Abstract
Rapeseed meal (RM) is produced in large quantities as a byproduct of oil extraction from rapeseeds. However, the efficient utilization of RM as animal feed is limited by its low metabolizable energy, poor palatability, and high levels of fiber and anti-nutritional components. Here, we investigate the potential of enriching RM with single-cell protein through fermentation with conventional and unconventional yeasts. The process of simultaneous saccharification and fermentation improved the parameters of the waste biomass, especially the protein content, while reducing the amount of crude fiber and enhancing the biotransformation of isoflavone compounds present in the waste. Fermentation yielded the highest protein gain for the Saccharomyces cerevisiae Ethanol Red strain (ΔN = 2.38%) at a biomass load of 12.5 g and for Scheffersomyces stipitis (ΔN = 2.34%) at an enzyme dose of 0.125 mL/10 g DM. The crude fiber content (CF) was reduced by 2.55–7.18%. The simultaneous saccharification and fermentation (SSF) process resulted in the conversion of isoflavones to forms with fewer adverse effects and a lower estrogenic activity. The results show the potential of using RM as a substrate for making a nutritionally improved feed components.
1. Introduction
Rapeseed meal (RM) is an important agro-industrial byproduct of oil extraction from rapeseeds. Global rapeseed oil production has increased in recent years [1]. As rapeseed oil production increases, so too does the production of waste material. Based on oil production data and market prices in the European Union, the estimated global value of RM waste biomass alone, without additional processing, is in the order of 6.36 billion EUR [1,2]. Biomass enrichment can significantly increase the nominal value of RM waste. The growing world population and Sustainable Development Goals require the valorization of waste by reusing, recycling, or composting and converting it into more useful materials, chemicals, fuels, or energy sources [3]. These activities fit perfectly into the definition of a circular economy.
Rapeseed meal is commonly used as organic fertilizer or biofuel. It can also be dried and used as a feed additive for livestock and poultry, because of its high protein content [4]. Rapeseed meal is mainly composed of protein, fat, fiber (celluloses, hemicelluloses, and lignins), pectin, and minerals with significant amounts of calcium, magnesium, zinc, and copper [5]. Of particular interest is the group of isoflavone compounds in RM, which exhibit a very broad spectrum of functionality. Notably, compounds such as daidzein, daidzin, genistein, genistin, glycitein, and glycitin show estrogenic, neuroprotective, cytostatic, and cytotoxic activities [6,7,8]. However, the efficient utilization of RM as animal feed is limited by its low metabolizable energy; poor palatability; and high levels of fiber and anti-nutritional components, such as glucosinolates, phytic acids, and phenolic compounds.
Agroresidues such as RM offer a cheap, eco-friendly substrate for the growth of microorganisms, which can be used as both a support matrix and a nutrient medium. An example is the production of single-cell protein (SCP) by fermentation with microorganisms [9]. However, the high content of protein, carbohydrates, and minerals in RM cannot be assimilated by the majority of microorganisms. These nutrients can be made more accessible for microorganisms through enzymatic hydrolysis. Microbial treatments can be used to detoxify RM and improve its digestibility during fermentation processes, as well as its functional properties [10,11]. For example, supplementation with lactic acid bacteria (Lactobacillus plantarum and Bacillus clausii) and yeasts (Saccharomyces cariocanus and Wickerhamomyces anomalus) can degrade free gossypol, improving the utilization efficiency of this substrate in the fermentation process [12]. Other studies show that fermentation with Saccharomyces cerevisiae or Saccharomyces boulardii reduces the content of antinutritive factors (e.g., glucosinolates, sinapine, sinapic acid, tannin, and phytic acid) and increases the protein content of the RM, without having major adverse effects on its overall nutritional value [13,14,15].
In this study, we investigate the enrichment of RM with SCP through cultivation with conventional and unconventional yeasts, to make a nutritionally-improved feed component. We determined the protein increment, free nitrogen content, and CF content with various biomass portions, and analyzed the microbial growth, protein increment, free nitrogen concentration, CF content, and flavonoid content after treatment with various enzyme doses. This research is a continuation of earlier work by Dygas et al. [16], which focused on biomass liquefaction, carbohydrate content, microbial growth, and amino acid profiles under various biomass doses.
2. Materials and Methods
2.1. Waste Material and Sample Preparation
The raw material for the research was rapeseed meal (RM) biomass after oil extraction. The waste material was provided by a rapeseed oil producer, Zakłady Tłuszczowe “Kruszwica” S.A. in Kruszwica, Poland (52°40′27″ N 18°19′35″ E). The dry material was hydrated before processing in order to achieve an optimal water content for enzymatic pre-hydrolysis and microbial growth. For this purpose, three different portions of waste biomass, 10 g (A), 12.5 g (B), and 15 g (C), were placed in conical flasks and filled with 90 mL of distilled water. The samples were sterilized at 121 °C for 15 min.
2.2. Enzymatic Pre-Hydrolysis and Simultaneous Saccharification and Fermentation (SSF)
RM biomass is rich in non-starch polysaccharides (pectins, hemicellulose, and cellulose). Moreover, the biomass absorbs added water, leading to a high-density mixture with a low water availability. Despite the increased cost, pre-hydrolysis is thus necessary to ensure efficient microbial growth. The sterilized RM samples were pre-hydrolyzed with the commercial enzymes Rohament® and Rohapect® (AB Enzyme, Darmstadt, Germany), at 50 °C for 4 h. Different doses of enzymes per dry mass of RM (0.5 mL/10 g DM (D); 0.25 mL/10 g DM (E); 0.125 mL/10 g DM (F)) were added to the samples. To avoid enzyme inactivation, the SSF process was used. To prevent the negative influence of ammonium sulfate, an alternative nitrogen source was added after pre-hydrolysis (0.3 g per sample: 0.3% m/m). At this stage, the samples were ready for inoculation and yeast cultivation. The yeast strains used are listed in Table 1.

Table 1.
List of tested yeast strains.
The samples were inoculated with the strains listed in Table 1. Subsequently, SSF was carried out for 48 h at an ambient temperature of approximately 21 °C.
2.3. Determination of the Protein Content
The Kjeldahl method was used to determine the protein fraction. For this purpose, the solid fraction was separated from the post-culture liquid by centrifugation (3000 RCF). The biomass samples were transferred to test tubes, filled with sulfuric acid (15 mL of a solution of 95% m/v), and 4 g of catalyst (3.5 g potassium sulfate and 0.5 g copper sulfate) was added. The samples were heated at 550 °C in a SpeedDigester K-425 for 2 h until a transparent liquid was obtained. The samples were neutralized (30% NaOH) and steam distilled in a KjelFlex K-360 instrument, then titrated automatically with 0.1 mol HCl solution in a TitroLine®5000.
2.4. Determination of the Free Nitrogen (FAN)
The liquid was separated from the solid fraction after culture and transferred to a test tube. To measure the free nitrogen content, the ninhydrin method was applied. Samples were diluted and heated with the addition of ninhydrin reagent. After cooling, the absorbance was measured with a spectrophotometer at a wavelength of λ = 570 nm, against glycine as a reference. Hydrolyzed unfermented rapeseed meal was used as a control. A detailed description of the methodology, including the preparation of reagents, can be found in the Eppendorf protocol [17].
2.5. Determination of the Crude Fiber (CF) Content
The CF content was determined through hot extraction with a 1.25% hydrochloric acid solution and 1.25% potassium hydroxide solution using a FOSS a FibertecTM 8000 apparatus. Drying and ashing were performed in a dryer and muffle furnace. The samples were prepared and the analysis was performed according to EN-ISO 6865:2000 [18].
2.6. Chromatographic Analysis of the Flavonoids
Chromatographic analysis of flavonoid compounds was performed according to the method described in detail by Sulyok et al. [19] and Steglińska et al. [20]. The samples were extracted with an extraction solvent (acetonitrile/water/formic acid of 79:20:1, v/v/v) for 90 min on a GFL rotary shaker (GFL, Burgwedel, Germany). Then, aliquots of the extracts were diluted 1:1 with a dilution solvent (acetonitrile/water/formic acid of 20:79:1, v/v/v). An Agilent 1290 Series HPLC System (Agilent Technologies, Waldbronn, Germany) coupled with a QTrap 5500 m (Sciex, Foster City, CA, USA) and equipped with a Turbo Ion Spray ESI source was used. The compounds were separated using a Gemini®C18 column, 150 × 4.6 mm i.d., 5 µm particle size from Phenomenex (Torrance, CA, USA) protected by a C18 security guard cartridge, 4 × 3 mm i.d. (Phenomenex, Torrance, CA, USA) at 25 °C. The eluent was a methanol/water gradient containing 1% acetic acid and 5 mM ammonium acetate. The elution rate was 1 mL/min. Scheduled multiple reaction monitoring (sMRM) was performed in both negative and positive mode after two separate chromatographic runs per sample for data acquisition. Confirmation of the identity of the metabolites was obtained by comparing the retention time and intensity ratio to a multianalyte standard (SANTE/12089/2016). External quantification was performed on the basis of the linear calibration curves from serial dilutions of a multianalyte standard.
2.7. Statistical Analysis
Statistical analysis was performed using analysis of variance (one-way ANOVA) at a significance level of p ≤ 0.05 using STATISTICA 14.0 (StatSoft, Tulsa, OK, USA) to determine the differences. Post-hoc analysis was performed when a statistical difference was detected (Tukey’s test, significance p ≤ 0.05).
3. Results
3.1. Influence of Different Biomass Loads on Various Parameters
3.1.1. Protein Content Increase with Various Biomass Loads
Hydrolyzed RM before cultivation was used as a control sample. Figure 1 presents the calculated increase in protein content after yeast cultivation (1–10) under various biomass loads (A, B, and C) compared to the control sample.
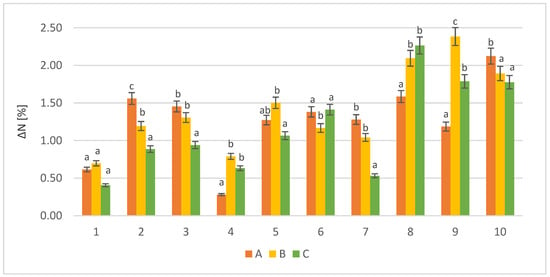
Figure 1.
Protein increase for various loads of waste biomass ΔN (%); a, b, and c—mean values for strains with different letters are significantly different (p < 0.05); A, B, and C—symbols of specific biomass portions (A, 10 g; B, 12.5 g; and C, 15 g); 1–10—yeast strain identifiers according to Table 1.
All of the tested yeast strains showed the ability to grow on RM biomass and increase its protein content. The applied biomass portions were favorable for the cultivation of S. cerevisiae strains (8, 9, and 10) compared with non-conventional yeasts (1–7). For the 10 g samples (A), the highest growth was recorded with S. cerevisiae Tokay (10) (ΔN = 2.12%). Increasing the biomass load to 12.5 g (B) resulted the highest amount of protein (ΔN = 2.38%) with S. cerevisiae Ethanol Red (9). Further increasing the biomass load up to 15 g (C) resulted in the highest biosynthesis of protein by S. cerevisiae TT (8) (ΔN = 2.26%).
3.1.2. Free Amino Nitrogen Content in Sample of Post-Culture Liquid
The centrifuged post-culture liquid was analyzed to determine FAN. Hydrolyzed, non-fermented RM samples were used as the controls. The results showed relatively stable FAN values for the control samples (308 ÷ 321 mg/L) under various biomass loads (A, B, and C) (Figure 2).
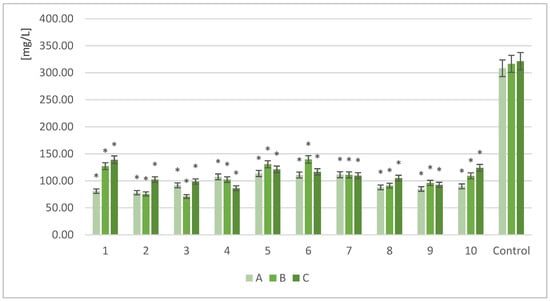
Figure 2.
Free amino nitrogen content in selected samples under various levels of biomass in the sample; *—indicator of statistical difference compared to the control samples (p < 0.05); A, B, and C—symbols of specific biomass portion (A, 10 g; B, 12.5 g; and C, 15 g); 1–10—yeast strain identifiers according to Table 1.
After yeast cultivation, the available FAN was significantly lower in all of the tested samples. All of the tested strains were able to assimilate the nitrogen compounds present in the RM media. For all of the tested strains, the 10 g biomass portion (A) allowed for a significant reduction in the content of nitrogen compounds. For this RM mass loading, the yeast strain Metschnikowia pulcherrima (2) provided the greatest nitrogen assimilation (78.1 mL/L). Similarly, Yarrowia lipolytica (1) was able to decrease nitrogen efficiently. In the samples with higher biomass portions (B and C), reductions in nitrogen assimilation were observed, especially for Y. lipolytica (1), S. cerevisiae TT (8), and S. cerevisiae Tokay (10).
3.2. Influence of Different Doses of Enzymatic Preparation on Production of Feeds
3.2.1. Measurement of Yeast Growth by the Plate Count Method
Yeast growth with different doses of enzymes was monitored using the pour plate method, similar to a previous phase of research by Dygas et al. [16]. Inoculated RM media before cultivation (time t = 0 h) were used as the control samples. Table 2 shows yeast growth (1–10) with different doses of enzymes (0.5 mL/10 g DM (D); 0.25 mL/10 g DM (E); 0.125 mL/10 g DM (F)) during the SSF process. The values in bold indicate a significant difference from the mean value of the control samples (p ≤ 0.05).

Table 2.
Numbers of cells produced by yeasts under the influence of various enzyme doses (CFU/mL).
All of the tested strains were able to grow on the hydrolyzed RM substrate. The highest cell number at the highest enzyme dose (D) was obtained for the strain Y. lipolytica (4.92 × 108 CFU/mL). Reducing the enzyme dose by half (E) provided optimal conditions for the cultivation of S. bayanus, resulting in a cell number of 2.92 × 108 CFU/mL. Further reducing the enzyme amount (F) led to a decrease in yeast growth. Under these conditions, the best growth was noted for the S. stipitis strain.
3.2.2. Protein Content under Different Doses of Enzymatic Preparation
In the next step of the study, we tested the influence of different doses of enzymes on the parameters of the final product. The protein content of the samples was also analyzed. The control sample was hydrolyzed not fermented RM biomass. Figure 3 shows the calculated increase in protein after cultivation with yeasts (1–10) and different enzyme doses (D, E, F) compared with the control samples.
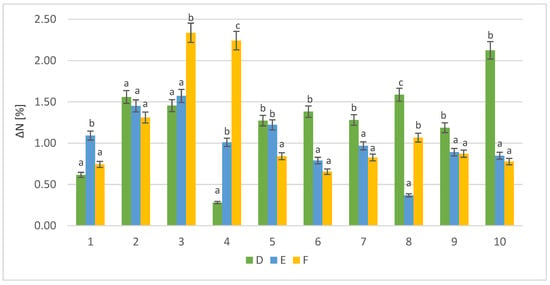
Figure 3.
Protein increase in the solid fraction after fermentation (%); D, E, and F—symbols of specific enzyme doses (D, 0.5 mL/10 g DM; E, 0.25 mL/10 g DM; and F, 0.125 mL/10 g DM); 1–10—strain identifiers according to Table 1; a, b, and c—indicators of statistically significant difference, mean values for strain with different letters are significantly different (p < 0.05).
In the case of the highest dose of enzyme (D), the most effective production of protein was noted for the strain S. cerevisiae Ethanol Red (ΔN = 2.12%). Reducing the volume of the enzyme dose by half (E) resulted in optimal environmental conditions for S. stipitis (ΔN = 1.57%). With the lowest enzyme dose (F), the highest protein increase (ΔN = 2.34%) was also noted for the strain S. stipitis. The combination of the lowest enzyme dose and S. stipitis provided the best conditions for protein biosynthesis.
3.2.3. Crude Fiber Content in Samples with Different Biomass Loads and Enzyme Doses
The CF content was analyzed in samples with different biomass loads and enzyme doses. The yeast strains presenting the greatest protein increase were analyzed. For easy comparison, the same strains were compared at variable enzyme doses. Figure 4 shows the CF content in the selected samples for S. stipitis (3), S. cerevisiae TT (8), S. cerevisiae Ethanol Red (9), and S. cerevisiae Tokay (10) after cultivation with different biomass loads (A, B, and C) and enzyme doses (D, E, and F).
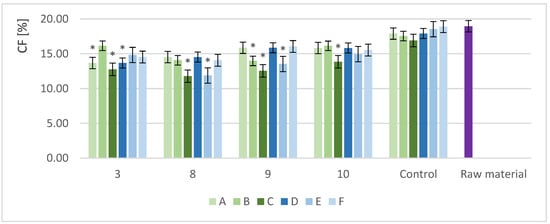
Figure 4.
CF content in the sample solid fraction after fermentation (%); *—indicator of statistical difference compared with the control sample (p < 0.05); 3, 8, 9, and 10—yeasts strain identifiers according to Table 1; A, B, and C—symbols of specific biomass portions (A, 10 g; B, 12.5 g; and C, 15 g); D, E, and F—symbols of specific enzyme doses (D, 0.5 mL/10 g DM; E, 0.25 mL/10 g DM; and F, 0.125 mL/10 g DM).
After fermentation, the biomass was separated from the liquid by centrifugation. The CF content in the solid fraction was determined. Raw RM was used as the reference material, and a pre-hydrolyzed non-fermented sample was used as the control sample. All of the samples showed a reduction in CF after pre-hydrolysis and SSF. The CF content ranged from 11.8% to 16.1% dry mass for the different biomass loads and from 11.9% to 15.9% for the different enzyme doses. Higher biomass loads had a positive effect on the efficiency of fiber degradation. The lowest amounts of CF were determined in the samples with RM biomass loads of 15 g (C) after the cultivation of S. cerevisiae TT (8). The results for biomass load (C) were statistically different from the control. Decreasing the load of biomass lowered the efficiency of fiber degradation in most of the cases. The results for the different enzyme doses ranged from 11.9% to 15.9%, with the lowest CF content also obtained for S. cerevisiae TT (E).
3.2.4. Flavonoid Content after Fermentation
The flavonoid content was analyzed according to the methodology described by Sulyok et al. [19]. The two samples with the greatest protein increment, S. stipitis with an enzyme dose of 0.125 mL/10 g DM (3 F) and S. cerevisiae Ethanol Red with a 12.5 g biomass load (9 B), were chosen for the analysis of the flavonoids content. Untreated raw RM biomass was used as a control, in order to compare the obtained product to the form commonly used as a feed additive (Table 3).

Table 3.
Concentration of flavonoids in the biomass samples (ng/g).
As a result of fermentation, the daidzein content increased by around 1.5 times compared with the control sample. A similar phenomenon was noticed for genistein, which increased by between 1.4 µg/g and 2.1 µg/g. The content of daidzin, a precursor of daidzein, was drastically reduced, around 250–460 times compared with the untreated biomass. A similar tendency was observed for genistin, a precursor of genistein, which was reduced by between 28 and 176 times compared with the control sample. The glycitein transformation was exceptional. Fermentation with 12.5 g of biomass and S. cerevisiae Ethanol Red resulted in a decrease of 206.1 ng/g. However, fermentation with 0.125 mL/10 g DM and S. stipitis led to an increase in glycitein content of 283.2 ng/g. The content of glycitin after fermentation was beneath the detection threshold (1.4 ng/g was the limit of detection (LOD)), from 53 mg/g before the process.
4. Discussion
Rapeseed meal is a waste product of the oil extraction process. Its composition varies depending on a range of factors, including origin, growing conditions, manufacturing processes, and the degree of oil extraction. Rapeseed meal has functional biological value as an animal feed, due to its protein and CF content [21]. However, because of the presence of anti-nutritional factors, RM is currently not fully used as a feed additive. Glucosinolates, phytic acid, tannin, sinapine, cellulose, and lignin are the main antinutritional factors in RM [22]. Biological methods appear to be the most researched strategy for improving RM quality. Microbiological methods refer to the use of microbial cell propagation (single cell protein) and secretion-related enzymes to effectively decompose antinutritional factors through fermentation. Fermentation of RM, either singly or in combination, can significantly improve the protein content, peptide hydrolysis, and detoxification effects.
In this study, we investigated the possibility of improving the nutritional value of RM in the SSF process, using different conventional and non-conventional yeasts. The greatest increase in protein content was observed in the RM samples after cultivation with Saccharomyces cerevisiae. This was probably due to the metabolic capabilities of this yeast, which is able to grow in conditions of reduced oxygen availability [23]. The lower protein content obtained after fermentation with unconventional strains was due to the limited adaptability of these yeasts to the conditions of low oxygen availability. Yarrowia lipolytica is able to metabolize different carbon sources [24]. However, our results indicate that it does not excel in its ability to increase the protein content of RM biomass. Candida spp. also did not increase the protein content dramatically. Its productivity depends not only on the composition of the medium, but also on the presence of oxygen in the environment [25,26]. A similar metabolism was demonstrated by Metschnikowia sp. However, the use of M. pulcherrima yeast, which inhibits the growth of fungal pathogens, can improve both the nutritional value of RM biomass and its microbial stability [27].
The increase in protein content of 2–3% dry matter obtained in this study may not seem very significant, but the change in the proportion of plant proteins in favor of yeast protein improves the feed value. RM is characterized by a high protein content, reaching almost 40% [21], but the degree of digestibility of this protein is about 68–70% [28]. Comparing this value with the digestibility of proteins of a microbial origin, which is over 94% for S. cerevisiae [29], clearly indicates the potential for improving the nutritional value of RM biomass as an animal feed. The fermentation of RM with yeast is important not only to increase protein digestibility, but also to improve the amino acid profile of the feed. Yeast biomass contains a rich pool of essential amino acids, as shown in a previous study by Dygas et al. [16].
The FAN content in the post-culture liquid was analyzed. Measuring the FAN content in the liquid fraction of the waste material after fermentation makes it possible to control the efficiency of nitrogen assimilation and protein synthesis. The FAN content showed statistically significant differences for all of the strains compared with the non-fermented sample. It can be concluded that the tested strains were able to assimilate nitrogen at different rates. The different amounts of residual nitrogen in the samples after fermentation may be due to the different biomass loads and nitrogen sources. There were two possible sources of nitrogen: ammonium sulphate, which was added in the sample preparation stage, and nitrogen from the RM biomass. Thus, increasing the load of RM biomass in the fermentation process increased the pool of available nitrogen for assimilation. The results show that the optimal RM dose needs to be selected individually for each strain of yeast. In the case of unconventional, highly aerobic yeasts, weaker nitrogen assimilation should be expected due to the low concentration of oxygen in the culture medium [30]. Generally, fermentation with mixed cultures seems to be more efficient than with monocultures. In mixed systems, aerobic unconventional yeasts can conduct fermentation first, before facultative anaerobic fermentation conducted by conventional S. cerevisiae strains.
To improve the culture conditions, it is worth considering enzymatic pretreatment of raw RM or conducting the process under SSF conditions. The combined fermentation of RM can not only improve the degree of protein hydrolysis in RM, but also enhance the solubility of proteins [31]. Some authors have used combined microbial fermentation with enzymatic hydrolysis to improve microbial growth and enzymatic activity [22]. In our study, lowering the dosage of the enzyme preparation during pre-hydrolysis decreased the number of cells produced by the tested yeasts. This is because lowering the enzyme dose led to a lower rate of depolymerization of the compounds present in the RM. With a lower dose of enzyme, the nutrients in RM are less available to microorganisms [32]. The increase in protein content was not strongly correlated with a decreasing enzyme dose. It can be assumed that the efficiency of nitrogen bioconversion is a strain-specific property. Depending on the environmental conditions, the strains used in the study reached their maxima of protein synthesis efficiency at different enzyme concentrations.
This study focused on the influence of SSF as an integral, non-separable stage of the fermentation process. The pre-hydrolysis process provided the initial decomposition of CF, which resulted in partial liquefaction of the RM biomass and resulted in environmental conditions more favorable for yeast growth. The SSF process successively led to the decomposition of CF in the RM, as a result of the action of enzymes in the applied commercial preparations and yeast catalysis. The tested yeast strains showed various enzymatic capabilities, as discussed in previous work by Dygas et al. [33]. Additional research is required to identify which enzymes had the greatest role in CF decomposition. The CF content is one of the most important parameters for the use of a waste material as a feed component. Reducing the CF content can increase the potential of using RM as an animal feed component by increasing its digestibility [34]. The CF content determines the use of a material as a feed for a particular animal. Depending on its life stage, an animal requires a different feed composition, including a specific CF content. A fibrous diet can reduce the feeding motivation of sows. However, it simultaneously extends the feeding time [35,36]. In the case of cattle, the supplementation of feed additives rich in CF may result in increased daily milk yield and improved milk quality [37].
The nutritional value of feed can be improved not only by increasing the protein content, but also by changing the content of anti-nutritional compounds, namely isoflavones. Genistein, daidzein, and glycitein show interesting biological activities in animals. They reside as glycosides with a low estrogenic activity compared with their deglycosylated form, also referred to as aglycone. Upon ingestion, these compounds are metabolically hydrolyzed by the intestinal microbiota to their aglycones [38]. However, several different factors may influence the biokinetics and bioavailability of isoflavones, such as the kind of animal, intestinal microflora, age, composition of feed, and duration of feed consumption [39]. The phenolic hydroxyl group in isoflavones can react with free radicals. The literature data show that eliminating soybean isoflavones from the diet may decrease the body weight and antioxidant capacity. Replenishing soybean isoflavones prevents a decrease in these parameters, indicating that isoflavones are beneficial for pig growth and play an essential role in antioxidation [40]. These isoflavones have also been shown to prevent atherosclerotic cardiovascular diseases in hypertensive rats [41].
After yeast fermentation, the daidzein and genistein content increased, indicating positive isoflavone conversion processes. Glycitein remained rather constant. Hu et al. showed that dietary supplementation with glycitein in sows during late pregnancy and lactation can elevate antioxidative indices, improving milk composition and enhancing the growth performance of sucking piglets [42]. The results for the total isoflavone content before and after yeast fermentation are also interesting. The content of these compounds was reduced about four-fold. Reducing the number of flavonoids and changing the proportions of glycosides and aglycones is thus more beneficial in terms of nutrition. After yeast fermentation, the ratios of glycosides:genistein and of daidzein:daidzin and genistin changed. Daidzein has a lower estrogenic activity compared with its deglycosylated forms. The glycitin content was reduced after fermentation to below the detection limit. This is of nutritional importance, because excessive isoflavones in the diet can have negative effects of animal reproduction. There are reports of negative outcomes after a high consumption of isoflavones, including an enhanced rate of endometriosis, the inability to become pregnant, and miscarriage [38]. Discrepancies between the content of isoflavone compounds in the control sample and the samples after fermentation were because the intermediate forms of flavonoids, such as dihydrodaidzein or dihydrogenistein, were not determined in the analysis [43].
5. Conclusions
Rapeseed meal is a very valuable waste product from rapeseed oil production, with a high management potential. Using a simple technology, it is possible to obtain a full-value product and increase profit margins greatly. The SSF process and properly selecting the yeast strains may improve the parameters of RM, especially the protein content, the amount of CF, and the transformation of anti-nutritional isoflavones. The biotransformation technology used changed both the content and quality of the available proteins, reduced the CF content, and changed the content of the anti-nutritional compounds. These improved parameters may increase the digestibility of RM and improve its nutritional value as a feed component. In the future, RM, with a high nutritional value and low toxicity, could play a greater role and make important contributions to alleviate the lack of protein resources in the feed sector.
Author Contributions
Conceptualization, J.B.; methodology, J.B. and D.D.; investigation, D.D., A.S., W.L. and M.S.; resources, D.D.; data curation, D.D.; writing—original draft preparation, D.D.; writing—review and editing, D.D. and J.B.; supervision, J.B. and D.K.; project administration, J.B.; funding acquisition, J.B. All authors have read and agreed to the published version of the manuscript.
Funding
The APC was funded by Lodz University of Technology.
Data Availability Statement
The data used in this study are included in the article.
Acknowledgments
This research was completed while the first three authors were doctoral candidates at the Interdisciplinary Doctoral School, Lodz University of Technology (Poland).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Shahbandeh, M. Rapeseed Oil: Global Production Volume 2012/13–2022/23; Statista: Hamburg, Germany, 2023; Available online: https://www.statista.com/statistics/613487/rapeseed-oil-production-volume-worldwide/ (accessed on 3 April 2023).
- Lemaire, A.; Limbourg, S. How Can Food Loss and Waste Management Achieve Sustainable Development Goals? J. Clean. Prod. 2019, 234, 1221–1234. [Google Scholar] [CrossRef]
- Zentrale Markt-und Preisinformationen Rapeseed Meal Current Stock Exchange Prices & Charts Rapeseed Meal. Available online: https://www.zmp.de/en/oilseeds/rapeseed-meal_future (accessed on 3 April 2023).
- Mattila, P.; Pap, N.; Järvenpää, E.; Kahala, M.; Mäkinen, S. Underutilized Northern Plant Sources and Technological Aspects for Recovering Their Polyphenols. Adv. Food Nutr. Res. 2021, 98, 125–169. [Google Scholar] [PubMed]
- De Corato, U.; Viola, E. Biofuel Co-Products for Livestock Feed. In Agricultural Bioeconomy; Academic Press: Cambridge, MA, USA, 2023; pp. 245–286. [Google Scholar]
- Rafii, F. The Role of Colonic Bacteria in the Metabolism of the Natural Isoflavone Daidzin to Equol. Metabolites 2015, 5, 56–73. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.-Y.; Ye, Y.; Xiao, L.; Rahman, K.; Xia, W.; Zhang, H. Daidzein: A Review of Pharmacological Effects. Afr. J. Tradit. Complement. Altern. Med. 2016, 13, 117. [Google Scholar] [CrossRef]
- Ahmed, T.; Javed, S.; Tariq, A.; Budzyńska, B.; D’Onofrio, G.; Daglia, M.; Fazel Nabavi, S.; Mohammad Nabavi, S. Daidzein and Its Effects on Brain. Curr. Med. Chem. 2017, 24, 365–375. [Google Scholar] [CrossRef]
- Tropea, A.; Ferracane, A.; Albergamo, A.; Potortì, A.G.; Lo Turco, V.; Di Bella, G. Single Cell Protein Production through Multi Food-Waste Substrate Fermentation. Fermentation 2022, 8, 91. [Google Scholar] [CrossRef]
- Gong, Y.; Liu, J.; Jiang, M.; Liang, Z.; Jin, H.; Hu, X.; Wan, X.; Hu, C. Improvement of Omega-3 Docosahexaenoic Acid Production by Marine Dinoflagellate Crypthecodinium Cohnii Using Rapeseed Meal Hydrolysate and Waste Molasses as Feedstock. PLoS ONE 2015, 10, e0125368. [Google Scholar] [CrossRef]
- Lomascolo, A.; Uzan-Boukhris, E.; Sigoillot, J.C.; Fine, F. Rapeseed and Sunflower Meal: A Review on Biotechnology Status and Challenges. Appl. Microbiol. Biotechnol. 2012, 95, 1105–1114. [Google Scholar] [CrossRef]
- Yusuf, H.A.; Piao, M.; Ma, T.; Huo, R.; Tu, Y. Effect of Lactic Acid Bacteria and Yeast Supplementation on Anti-Nutritional Factors and Chemical Composition of Fermented Total Mixed Ration Containing Cottonseed Meal or Rapeseed Meal. Anim. Biosci. 2022, 35, 556–566. [Google Scholar] [CrossRef]
- Vlassa, M.; Filip, M.; Țăranu, I.; Marin, D.; Untea, A.E.; Ropotă, M.; Dragomir, C.; Sărăcilă, M. The Yeast Fermentation Effect on Content of Bioactive, Nutritional and Anti-Nutritional Factors in Rapeseed Meal. Foods 2022, 11, 2972. [Google Scholar] [CrossRef]
- Bau, H.-M.; Villaume, C.; Lin, C.-F.; Evrard, J.; Quemener, B.; Nicolas, J.-P.; Méjean, L. Effect of a Solid-state Fermentation Using Rhizopus Oligosporus Sp.T-3 on Elimination of Antinutritional Substances and Modification of Biochemical Constituents of Defatted Rapeseed Meal. J. Sci. Food Agric. 1994, 65, 315–322. [Google Scholar] [CrossRef]
- Mandiki, S.N.M.; Derycke, G.; Bister, J.L.; Mabon, N.; Wathelet, J.P.; Marlier, M.; Paquay, R. Chemical Changes and Influences of Rapeseed Antinutritional Factors on Gestating and Lactating Ewes 1. Animal Performances and Plasma Hormones and Glucose. Anim. Feed Sci. Technol. 2002, 98, 25–35. [Google Scholar] [CrossRef]
- Dygas, D.; Janicka, P.; Berłowska, J.; Kręgiel, D. Conventional and Unconventional Yeasts Able to Grow on Rapeseed Meal Hydrolysates. BioResources 2022, 17, 3082–3094. [Google Scholar] [CrossRef]
- Geisler, J.; Weiß, N. Free Amino Nitrogen (FAN) Measurement in Beer. Short Protoc. 2015, 9, 10–12. [Google Scholar]
- ISO 6865:2000; Animal Feeding Stuffs—Determination of Crude Fibre Content—Method with Intermediate Filtration. ISO: Geneva, Switzerland, 2000; p. 10.
- Sulyok, M.; Stadler, D.; Steiner, D.; Krska, R. Validation of an LC-MS/MS-Based Dilute-and-Shoot Approach for the Quantification of > 500 Mycotoxins and Other Secondary Metabolites in Food Crops: Challenges and Solutions. Anal. Bioanal. Chem. 2020, 412, 2607–2620. [Google Scholar] [CrossRef]
- Steglińska, A.; Sulyok, M.; Janas, R.; Grzesik, M.; Liszkowska, W.; Kręgiel, D.; Gutarowska, B. Metabolite Formation by Fungal Pathogens of Potatoes (Solanum tuberosum L.) in the Presence of Bioprotective Agents. Int. J. Environ. Res. Public Health 2023, 20, 5221. [Google Scholar] [CrossRef]
- Bell, J.M. Nutrients and Toxicants in Rapeseed Meal: A Review. J. Anim. Sci. 1984, 58, 996–1010. [Google Scholar] [CrossRef]
- Yang, Z.; Huang, Z.; Cao, L. Biotransformation technology and high-value application of rapeseed meal: A review. Bioresour. Bioprocess. 2022, 9, 103. [Google Scholar] [CrossRef]
- Parapouli, M.; Vasileiadis, A.; Afendra, A.-S.; Hatziloukas, E.; Parapouli, M.; Vasileiadis, A.; Afendra, A.-S.; Hatziloukas, E. Saccharomyces Cerevisiae and Its Industrial Applications. AIMS Microbiol. 2020, 6, 1–31. [Google Scholar] [CrossRef]
- Liu, H.H.; Ji, X.J.; Huang, H. Biotechnological Applications of Yarrowia Lipolytica: Past, Present and Future. Biotechnol. Adv. 2015, 33, 1522–1546. [Google Scholar] [CrossRef]
- Postma, E.; Kuiper, A.; Tomasouw, W.F.; Scheffers, W.A.; Van Dijken, J.P. Competition for Glucose between the Yeasts Saccharomyces Cerevisiae and Candida Utilis. Appl. Environ. Microbiol. 1989, 55, 3214–3220. [Google Scholar] [CrossRef]
- Carranza-Méndez, R.C.; Chávez-González, M.L.; Sepúlveda-Torre, L.; Aguilar, C.N.; Govea-Salas, M.; Ramos-González, R. Production of Single Cell Protein from Orange Peel Residues by Candida Utilis. Biocatal. Agric. Biotechnol. 2022, 40, 102298. [Google Scholar] [CrossRef]
- Freimoser, F.M.; Rueda-Mejia, M.P.; Tilocca, B.; Migheli, Q. Biocontrol Yeasts: Mechanisms and Applications. World J. Microbiol. Biotechnol. 2019, 35, 154. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, H.; Han, B.; Li, H.Y.; Liu, X.L. Improvement of Nutritional Value, Molecular Weight Patterns (Soluble Peptides), Free Amino Acid Patterns, Total Phenolics and Antioxidant Activity of Fermented Extrusion Pretreatment Rapeseed Meal with Bacillus subtilis YY-1 and Saccharomyces cerevisiae Y. LWT 2022, 160, 113280. [Google Scholar] [CrossRef]
- Bertolo, A.P.; Biz, A.P.; Kempka, A.P.; Rigo, E.; Cavalheiro, D. Yeast (Saccharomyces cerevisiae): Evaluation of Cellular Disruption Processes, Chemical Composition, Functional Properties and Digestibility. J. Food Sci. Technol. 2019, 56, 3697–3706. [Google Scholar] [CrossRef]
- Uçkun Kiran, E.; Salakkam, A.; Trzcinski, A.P.; Bakir, U.; Webb, C. Enhancing the Value of Nitrogen from Rapeseed Meal for Microbial Oil Production. Enzyme Microb. Technol. 2012, 50, 337–342. [Google Scholar] [CrossRef]
- Poulsen, H.D.; Blaabjerg, K. Fermentation of rapeseed meal, sunflower meal and faba beans in combination with wheat bran increases solubility of protein and phosphorus. J. Sci. Food Agric. 2017, 97, 244–251. [Google Scholar] [CrossRef]
- Jeong, H.S.; Kim, H.Y.; Ahn, S.H.; Oh, S.C.; Yang, I.; Choi, I.G. Optimization of Enzymatic Hydrolysis Conditions for Extraction of Pectin from Rapeseed Cake (Brassica napus L.) Using Commercial Enzymes. Food Chem. 2014, 157, 332–338. [Google Scholar] [CrossRef]
- Dygas, D.; Nowak, S.; Olszewska, J.; Szymańska, M.; Mroczyńska-Florczak, M.; Berłowska, J.; Dziugan, P.; Kręgiel, D. Ability of Yeast Metabolic Activity to Reduce Sugars and Stabilize Betalains in Red Beet Juice. Fermentation 2021, 7, 105. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, L.; Zhang, Z.; Ding, L.; Hang, S. Combination of Fiber-Degrading Enzymatic Hydrolysis and Lactobacilli Fermentation Enhances Utilization of Fiber and Protein in Rapeseed Meal as Revealed in Simulated Pig Digestion and Fermentation in Vitro. Anim. Feed. Sci. Technol. 2021, 278, 115001. [Google Scholar] [CrossRef]
- Ramonet, Y.; Meunier-Salaün, M.C.; Dourmad, J.Y. High-Fiber Diets in Pregnant Sows: Digestive Utilization and Effects on the Behavior of the Animals. J. Anim. Sci. 1999, 77, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Guillemet, R.; Hamard, A.; Quesnel, H.; Père, M.C.; Etienne, M.; Dourmad, J.Y.; Meunier-Salaün, M.C. Dietary Fibre for Gestating Sows: Effects on Parturition Progress, Behaviour, Litter and Sow Performance. Animal 2007, 1, 872–880. [Google Scholar] [CrossRef] [PubMed]
- Grigorev, M.; Grigoreva, A.; Sharvadze, R.; Chernogradskaya, N.; Stepanova, S. The Effectiveness of Unconventional Feed Additives at Feeding Cattle in Conditions Yakutia. Lect. Notes Netw. Syst. 2023, 574, 156–166. [Google Scholar] [CrossRef]
- Grgic, D.; Varga, E.; Novak, B.; Müller, A.; Marko, D. Isoflavones in Animals: Metabolism and Effects in Livestock and Occurrence in Feed. Toxins 2021, 13, 836. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, X.; Cai, L.; Zhang, Y.; Ding, H.; Yin, J.; Li, X. Effects of Daidzein on Antioxidant Capacity in Weaned Pigs and IPEC-J2 Cells. Anim. Nutr. 2022, 11, 48–59. [Google Scholar] [CrossRef]
- Li, Y.P.; Jiang, X.R.; Wei, Z.X.; Cai, L.; Yin, J.D.; Li, X.L. Effects of Soybean Isoflavones on the Growth Performance, Intestinal Morphology and Antioxidative Properties in Pigs. Animal 2020, 14, 2262–2270. [Google Scholar] [CrossRef]
- Pan, W.; Ikeda, K.; Takebe, M.; Yamori, Y. Genistein, Daidzein and Glycitein Inhibit Growth and DNA Synthesis of Aortic Smooth Muscle Cells from Stroke-Prone Spontaneously Hypertensive Rats. J. Nutr. 2001, 131, 1154–1158. [Google Scholar] [CrossRef]
- Hu, Y.J.; Gao, K.G.; Zheng, C.T.; Wu, Z.J.; Yang, X.F.; Wang, L.; Ma, X.Y.; Zhou, A.G.; Jiang, Z.J. Effect of Dietary Supplementation with Glycitein during Late Pregnancy and Lactation on Antioxidative Indices and Performance of Primiparous Sows. J. Anim. Sci. 2015, 93, 2246–2254. [Google Scholar] [CrossRef]
- Smith, B.N.; Dilger, R.N. Immunomodulatory Potential of Dietary Soybean-Derived Isoflavones and Saponins in Pigs. J. Anim. Sci. 2018, 96, 1288–1304. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).