently limited information on formic acid stability in hydrogen gas; therefore, part of this study aimed at evaluating the long-term stability of formic acid amount fraction in hydrogen matrix in gas cylinder (several months) and at various conditions (low temperature and low pressure), thus providing much needed new information on formic acid behaviour in hydrogen.
Moreover, if formic acid is stable in hydrogen, a gas calibrant can be easily transported, stored, and used for various instruments or applications. The importance of producing reliable RM with a shelf life compatible with industry requirement (>1 year) would allow for the validation of new analytical methods for measuring formic acid in hydrogen at the ISO 14687 threshold. Simultaneously, it will support the degradation studies on fuel cells and fuel cell stacks, which are helping to understand the effect of impurities in transportation applications.
The purpose of this paper is to describe the production of formic acid in hydrogen reference materials using the gravimetric approach. This paper also investigates the stability of these RMs from 0.7 to 100 µmol·mol−1 to underpin traceable purity measurements for quality assurance of fuel cell hydrogen. Finally, formic acid stability in hydrogen will be revised based on the new data obtained.
2. Materials and Methods
2.1. Preparation of Formic Acid Gas Standards in Hydrogen
2.1.1. Preparation of 40 and 100 µmol·mol−1 Formic Acid Gas Standards in Hydrogen
Primary reference standards of formic acid of 40 and 100 µmol·mol−1 were prepared from pure formic acid in the liquid phase (purity > 95%, Sigma-Aldrich, Gillingham, UK) in accordance with ISO 6142-1 [19]. The pure formic acid solution contained less than 2.4% of water as stabilising agent. Pure formic acid was added to a transfer loop via syringe injection. The transfer loop consisted in a piece of 1/8-inch external diameter tubing with Swagelok fittings on one end and a three-way valve on the other. All the parts of the transfer loop were made of Swagelok alloy 400 (Monel®). The transfer loop and the valve were evacuated to a pressure of 1 × 10−6 mbar to ensure no contaminants or air were present in the system. The evacuated transfer loop and valve were then weighed on a balance (Sartorius Research, Epsom, UK). Pure formic acid was then transferred into a syringe equipped with a two-way valve (Hamilton, Giarmata, Romania). The syringe was connected to the three-way valves on the transfer loop. The volume within the connection between the three-way valve and the syringe was evacuated to a pressure of 1 × 10−6 mbar to remove contaminants and air. The formic acid was then transferred from the syringe into the transfer loop. The transfer loop, filled with formic acid, was then weighed again. An empty gas cylinder (10 L aluminium with spectraseal passivation, BOC, Woking, UK), with an NPL-designed outlet diaphragm valve (Rotarex Ceodeux, Luxembourg) was evacuated below 5 × 10−7 mbar using a turbo molecular pump (Leybold Vacuum, Chessington, UK) for at least 12 h. The cylinder valve included an internal screw thread to minimise dead volume. The gas cylinder was connected to the transfer loop using a minimum dead volume connection. The volume within the connection between the transfer loop and the minimum dead volume fitting was evacuated to a pressure of 1 × 10−6 mbar to ensure no contaminants or air were present. The formic acid was then transferred from the transfer loop into the cylinder. After the transfer, the transfer loop was weighed again. The cylinder was weighed on a balance (XPE26003LC, Mettler Toledo, Leicester, UK) using an automated weighing facility (KRISS, SK). The cylinder was filled with 60 to 100 bar of hydrogen (purity > 99.9999%, BIP+, Air Products, Walton-on-Thames, UK). The pure hydrogen had less than 10 nmol/mol of water amount fraction. The cylinder was then weighed and homogenised through rolling for a further two hours.
2.1.2. Preparation of 0.7 and 4 µmol·mol−1 Formic Acid Gas Standards in Hydrogen
Due to the limitation of the mass of pure formic acid that can be transferred using the method described in Section 2.1.1, the reference materials of formic acid at 0.7 and 4 µmol·mol−1 in hydrogen gas were prepared via dilution. These were prepared in the same cylinder type as the higher amount fraction gas standards. The gas cylinders were conditioned using a NPL proprietary treatment. The compounds were added to the cylinder being filled by direct transfer of a NPL primary reference materials (prepared as described in Section 2.1.1) via a length of 1/16 inch stainless steel tubing (Swagelok, Kings Langley, UK) that had undergone Silcosteel® passivation (Thames Restek, Saunderton, UK). The cylinder was weighed on balance (XPE26003LC, Mettler Toledo, Leicester, UK) after each transfer, and finally filled with 60 to 100 bar of hydrogen (purity > 99.9999%, BIP+, Air Products, Walton-on-Thames, UK). Upon completion of preparation, the gas standards were homogenised by rolling along the vertical axis for two hours.
The compositions of the gas standards (amount fraction and associated uncertainty) were calculated from the masses of formic acid and hydrogen introduced in each cylinder using the software package ‘GravCalc2’ [20] (NPL, Teddington, UK). The formic acid amount fraction in each gas mixture were summarised in Table 1. The purity of formic acid in the mixture was assessed by FT-IR.

Table 1.
List of formic acid amount fraction in hydrogen gas standards prepared for this study with associated uncertainties.
2.2. Stability Study of Formic Acid in Hydrogen
2.2.1. Long-Term Stability Study of Formic Acid in Hydrogen—Isochronous Study
The stability of formic acid in hydrogen at 4, 40 and 100 µmol mol−1 was monitored over an eight-month period. To minimise the impact of day-to-day analysis variation, an isochronous study was performed. This involved producing fresh formic acid in hydrogen gas standards at different time (i.e., 0 month, 6 months, 7 months and 8 months after preparation of the first mixture) to act as reference. All gas standards prepared in hydrogen (see Table 1) were analysed under repeatable condition. This allowed any variations between the gas standards in hydrogen and the standard caused by instability to be established.
2.2.2. Low-Temperature Stability Study of Formic Acid in Hydrogen
The stability of gas standards D (Table 1) was monitored over 1 week at low temperature (5 ± 1 °C). The cylinder was immerged in a water tank with a cooling system set at 4 °C (Figure 1). The gas cylinder was connected to the Fourier-transform infrared (FT-IR) spectrometer for the whole duration of the test.
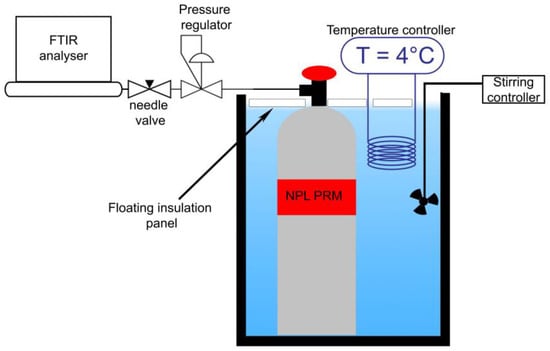
Figure 1.
Schematic of the experimental setup to perform low-temperature study of formic acid in hydrogen matrix.
The mixture was analysed at room temperature before starting the cooling and then on days 1, 2, 3, 4, 5 after preparation using FT-IR set-up. The temperature was monitored using a laser thermometer aimed directly to the cylinder surface.
2.3. Analytical Methods
2.3.1. Fourier-Transform Infrared (FT-IR) Spectroscopy
FT-IR spectroscopic measurements of formic acid in hydrogen standards were made on a benchtop FT-IR spectrometer (Nicolet 6700, Thermo Fisher Scientific, Renfrew, UK) equipped with N2 purged multi-range optics, a KBr beam splitter and a liquid-nitrogen-cooled MCT-A detector. The spectrometer was fitted with a “White”-type gas cell (Cyclone C5, Specac, Orpington, UK), with nominal path length of 8 m and volume of 1.33 L, equipped with a borosilicate glass body and KBr windows. Absorption spectra of the formic acid in hydrogen gas standards and the background spectra of pure hydrogen (Hydrogen BIP®+, Air Products, Walton-on-Thames, UK) were made at a temperature of (29 ± 1) °C, pressure of 1050 mbar with a sample flow rate of 0.5 L/min. The instrumental spectral resolution was 0.5 cm−1 and the spectral window ranged from 4000 to 640 cm−1. For low amount fraction of formic acid, the spectral window selected was from 1925–1558 cm−1. The peak 1778–1774 cm−1 was used for the integration. For high amount fraction of formic acid, the spectral window selected was from 2579–1988 cm−1. The peak 2229–2157 cm−1 was used for integration.
2.3.2. Selected Ion Flow Tube Mass Spectrometry (SIFT-MS)
Analysis of the formic acid gas standards was carried out on a Syft Technolgies’ Voice 200ultra SIFT-MS (Syft Technologies, Christchurch, New Zealand). The sample was introduced in the instrument via a heated transfer line at 120 °C, using a flow passed configuration. The SIFT-MS instrument passively sampled at approximately 30 mL·min−1, with the flow controlled by a narrow-bore capillary. The samples were delivered to the instrument using 1/8-inch stainless steel tubing attached to the transfer line via a tee piece union. The flow from the cylinder was kept above 50 mL·min−1, with the instrument sampling at the required rate, and any excess flow vented via the tee piece. The single ion signal was recorded for formic acid using H3O+ as the reagent ion and HCOOH2+ (m/z = 47 a.m.u.) as the product ion, along with the secondary adduct HCOOH2+·H2O (m/z = 65 a.m.u.). Whilst SIFT-MS is inherently quantitative, the quantitation relies on accurate values of the rate constants (kr) for the ion-molecule reactions taking place in the flow tube. There are numerous literature sources for this data [19,20,21]; however, there is very limited data on rate constants in hydrogen gas matrices. The kr-values used in the calculations in this paper were derived from analytes in helium. These quantified the amount fraction of formic acid in hydrogen to within 5% of the expected value, thus confirming that kr values in helium (2.2 × 10−9 cm3·s−1) [22] are applicable to a hydrogen matrix.
For the analysis of low-level formic acid (i.e., 0.7 µmol·mol−1), dynamically generated standards were produced. These were prepared by dilution of NPL high amount fraction PRM (i.e., 40 µmol·mol−1) in high purity hydrogen (H2 BIP+®, Air product, Walton-on-Thames, UK) using two mass flow controllers (EL-FLOW® Bronkhorst, Ruurlo, The Netherlands) calibrated for hydrogen gas. The dynamic dilutions were used to generate a calibration curve at sub-μmol·mol−1 amount fractions, which was then used to quantify the amount fraction of formic acid in the gas standards under test using the XLGenline version 2 software [23].
2.4. Data Treatment and Evaluation
2.4.1. Normalisation
For the long-term stability study of formic acid in hydrogen, a specific data treatment was realized. As the formic acid gas standards were prepared at different times, the amount fraction of formic acid may vary between them. Normalisation of the measured amount fraction by the gravimetric value was performed as described in Equation (1).
: normalised formic acid amount fraction y at time t;
: measured amount fraction for gas cylinder y at time t;
: gravimetric amount fraction for the reference mixture y.
The normalised value () was then multiplied by a constant to scale it back to a nominal value (i.e., multiplied by 40 for the 40 µmol·mol−1) for the graphical representation.
The uncertainty reported was the combination of the measurement uncertainties of the sample and of the gravimetric reference amount fraction.
2.4.2. Stability
Due to the intrinsic variation of measurement results, no study can rule out the degradation of materials completely, even in the absence of statistically significant trends. It is therefore necessary to quantify the potential degradation that could be hidden by the method’s repeatability, i.e., to estimate the uncertainty of stability. This means, even under ideal conditions, the outcome of a stability study can only be in the form of “degradation is (0 ± x)% per unit time”.
Uncertainties of stability during dispatch and storage were estimated as described in [24] for each parameter. For this approach, the uncertainty of the linear regression line with a slope of zero is calculated. The uncertainty contribution of short term (usts) and long term (ults) uncertainties are calculated as the product of the chosen transport time/shelf life and the uncertainty of the regression lines as:
RSD: relative standard deviation of all results of the stability study;
xi: result at time point i;
: mean results for all time points;
tsl: chosen shelf life (52 weeks at 18 °C).
The following uncertainties were estimated:
ults,rel, the stability during storage. This uncertainty contribution was estimated from the (18 ± 2) °C study. The uncertainty contribution describes the possible degradation for 1 year of storage at 18 °C.
usts,rel, the stability during transport. This uncertainty contribution was estimated from the (5 ± 1) °C study. The uncertainty contribution describes the possible degradation for 4 weeks of storage at 5 °C.
3. Results and Discussion
3.1. Validation of Formic Acid in Hydrogen Primary Gas Standards
FT-IR spectroscopy was used to identify the compounds present in each mixture. The first objective was to confirm the purity of formic acid. After preparation, the FT-IR spectra of each mixture (Figure 2) clearly matched the scaled reference spectra from the Pacific Northwestern National Laboratory (PNNL) database [25].
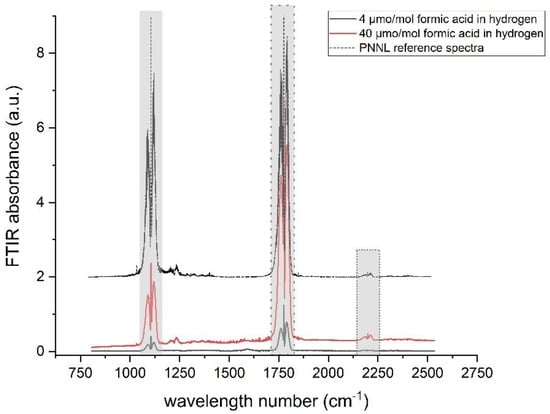
Figure 2.
Comparison of experimental FT-IR spectra for 4, 40 µmol·mol−1 formic acid in hydrogen gas mixture (black and red, respectively) and spectra calculated from PNNL reference spectra (black dotted line). The grey area highlights the formic acid absorbance peak, the large, dotted grey area represents the peak used for low amount fraction of formic acid, and the small, dotted line represents the peak used for the high amount fraction of formic acid.
The RMs produced at 4, 40 and 100 µmol·mol−1 of formic acid in hydrogen were validated against FT-IR spectra (PNNL FT-IR database [24]). The comparison between the FT-IR spectra and experimental spectra confirmed that formic acid was present in the gas phase (see Figure 2). There was also visible trace of water vapour in the spectra, which may be attributed to residual water vapour in the sampling system and/or to the pure formic acid source that contained trace of water as a stabilizing agent.
A quantification using PNNL reference spectra was produced based on the instrument parameters and conditions (standardless) to confirm the trueness of the reference materials. The relative difference between the gravimetric formic acid amount fraction and the value obtained from FT-IR measurements based on PNNL spectra were below 10%. This difference may be due to the analytical method, or to the difference between real sample and PNNL spectra.
No by-products were observed in the different primary reference standards by FT-IR. The entire FT-IR spectrum was checked without finding significant traces of additional contaminants except for trace water, which was present in the pure formic acid solution. This spectral analysis indicates that formic acid, water and no other impurities or degradation products were present in the gas standards at all stages of the analysis.
3.2. Long-Term Stability of Formic Acid in Hydrogen
3.2.1. Evolution of Formic Acid Amount Fraction in Hydrogen over Time at µmol·mol−1 Level
The evolution of the formic acid amount fraction in hydrogen was monitored over time using FT-IR and SIFT-MS. As described above, cylinders of formic acid in hydrogen of different ages were analysed over two consecutive days (isochronous study) as shown in Figure 3A–C.
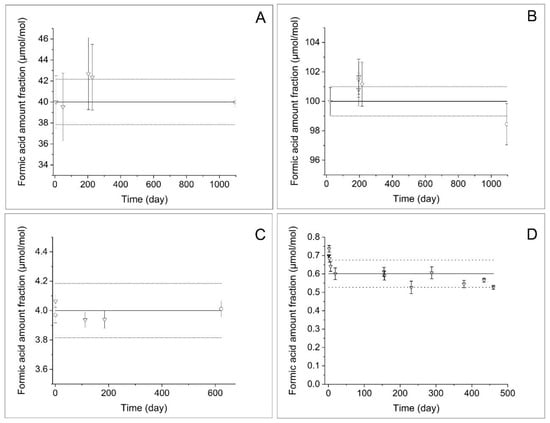
Figure 3.
Normalised formic acid amount fraction as a function of the age of the reference materials. (A) The isochronous stability study of 40 µmol·mol−1 of formic acid in hydrogen (triangle point). An additional measurement outside the isochronous study is presented (circle point) to highlight long-term stability. (B) The isochronous stability study of 100 µmol·mol−1 of formic acid in hydrogen (triangle point). An additional measurement outside the isochronous study is presented (circle point) to highlight long-term stability. (C) Two isochronous stability studies of 4 µmol·mol−1 of formic acid in hydrogen. (D) Stability study of 0.7 µmol·mol−1 of formic acid in hydrogen. Uncertainty is reported with k = 1. The black line represents the reference value, and the dotted line the represents the upper and lower stability uncertainty for a 1-year shelf life.
To certify gas standards, it is important to determine the uncertainty of the stability as defined in ISO Guide 34 [26]. For this reason and to minimise the impact of day-to-day variations, an isochronous study was performed. Standards prepared on different dates were analysed under repeatable conditions to observe deviations from the nominal amount fraction due to instability. For this study, the time 0 was defined as the day of preparation, and the age of each mixture was then calculated as the difference between the analysis and production date. All RMs (A to D, A′ to E′, A″ to C″ and D″ to E″) were analysed on the same day: No outliers were observed using Grubb’s test at the 95 and 99% confidence levels in all study. The slope of the linear regression was significant at the 95% confidence level for the study at 4, 40 and 100 µmol·mol−1; however, the slope was positive for the study at 40 and 100 µmol·mol−1, indicating a production of formic acid in the cylinder, which is highly unexpected. Therefore, the statistical evaluation excluded any degradation in the calculation of stability uncertainty. For a given shelf life of 52 weeks, the relative stability uncertainty was estimated at 0.5% (k = 1) and 2.7% for 100 and 40 µmol·mol−1 of formic acid in hydrogen, respectively.
Additional stability measurements were performed between two cylinders made more than 3 years apart following the methodology described in Section 2.1.1. For the 100 µmol·mol−1 formic acid in hydrogen reference materials, the difference between formic acid amount fraction in cylinder E and Erec was less than 1.6%, considering an expanded measurement uncertainty of 2.8%. The two reference materials were considered not significantly different at the 95% confidence level within the measurement uncertainty according to Application Note 1 [24]; therefore, formic acid in hydrogen was stable for more than 3 years at 100 µmol·mol−1. Furthermore, it demonstrated that the assumption of 0.5% (k = 1) relative uncertainty is slightly conservative. For 40 µmol·mol−1 formic acid in hydrogen reference materials, the difference between formic acid amount fraction in cylinder C and Crec was less than 0.6%, considering an expanded measurement uncertainty of 1.1%. The two reference materials were considered not significantly different at the 95% confidence level within the measurement uncertainty. Formic acid in hydrogen was stable for more than 3 years at 40 µmol·mol−1. Furthermore, it demonstrated that the assumption of 2.7% (k = 1) relative uncertainty is conservative.
For the 4 µmol·mol−1 study, Figure 3C (study represented with crosses) showed that there is a drop between the time point 0 and the other points later in time, prompting a potential decay. No outliers were observed using Grubb’s test at the 95 and 99% confidence levels. For a given shelf life of 52 weeks, the relative stability uncertainty was estimated at 4.4% (k = 1), including the decay rate; however, a second study comparing only two gas standards aged 1 day and 622 days suggested that there was no significant decay. Therefore, a less conservative stability uncertainty could be estimated to 2.3% (k = 1), discarding the decay observed in the first study.
3.2.2. Evolution of Formic Acid Amount Fraction in Hydrogen over Time at nmol·mol−1 Level
The stability study of sub-µmol·mol−1 formic acid in hydrogen was limited by the measurement method capacity; therefore, a study at 0.2–0.4 µmol·mol−1 was attempted, but the measurement uncertainty reached 5–10% (k = 1). Due to the complexity of preparing these gas standards, an isochronous study was not performed as the large uncertainties precluded the detection of any stability variation; therefore, the study at 0.7 µmol·mol−1 in Figure 3D represented the lowest amount fraction in this study. The first observation is the quick decay in less than 20 days from 0.7 µmol·mol−1 to 0.6 µmol·mol−1. After this quick initial drop, the value stabilized and decayed slowly over time. The quick decay was considered an initial reaction time between the gas container surface and the gas mixture, resulting in a small adsorption of formic acid onto the cylinder wall. Therefore, the stability study was only considered from day 20 to 460. No outliers were observed using Grubb’s test at the 95 and 99% confidence levels in all study. The slope of the linear regression was significant at the 95% confidence level. Its decrease was consistent with a slow decay. For a given shelf life of 1 year, the relative stability uncertainty was estimated at 6.2% (k = 1), including the decay rate. Considering the initial drop and the following slow decay, it is therefore possible to certify the gas standard for formic acid in hydrogen at sub- µmol·mol−1 level if the value is certified against a traceable measurement 20 days after the preparation of the reference material.
Based on the hypothesis that the decay of formic acid amount fraction in hydrogen may be related to adsorption on the gas container wall, it is advisable to evaluate other types of gas containers, as different treatment of internal surfaces may be more suitable (i.e., sulfinert stainless steel, aluminium SGS from Luxfer).
3.3. Low-Temperature Stability Study
To evaluate the impact of transport onto the stability of formic acid in hydrogen, a short-term stability study was performed at low temperature (5 °C) (see Figure 4A). During the study, the pressure was estimated based on the gas use for each FT-IR measurement (Figure 4B). No outliers were observed using Grubb’s test at the 95 and 99% confidence levels. The slope of the linear regression was not significant at the 95% confidence level.
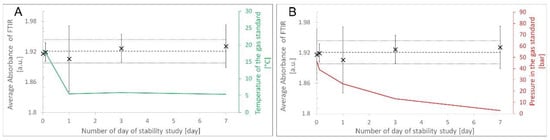
Figure 4.
Stability of formic acid in hydrogen at low temperature (A) and decreasing pressure (B). (A) presents the formic acid average absorbance (cross on graphic) in function of day. The dotted line represent stable result over time and the plain line are uncertainties. The green line presents the temperature of the cylinder over time. On (B), the red line presents the evolution of pressure in the cylinder over time.
No change in formic acid amount fraction was observed during the variation of temperature or pressure. This indicates that the formic acid gas standards in hydrogen are stable and not affected by temperature or pressure within the measurement uncertainty of the study; therefore, a short-term stability uncertainty (for transport) can be assigned at 2.17% for 28 days at 5 °C.
3.4. Thermodynamic Calculations
The stability of formic acid in a hydrogen matrix is supported by thermodynamic calculations. Formic acid can undergo reaction with molecular hydrogen to produce formaldehyde and water [27], as shown in Equation (3):
HCOOH + H2 → HCHO + H2O
A previous study [28] has showed how formaldehyde is unstable in a hydrogen matrix, so reaction (1) could potentially represent the first step of the degradation of formic acid into secondary products once formaldehyde is formed, as indicated by Equation (4):
HCHO + H2 → CH3OH
However, calculations using thermodynamic data from the NIST database [29], following the approach by [27], show that the enthalpy of Equation (3), ΔrH, is +21.3 kJ mol−1, which indicates that the reagents are more stable than the products. In addition, the change in Gibbs energy, ΔrG, at room temperature (25 °C) is also positive (12.8 kJ mol−1), which indicates the reaction is non-spontaneous, and the equilibrium constant, Keq, also at room temperature, is 5.70 × 10−3, indicating that the equilibrium favours the reagents.
By contrast, ΔrH for Equation (4) is −89 kJ mol−1 (products more stable than reagents), ΔrG is −56 kJ mol−1 (spontaneous reaction) and Keq is 7.69 × 109 (products favoured over reagents). This indicates that formaldehyde is thermodynamically unstable in hydrogen, which was confirmed by observations [27].
We conclude that formic acid in hydrogen is thermodynamically stable and that conversion to formaldehyde is unfavourable.
3.5. Recommendations to the Hydrogen Industry
This study has demonstrated that formic acid is stable in hydrogen at high amount fraction (from 4 to 100 µmol·mol−1). At nmol·mol−1 amount fractions, an initial adsorption on the internal surface of the gas cylinder may occur. After this initial loss, the remaining formic acid amount fraction in the gas phase was stable over long periods. Unlike formaldehyde, which is unstable in hydrogen [28], formic acid is likely to remain as formic acid; therefore, the absence of formic acid in a hydrogen sample may not be linked to reactivity. Further studies are required to investigate the behaviour of formic acid at low amount fraction (nmol·mol−1) and its presence in real samples from the steam methane reforming process, which are more likely to contain formic acid impurities. The current study demonstrates that it is possible to prepare accurate and traceable reference material for formic acid in hydrogen. These new reference materials may be relevant to evaluate the real impact of formic acid on fuel cell. Moreover, this study was unable to observe any by-products.
4. Conclusions
This manuscript presents the first stability study of formic acid in hydrogen matrix in gas cylinder. NPL successfully prepared more than fifteen gas reference materials of formic acid in hydrogen at 4–100 µmol·mol−1. These gas standards were stable for 52 weeks within an expanded uncertainty below 5% (k = 1). No degradation products were identified during the 8 months of the stability study, and neither after low temperature storage nor for the decreasing internal cylinder. Further measurements extended the stability period over 2 years. For this study, aluminium cylinders with a surface passivation treatment (SPECTRA-SEAL®, BOC, Woking, UK) were used as the current state-of-the-art. Primary Reference Material of formic acid in hydrogen can indeed be prepared and transported within an uncertainty 3% (k = 1) including 2-year lifetime and transportation without stringent requirement (T > 5 °C). Thermodynamic investigation agreed with the experimental observation concluding on the stability of formic acid in hydrogen gas.
The stability study of formic acid at sub- µmol·mol−1 showed some decay that was potentially related to adsorption on the gas container wall; however, it is possible to certify the formic acid amount fraction after a period of 20 days and then ensure the certified value validity for 1 year with an uncertainty below 7% (k = 1). To provide primary reference material, further studies are needed investigating other type of gas container to determine if there is better stability with alternative surface treatments (i.e., sulfinert stainless steel, aluminium SGS from Luxfer).
The novel reference materials produced in this study may be required for routine analysis of hydrogen quality or to investigate the impact of formic acid on various applications using traceable and reliable reference values. Further studies are required to evaluate the long-term stability, the behaviour of formic acid in hydrogen gas at nmol·mol−1. Due its reactive nature, future investigation of the interaction of formic acid in the gas phase with different type of cylinders surface or other compounds is vital to further understand its behaviour in the gas phase.
Author Contributions
Conceptualization, T.B., A.S.O.M., R.U. and M.K.M.W.; methodology, T.B., A.S.O.M., R.U. and M.K.M.W.; samples, T.B., A.S.O.M., M.H. and Y.H.; formal analysis, T.B., A.S.O.M., M.K.M.W. and M.P.; investigation, T.B., A.S.O.M., M.H. and M.K.M.W.; thermodynamics, V.F.; data curation, T.B. and A.S.O.M.; writing—original draft preparation M.H., M.K.M.W. and T.B.; writing—review and editing, T.B., Y.H., M.H., V.F. and A.M.; supervision, T.B. and A.M.; project administration, T.B.; funding acquisition, T.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data presented in this study are available in this article: Formic acid in hydrogen: is it stable in a gas container?
Conflicts of Interest
The authors declare no conflict of interest.
References
- IEA. Global Hydrogen Review 2022; IEA: Paris, France, 2022; Available online: https://www.iea.org/reports/global-hydrogen-review-2022 (accessed on 12 March 2023).
- Riemer, M.; Zheng, L.; Pieton, N.; Eckstein, J.; Kunze, R.; Wietschel, M. Future Hydrogen Demand: A Cross-Sectoral, Multiregional Meta-Analysis; HYPAT Working Paper 04/2022; Fraunhofer ISI: Karlsruhe, Germany, 2022. [Google Scholar]
- Viitakangas, J.; Ihonen, J.; Koski, P.; Reinikainen, M.; Aarhaug, T.A. Study of Formaldehyde and Formic Acid Contamination Effect on PEMFC. J. Electrochem. Soc. 2018, 165, F718. [Google Scholar] [CrossRef]
- Borup, R.; Meyers, J.; Pivovar, B.; Kim, Y.S.; Mukundan, R.; Garland, N.; Myers, D.; Wilson, M.; Garzon, F.; Wood, D.; et al. Scientific aspects of polymer electrolyte fuel cell durability and degradation. Chem. Rev. 2007, 107, 3904–3951. [Google Scholar] [CrossRef] [PubMed]
- Tedsree, K.; Li, T.; Jones, S.; Chan, C.W.A.; Yu, K.M.K.; Bagot, P.A.J.; Marquis, E.A.; Smith, G.D.W.; Tsang, S.C.E. Hydrogen production from formic acid decomposition at room temperature using a Ag–Pd core–shell nanocatalyst. Nat. Nanotechnol. 2011, 6, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Müller, K.; Brooks, K.; Autrey, T. Hydrogen Storage in Formic Acid: A Comparison of Process Options. Energy Fuels 2017, 31, 12603–12611. [Google Scholar] [CrossRef]
- Lopez, J.A.P.; Somiari, I.; Manousiouthakis, V.I. Hydrogen/formic acid production from natural gas with zero carbon dioxide emissions. J. Nat. Gas Sci. Eng. 2018, 49, 84–93. [Google Scholar] [CrossRef]
- ISO 14687; Hydrogen Fuel Quality—Product Specification. International Organization for Standardization: Geneva, Switzerland, 2019.
- EN 17124:2018; Hydrogen Fuel. Product Specification and Quality Assurance. Proton Exchange Membrane (PEM) Fuel Cell Applications for Road Vehicles. European Committee on Standardisation: Bruxelles, Belgium, 2018.
- J2719_202003; Hydrogen Fuel Quality for Fuel Cell Vehicles. SAE International: Warrendale, PA, USA, 2020.
- Molter, T. Final Report—Effects of Impurities on Fuel Cell Performance and Durability; The University of Connecticut: Mansfield, CT, USA, 2012. [Google Scholar]
- Narusawa, K.; Hayashida, M.; Kamiya, Y.; Roppongi, H.; Kurashima, D.; Wakabayashi, K. Deterioration in fuel cell performance resulting from hydrogen fuel containing impurities: Poisoning effects by CO, CH4, HCHO and HCOOH. JSAE Rev. 2003, 24, 41–46. [Google Scholar] [CrossRef]
- Directive 2014/94/EU of the European Parliament and of the Council of 22 October 2014 on the Deployment of Alternative Fuels Infrastructure. Off. J. Eur. Union 2014. Available online: http://eur-lex.europa.eu/legalcontent/EN/TXT/PDF/?uri=CELEX:32014L0094&from=EN (accessed on 10 December 2022).
- Murugan, A.; Brown, A.S. Review of purity analysis methods for performing quality assurance of fuel cell hydrogen. Int. J. Hydrogen Energy 2015, 40, 4219–4233. [Google Scholar] [CrossRef]
- Arrhenius, K.; Alexandersson, A.; Haleh, Y.; Engelbrektsson, J.; Stromberg, N. Analysis of Hydrogen Quality According to Standard ISO/DIS 14687-2 Pre Study; Energieforsk AB Report 2015:177; Energieforsk: Stockholm, Sweden, 2015; ISBN 978-91-7673-177-2. Available online: https://energiforskmedia.blob.core.windows.net/media/18581/analysis-of-hydrogen-quality-energiforskrapport-2015-177.pdf (accessed on 2 April 2022).
- Beurey, C.; Gozlan, B.; Carré, M.; Bacquart, T.; Morris, A.; Moore, N.; Arrhenius, K.; Meuzelaar, H.; Persijn, S.; Rojo, A.; et al. Review and Survey of Methods for Analysis of Impurities in Hydrogen for Fuel Cell Vehicles According to ISO 14687:2019. Front. Energy Res. 2021, 8, 615149. [Google Scholar] [CrossRef]
- ASTM D7653-10; Standard Test Method for Determination of Trace Gaseous Contaminants in Hydrogen Fuel by Fourier Transform Infrared (FTIR) Spectroscopy. ASTM International: West Conshohocken, PA, USA, 2015.
- Veres, P.; Gilman, J.B.; Roberts, J.M.; Kuster, W.C.; Warneke, C.; Burling, I.R.; de Gouw, J. Development and validation of a portable gas phase standard generation and calibration system for volatile organic compounds. Atmos. Meas. Tech. 2010, 3, 683–691. [Google Scholar] [CrossRef]
- ISO 6142-1; Gas analysis—Preparation of Calibration Gas Mixtures—Gravimetric Method for Class I Mixtures. International Organization for Standardization: Geneva, Switzerland, 2015.
- Brown, A.S. GravCalc2 (Version 2.3.0) User Guide; NPL Report AS 42; National Physical Laboratory: Teddington, UK, 2009. [Google Scholar]
- Ŝpaněl, D.; Ji, Y.; Smith, D. SIFT studies of the reactions of H3O+, NO+ and O2+ with a series of aldehydes and ketones. Int. J. Mass Spectrom. Ion Process. 1997, 165–166, 25–37. [Google Scholar] [CrossRef]
- Ŝpaněl, P.; Smith, D. SIFT studies of the reactions of H3O+, NO+ and O+2 with a series of volatile carboxylic acids and esters. Int. J. Mass Spectrom. Ion Process. 1998, 172, 137–147. [Google Scholar] [CrossRef]
- Smith, I.M.; Onakunle, F.O. SSfM-3 1.6.1—XLGENLINE, Software for Generalised Least-Squares Fitting, Developed by the (NPL); NPL Document Reference: CMSC/M/06/657; National Physical Laboratory: Teddington, UK, 2007. [Google Scholar]
- Linsinger, T.P.J.; Pauwels, J.; Lamberty, A.; Schimmel, H.; van der Veen, A.M.H.; Siekmann, L. Estimating the Uncertainty of Stability for Matrix CRMs. Fres. J. Anal. Chem. 2001, 370, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Sharpe, S.W.; Johnson, T.J.; Sams, R.L.; Chu, P.M.; Rhoderick, G.C.; Johnson, P.A. Gas-Phase Databases for Quantitative Infrared Spectroscopy. Appl. Spectrosc. 2004, 58, 1452–1461. [Google Scholar] [CrossRef] [PubMed]
- ISO Guide 34; General Requirements for the Competence of Reference Material Producers. International Organization for Standardization: Geneva, Switzerland, 2009.
- Tsurusaki, A.; Murata, K.; Onishi, N.; Sordakis, K.; Laurenczy, G.; Himeda, Y. Investigation of Hydrogenation of Formic Acid to Methanol using H2 or Formic Acid as a Hydrogen Source. ACS Catal. 2017, 7, 1123–1131. [Google Scholar] [CrossRef]
- Bacquart, T.; Perkins, M.; Ferracci, V.; Martin, N.A.; Resner, K.; Ward, M.K.M.; Cassidy, N.; Hook, J.B.; Brewer, P.J.; Irvine, J.T.C.; et al. Production and stability of low amount fraction of formaldehyde in hydrogen gas standards. Int. J. Hydrogen Energy 2018, 43, 6711–6722. [Google Scholar] [CrossRef]
- NIST Chemistry WebBook. NIST Standard Reference Database Number 69. Available online: https://webbook.nist.gov/chemistry/ (accessed on 20 February 2023).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).