Abstract
Chamaecyparis obtusa (Siebold & Zucc.) Endl. (Cupressaceae) is known to produce a variety of antimicrobial substances. In the present study, components of three lots of essential oil from C. obtusa were analyzed by GCMS. It was confirmed that thujopsene and pinene were common markers. In addition, we report indoor space disinfectant effects of products containing C. obtusa essential oil (PO100, PO500, PO1000). It was confirmed that PO100 and PO500 could effectively remove airborne microorganisms in indoor spaces. Results of our study suggest that C. obtusa essential oil is effective in reducing contamination by infectious microorganisms in confined spaces.
1. Introduction
In the global pandemic situation, the prevention of pathogenic microbes in indoor air spaces and on various surfaces attract significant attention. Recently, as interest in disinfection methods or antibacterial materials increases, interest in antibacterial materials as natural disinfectants is also increasing [1]. The disinfectants should not only be effective in reducing the microbes present in the air but should also not be toxic to humans, allowing their continuous application [2].
Bacteria can be released into the air in a variety of natural and anthropogenic environments. When infectious bacteria are released into the air, they can be easily transferred to susceptible individuals via bioaerosols. Representative microorganisms among airborne bacteria include Legionella, Mycobacterium, Escherichia, Enterobacter, Pseudomonas, Aspergilius, and Acinetobacter species. These infectious microorganisms cause diseases such as legionellosis, pneumonia, tuberculosis, and leprosy [3].
Essential oils are mixtures of volatile and non-volatile compounds and can be obtained from natural plants by organic solvent extraction, hydrodistillation, and compression [4]. Essential oils have been shown to have antimicrobial, antifungal, and antiviral activities [5]. The antimicrobial effects of essential oils are explained by their composition and cytotoxic effects, which cause cell membrane damage. For example, essential oils containing carvacrol, cinnamaldehyde, and thymol showed antibacterial activity against E. coli and S. aureus in vitro and exhibited antibacterial activity through inhibition of cell membrane formation. The cytotoxicity of the essential oils tested was lower than that of chlorhexidine. Therefore, carvacrol, cinnamaldehyde, and thymol appear to be natural agents that can be used effectively against E. coli and S. aureus-borne infections [6].
Several scientific studies on disinfection methods using the antibacterial effects of essential oils have been reported. The potential use of Melaleuca alternifolia (Tea tree) oil as a disinfectant has been shown in a previous report for control bacteria [7] due to terpinen-4-ol (35–45%) and 1,8-cineole (1–6%) [8,9].
Eucalyptus oil is usually rich in sesquiterpenes that show antimicrobial activities against gram-positive and negatives [10]. Tea tree oil and eucalyptus oil have been reported to have significant activity against airborne pathogenic bacteria in a short period of time [11].
Several papers have reported that the antimicrobial efficacy of essential oil vapor is more effective than essential oils in liquid form [12]. Therefore, empirical experiments on the indoor space control effect of various essential oil components are needed, and the need to present the basis for the development of various natural disinfectants based on empirical data is increasing.
The antimicrobial effect of an essential oil mixture in a small chamber was reported by Chaoet et al. [13]. Chaoet et al. evaluated the antimicrobial activity of blended essential oils of cinnamon, clove, rosemary, eucalyptus, and lemon in a closed hood with an area of 0.4 m3. For further empirical studies, Lanzerstorfer et al. reported that a mixture of Citrus limon essential oil and Abies alba essential oil removed 40% of bacteria using an ultrasonic vaporizer in a hospital room [14]. There have been reports on demonstration tests of various essential oil mixtures using ultrasonic vaporizers; however, there have been no demonstration tests on Chamaecyparis obtusa essential oil.
In 1926, Chamaecyparis obtusa was adopted as an afforestation species in southern regions of Korea. In 1960, it began to be cultivated in Jeju Island, Gyeongnam, Jeollanam-do, Korea. Among them, the C. obtusa forest in Jangheung is the oldest. In Jeollanam-do, C. obtusa occupies about 78,000 ha, with 65% of the nation’s reforestation concentrated in this area. C. obtusa is a species of growing interest in Korea as it has excellent effects on environmental and infectious diseases [15,16]. Previously, we have determined the efficacy and components of C. obtusa essential oil to confirm the value of Jangheung C. obtusa. Some studies have been reported on the effect of C. obtusa essential oil on the human body and animal inhalation experiments. One clinical trial of C. obtusa essential oil was reported. Chen et al. extracted C. obtusa essential oil and C. formosensis essential oil. Physiological and psychological effects were investigated by inhaling essential oils for 5 min in 16 healthy adults. After inhalation of C. obtusa essential oil, systolic blood pressure, heart rate, and parasympathetic activity decreased while sympathetic nervous system activity increased. Additionally, in the profile of mood states test, C. obtusa essential oil stimulated a pleasant mood state. Chen’s results indicated that C. obtusa essential oil was helpful in controlling sympathetic nervous system dysfunction.
As a result, it showed excellent effects on general microorganisms and antibiotic-resistant bacteria [15]. Although the antimicrobial effect of C. obtusa has been reported previously, there has been no empirical study on the antimicrobial effect of the essential oil of C. obtusa. Thus, the objective of the present study was to analyze the bioactive components of three lots of essential oil. The antibacterial excellence of a product containing essential oil was identified through an indoor space disinfection test.
2. Materials and Methods
2.1. Essential Oil Preparation and Chemical Analysis
C. obtusa essential oil was produced three times (June 2014, December 2014, and April 2015) from leaves of C. obtusa and supplied by Earthmate Jungamjin Co (Jangheung, Republic of Korea). Its active ingredient was measured by GCMS. GCMS analysis analyzed the components of essential oil using the method used by Bae et al. [15]. Agilent 7890 gas chromatography (GC) and Agilent 5975 quadrupole mass spectrometry (MS) systems (Agilent Technologies, Palo Alto, CA, USA) were utilized to analyze molecular mass fragments (50–550 amu) of the Lot 1~3, with Agilent HP-5MS fused silica capillary column (30 mm l. × 0.25 mm i.d., 0.25 μm film thickness, Agilent Technologies, Palo Alto, CA, USA). The mass fragments were ionized under electron ionization (EI) conditions. A GC oven was isothermally programmed at 65 °C for 10 min at 10 to 300 min−1 with helium (He) as a carrier gas. All the data were compared with the system library (NIST 2017). Three lots were analyzed for essential oil components, and common components between lots were selected.
2.2. Space Disinfectant Preparation and Space Disinfectant Test
A space disinfectant test sample (HINOPHY PO) was provided by Earthmate Jungamjin (Jangheung, Republic of Korea) by diluting C. obtusa essential oil (LOT3) 100 times, 500 times, and 1000 times (Product code, PO100-001, PO500-001, PO1000-001) to make test product. The product did not contain any synthetic ingredients other than essential oil. For the disinfectant test, an actual space was selected to confirm the degree of control efficiency of essential oil-containing products. For actual space selection, the space (average 8 h, 20–40 actual users) was selected so that it could be applied to offices and public institutions. The area of the test space was 60 m2 for Room 1 (R1) and 90 m2 for Room 2 (R2) and Room 3 (R3). PO100, PO500, and PO1000 were supplied to the selected space, and 0.25 L per space was evenly sprayed over 15 min. For measuring falling bacteria, 4 points were selected for each space. At each point, a solid medium (nutrient agar, BD DIFCO. Co., Franklin Lakes, NJ, USA) in a 10 cm petri dish (SPL life science, Seoul. Republic of Korea) was exposed to the space for 8 h to recover the falling bacteria. The recovered strain was cultured at 37 °C for 24–48 h, and the number of falling cells was counted. The falling bacteria were visually counted by the number of colonies before and after the treatment of the PO series. We only considered the collection of falling bacteria when the petri dish was left in the test space after sample processing. Indoor temperature and humidity were not recorded (Figure 1).
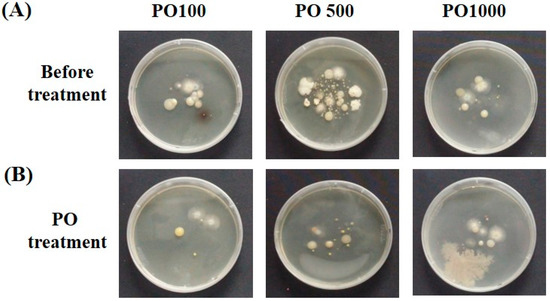
Figure 1.
Visualization of falling bacterial strain. Example, (A) Falling bacteria pattern before PO treatment; (B) Falling bacteria pattern after space disinfection.
3. Results and Discussion
3.1. C. obtusa Essential Oil Component Analysis
In lot 1, several components, such as limonene (6%), thujopsene (3%), alpha-pinene (2.3%), and phellandrane (1.7%), were detected (Table 1). In lot 2, thujopsene (5.2%), limonene (4%), (+)-Epi-bicyclosesquiphellandrene (2.4%), cedrol (2%), and alpha-pinene (1.5%) were detected. In lot 3, alpha-pinene (1.5%), phellandrane (3.3%), (+)-4-Carene (2.1%), cedrol (1.55%), thujopsene (5.1%), and (+)-Epi-bicyclosesquiphellandrene (2.6%) were detected. Lots 2 and 3 also showed similar results. Since the manufacturing date of each source is different, there are slight differences in some components; however, the main components are similar.

Table 1.
Example of GC-MS analysis of essential oil from C. obtusa (Lot 1).
To summarize representative active ingredients of lots 1 to 3, thujopsene and pinene are common ingredients in the analysis. Thujopsene has shown potent antibacterial activity, for example, against Phytophthora ramorum at 2.0~3.0 ppm [17]. Limonene also can be utilized as one of the solutions to the problem of antimicrobial resistance. Advantageous contributions have been made by various research groups in the study of the antimicrobial properties of limonene. Previous studies have shown that limonene inhibits disease-causing pathogenic microbes [18]. In addition, it was observed positive results for the isolated antibacterial action of α-pinene. Of these, the strains that worked were Escherichia coli ATCC, Staphylococcus aureus ATCC and Salmonella enterica, revealing their susceptibility to the α-pinene [19]. The recently extracted lot 2 and lot 3 showed almost similar component detection, such as thujopsene, limonene, and α-pinene and content patterns. It seems desirable to set the standard for quality control based on active ingredients of lots 1~3 in the future (Figure 2).
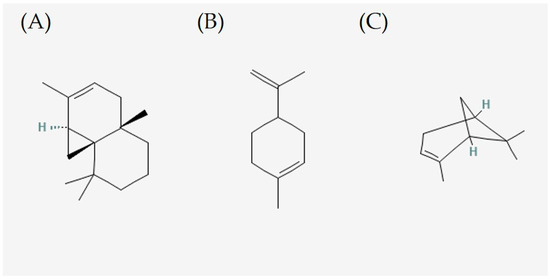
Figure 2.
Biomarkers of C. obtusa essential oil. (A) thujopsene, (B) limonene, and (C) α-pinene.
3.2. Indoor Space Disinfectant Test
As shown in Figure 3, we measured the number of falling bacteria before and after spraying PO100, PO500, and PO1000 into three spaces. Since the distribution of dropping bacteria varied by location, 4~6 points were designated per room to measure falling bacteria. The average number of falling bacteria per room was calculated. On average, falling bacteria in R1 and R2 before PO treatment were 50~60 CFU/plate. When PO100 and 500 were used for treatment, a statistically significant reduction in falling bacteria was shown in R1 and R2. When treated with PO100, falling bacteria were reduced by 12 to 65% in R1 and 10 to 55% in R2. In R3, 42 to 67% of falling bacteria were reduced. The area of R2 is the same as that of R3. The reason for the slightly different decrease in falling bacteria is thought to be that the distribution of airborne bacteria is different depending on the number of people and their activities. When PO500 was used for treatment, the number of falling bacteria decreased by 21 to 63.6% in R1, by 11.3 to 43.8% in R2, and by 3.5 to 31.8% in R3. When PO1000 was used for treatment, the number of falling bacteria decreased by 44 to 61% in R1, by 6 to 25% in R2, and by 8.4 to 68.2% in R3. The statistical significance of the PO1000 treatment group was not observed. Experimental results revealed that PO100 and PO500 showed statistically significant indoor space disinfectant effects, probably due to the volatilization of essential oil components in PO products and the antibacterial power of non-volatile substances. In particular, non-volatile substances that remain in the indoor space are presumed to inhibit the growth of microorganisms distributed in the space. Previously, we reported the study on the essential oil and non-volatile residues derived from C. obtusa leaves showed broad antimicrobial activities. Thus, both essential oil and non-volatile residues may be eco-friendly disinfectants for the prevention of skin disorders caused by infectious bacteria [15]. There have been no studies on the control of disinfectants containing C. obtusa essential oils in real indoor spaces. For effective product development in the future, research on deriving the optimal blending ratio between the existing indoor space disinfectant and C. obtusa essential oil and a community study of controlled microbes should be conducted.
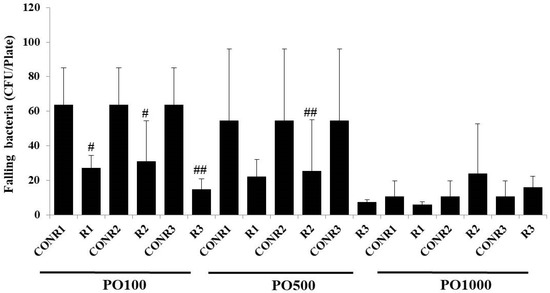
Figure 3.
Inhibition of falling bacteria of PO100, PO500, PO1000 containing C. obtusa essential oil. CONR1~3 means the colonies of falling bacteria before not treated with PO series in the experimental space (R1~3), p value, # < 0.05 and ## < 0.02.
4. Conclusions
In the present study, components and indoor space disinfectant efficacy of C. obtusa essential oil were evaluated. As a result of the analysis, it was found that common ingredients such as thujopsene, limonene, and α-pinene were identified for lots 1~3. Anti-bacterial efficacy was also confirmed. In the case of the GCMS analysis method, if 2~3 markers were selected, it was considered appropriate as an analysis method for quality control. In addition, through an indoor space disinfectant test of products containing C. obtusa essential oil, it was thought that essential oil could efficiently remove airborne microorganisms. In conclusion, our study showed the possibility of practical use of C. obtusa essential oil by demonstrating that C. obtusa essential oil could inhibit the growth of various microorganisms and reduce human exposure to infectious microorganisms.
Author Contributions
Conceptualization, S.-S.C. and D.-H.P.; methodology and investigation, S.-Y.S., S.-H.L., J.-H.S., G.Y., J.-W.P., C.-Y.C. and M.-S.B.; writing and editing, S.-S.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (No. 2022R1A5A8033794), and this work was supported by a grant from the National Institute of Environment Research (NIER), funded by the Ministry of Environment (MOE) of the Republic of Korea (NIER-2021-03-03-007).
Data Availability Statement
Data available on request.
Conflicts of Interest
The authors declared no potential conflict of interest with respect to the research, authorship, and/or publication of this article.
References
- Mirskaya, E.; Agranovski, I.E. Control of Airborne Microorganisms by Essential Oils Released by VaxiPod. Atmosphere 2021, 12, 1418. [Google Scholar] [CrossRef]
- Musee, N.; Ngwenya, P.; Motaung, L.K.; Moshuhla, K.; Nomngongo, P. Occurrence, effects, and ecological risks of chemicals in sanitizers and disinfectants: A review. Environ. Chem. Ecotoxicol. 2023, 5, 62–78. [Google Scholar] [CrossRef]
- Stetzenbach, L.D. Airborne Infectious Microorganisms. Encycl. Microbiol. 2009, 52, 175–182. [Google Scholar] [CrossRef]
- Rai, M.; Zacchino, S.; Derita, M. Essential Oils and Nanotechnology for Treatment of Microbial Diseases; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Tariq, S.; Wani, S.; Rasool, W.; Shafi, K.; Bhat, M.A.; Prabhakar, A.; Shalla, A.H.; Rather, M.A. A comprehensive review of the antibacterial, antifungal and antiviral potential of essential oils and their chemical constituents against drug-resistant microbial pathogens. Microb. Pathog. 2019, 134, 103580. [Google Scholar] [CrossRef] [PubMed]
- García-Salinas, S.; Elizondo-Castillo, H.; Arruebo, M.; Mendoza, G.; Irusta, S. Evaluation of the Antimicrobial Activity and Cytotoxicity of Different Components of Natural Origin Present in Essential Oils. Molecules 2018, 23, 1399. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, J.M.; Cavanagh, H.M. Antibacterial activity of essential oils from Australian native plants. Phytother. Res. PTR 2005, 19, 643–646. [Google Scholar] [CrossRef]
- May, J.; Chan, C.H.; King, A.; Williams, L.; French, G.L. Time-kill studies of tea tree oils on clinical isolates. J. Antimicrob. Chemother. 2000, 45, 639–643. [Google Scholar] [CrossRef] [PubMed]
- Carson, C.F.; Hammer, K.A.; Riley, T.V. Melaleuca alternifolia (Tea Tree) oil: A review of antimicrobial and other medicinal properties. Clin. Microbiol. Rev. 2006, 19, 50–62. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, L.C.A.; Filomeno, C.A.; Teixeira, R.R. Chemical variability and biological activities of Eucalyptus spp. essential oils. Molecules 2016, 21, 1671. [Google Scholar] [CrossRef]
- Usachev, E.V.; Pyankov, O.V.; Usacheva, O.V.; Agranovski, I.E. Antiviral activity of tea tree and eucalyptus oil aerosol and vapour. J. Aerosol Sci. 2013, 59, 22–30. [Google Scholar] [CrossRef]
- Tian, F.; Lee, S.Y.; Chun, H.S. Comparison of the Antifungal and Antiaflatoxigenic Potential of Liquid and Vapor Phase of Thymus vulgaris Essential Oil against Aspergillus flavus. J. Food Prot. 2019, 82, 2044–2048. [Google Scholar] [CrossRef] [PubMed]
- Chao, S.C.; Young, D.G.; Oberg, C.J. Effect of a diffused essential oil blend on bacterial bioaerosols. J. Essent. Oil Res. 1998, 10, 517–523. [Google Scholar] [CrossRef]
- Lanzerstorfer, A.; Hackl, M.; Schlömer, M.; Rest, B.; Deutsch-Grasl, E.; Lanzerstorfer, C. The influence of air-dispersed essential oils from lemon (Citrus limon) and silver fir (Abies alba) on airborne bacteria and fungi in hospital rooms. J. Environ. Sci. Health Part A 2019, 54, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Bae, M.S.; Park, D.H.; Choi, C.Y.; Kim, G.Y.; Yoo, J.C.; Cho, S.S. Essential Oils and Non-volatile Compounds Derived from Chamaecyparis obtusa: Broad Spectrum Antimicrobial Activity against Infectious Bacteria and MDR(multidrug resistant) Strains. Nat. Prod. Commun. 2016, 11, 693–694. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-S.; Han, S.-R.; Yang, M. Evaluations on the Deodorization Effect and Antibacterial Activity of Chamaecyparis obtusa Essential Oil. J. Odor Indoor Environ. 2009, 8, 101–107. [Google Scholar]
- Manter, D.K.; Kelsey, R.G.; Karchesy, J.J. Antimicrobial activity of extractable conifer heartwood compounds toward Phytophthora ramorum. J. Chem. Ecol. 2007, 33, 2133–2147. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Jeyakumar, E.; Lawrence, R. Journey of Limonene as an Antimicrobial Agent. J. Pure Appl. Microbiol. 2021, 15, 1094–1110. [Google Scholar] [CrossRef]
- Borges, M.F.d.A.; Lacerda, R.d.S.; Correia, J.P.d.A.; de Melo, T.R.; Ferreira, S.B. Potential Antibacterial Action of α-Pinene. Med. Sci. Forum 2022, 12, 11. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).