Hydrothermal Leaching of Amylose from Native, Oxidized and Heat-Treated Starches
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods of Modification of Starch
2.3. Microscopic Studies of Starch
2.4. Methodology of Kinetic Measurements
2.5. Thin-Layer Chromatographic Studies
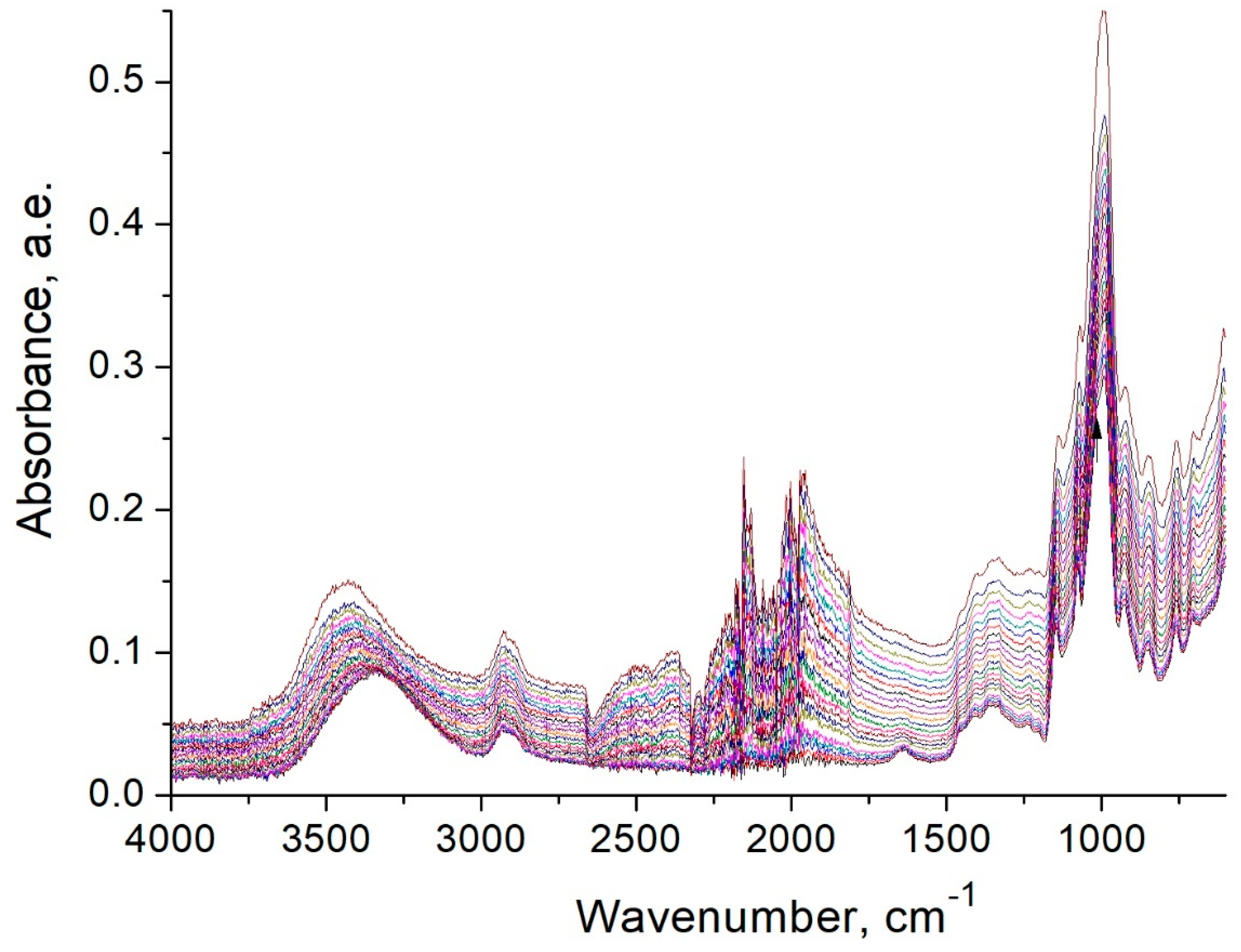
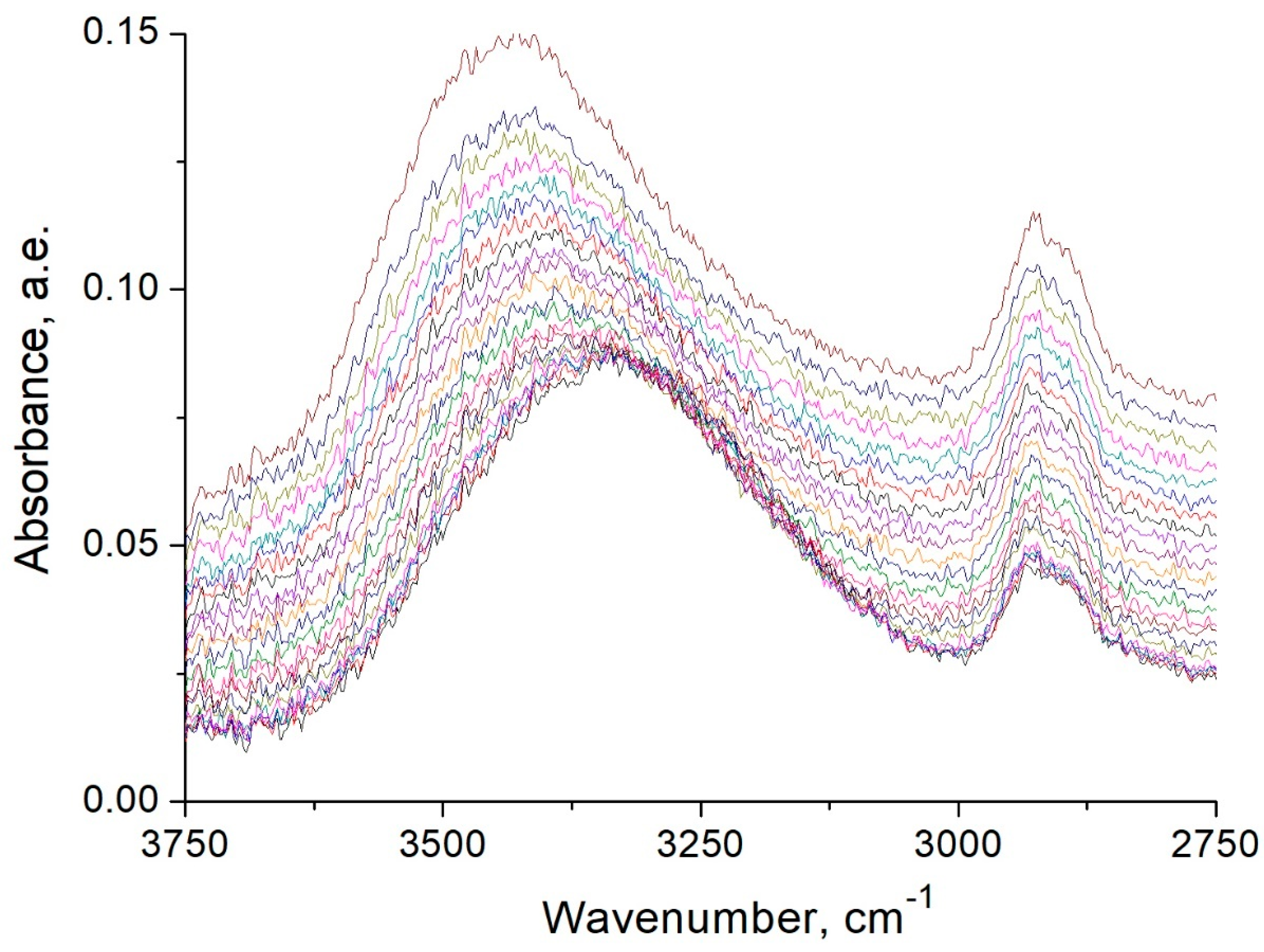
2.6. Thermal Methods of Analysis and FTIR Spectroscopy Studies
2.7. Statistical Analysis
3. Results
3.1. Microscopic Studies
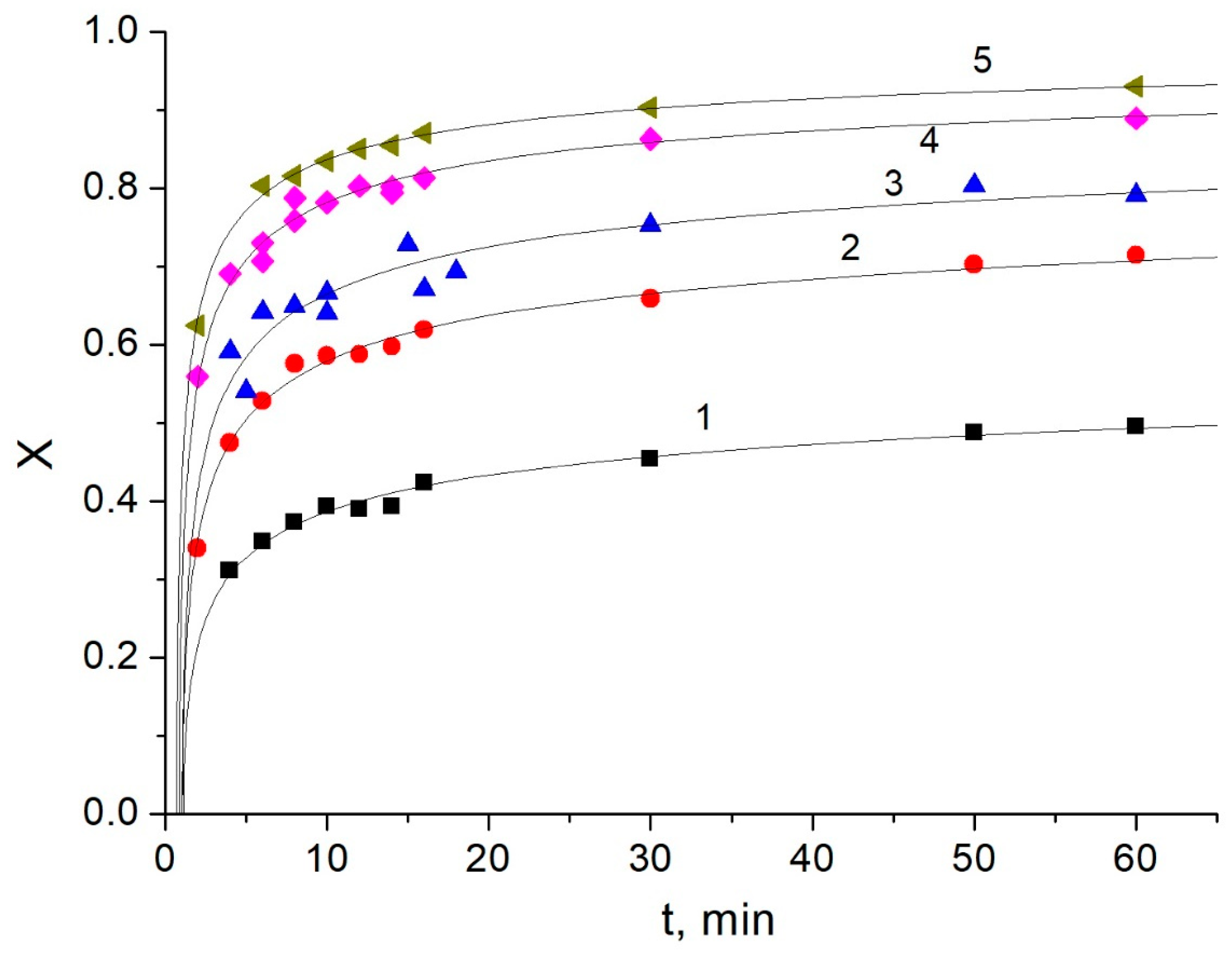
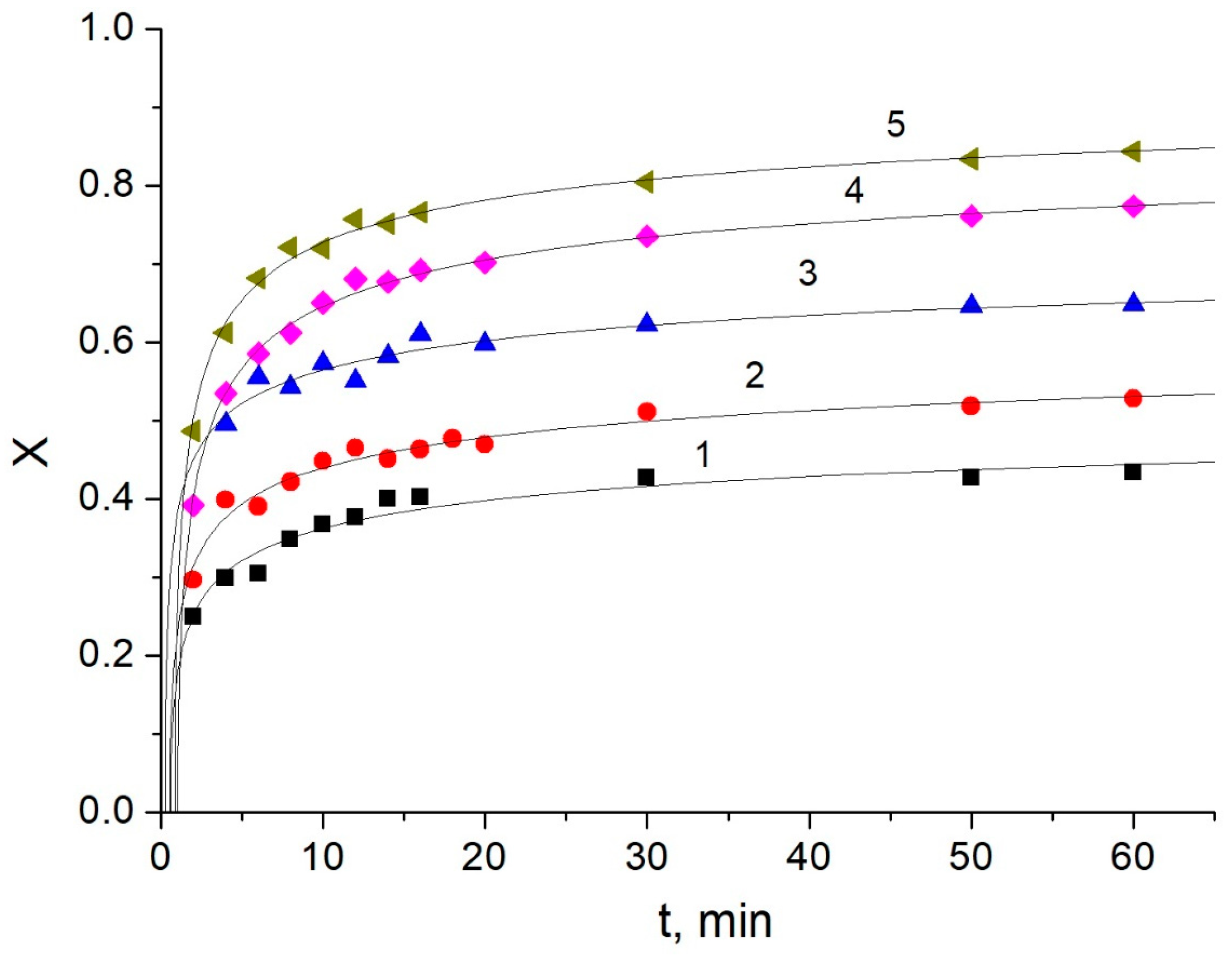
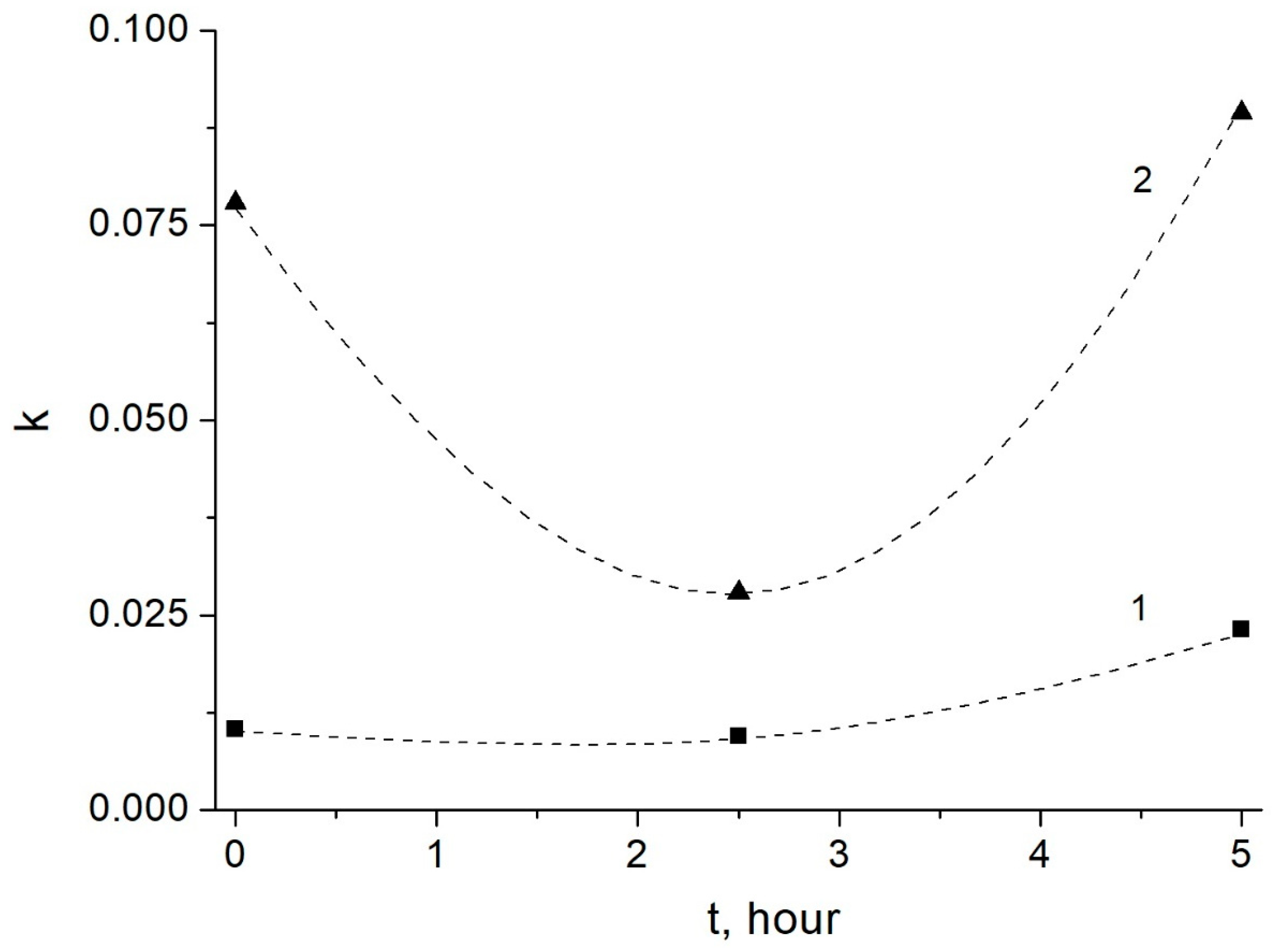
3.2. Kinetic Studies of Amylose Leaching
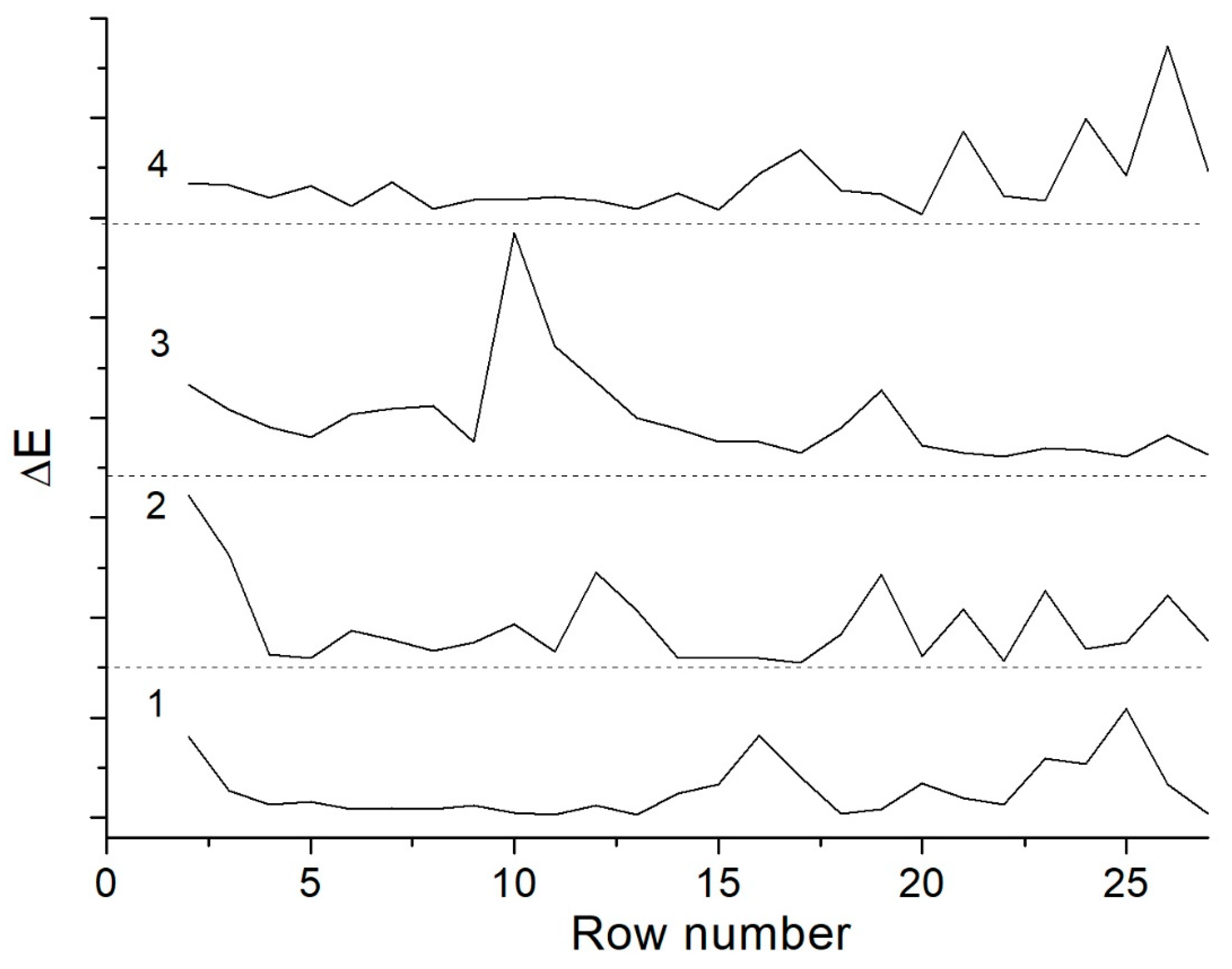
3.3. Study of Molecular Mass Distribution of Amylose Macromolecules
3.4. TG and DSC Studies of Starch Heat Treatment Process
3.5. FTIR Spectroscopy Studies of Starch Heat Treatment Process
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Apriyanto, A.; Compart, J.; Fettke, J. A review of starch, a unique biopolymer—Structure, metabolism and in planta modifications. Plant Sci. J. 2022, 318, 111223. [Google Scholar] [CrossRef]
- Kaur, P.; Kaur, K.; Basha, S.J.; Kennedy, J.F. Current trends in the preparation, characterization and applications of oat starch—A review. Int. J. Biol. Macromol. 2022, 212, 172–181. [Google Scholar] [CrossRef]
- Fan, Y.; Picchioni, F. Modification of starch: A review on the application of “green” solvents and controlled functionalization. Carbohydr. Polym. 2020, 241, 116350. [Google Scholar] [CrossRef] [PubMed]
- Nizam, N.H.M.; Rawi, N.F.M.; Ramle, S.F.M.; Aziz, A.A.; Abdullah, C.K.; Rashedi, A.; Kassim, M.H.M. Physical, thermal, mechanical, antimicrobial and physicochemical properties of starch based film containing aloe vera: A review. J. Mater. Res. Technol. 2021, 15, 1572–1589. [Google Scholar] [CrossRef]
- Adewale, P.; Yancheshmeh, M.S.; Lam, E. Starch modification for non-food, industrial applications: Market intelligence and critical review. Carbohydr. Polym. 2022, 291, 119590. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.-J.; Lim, H.S.; Lim, S.-T. Effect of partial gelatinization and retrogradation on the enzymatic digestion of waxy rice starch. J. Cereal Sci. 2006, 43, 353–359. [Google Scholar] [CrossRef]
- Donmez, D.; Pinho, L.; Patel, B.; Desam, P.; Campanella, O.H. Characterization of starch–water interactions and their effects on two key functional properties: Starch gelatinization and retrogradation. Curr. Opin. Food Sci. 2021, 39, 103–109. [Google Scholar] [CrossRef]
- Majzoobi, M.; Farahnaky, A. Granular cold-water swelling starch; properties, preparation and applications, a review. Food Hydrocoll. 2021, 111, 106393. [Google Scholar] [CrossRef]
- Chavan, P.; Sinhmar, A.; Nehra, M.; Thory, R.; Pathera, A.K.; Sundarraj, A.A.; Nain, V. Impact on various properties of native starch after synthesis of starch nanoparticles: A review. Food Chem. 2021, 364, 130416. [Google Scholar] [CrossRef]
- Amagliani, L.; O’Regan, J.; Kelly, A.L.; O’Mahony, J.A. Chemistry, structure, functionality and applications of rice starch. J. Cereal Sci. 2016, 70, 291–300. [Google Scholar] [CrossRef]
- Li, C. Recent progress in understanding starch gelatinization—An important property determining food quality. Carbohydr. Polym. 2022, 293, 119735. [Google Scholar] [CrossRef] [PubMed]
- Renzetti, S.; van den Hoek, I.A.F.; van der Sman, R.G.M. Mechanisms controlling wheat starch gelatinization and pasting behaviour in presence of sugars and sugar replacers: Role of hydrogen bonding and plasticizer molar volume. Food Hydrocoll. 2021, 119, 106880. [Google Scholar] [CrossRef]
- Miles, M.J.; Morris, V.J.; Orford, P.D.; Ring, S.G. The roles of amylose and amylopectin in the gelation and retrogradation of starch. Carbohydr. Res. 1985, 135, 271–281. [Google Scholar] [CrossRef]
- Putseys, J.A.; Gommes, C.J.; Van Puyvelde, P.; Delcour, J.A.; Goderis, B. In situ SAXS under shear unveils the gelation of aqueous starch suspensions and the impact of added amylose-lipid complexes. Carbohydr. Polym. 2011, 84, 1141–1150. [Google Scholar] [CrossRef]
- Vermeylen, R.; Derycke, V.; Delcour, J.A.; Goderis, B.; Reynaers, H.; Koch, M.H.J. Gelatinization of starch in excess water: beyond the melting of lamellar crystallites. A combined wide- and small-angle X-ray scattering study. Biomacromolecules 2006, 7, 2624–2630. [Google Scholar] [CrossRef]
- Turhan, M.; Gunasekaran, S. Kinetics of in situ and in vitro gelatinization of hard and soft wheat starches during cooking in water. J. Food Eng. 2002, 52, 1–7. [Google Scholar] [CrossRef]
- Doblado-Maldonado, A.; Gomand, S.; Goderis, B.; Delcour, J. The extent of maize starch crystal melting as a critical factor in the isolation of amylose via aqueous leaching. Food Hydrocoll. 2016, 61, 36–47. [Google Scholar] [CrossRef]
- Doblado-Maldonado, A.; Janssen, F.; Gomand, S.; Ketelaere, B.; Goderis, B.; Delcour, J. A response surface analysis of the aqueous leaching of amylose from maize starch. Food Hydrocoll. 2017, 63, 265–272. [Google Scholar] [CrossRef]
- Palav, T.; Seetharaman, K. Mechanism of starch gelatinization and polymer leaching during microwave heating. Carbohydr. Polym. 2006, 65, 364–370. [Google Scholar] [CrossRef]
- Cabrera, E.; Pineda, J.C.; Duran De Bazua, C.; Segurajauregeui, J.S.; Vernon, E.J. Kinetics of water diffusion and starch gelatinization during corn nixtamalization. In Engineering and Food; McKenna, B.M., Ed.; Engineering sciences in the food industry; Elsevier: London, UK, 1984; Volume 1, pp. 117–125. [Google Scholar]
- Zhu, Y.; Cui, B.; Yuan, C.; Lu, L.; Li, J. A new separation approach of amylose fraction from gelatinized high amylose corn starch. Food Hydrocoll. 2022, 131, 107759. [Google Scholar] [CrossRef]
- Naguleswaran, S.; Vasanthan, T.; Hoover, R.; Bressler, D. Amylolysis of amylopectin and amylose isolated from wheat, triticale, corn and barley starches. Food Hydrocoll. 2014, 35, 686–693. [Google Scholar] [CrossRef]
- Li, H.; Dhital, S.; Flanagan, B.; Mata, J.; Gilbert, E.; Gidley, M. High amylose wheat and maize starches have distinctly different granule organization and annealing behaviour: A key role for chain mobility. Food Hydrocoll. 2020, 105, 105820. [Google Scholar] [CrossRef]
- Li, Y.; Liu, P.; Ma, C.; Zhang, N.; Shang, X.; Wang, L.; Xie, F. Structural disorganization and chain aggregation of high-amylose starch in different chloride salt solutions. ACS Sustain. Chem. Eng. 2020, 8, 483–4847. [Google Scholar] [CrossRef]
- Shi, H.; Yin, Y.; Hu, X.; Jiao, S. Determination of molecular weight and structure characterization of canna amylose purified using the method of n-butanol recrystallization. Adv. Mat. Res. 2012, 554–556, 1216–1222. [Google Scholar] [CrossRef]
- Li, Y.; Wu, Z.; Wan, N.; Wang, X.; Yang, M. Extraction of high-amylose starch from Radix Puerariae using high-intensity low-frequency ultrasound. Ultrason. Sonochem. 2019, 59, 104710. [Google Scholar] [CrossRef]
- Setyawati, Y.; Ahsan, S.; Ong, L.; Soetaredjo, F.; Ismadji, S.; Ju, Y. Production of glutinous rice flour from broken rice via ultrasonic assisted extraction of amylose. Food Chem. 2016, 203, 158–164. [Google Scholar] [CrossRef]
- Yang, F.; Du, Q.; Miao, T.; Zhang, X.; Xu, W.; Jia, D. Interaction between potato starch and Tremella fuciformis polysaccharide. Food Hydrocoll. 2022, 127, 107509. [Google Scholar] [CrossRef]
- Yuris, A.; Goh, K.; Hardacre, A.; Matia-Merino, L. Understanding the interaction between wheat starch and Mesona chinensis polysaccharide. LWT 2017, 84, 212–221. [Google Scholar] [CrossRef]
- Sun, L.; Xu, Z.; Song, L.; Ma, M.; Zhang, C.; Chen, X.; Xu, X.; Sui, Z.; Corke, H. Removal of starch granule associated proteins alters the physicochemical properties of annealed rice starches. Int. J. Biol. Macromol. 2021, 185, 412–418. [Google Scholar] [CrossRef]
- Chen, R.; Williams, P.; Shu, J.; Luo, S.; Chen, J.; Liu, C. Pectin adsorption onto and penetration into starch granules and the effect on the gelatinization process and rheological properties. Food Hydrocoll. 2022, 129, 107618. [Google Scholar] [CrossRef]
- Hou, C.; Zhao, X.; Tian, M.; Zhou, Y.; Yang, R.; Gu, Z.; Wang, P. Impact of water extractable arabinoxylan with different molecular weight on the gelatinization and retrogradation behavior of wheat starch. Food Chem. 2020, 318, 126477. [Google Scholar] [CrossRef] [PubMed]
- Fonseca-Florido, H.; Gomez-Aldapa, C.; Velazquez, G.; Hernandez-Hernandez, E.; Mata-Padilla, J.; Solís-Rosales, S.; Mendez-Montealvo, G. Gelling of amaranth and achira starch blends in excess and limited water. LWT 2017, 81, 265–273. [Google Scholar] [CrossRef]
- Spigno, G.; De Faveri, D. Gelatinization kinetics of rice starch studied by non-isothermal calorimetric technique: Influence of extraction method, water concentration and heating rate. J. Food Eng. 2004, 62, 337–344. [Google Scholar] [CrossRef]
- Suzuki, K.; Kubota, K.; Omich, M.; Hosaka, H. Kinetic studies on cooking of rice. J. Food Sci. 1976, 41, 1180–1185. [Google Scholar] [CrossRef]
- Bakshi, A.S.; Singh, R.P. Kinetics of water diffusion and starch gelatinization during rice parboiling. J. Food Sci. 1980, 45, 1387–1392. [Google Scholar] [CrossRef]
- Birch, G.; Priestley, R. Degree of gelatinization of cooked rice. Starch 1973, 25, 98–100. [Google Scholar] [CrossRef]
- Kubota, K. Studies on the soaking and cooking rate equations of soybean. J. Fac. Appl. Biol. Sci. Hiroshima Univ. 1979, 18, 1–9. [Google Scholar] [CrossRef]
- Okechukwu, P.; Rao, M. Kinetics of cowpea starch gelatinization based on granule swelling. Starch 1996, 48, 43–47. [Google Scholar] [CrossRef]
- Shiotsubo, T. Starch gelatinization at different temperatures as measured by enzymatic digestion method. Agric. Biol. Chem. 1983, 47, 2421–2425. [Google Scholar] [CrossRef]
- Kubota, K.; Hosokawa, Y.; Suzuki, K.; Hosaka, H. Studies on the gelatinization rate of rice and potato starches. J. Food Sci. 1979, 44, 1394–1397. [Google Scholar] [CrossRef]
- Zhou, Z.; Robards, K.; Helliwell, S.; Blanchard, C. Effect of storage temperature on rice thermal properties. Int. Food Res. J. 2010, 43, 709–715. [Google Scholar] [CrossRef]
- Resio, A.C.; Suarez, C. Gelatinization kinetics of amaranth starch. Int. J. Food Sci. Technol. 2001, 36, 441–448. [Google Scholar] [CrossRef]
- Ojeda, C.; Tolaba, M.; Suarez, C. Modeling starch gelatinization kinetics of milled rice flour. Cereal Chem. 2000, 77, 145–147. [Google Scholar] [CrossRef]
- Yeh, A.; Li, J. Kinetics of phase transition of native, cross-linked and hydroxipropilated rice starches. Starch 1996, 48, 17–21. [Google Scholar] [CrossRef]
- Ahmed, J. Rheometric non-isothermal gelatinization kinetics of mung bean starch slurry: Effect of salt and sugar—Part 1. J. Food Eng. 2012, 109, 321–328. [Google Scholar] [CrossRef]
- Li, C.; Hu, Y. A kinetics-based decomposition approach to reveal the nature of starch asymmetric gelatinization thermograms at non-isothermal conditions. Food Chem. 2021, 344, 128697. [Google Scholar] [CrossRef]
- Lund, D.B.; Wirakartakusumah, M. A Model for Starch Gelatinization Phenomena. In Engineering and Food; McKenna, B.M., Ed.; Elsevier: London, UK, 1984; Volume 1, pp. 425–432. [Google Scholar]
- Popov, D.; Buléon, A.; Burghammer, M.; Chanzy, H.; Montesanti, N.; Putaux, J.-L.; Potocki-Véronèse, G.; Riekel, C. Crystal structure of A-amylose: A revisit from synchrotron microdiffraction analysis of single crystals. Macromolecules 2009, 42, 1167–1174. [Google Scholar] [CrossRef]
- Sattelle, B.; Almond, A. Microsecond kinetics in model single- and double-stranded amylose polymers. Phys. Chem. Chem. Phys. 2014, 16, 8119–8126. [Google Scholar] [CrossRef]
- Xu, J.; Li, Z.; Zhong, Y.; Zhou, Q.; Lv, Q.; Chen, L.; Blennow, A.; Liu, X. The effects of molecular fine structure on rice starch granule gelatinization dynamics as investigated by in situ small-angle X-ray scattering. Food Hydrocoll. 2021, 121, 107014. [Google Scholar] [CrossRef]
- Dickinson, C.F.; Heal, G.R. Solid-liquid diffusion controlled rate equations. Thermochim. Acta 1999, 340–341, 89–103. [Google Scholar] [CrossRef]
- Li, H.; Li, J.; Guo, L. Rheological and pasting characteristics of wheat starch modified with sequential triple enzymes. Carbohydr. Polym. 2020, 230, 115667. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, E.; Fan, X.; Yang, C.; Ma, H.; Gilbert, R.G. The effects of the chain-length distributions of starch molecules on rheological and thermal properties of wheat flour paste. Food Hydrocoll. 2020, 101, 105563. [Google Scholar] [CrossRef]
- Pigłowska, M.; Kurc, B.; Rymaniak, Ł.; Lijewski, P.; Fuć, P. Kinetics and thermodynamics of thermal degradation of different starches and estimation the OH group and H2O content on the surface by TG/DTG-DTA. Polymers 2020, 12, 357. [Google Scholar] [CrossRef] [PubMed]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikolenko, M.V.; Myrhorodska-Terentieva, V.D.; Sakhno, Y.; Jaisi, D.P.; Likozar, B.; Kostyniuk, A. Hydrothermal Leaching of Amylose from Native, Oxidized and Heat-Treated Starches. Processes 2023, 11, 1464. https://doi.org/10.3390/pr11051464
Nikolenko MV, Myrhorodska-Terentieva VD, Sakhno Y, Jaisi DP, Likozar B, Kostyniuk A. Hydrothermal Leaching of Amylose from Native, Oxidized and Heat-Treated Starches. Processes. 2023; 11(5):1464. https://doi.org/10.3390/pr11051464
Chicago/Turabian StyleNikolenko, Mykola V., Viktoriia D. Myrhorodska-Terentieva, Yuriy Sakhno, Deb P. Jaisi, Blaž Likozar, and Andrii Kostyniuk. 2023. "Hydrothermal Leaching of Amylose from Native, Oxidized and Heat-Treated Starches" Processes 11, no. 5: 1464. https://doi.org/10.3390/pr11051464
APA StyleNikolenko, M. V., Myrhorodska-Terentieva, V. D., Sakhno, Y., Jaisi, D. P., Likozar, B., & Kostyniuk, A. (2023). Hydrothermal Leaching of Amylose from Native, Oxidized and Heat-Treated Starches. Processes, 11(5), 1464. https://doi.org/10.3390/pr11051464










