1. Introduction
The
Mikania genus belongs to the Asteraceae (Daisy) family and it is reported to have around 450 subspecies in the Central America and Asia–Pacific regions [
1]. Traditionally, the decoction of
M. micrantha leaves has been used indigenously to treat tumors by the ethnic people of Assam, India [
2,
3]. Moreover, the Mizoram tribes in India have traditionally used
M. micrantha juice to treat cuts and open wounds [
4].
M. cordata has been used indigenously in Bangladesh to treat various ailments, such as bronchitis, cough, diabetes, fever, influenza, jaundice, muscle spasms, septic sores, and snake bites [
5]. Da Silva et al. [
6] have reviewed the pharmacological properties of the
Mikania genus and reported that it possesses antibacterial, antidiarrheal, antifungal, anti-inflammatory, antinociceptive, antiophidian, antiparasitic, antiprotozoal, antispasmodic, antiulcerogenic, antiviral, bronchodilating, cytotoxic, mutagenic, and vasodilating properties. Recently, Radhakrishnan et al. [
7] have reported the mosquitocidal activity of
M. scandens.
Our research team identified 26 ligands of the phytoconstituents of
Mikania species during the development of mosquito repellents [
7]. The present study focuses on
Mikania species to demonstrate the relationships among their pharmacological actions and the phytochemicals. Recently, species of
Mikania have attracted the interest of researchers due to their numerous pharmacological actions [
6]. In this work, therefore, we conducted a docking study with the phytoconstituents of
Mikania species, viz., mikamicranolide (sesquiterpene dilactone), kaurenoic acid (diterpenoid), stigmasterol (phytosterol), grandifloric acid (diterpenoid), kaurenol (diterpenoid), spathulenol (sesquiterpenoid), caryophyllene oxide (sesquiterpenoid oxide), syringaldehyde (hydroxybenzaldehyde), dihydrocoumarin (benzopyrone), o-coumaric acid (hydroxycinnamic acid), taraxerol (triterpenoid), melilotoside (phenylpropanoid), patuletin (flavonol), methyl-3,5-di-
O-caffeoyl quinate (cyclitol derivative), 3,3′,5-trihydroxy-4′,6,7-trimethoxyflavone (flavonol), psoralen (furanocoumarin), curcumene (sesquiterpenoid), herniarin (coumarin), 2,6-dimethoxyquinone (quinone derivative), bicyclogermacrene (sesquiterpenoid),
α-bisabolol (monocyclic sesquiterpene),
γ-elemene (triterpenoid), provincialin (sesquiterpene lactone), dehydrocostus lactone (sesquiterpene lactone), mikanin-3-
O-sulfate (flavonoid sulfate), and nepetin (flavonoid). The above-mentioned phytoconstituents of
Mikania species were investigated for docking with (i) cyclooxygenase 2 (COX 2), (ii) human neutrophil elastase (HNE), (iii) lipoxygenase (LOX), matrix metalloproteinase ((iv) MMP 2 and (v) MMP 9), and (vi) microsomal prostaglandin E synthase 2 (mPGES 2), with an examination of the enzymes’ apparent binding sites using Discovery Studio (in the case of LOX, the Autodock method was applied). Furthermore, STITCH (Search Tool for Interacting Chemicals), physicochemical, drug-likeness, and ADMET analyses were conducted utilizing the STITCH web server, the Mol-inspiration web server, and Discovery Studio, respectively.
2. Results and Discussion
Computational approaches have been emerging as a new tool for evaluating the therapeutic potential of medicinal plants. In particular, molecular docking is used to select protein (enzymes/biomarkers) targets of interest and to identify the docking behavior of particular phytoconstituents on these targets [
8]. Computational approaches have great potential for drug repositioning, target identification, ligand profiling, and receptor de-orphanization [
9].
Da Silva et al. [
6] have demonstrated the anti-inflammatory activity of the
Mikania genus and they further reported that
Mikania scandens (leaf extract) possesses stronger anti-inflammatory activity than
M. scandens (stem extract). Suyenaga et al. [
10] have shown the anti-inflammatory activity of
Mikania laevigata (leaf decoction) under an in vivo (animal model) approach. Perez-Amador et al. [
11] have described the anti-inflammatory activity of
Mikania micrantha ethyl acetate (EA) extract in a TPA (12-O-tetradecanoylphorbol-13-acetate)-induced animal model in an in vivo experiment. Della Pasqua et al. [
12] have demonstrated that
M. laevigata (leaf aqueous extract) possesses superior anti-inflammatory activity compared to
M. glomerata (leaf aqueous extract). Thus, the above-summarized anti-inflammatory studies were evaluated to perform the present study.
The search tool for interacting chemicals (STITCH) free web server provides comprehensive particulars regarding: (i) metabolic pathways of interactions, (ii) crystal structure information, (iii) binding investigations, and (iv) target–drug correlations [
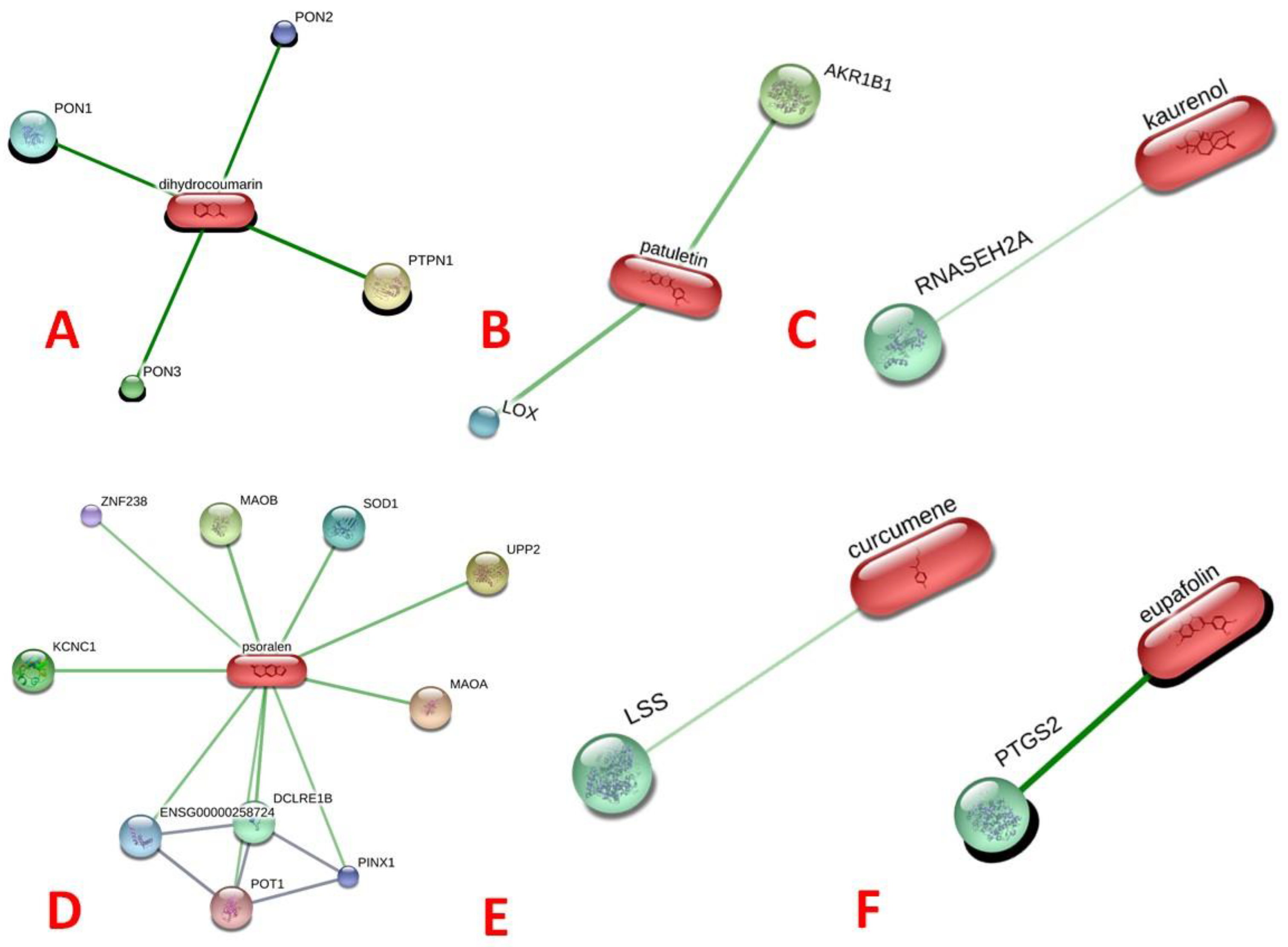
13]. In the present study, the STITCH analysis revealed that only six ligands, namely (a) dihydrocoumarin, (b) patuletin, (c) kaurenol, (d) psoralen, (e) curcumene, and (f) nepetin (eupafolin), showed interactions with human proteins (
Figure 1). Interestingly, patuletin interacted with the human lipooxygenase (LOX, inflammatory) protein, as presented in
Figure 1b.
Prior to the docking experiments, it is vital to understand the (i) physicochemical, (ii) drug- likeness/bioactivity score, (iii) ADME, and finally, (iv) the toxicity of the 26 chosen phytoconstituents of the
Mikania species. These analyses have been shown to help in the computer-aided drug development (CADD) process [
14]. Regarding the physicochemical properties, six ligands (stigmasterol, taraxerol, curcumene, bicyclogermacrene, γ-elemene, and provincialin) showed one violation, while only one ligand (3,5-methyl-di-O-caffeoyl quinate) displayed three violations for the rule of five (
Table 1). Similarly, with reference to supporting the drug-likeness or the score of the bioactivity analysis, only one ligand (mikamicranolide) revealed a bioactivity score of >0 towards the six descriptors; on the other hand, the other ligands showed a bioactivity score range of active to moderate. Moreover, the other 26 selected ligands showed an inactive score (<−5.0) (
Table 2).
Before docking, it is vital to know a compound’s/ligand’s properties, such as (i) physicochemical, (ii) drug-likeness or score of bioactivity, and (iii) ADMET, along with its (iv) toxicity. Moreover, standardized rule (Lipinski’s rule of five) and ADMET are available for determining such properties [
15]. Concerning ADMET analysis, eleven ligands (mikamicranolide, spathulenol, caryophyllene oxide, patuletin, 3,3′,5-trihydroxy-4′,6,7-trimethoxyflavone, psoralen, herniarin, 2,6-dimethoxyquinone, dehydrocostus lactone, mikanin-3-
O-sulfate, and nepetin) have hepatotoxic properties, as displayed in
Table 3.
Regarding the toxicological screening of 26 ligands, as illustrated in
Table 4, 5 ligands (dihydrocoumarin, patuletin, 3,3′,5-trihydroxy-4′,6, 7-trimethoxyflavone, 3-
O-mikanin-sulfate along with nepetin) are non-degradable in terms of aerobic biodegradability nature. Two ligands (patuletin and 3, 3′, 5-trihydroxy-4′, 6, 7-trimethoxyflavone) are predicated as mutagens.
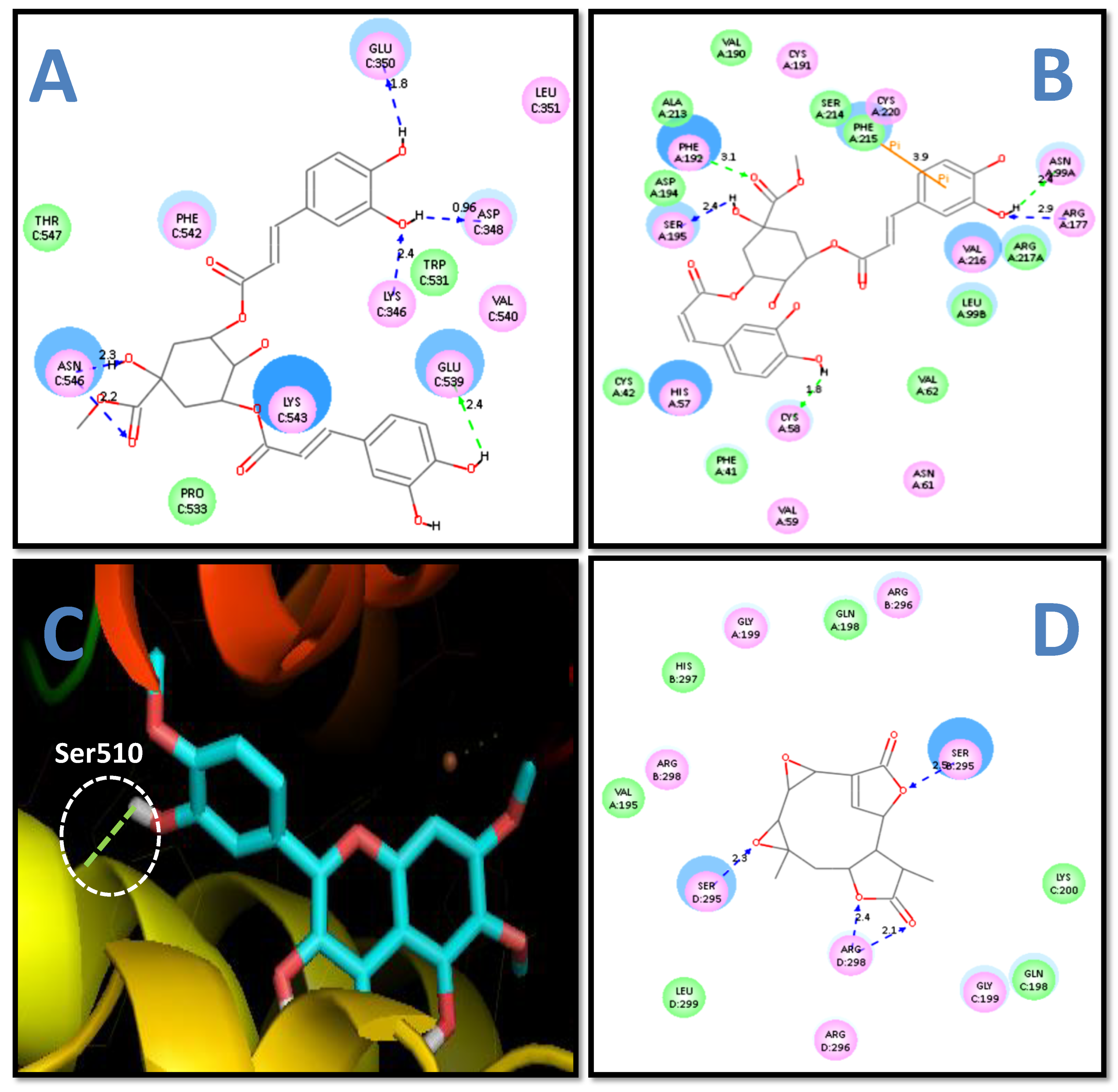
The C-docking study and free energy binding analysis (
Table 5) showed that 3,5-methyl-di-O-caffeoyl quinate possesses the maximum energy interaction (−42.51 kcal/mol) with the COX 2 enzyme (as presented in
Figure 2a). In contrast, psoralen revealed the least interaction energy (−15.57 kcal/mol). Moreover, eight ligands (grandifloric acid, kaurenol, o-coumaric acid, melilotoside, patuletin, 3,5-methyl-di-
O-caffeoylquinate, mikanin-3-
O-sulfate, and nepetin) showed interaction with the Glu539 residues of the COX 2 enzyme, as displayed in
Table 5. The present results were in good conformity with our previous findings where 4-hydroxyisoleucine (4-HIL) and phytic acid (PA) showed interaction with (i) Glu539; (ii) Glu350; (iii) Asn546; and (iv) Trp531 amino acid (AA) residues of the COX 2 enzyme [
16].
Stigmasterol has been described to inhibit thromboxane B
2 (TXB
2) production, which afterwards leads to inhibition of cyclooxygenase 1 (COX 1) activity [
17]. However, no reports are available for stigmasterol’s cyclooxygenase 2 (COX 2) inhibition activity. Additionally, caryophyllene has been reported to exhibit cyclooxygenase-2 (COX-2) inhibition activity in THP-1 (human monocytic) cells [
18]. Psoralen, spathulenol, syringaldehyde, and taraxerol acetate have been found to exhibit cyclooxygenase-2 (COX-2) inhibition activity [
19,
20,
21,
22,
23]. All the above findings were in agreement with our results on cyclooxygenase 2 (COX 2) inhibition activity.
The HNE is an additional targeted enzyme whose docking analysis and free energy binding analysis showed that 3,5-methyl-di-
O-caffeoyl quinate displayed the maximum energy of interactions (−54.66 kcal/mol), as presented in
Figure 2b. Thirteen ligands (kaurenol, syringaldehyde, o-coumaric acid, melilotoside, patuletin, 3,5-methyl-di-O-caffeoyl quinate, trihydroxy-3,3′,5-trimethoxy-4′,6,7-flavone, psoralen, herniarin, 2,6-dimethoxyquinone, provincialin, mikanin-3-
O-sulfate, and nepetin) exhibited interaction with Ser195 amino acid residue of HNE, as shown in
Table 6. The present finding was in good agreement with our previous study, where phytic acid (PA) and 4-hydroxyisoleucine (4-HIL) demonstrated interaction with (i) Ser195; (ii) Arg147; (iii) Cys191; (iv) Phe192; (v) Gly193; (vi) Asp194; and (vii) Ser214 amino acid (AA) residues of the HNE enzyme [
16].
Five sesquiterpene lactones, namely (15- (3′-Hydroxy)-methacryloyloxy-micrantholide, isobutyryloxy-15-(2′,3′-Epoxy) -micrantholide, isobutyryloxy-15-(2′-Hydroxy)-micrantholide, 4α hydroxy-1β-Acetoxy-15- eudesma-isobutyryloxy-12-8β-olide11-13-en from
M. cordifolia, and Scandenolide from
M. micrantha have been reported to exhibit human neutrophil elastase (HNE) inhibition activity [
24]. Similarly,
p-coumaric acid and di-
O-caffeoyl-3,5-quinic acid, two phytochemicals, were described as possessing human neutrophil elastase (HNE) inhibition activity [
25]. Both reports were in close agreement with the present findings on the human neutrophil elastase (HNE) inhibition activity.
The docking study and free binding energy analysis showed that 3,3′,5-trihydroxy-4′,6,7-trimethoxyflavone (
Figure 2c) had the least binding energy (−9.71 kcal/mol) (
Table 7). Moreover, five ligands (mikamicranolide, syringaldehyde, patuletin, 2,6-dimethoxyquinone, and mikanin-3-
O-sulfate) exhibited interactions with the His518 amino acid residue of LOX. The current finding was in good accord with our previous study, where the compound-3e (Geranylacetophenone derivative) showed interaction with His518 amino acid residue of the LOX enzyme [
26]. Similarly, our earlier study also displayed that 4-hydroxyisoleucine (4-HIL) showed interaction with (i) Ser510; (ii) His513; and (iii) Gln716 amino acid (AA) residues of the LOX enzyme [
16].
Mikania micrantha (leaves and stems—ethyl acetate extract) [
11],
Mikania lindleyana (aerial parts of the plant—methanolic extract), and
Mikania cordata (root—methanolic extract) have been described to have anti-inflammatory properties [
28,
29], whereas three other
Mikania species (
M. glomerata,
M. hirsutissima, and
M. laevigata) have been reported to inhibit 5- lipoxygenase (5-LOX) activity in a dose-dependent manner [
30,
31]. Jyothi Lakshmi [
32] reported the cyclooxygenase (COX), lipoxygenase (LOX), and nitric oxide synthase (iNOS) inhibition activities of
Mikania micrantha (leaf and flower extract). Similarly, (i) 6,7-dihydroxy coumarin, (ii) β- caryophyllene, and (iii) β- caryophyllene oxide have been reported to inhibit 5- lipoxygenase (5-LOX) activity [
33], whereas stigmasterol has been described to inhibit 15- lipoxygenase (15-LOX) activity [
34]. Kaurenoic acid has been reported to have weak lipoxygenase (LOX) inhibition activity [
35]. All the above-mentioned studies are in good correlation with the current results on lipoxygenase (LOX) inhibition activity.
The docking study and binding free energy analysis with MMP 2 showed that 3,5-methyl-di-
O-caffeoylquinate possessed the maximum interaction energy (−83.34 kcal/mol), and five ligands (syringaldehyde, o-coumaric acid, 3,5-methyl-di-
O-caffeoylquinate, 3-
O-mikanin-sulfate, and nepetin) showed interaction with the MMP2 amino acid residue Glu-202 (
Table 8). This observation was in agreement with previous findings, where 4-hydroxyisoleucine (4-HIL) has shown interaction with the (i) Glu202; (ii) Ala165; and (iii) His201 amino acid (AA) residues of the MMP 2 enzyme [
16].
Similarly, in the C-docking study and binding energy analysis with MMP 9, 3,5-methyl-di-
O-caffeoyl quinate exhibited the maximum binding energy (−81.65 kcal/mol), and three ligands (3,5-methyl-di-O-caffeoylquinate, curcumene, and 2,6-dimethoxyquinone) displayed an interaction with His226 amino acid (AA) residue of MMP 9 (
Table 9). The current result was in good correlation with our preceding study, where 3-phenyllactic acid (3-PLA) showed interaction with His226 amino acid (AA) residues of the MMP 9 enzyme [
36]. Stigmasterol has been reported to reduce matrix metalloproteinase 3 (MMP 3) mRNA expression in humans and mouses, MMP 3 protein in mice, and matrix metalloproteinase 13 (MMP 13) mRNA expression in humans and mice [
37]. However, in the present study, stigmasterol failed to dock with both enzymes (MMP 2 and 9).
Docking and energy binding analysis (
Table 10) shows that the provincialin had maximum energy binding (−54.18 kcal/ mol) with the mPGES 2 enzyme (as illustrated in
Figure 2d) and twelve ligands (syringaldehyde, o-coumaric acid, melilotoside, patuletin, 3,5-methyl-di-
O-caffeoylquinate, 4′,6,7-trimethoxyflavone-3,3′,5-trihydroxy, psoralen, herniarin, provincialin, dehydrocostus lactone, mikanin-3-
O-sulfate, and nepetin) had interaction with Arg298 amino acid (AA) residue of mPGES 2. Interestingly, in the present study, all 25 ligands (except for 2,6-dimethoxyquinone) showed docking and binding affinities with microsomal prostaglandin E synthase 2 (mPGES 2). Maione et al. [
38] have reported that the amino acids (i) Cys110, (ii) His241, (iii) His244, (iv) Ser247, (v) Arg292, and (vi) Arg296 are the key binding residues for mPGES 2. However, there are no reports on their mPGES 2 inhibition activity.