Digital Twin Implementation for Manufacturing of Adjuvants
Abstract
1. Introduction
2. Materials and Methods
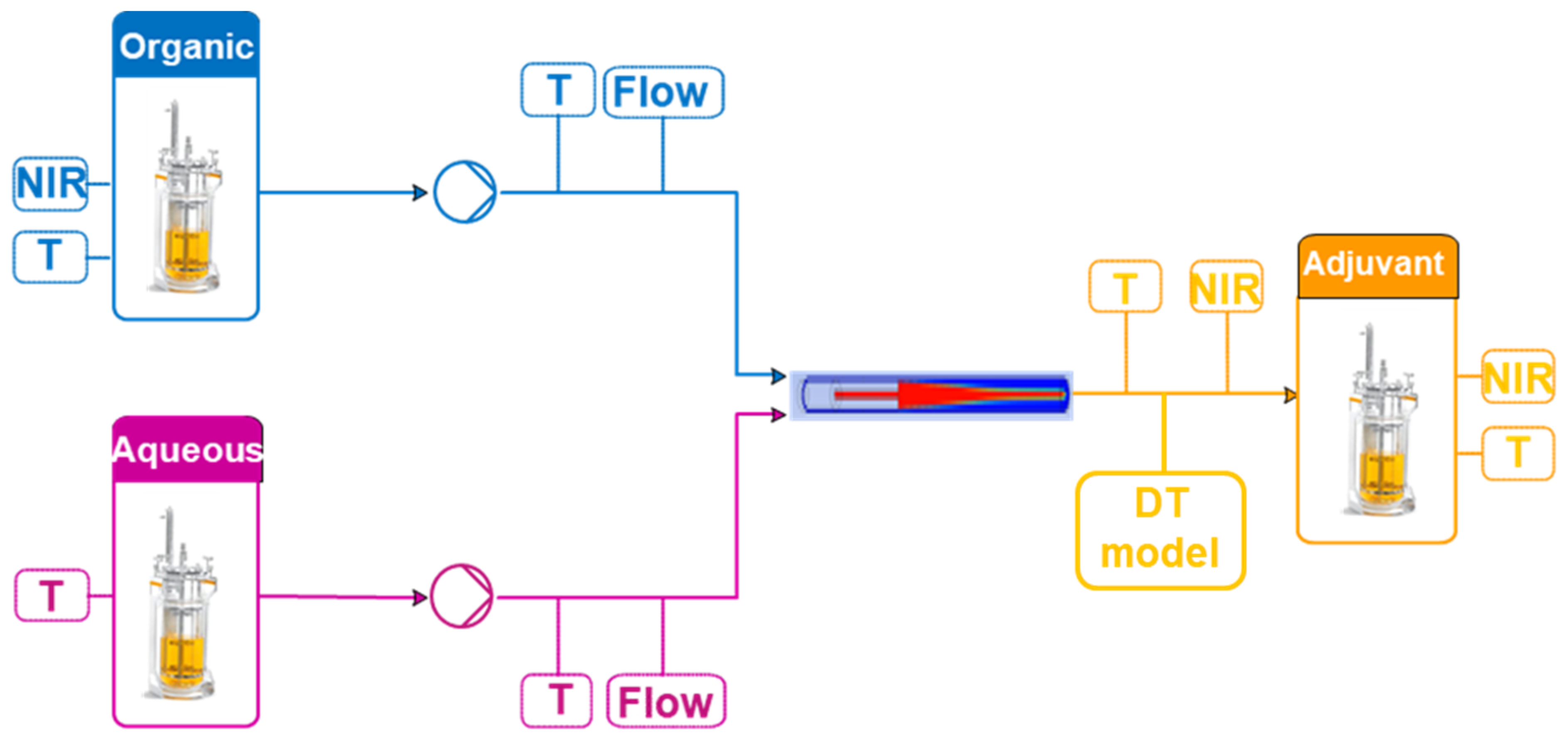
2.1. Process Description
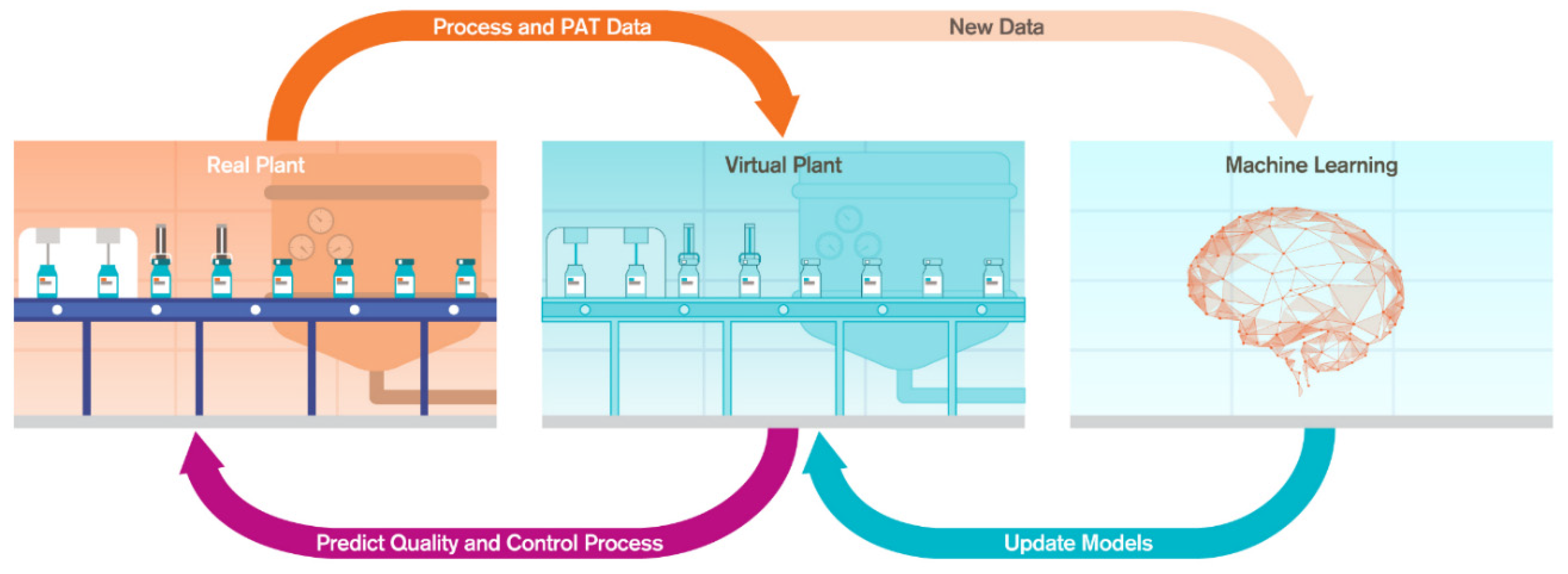
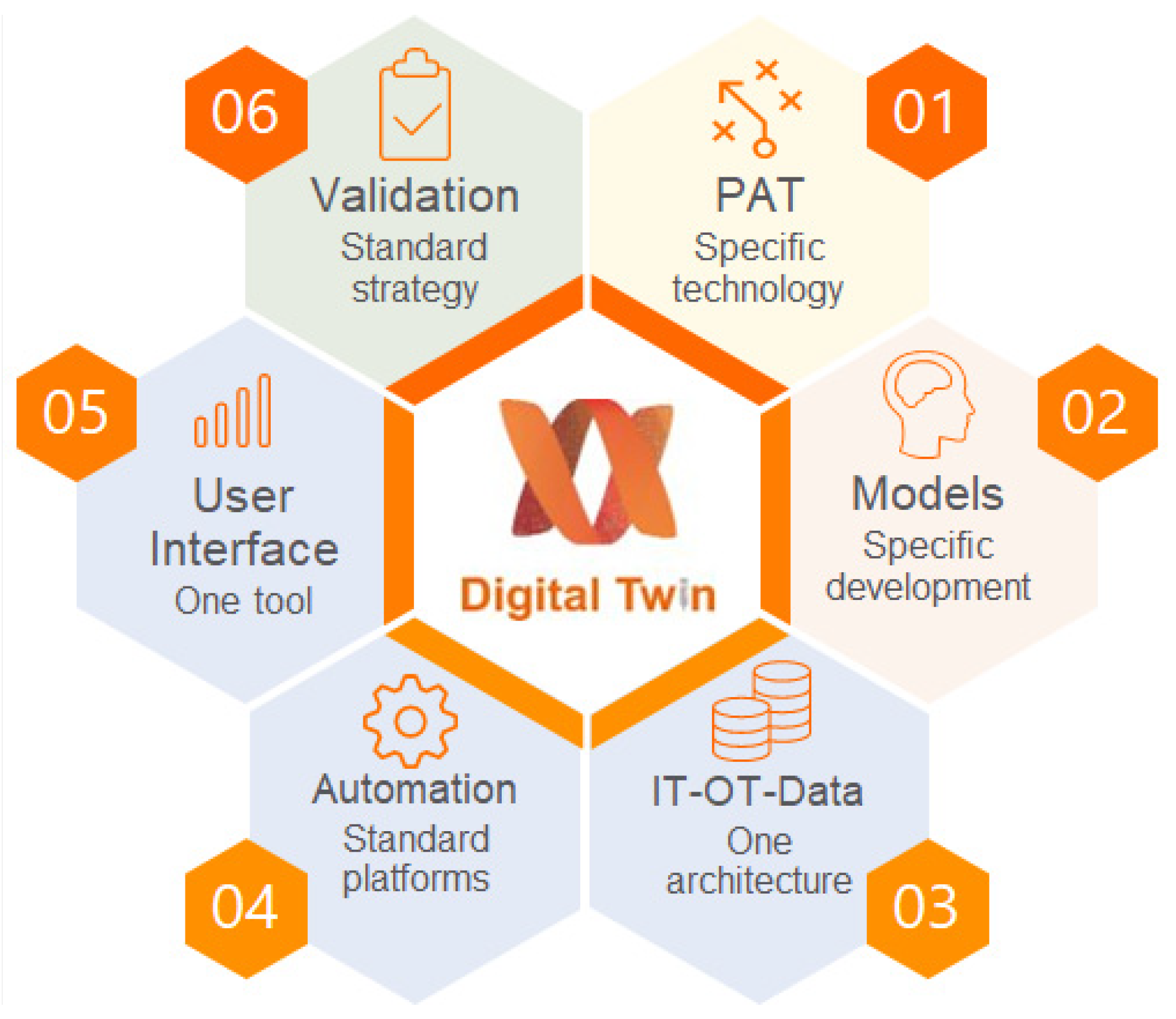
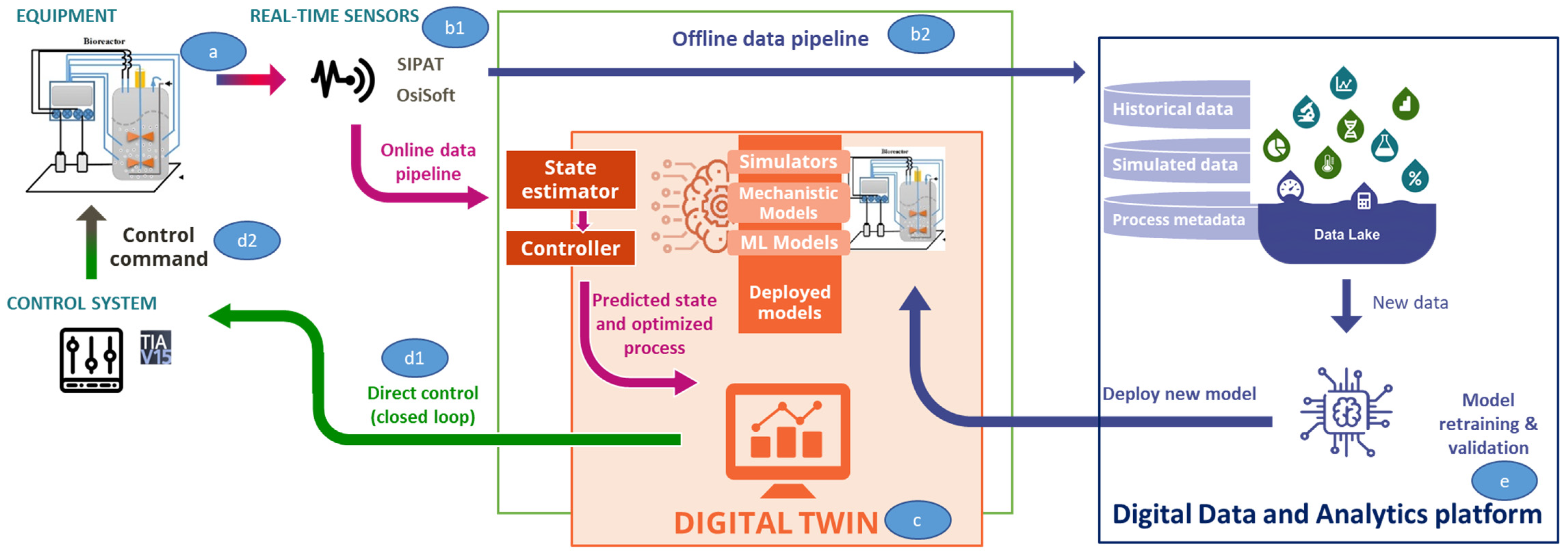
2.2. Digital Twin Components
2.2.1. PAT Tools
2.2.2. Process Models
2.2.3. Control Models
2.2.4. Experimental Data Generation and Model Validation
2.2.5. IT-OT Architecture
Lab Floor
- Lab equipment
- Programmable logic controllers (PLCs): ruggedized industrial computer used to perform control actions on the lab process
- I/O sensors: devices connected to the equipment that detect events or changes and send the information to the data historian
- PAT sensors: PAT capable of measuring in real-time raw materials, intermediates, and products, providing insights on how process variables affect chemistry, bioprocess, or particle-based systems
IT and Automation Systems
- Open platform communication (OPC) server: it allows communication to automation controllers, I/O sensors, and field devices
- Data historian: automation software that records process data in a time-series fashion. In our context, it is used to record equipment and I/O sensors data, as well as ML models predictions and control actions
- PAT software: controls PAT sensors, calibrating them and managing alarms, collects raw data measured by PATs, runs/integrates with statistical and/or physical models to calculate a multivariate quantitative or qualitative representation of the process operation or the products quality attributes, interprets statistical results and exchanges data with the OT data streaming platform
- OT data streaming platform: provides near-real-time data transmission, ingestion and processing within the OT network. It serves as a single point of connection for the different data producers (i.e., data historian, PAT software, etc.) and data consumers (i.e., ML models) and transforms data to match a defined logical data model, minimizing the impact of changes in one system to others. As a result, the digital twin becomes agnostic of the underlying technological components and horizontal, simplifying its application to other labs
- Data pipelines: from the lab to the models to the data lake, through the data streaming platform, both in an online fashion for process monitoring and control and in an offline fashion, for user training and process simulation
Digital Equivalent of the Physical Process
- ML models: State estimator model combined with control models establish the behavior of the physical process which can be solved in real-time
- Docker container: where the models are deployed for process monitoring and control
Closed Loop Systems
- OPC server: see point IT and automation systems above
- Control system: acts on the physical process and implements the identified control actions
- Monitoring and control user interface: shows, in near-real time, the process performances and the control actions
On Edge Cloud Platform
- Data lake: where the process data, predictions and control actions are stored and made available to the end users for further analysis, process simulation and end-user training
- Model versioning and retraining [24]
- Simulation user interface: allows the end users to execute the digital twin models offline and to perform in silico experimentation
2.2.6. User Interface
3. Results
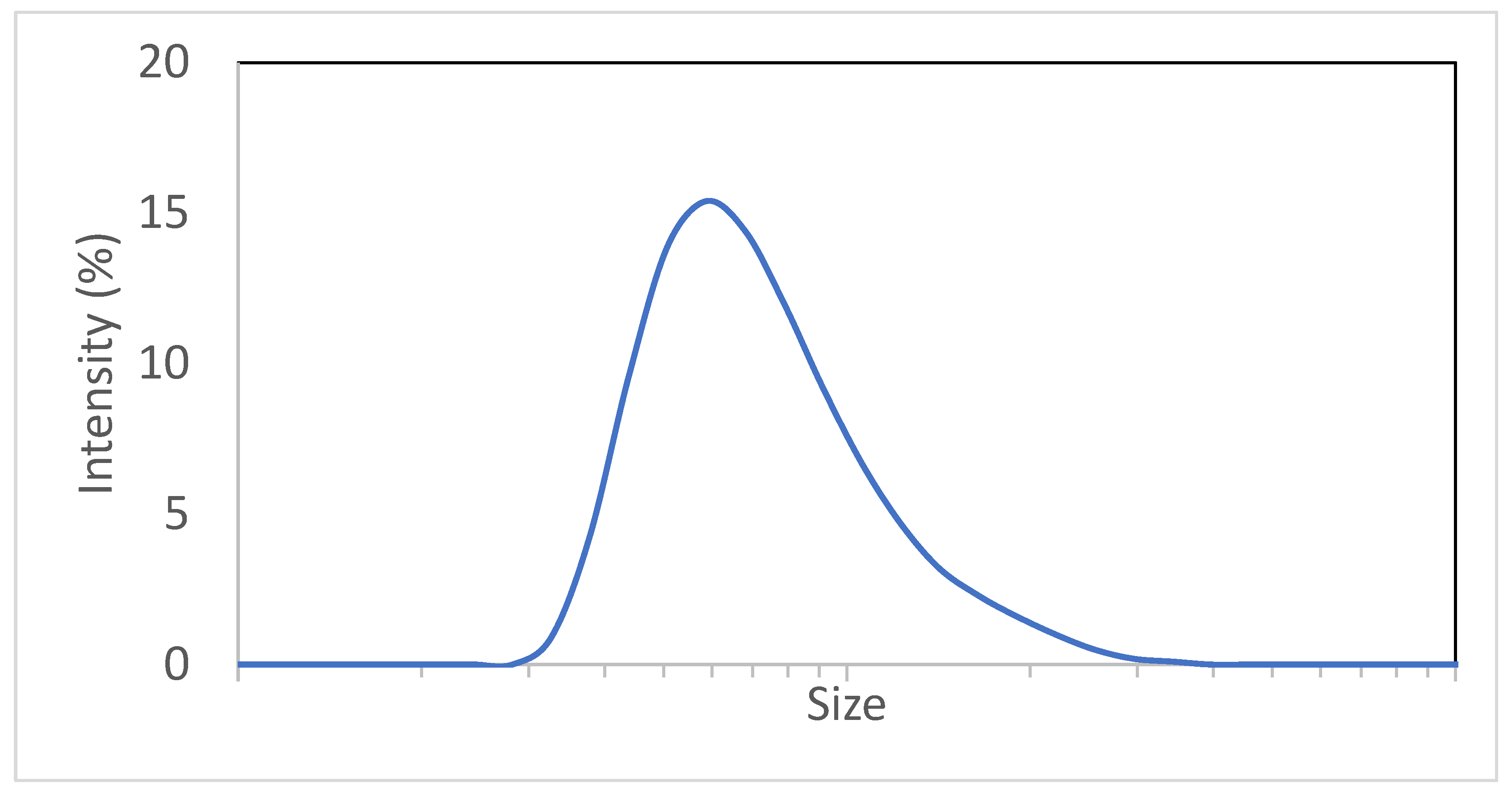
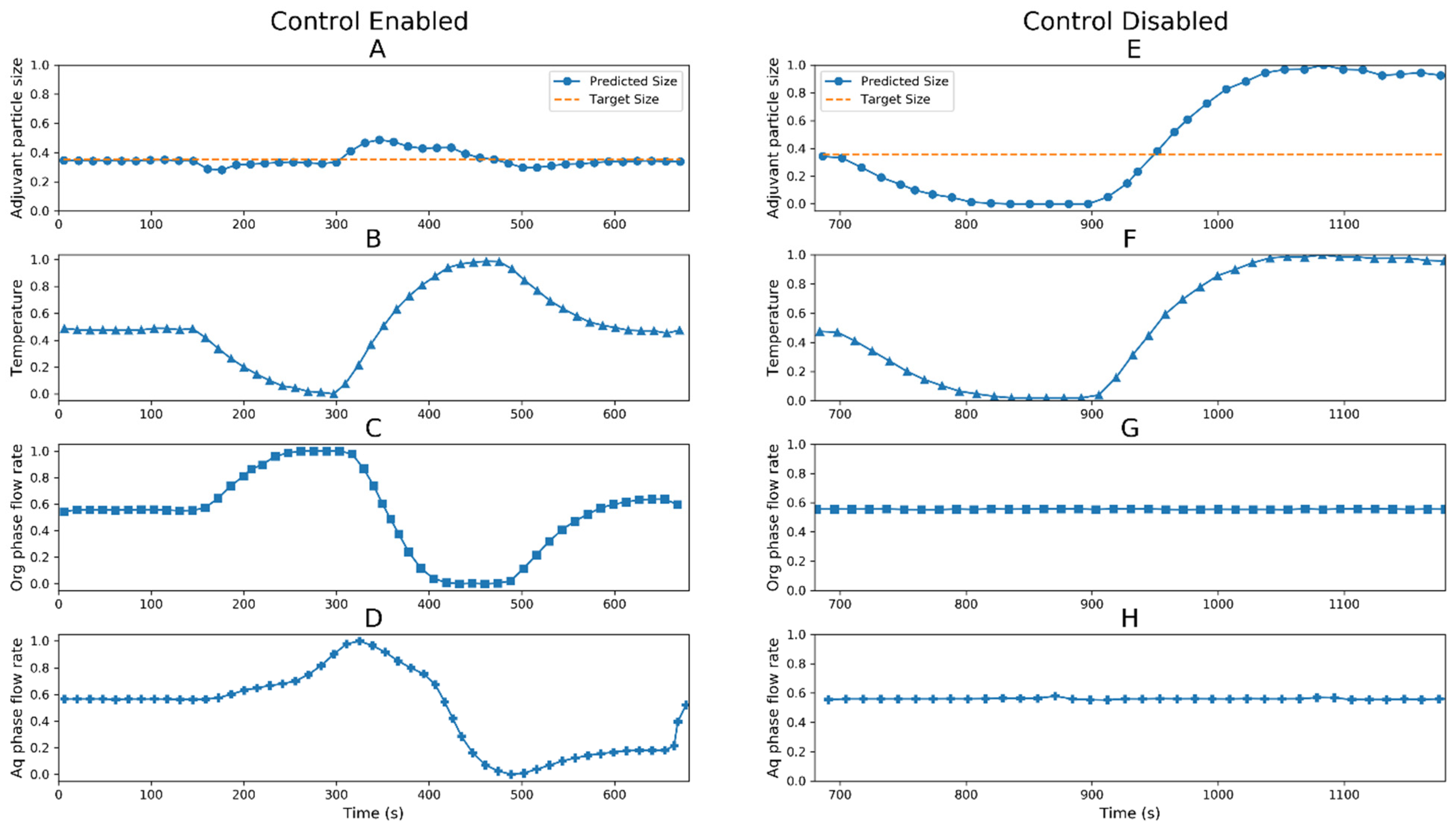
3.1. Digital Twin Proof of Concept Run
3.2. Digital Twin Engineering Run
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sniderman, B.; Mahto, M.; Cotteleer, M. Industry 4.0 and manufacturing ecosystems: Exploring the world of connected enterprises. Deloitte Consult. 2016, 1, 3–14. [Google Scholar]
- Tao, F.; Qi, Q.; Liu, A.; Kusiak, A. Data-driven smart manufacturing. J. Manuf. Syst. 2018, 48, 157–169. [Google Scholar] [CrossRef]
- O’Donovan, P.; Leahy, K.; Bruton, K.; O’Sullivan, D.T. An industrial big data pipeline for data-driven analytics maintenance applications in large-scale smart manufacturing facilities. J. Big Data 2015, 2, 25. [Google Scholar] [CrossRef]
- Lee, J.; Lapira, E.; Yang, S.; Kao, A. Predictive manufacturing system-Trends of next-generation production systems. Ifac Proc. Vol. 2013, 46, 150–156. [Google Scholar] [CrossRef]
- Litster, J.; Bogle, I.D.L. Smart process manufacturing for formulated products. Engineering 2019, 5, 1003–1009. [Google Scholar] [CrossRef]
- Rosen, R.; Von Wichert, G.; Lo, G.; Bettenhausen, K.D. About the importance of autonomy and digital twins for the future of manufacturing. Ifac-Papersonline 2015, 48, 567–572. [Google Scholar] [CrossRef]
- Haag, S.; Anderl, R. Digital twin–Proof of concept. Manuf. Lett. 2018, 15, 64–66. [Google Scholar] [CrossRef]
- Portela, R.; Varsakelis, C.; Richelle, A.; Giannelos, N.; Pence, J.; Dessoy, S.; von Stosch, M. When is an in silico representation a digital twin? A biopharmaceutical industry approach to the digital twin concept. In Digital Twins. Advances in Biochemical Engineering/Biotechnology; Herwig, C., Pörtner, R., Möller, J., Eds.; Springer: Cham, Switzerland, 2020; Volume 176, pp. 35–55. [Google Scholar] [CrossRef]
- Tao, F.; Cheng, J.; Qi, Q.; Zhang, M.; Zhang, H.; Sui, F. Digital twin-driven product design, manufacturing and service with big data. Int. J. Adv. Manuf. Technol. 2018, 94, 3563–3576. [Google Scholar] [CrossRef]
- Shao, G.; Helu, M. Framework for a Digital Twin in Manufacturing: Scope and Requirements. Manuf. Lett. 2020, 24, 105–107. [Google Scholar] [CrossRef]
- Zobel-Roos, S.; Schmidt, A.; Mestmäcker, F.; Mouellef, M.; Huter, M.; Uhlenbrock, L.; Kornecki, M.; Lohmann, L.; Ditz, R.; Strube, J. Accelerating Biologics Manufacturing by Modeling or: Is Approval under the QbD and PAT Approaches Demanded by Authorities Acceptable without a Digital-Twin? Processes 2019, 7, 94. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, O.; Sampat, C.; Bhalode, P.; Ramachandran, R.; Ierapetritou, M. Digital twins in pharmaceutical and biopharmaceutical manufacturing: A literature review. Processes 2020, 8, 1088. [Google Scholar] [CrossRef]
- Krippl, M.; Kargl, T.; Duerkop, M.; Dürauer, A. Hybrid modeling reduces experimental effort to predict performance of serial and parallel single-pass tangential flow filtration. Sep. Purif. Technol. 2021, 276, 119277. [Google Scholar] [CrossRef]
- Möller, J.; Kuchemüller, K.B.; Steinmetz, T.; Koopmann, K.S.; Pörtner, R. Model-assisted design of experiments as a concept for knowledge-based bioprocess development. Bioprocess Biosyst. Eng. 2019, 42, 867–882. [Google Scholar] [CrossRef]
- Baur, D.; Angelo, J.; Chollangi, S.; Muller-Spath, T.; Xu, X.; Ghose, S.; Li, Z.J.; Morbidelli, M. Model-assisted process characterization and validation for a continuous two-column protein A capture process. Biotechnol. Bioeng. 2019, 116, 87–98. [Google Scholar] [CrossRef]
- Mouellef, M.; Vetter, F.L.; Zobel-Roos, S.; Strube, J.J.P. Fast and versatile chromatography process design and operation optimization with the aid of artificial intelligence. Processes 2021, 9, 2121. [Google Scholar] [CrossRef]
- Gerogiorgis, D.I.; Castro-Rodriguez, D. A Digital Twin for Process Optimisation in Pharmaceutical Manufacturing. In Computer Aided Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2021; Volume 50, pp. 253–258. [Google Scholar]
- Davidopoulou, C.; Ouranidis, A.J.P. Pharma 4.0-Artificially Intelligent Digital Twins for Solidified Nanosuspensions. Pharmaceutics 2022, 14, 2113. [Google Scholar] [CrossRef]
- Bhalode, P.; Chen, Y.; Ierapetritou, M. Hybrid Modelling Strategies for Continuous Pharmaceutical Manufacturing within Digital Twin Framework. In Computer Aided Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2022; Volume 49, pp. 2125–2130. [Google Scholar]
- Sokolov, M.; von Stosch, M.; Narayanan, H.; Feidl, F.; Butté, A. Hybrid modeling—A key enabler towards realizing digital twins in biopharma? Curr. Opin. Chem. Eng. 2021, 34, 100715. [Google Scholar] [CrossRef]
- Klepzig, L.S.; Juckers, A.; Knerr, P.; Harms, F.; Strube, J.J.P. Digital twin for lyophilization by process modeling in manufacturing of biologics. Processes 2020, 8, 1325. [Google Scholar] [CrossRef]
- Schmidt, A.; Helgers, H.; Vetter, F.L.; Juckers, A.; Strube, J. Digital Twin of mRNA-Based SARS-COVID-19 Vaccine Manufacturing towards Autonomous Operation for Improvements in Speed, Scale, Robustness, Flexibility and Real-Time Release Testing. Processes 2021, 9, 748. [Google Scholar] [CrossRef]
- Di Pasquale, A.; Preiss, S.; Tavares Da Silva, F.; Garcon, N. Vaccine Adjuvants: From 1920 to 2015 and Beyond. Vaccines 2015, 3, 320–343. [Google Scholar] [CrossRef]
- Canzani, E.; Timmer, S. Beyond building predictive models: TwinOps in biomanufacturing. TechRxiv 2021. preprint. [Google Scholar]
- Alber, M.; Buganza Tepole, A.; Cannon, W.R.; De, S.; Dura-Bernal, S.; Garikipati, K.; Karniadakis, G.; Lytton, W.W.; Perdikaris, P.; Petzold, L. Integrating machine learning and multiscale modeling—Perspectives, challenges, and opportunities in the biological, biomedical, and behavioral sciences. NPJ Digit. Med. 2019, 2, 115. [Google Scholar] [CrossRef]
- Peng, G.C.; Alber, M.; Buganza Tepole, A.; Cannon, W.R.; De, S.; Dura-Bernal, S.; Garikipati, K.; Karniadakis, G.; Lytton, W.W.; Perdikaris, P. Multiscale modeling meets machine learning: What can we learn? Arch. Comput. Methods Eng. 2021, 28, 1017–1037. [Google Scholar] [CrossRef] [PubMed]
- Doddridge, G.; Doherty, S.; Shi, Z.; Huang, T.-K.; Cauchon, N.; Wang, T.; Wisniak, O.; Hutchens, C.; Lequeux, I. Industry Proposal: Regulatory Submission and Lifecycle Management Strategy of Models Used in the Manufacture of Pharmaceuticals and Biological Products. Biophorum 2021, 2021. [Google Scholar] [CrossRef]
- Erdmann, N.; Blumenthal, R.; Baumann, I.; Kaufmann, M. AI Maturity Model for GxP Application: A Foundation for AI Validation. ISPE 2022, 2022. [Google Scholar]
- Food and Drug Amendments of 2022. 2022. Available online: https://republicans-energycommerce.house.gov/wp-content/uploads/2022/05/UFAs-reauth_01_xml.pdf (accessed on 1 June 2022).
- Pardoe, D.; Stone, P. Boosting for regression transfer. In Proceedings of the 27th International Conference on International Conference on Machine Learning Committee for Medicinal Products for Human Use (CHMP), London, UK, 19–22 July 2010; pp. 863–870. Available online: https://www.ema.europa.eu/en/documents/minutes/chmp-prom-minutes-meeting-6-december-2021_en.pdf (accessed on 1 June 2022).
- Artificial Intelligence/Machine Learning (AI/ML)-Based Software as a Medical Device (SaMD) Action Plan. Available online: https://www.fda.gov/media/145022/download (accessed on 1 June 2022).
- Danish Medicines Agency, Questions to Critical GxP AIML Applications Based on Static AIML Algorithms and Supervised Learning V.0.9.4. 2021. Available online: https://laegemiddelstyrelsen.dk/en/licensing/supervision-and-inspection/inspection-of-authorised-pharmaceutical-companies/using-aiml-algorithms-in-gxp/~/media/B02C888935984271BF61BD756ADDAB6B.ashx (accessed on 1 June 2022).


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phalak, P.; Tomba, E.; Jehoulet, P.; Kapitan-Gnimdu, A.; Soladana, P.M.; Vagaggini, L.; Brochier, M.; Stevens, B.; Peel, T.; Strodiot, L.; et al. Digital Twin Implementation for Manufacturing of Adjuvants. Processes 2023, 11, 1717. https://doi.org/10.3390/pr11061717
Phalak P, Tomba E, Jehoulet P, Kapitan-Gnimdu A, Soladana PM, Vagaggini L, Brochier M, Stevens B, Peel T, Strodiot L, et al. Digital Twin Implementation for Manufacturing of Adjuvants. Processes. 2023; 11(6):1717. https://doi.org/10.3390/pr11061717
Chicago/Turabian StylePhalak, Poonam, Emanuele Tomba, Philippe Jehoulet, André Kapitan-Gnimdu, Pablo Martin Soladana, Loredana Vagaggini, Maxime Brochier, Ben Stevens, Thomas Peel, Laurent Strodiot, and et al. 2023. "Digital Twin Implementation for Manufacturing of Adjuvants" Processes 11, no. 6: 1717. https://doi.org/10.3390/pr11061717
APA StylePhalak, P., Tomba, E., Jehoulet, P., Kapitan-Gnimdu, A., Soladana, P. M., Vagaggini, L., Brochier, M., Stevens, B., Peel, T., Strodiot, L., & Dessoy, S. (2023). Digital Twin Implementation for Manufacturing of Adjuvants. Processes, 11(6), 1717. https://doi.org/10.3390/pr11061717





