Abstract
Un-doped (uZO) and silver-doped zinc oxide (SZO) films were prepared by oblique incidence sputtering deposition under different process parameters. The crystalline structure, chemical composition, and surface morphology were correlated with the optical properties, as well as with the wettability of the films. In the case of uZO films, the orientation, inclination, and morphology of the columnar structure determined the wettability of the layer, moving from a hydrophilic- to hydrophobic-like character. In the case of SZO films, although almost all of them displayed hydrophobic behavior, the hydrophobic character increased with the Ag content. The most hydrophobic surface was obtained when the Ag content in the layers was greater than 7 at.% and, in these cases, the structural results indicate that the layers were formed by a disordered mixture of Zn and Ag oxides.
1. Introduction
Zinc oxide is a highly researched functional semiconductor with a wide range of properties, including high photo-activity, n-type conductivity, wide band gap energy, strong oxidizing power, non-toxicity, high transparency in the visible and near-UV range, piezoelectricity, chemical stability and biocompatibility, among others [1,2,3,4,5]. Through compositional and structural modification, its properties can be customized to suit various applications, such as piezoelectric transducers, chemical gas sensors, and solar cells [6,7]. Recently, self-cleaning and transparent surfaces have been extensively studied for use in smart windows, solar panels, camera lenses, and other optoelectronic devices [8,9]. These surfaces can extend product lifetime, reduce the need for chemical detergents and labor costs, and inhibit dirt accumulation and bacteria growth. To achieve water and dust repellency on device surfaces, research on the hydrophobicity of ZnO thin films is essential. It is also noteworthy that most natural and synthetic superhydrophobic surfaces are opaque, limiting their use in the optical industry. To enable the use of superhydrophobic surfaces in optical and electronic devices (e.g., automobile glass, smart windows, and electronic screens), it is critical to maintain high optical transparency. Transparent and self-cleaning surfaces are expected to be a key advance in future optical and electronic devices. Surface roughness and surface chemistry are the two key factors that determine surface wettability [10]. Generally, the rougher the surface, the larger the contact angle. In addition, surfaces usually carry a specific energy, also referred to as surface tension, because atoms or molecules on liquids and solids have less chemical bonding at the surface. The chemical structure of the materials defines the surface tension, which in return partially determines the wettability and adhesion of the materials [11]. Micro- or nanostructures have commonly been used to enhance the surface wettability of ZnO films and, in some cases, to obtain superhydrophobic surfaces [12]. Feng et al. [13] synthesized ZnO nanorods with reversible surface wettability, exhibiting both super-hydrophobicity and super-hydrophilicity. Zhang et al. [14] found that nanotip structures had the potential to improve the wetting properties of ZnO films. However, there have been few studies that have examined the impact of Ag integration into the ZnO structure, surface morphology, and surface wettability. Sputtering deposition is a widely used method for thin film growth due to its ability to cover large surface areas, making it easy to scale to an industrial level at a relatively low cost. Furthermore, by utilizing two separate, highly pure and independent targets of Ag and Zn, we were able to obtain high control of the thin film composition. Additionally, oblique angle deposition (OAD) has garnered significant attention as it can be used to design the morphology of thin films with inclined porous columnar nanostructures due to the atomic shadowing effect [15]. Here, the tilted angle of the substrate with respect to the incoming particle flux allows changes in the columnar growth mode, resulting in preferential growth directions (anisotropy).
In this study, thin films of un-doped (uZO) and silver-doped (SZO) ZnO were deposited via magnetron sputtering co-deposition in an OAD with varying process parameters. We explored the correlation between the chemical composition, crystalline structure, and surface morphology of uZO and SZO thin films, obtained by adjusting the process parameters, and their wettability and optical properties.
2. Materials and Methods
uZO and SZO thin films were deposited on (100) oriented silicon and α-Al2O3 (0001) substrates by DC magnetron co-sputtering of highly pure Zn (99.99%) and Ag (99.99%) targets with a mixture of argon and oxygen gas at different working pressures. After pre-sputtering the targets for five minutes, the deposition process was carried out. The power applied to the Zn cathode (WZn) remained constant at 100 W throughout, while the power applied to the Ag cathode (WAg) varied at 5 and 20 W for SZO deposition. The reaction chamber consisted of a cylindrical vacuum chamber with two circular planar magnetrons (3 in diameter) located in a confocal configuration at at 60° angle with each other. The distance between the center of each target and the substrate was set at 15 cm in all depositions. The base pressure was around 3.6 × 10−6 mbar before the deposition process. A load-lock chamber attached to the system was utilized for introducing the substrates, which were chemically cleaned beforehand in a sequential process involving acetone and propanol, followed by drying with high purity N2 gas, prior to loading them into the reaction chamber.
The crystalline structure of the samples was identified using grazing incidence X-ray diffraction (XRD) at 0.5° incidence angle (PANalytical Multi-Purpose Diffractometer model X’Pert PRO MRD). The electronic structure was studied with X-ray absorption near-edge structure (XANES). This technique provides short-range information of electronic states for each individual element, being a powerful technique to study complex multi-element structures. XANES measurements were carried out with the dipole beamline PM3 at the synchrotron facility BESSY-II of Helmholtz-Zentrum Berlin (HZB). In order to determine compositional profiles, Rutherford backscattering spectroscopy (RBS) was performed at the 5 MeV HVEE Tandetron accelerator located at the Centro de Micro-Análisis de Materiales of the Universidad Autónoma de Madrid [16]. RBS experiments were performed with 4He+ projectiles for a dose of 10 µC with an energy of 1.8 MeV. The backscattered particles were detected with a silicon detector located at a scattering angle of 170°. The chemical composition of the samples was extracted using the SIMNRA simulation software [17]. The morphology of the samples was analyzed with a field emission scanning electron microscope (FE-SEM) (Verios-460) operated at 20 kV. Atomic force microscopy (AFM) images were collected with Nano-Observer equipment from Scientec (France). The images were processed and analyzed with the open-source software Gwyddion [18]. The contact angle (CA) was measured with a KSV CAM 200 system using the sessile drop method [19]. Distilled water droplets with a volume of ~0.7 µL were deposited at the surface at room temperature and imaged after stabilization using an optical CCD camera. The drop profile was analyzed by means of the system software in order to determine the wetting properties. The optical transmission characterization was performed by using a Shimadzu SolidSpec-3700 spectrophotometer in the 200–900 nm wavelength range. The signal from the substrate was eliminated by measuring the corresponding bare substrate. The thickness of the films was determined by a Veeco Dektak 150 profilometer.
3. Results and Discussion
3.1. Un-Doped ZnO Thin Films (uZO)
uZO films were deposited under the above experimental conditions by OAD with a deposition angle, α, between the target and substrate normal of 80° (uZO-80) at different process pressures. Deposition parameters, deposition rate, and atomic composition of uZO films are shown in Table 1. It is important to mention that, at low sputtering pressure, more sputtered atoms reached the substrate as a result of the increase in mean free path causing a lower number of collisions in the gas phase. Hence, the relatively larger thickness under this condition is not surprising (400 nm or a deposition rate of ~6.7 ± 0.3 nm/min). The combination of the areal density extracted from the RBS spectra (expressed in 1015 at/cm2) and the thickness values, measured by profilometry and further confirmed by cross-section SEM, can be used to estimate the atomic density. RBS fitting shows that lowering the sputtering pressure from 7.4 to 3.0 (×10−3 mbar) caused no significant changes in the film composition. However, for sputtering pressure of 1.4 × 10−3 mbar it is possible to appreciate a considerable decrease of the atomic density from 5.0 to 3.9 ± 0.5 g/cm3 as well as an excess of oxygen content. Both trends can be related to the formation of voids during the growth process where the oxygen molecules may be trapped [20].

Table 1.
Deposition conditions and atomic composition of uZO films with varying sputtering pressure.
Cross-section SEM images of the uZO-80 films deposited at different sputtering pressures are displayed in Figure 1a. By comparing the cross-section morphology, uZO-80 films grown at low sputtering pressures (3.0 and 1.4 × 10−3 mbar) show oblique columns with an inclination (β) of approximately 18 and 45° with respect to the sample normal. In contrast, the sample grown at the highest sputtering pressure (7.4 × 10−3 mbar) exhibited a columnar growth structure perpendicular to the silicon substrate (not inclined). In addition, the oblique columns present a conical shape with a narrower width during their early growth stage but wider as the deposition proceeded, similarly to the behavior reported by S. Bairagi et al. [21]. The relation between the inclination of the columns (β) and the glancing angle, α, has been theoretically studied. The tangent rule (2tan (β) ≈ tan (α)) developed by Nieuwenhuizen et al. [22] is known for providing good estimations of β applicable for films deposited at low α (<50°). For higher α, Tait et al. [23] proposed a purely ballistic model with an expression [2sin (α − β) = 1 − cos (α)] that predicts β of 50° for α of 80°, i.e., consistent with our experiments at low pressures (1.4 × 10−3 mbar). However, it is worth mentioning that many other sputtering parameters may influence the inclination angle of the columns during deposition, such as the target–substrate distance [24] and the substrate temperature [25], among others. As the pressure increases, the growth becomes less directional due to the promotion of gas-phase collisions, and the inclination of the columns becomes less pronounced. At the highest value (7.4 × 10−3 mbar), the growth mode enters a diffusive regime with no preferential direction and the columns grow perpendicular to the substrate surface. Typical surface AFM images of uZO-80 films deposited at different sputtering pressures are presented in Figure 1b. It is clear that the surface roughens and, consequently, the specific surface area of the film increases for a lower process pressure as a consequence of the inclination of the columns (shadowing regime). The increase in roughness becomes one order of magnitude for the samples grown at the lowest pressure (from 1.6 to 12.6 nm rms roughness for 7.4 and 1.3 (×10−3 mbar), respectively). The hydrophilicity of the uZO samples was characterized by measuring the CA, defined as the angle between the tangent of the water droplet at the contact point and the surface normal at this point. CA values less than 90° are associated with hydrophilic surfaces, while hydrophobic surfaces have CA larger than 90°. The images of water drops on different uZO surfaces are shown in Figure 1c. Typically, the presence of topographical features and, hence, increase in roughness (lotus effect) is associated with hydrophobic character [26]. Hence, in our case, chemical effects seem to play a relevant role in the wettability. These obtained results clearly show how the wettability of the uZO films changes from hydrophobic (110 ± 2°) to hydrophilic (80 ± 2°) behavior by modifying the column inclinations with the sputtering pressure.
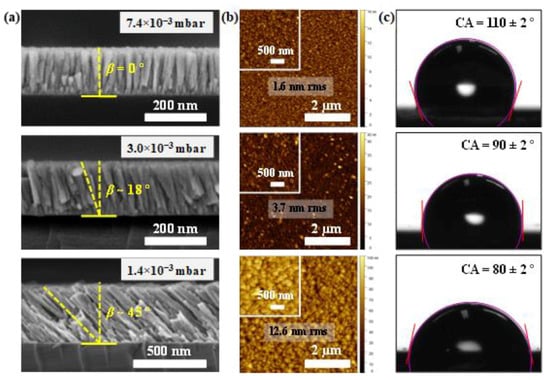
Figure 1.
(a) Cross-sectional SEM images; (b) AFM topography images (5 × 5 µm2) indicating the (rms) roughness; (c) contact angle (CA) measurement of a 0.75 µL distilled water droplet on the uZO films grown at different pressures. The purple contour corresponds to the droplet boundary and the red lines are the tangent to the water droplet.
The effect of the inclination of the columns on the wettability of the samples is more evident when we investigate how the CA varies with respect to the in-plane axis of the surface (i.e., parallel or perpendicular to the deposition direction) [27]. When the CA is measured perpendicularly to the deposition flux direction (no inclination of the columns in the cross-section view), the CA is slightly reduced with sputtering pressure (110 > 108 > 104°) and, in all the cases, the surfaces have a hydrophobic character (CA > 90°). However, when CA is measured in the plane parallel to the growth direction of the films (here, columnar inclination is observed in the cross-section view), the CA varies strongly with pressure and the surface becomes hydrophilic (see Figure 2).
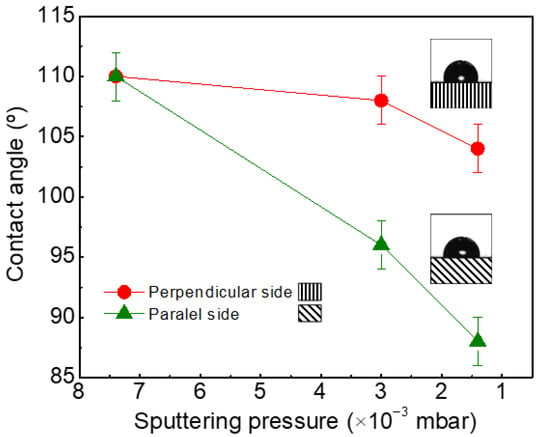
Figure 2.
Variation of wettability with the direction of measurement of the contact angle (CA).
XRD patterns of the uZO films deposited at different sputtering pressures are shown in Figure 3. All the samples have a hexagonal wurtzite structure with preferred (002) orientation with the c-axis normal to the substrate. This preferential orientation is partially lost at 1.4 × 10−3 mbar, since the presence of multiple reflections indicates the random orientation of the crystalline grains. One possible explanation for the reduction in crystal quality at low pressures may be the incorporation of molecular oxygen in the structure during growth, as previously reported by M. Yuste et al. [28] and according to the excess of oxygen in the atomic composition detected by RBS (see Table 1). Under this schema, the morphology, crystalline quality, and density of the uZO films, as well as the defects caused by the embedded oxygen, seem to determine the hydrophobic/hydrophilic behavior of uZO films.
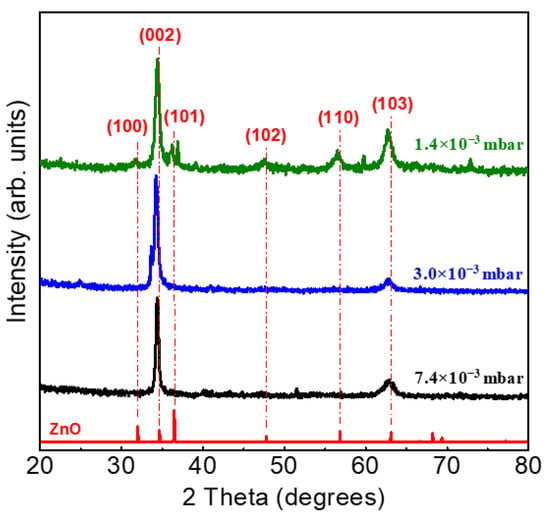
Figure 3.
Diffraction patterns of the uZO-80 films with varying sputtering pressure.
3.2. Silver Doped Zinc Oxide Thin Films (SZO)
SZO films with different Ag/Zn relative content were deposited using two independent Zn and Ag cathodes connected to two DC power sources and in a co-sputtering configuration shown in Figure 4. As an additional parameter, the α’ angle of the substrate holder was also varied. The working pressure was 7.4 × 10−3 mbar and the power applied to the Zn cathode (WZn) was kept constant at 100 W and the power applied to the Ag cathode (WAg) was set at 5 and 20 W. These power values are within the range determined in our previous report [29] to produce silver doping levels in ZnO and mixed ternary oxides, respectively. Sample labels, deposition conditions, thicknesses, and atomic composition are indicated in Table 2. Samples are named as SZO-x, where x refers to the silver content (expressed in at.%).
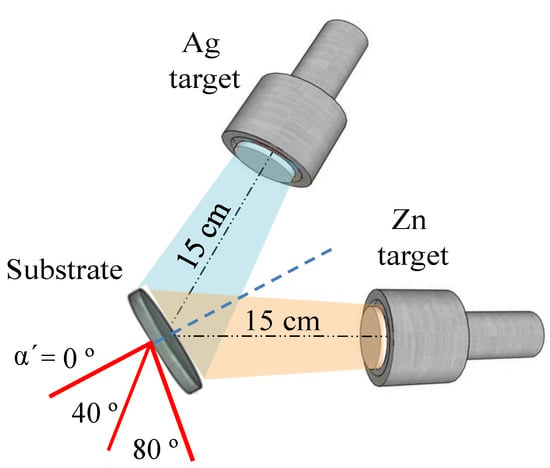
Figure 4.
Scheme of the geometrical arrangement of the substrate with respect to the co-sputtering targets for growth of SZO films. The dotted blue line indicates the normal incidence angle (the substrate is at 45° for both magnetrons, α’ = 0°) and the red lines show the substrate position during the co-sputtering process.

Table 2.
Deposition conditions, thicknesses, and atomic composition of SZO thin films with different silver concentration grown by co-sputtering process. The deposition time was 60 min for all samples.
SEM cross-section views of the SZO films with low Ag concentration (≤7 at.%) are shown in Figure 5a. Typical ZnO columnar growth was observed in all the cases, with significant amount of particles (brighter areas) taped throughout the columnar structures and that we associate with the segregation of silver-rich regions. It is clear that surface morphology changes considerably with the incidence angle, as appreciated in the top-view SEM images displayed in Figure 5b. SZO films present a granular structure composed of relatively large grains (40 ± 5 nm) that can be ascribed to the column tips. It can also be observed that the grains become progressively smaller and closer to each other by increasing the deposition angle. The RBS spectra of SZO films samples with low silver content (Ag ≤ 7 at.%) are shown in Figure 5c. The spectra display the individual contributions of the different elements (color lines) to the overall simulated spectra. The spectra clearly indicate an increase in the Ag content with α’. Simultaneously, the film thickness (atomic incorporation rate) decreases with α’ as derived by the lower energy span of the film containing elements. For very low Ag contents (≤4 at.%), the composition seems to be homogeneous along the film thickness as the spectra can be nicely fitted with a single-layer model. However, the spectrum of the SZO-7 film can be fitted with a two-layer model with a slightly higher Ag concentration in the inner part of the sample. The quantitative values of the above trends are shown in Table 2, as extracted from the fitting results with the SIMNRA software [17]. Both the decrease in thickness and the increase in the Ag content with α’ can be correlated to a higher (lower) deposition angle with respect to the Zn (Ag) target normal. It should be noted that each α’ step accounts for nearly half (double) the thickness (Ag content) in the films.
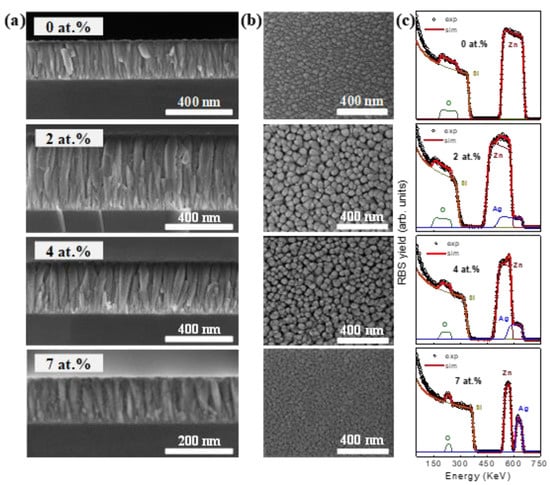
Figure 5.
(a) Cross-sectional; (b) Top-view SEM images; (c) RBS spectra of SZO samples growth with different Ag content.
The SEM images with cross-section views of the SZO films with high Ag concentration (≥7 at.%) are shown in Figure 6a. The topography and microstructure that they present are very different with respect to the images shown in Figure 5a. In particular, no columnar structure is observed in any cases and the grains are already agglomerated (see Figure 6b). In all the cases, it was impossible to obtain images with well-defined features due to the accumulated charge in the sample. Figure 6c shows the RBS spectra of the SZO films with high Ag concentration. As in the previous case, the contribution of the different elements is highlighted by the different color lines. Contrary to the results shown in Figure 5c and in agreement with the SEM images, the spectra display complex compositional profiles except for the highest concentration (30 at.%). This statement can be clearly extracted from the relative variations of the Ag yield. In the particular case of SZO-9, the SEM images show that the film was not homogeneous throughout the thickness, existing at least two distinct layers with presumably a different composition or structure as evidenced by the change in the image texture. Taking into account the difference in contrast, the surface layer should present a higher Ag concentration (heavier element). This latter assumption is confirmed by the RBS measurements shown in Figure 6c. For SZO with Ag content up to 9 at.% (SZO-22 and SZO-30), the Ag distribution is more complex, where at least four layers are required to fit the spectral lineshape. The formation of this layered structure is in agreement with SEM and could be attributed to Ag out-diffusion (after growth). In this case, the RBS analysis reveals a marked Ag surface enrichment (see the intense high-energy peak in the Ag yield in Figure 6c), followed by a relatively thin depleted region with lower Ag content and, finally, different homogeneous layers with intermediate Ag contents. In any case, despite such Ag distribution, the changes in composition are within a few at.% since Ag is a relatively heavy element and a small variation induces a significant change in the RBS yield. A similar scheme can be extracted for the SZO-22 film although, in this case, the surface and depleted layers seem to be thinner than for SZO-9. Finally, although several layers may be hintered in the SZO-30 film, the compositional profile seems to be more homogeneous. Hence, Ag out-diffusion seems to depend on the Ag content and is more evident for values around 10 at.%.
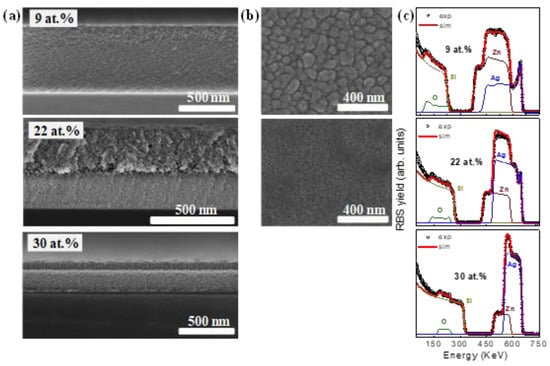
Figure 6.
(a) Cross-sectional; (b) top-view SEM images; (c) RBS spectra of SZO samples grown with different Ag content.
The XRD patterns of SZO films deposited with different Ag contents and several reference spectra (ZnO and Ag2O) are shown in Figure 7. An interesting point is that, for low doping concentration of Ag (≤7 at.%) (see Figure 7a), the hexagonal wurtzite structure with preferred orientation (002) was not altered as Ag was incorporated into the lattice. Moreover, no signal related to metallic silver or silver oxides was detected, indicating that Ag was effectively incorporated in the ZnO matrix. The shift of the (002) reflection with respect to pure ZnO indicates that Ag+ ions were incorporated into the ZnO structure at substitutional sites. Figure 7b shows the XRD spectra for high Ag enriched SZO. In the case of SZO-9 film, the hexagonal wurtzite structure is almost lost and peaks corresponding to Ag2O phase are detected (see inset in Figure 7b). It seems that the film displays a disordered structure with the predominance of mixed oxide Zn and Ag phases and a minor content of nanocrystalline wurtzite ZnO (see inset). Higher Ag concentrations produce an amorphization of the samples and only a very broad band located about 32° is detected, indicating a very disordered structure.
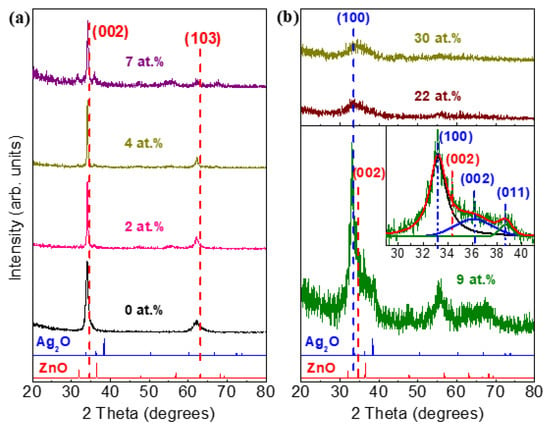
Figure 7.
XRD pattern of SZO films deposited with different Ag contents: (a) low doping (≤7 at.%); (b) high doping (≥7 at.%). Inset in (b) shows the enlarged Ag2O peaks region of the SZO-9 film.
The ionic radius and valence state are two important parameters for determining the solubility limit of the dopant [30]. In our case, the Ag+ ions have a different valence than Zn2+ and the ionic radius is larger (144 and 135 pm, respectively). These facts suggest that when the silver concentration exceeds the limit of ~10 at.%, the hexagonal wurtzite structure is completely lost and Ag atoms tend to emigrate to the surface, as observed by XRD and RBS. At the largest Ag concentrations, as shown by RBS, the formation of the w-ZnO structure was inhibited, resulting in nearly homogeneous composition profiles. Similar behavior using titanium as dopant was reported by M. Yuste et al. [31].
The bonding structure of SZO films can be rather complex due to the mixture of different oxide phases. XANES can provide some insights in this respect since it provides site-selective information with local order range. The XANES spectra of the O1s and Zn2p edges for the SZO films with different Ag contents are shown in Figure 8. The reference spectra from bulk ZnO are also shown for discussion. In line with XRD, data for the uZO film (0 at.% Ag) clearly resemble the spectra of ZnO at both edges. However, there is a prominent peak around 531 eV at the O1s edge that it is absent in the reference case. This feature can be ascribed to the contribution of O-O bonds [32] (i.e., embedded O2 molecules), as previously reported in pure ZnO films grown by our group [27]. The presence of O2 is also an indication of porosity in the films and supports the above discussion of the film density variation as a function of the working pressure. The edges of the SZO-2 film are similar to the uZO film, hence indicating that the wurtzite structure is preserved with Ag content of a few at.%. In this case, the O2 contribution is less than in the uZO film probably due to the higher density of SZO. However, contrary to XRD, the wurtzite structure is lost for Ag content equal or above 4 at.%, especially, as revealed by the O1s edge. This can be interpreted as a dominant contribution of Ag oxides in the XANES signal, supported by the spectral lineshape with an intensive and narrow pre-edge peak around 531 eV followed by a broad structure around 540 eV as reported in O2/Ag (110) [33] and bulk Ag(I) oxides [34]. It should be noted that XANES is surface sensitive and, hence, discrepancy with the XRD results can arise from a very thin surface layer enriched with Ag of a few tens of nms. In this case, RBS would not be able to resolve such a thin surface layer. Last, but not least, the XANES analysis also reveals that for the largest Ag contents the surface still contains a significant amount of Zn atoms. In this case, the Zn2p edge is rather noisy due to the mitigated Zn contribution.
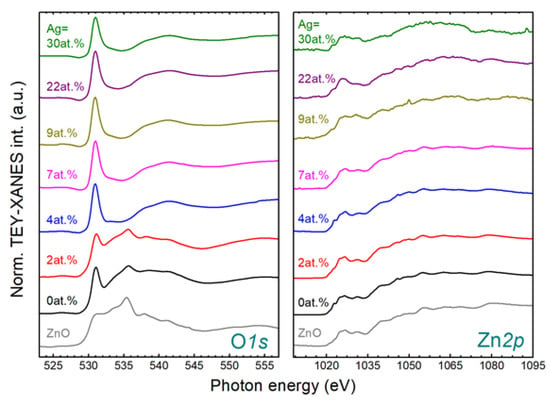
Figure 8.
XANES O1s (left) and Zn2p (right) spectra of SZO films deposited with different silver concentrations. Characteristic spectral features have been labelled for discussion.
Figure 9 shows the results obtained in the wetting measurements with SZO samples with increasing silver content. For reference, the CA for a bare silicon substrate was also measured, finding a value of 78 ± 2° in agreement with previous reported values of Si with a native oxide layer [35]. After the growth of uZO thin film, the surface had a more hydrophilic behavior than the substrate, with a CA of 50 ± 2°. It is interesting to note that the CA of the SZO films shows an increasing trend of hydrophobicity by increasing the silver concentration added to ZnO, reaching a maximum value of 125 ± 2° for SZO-30. The optical transmittance spectra in the 300–900 nm range for uZO and SZO thin films with different silver concentrations are shown inset in Figure 9. The average optical transmission is over 75% in the whole range of visible wavelength at low doping concentration of Ag (≤7 at.%). However, high Ag content (≥7 at.%) induced an appreciable decrease of transparency down to a transmittance of 60%.
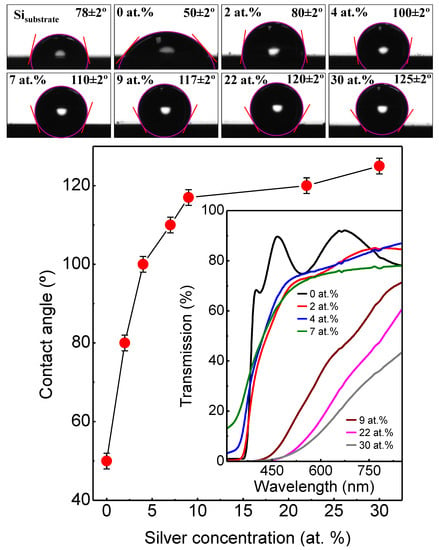
Figure 9.
Contact angle measurements of distilled water on Si substrate, uZO and SZO films. Inset shows the optical transmission.
4. Conclusions
Un-doped (uZO) and silver-doped zinc oxide (SZO) films were prepared by oblique incidence sputtering deposition under different process parameters. All uZO samples had a hexagonal wurtzite structure with preferred orientation (002) along the c-axis normal to the substrate. The wettability of the uZO films changes from hydrophobic (110 ± 2°) to hydrophilic (80 ± 2°) behavior by modifying the columns’ inclination with the sputtering pressure. According to the obtained results, the morphology, crystalline quality, and density of the uZO films, as well as the defects caused by the embedded oxygen, seem to determine their hydrophobic/hydrophilic behavior. In the case of SZO samples, the structure, morphology and wettability depend strongly on the Ag content in the films. It is interesting to note that the contact angle of the SZO films showed an increasing trend of hydrophobicity with increasing the silver concentration added to the ZnO films, reaching a maximum value of 125 ± 2° (for 30 at.% Ag). The average optical transmission was over 75% in the whole range of visible wavelength, however, high Ag content induced an appreciable decrease of transparency, down to a transmittance of 60%.
Author Contributions
Conceptualization, L.Á.-F., R.G. and O.S.; methodology, L.Á.-F. and O.S.; validation, L.Á.-F., R.G. and O.S.; formal analysis, L.Á.-F., R.G., J.d.J.A. and J.A.; investigation, L.Á.-F., J.d.J.A. and J.A.; resources, R.G., I.J. and O.S.; data curation, L.Á.-F., R.G. and O.S.; writing—original draft preparation, L.Á.-F. and O.S.; writing—review and editing, L.Á.-F., R.G. and O.S.; visualization, L.Á.-F.; supervision, R.G. and O.S.; funding acquisition, R.G., I.J. and O.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by grant TED2021-129876B-I00 of the Ministerio de Ciencia, Innovación y Universidades (Spain).
Data Availability Statement
The data presented in this study are available in this article.
Acknowledgments
The authors acknowledge the service from the MiNa Laboratory at IMN, and funding from CM (project S2018/NMT-4291 TEC2SPACE), MINECO (project CSIC13-4E-1794) and EU (FEDER, FSE). The authors are also thankful to L. García-Pelallo and M. Manso-Silvan for providing access to conduct the contact angle measurements. J.d.J.A. acknowledged the support by CONACYT- MX for his sabbatical stay 2023.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pan, L.; Sun, S.; Chen, Y.; Wang, P.; Zhang, X.; Zou, J.-J.; Wang, Z.L. Advances in piezo-phototronic effect enhanced photocatalysis and photoelectrocatalysis. Adv. Energy Mater. 2020, 10, 2000214. [Google Scholar] [CrossRef]
- Elsellamy, L.; Djeridi, W. Charge transfer modulation (e−/h+) between TiO2, ZnO, and Ag for a superior photocatalytic performance. J. Mater. Sci. Res. 2022, 37, 897–908. [Google Scholar] [CrossRef]
- Weldegebrieal, G.K. Synthesis method, antibacterial and photocatalytic activity of ZnO nanoparticles for azo dyes in wastewater treatment: A review. Inorg. Chem. Commun. 2020, 120, 108140. [Google Scholar] [CrossRef]
- Ali, T.A.; Pilz, J.; Schäffner, P.; Kratzer, M.; Teichert, C.; Stadlober, B.; Coclite, A.M. Piezoelectric properties of zinc oxide thin films grown by plasma-enhanced atomic layer deposition. Phys. Status Solidi A 2020, 217, 2000319. [Google Scholar] [CrossRef]
- Nain, V.; Kaur, M.; Sandhu, K.S.; Thory, R.; Sinhmar, A. Development, characterization, and biocompatibility of zinc oxide coupled starch nanocomposites from different botanical sources. Int. J. Biol. Macromol. 2020, 162, 24–30. [Google Scholar] [CrossRef]
- Hu, Y.-C.; Lee, T.-H.; Chang, P.-Z.; Su, P.-C. High power Co3O4/ZnO p-n type piezoelectric transducer. Thin Solid Film. 2015, 584, 112–115. [Google Scholar] [CrossRef]
- Shukla, V.; Patel, A. Effect of doping concentration on optical and electrical properties of intrinsic n-type ZnO (i-ZnO) and (Cu, Na and K) doped p-type ZnO thin films grown by chemical bath deposition method. J. Nanosyst. Phys. Chem. Math. 2020, 11, 391–400. [Google Scholar] [CrossRef]
- Syafiq, A.; Balakrishnan, V.; Ali, M.S.; Dhoble, S.J.; Rahim, N.A.; Omar, A.; Bakar, A.H.A. Application of transparent self-cleaning coating for photovoltaic panel: A review. Curr. Opin. Chem. Eng. 2022, 36, 100801. [Google Scholar] [CrossRef]
- Lee, Y.; You, E.-A.; Ha, Y.-G. Transparent, self-cleaning and waterproof surfaces with tunable micro/nano dual-scale structures. Nanotechnology 2016, 27, 355701. [Google Scholar] [CrossRef]
- Fei, L.; He, Z.; LaCoste, J.D.; Nguyen, T.H.; Sun, Y. A mini review on superhydrophobic and transparent surfaces. Chem. Rec. 2020, 20, 1257–1268. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.-H.; Chi, P.-W.; Wei, D.-H. Investigations on the crystallographic orientation induced surface morphology evolution of ZnO thin films and their wettability and conductivity. J. Phys. Chem. C 2016, 120, 8210–8219. [Google Scholar] [CrossRef]
- Li, B.J.; Huang, L.J.; Zhou, M.; Ren, N.F. Morphology and wettability of ZnO nanostructures prepared by hydrothermal method on various buffer layers. Appl. Surf. Sci. 2013, 286, 391–396. [Google Scholar] [CrossRef]
- Feng, X.; Feng, L.; Jin, M.; Zhai, J.; Jiang, L.; Zhu, D. Reversible super-hydrophobicity to super-hydrophilicity transition of aligned ZnO nanorod films. J. Am. Chem. Soc. 2004, 126, 62–63. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Chen, H.; Zhong, J.; Saraf, G.; Lu, Y. Fast and reversible wettability transitions on ZnO nanostructures. J. Electron. Mater. 2007, 36, 895–899. [Google Scholar] [CrossRef]
- Barranco, A.; Borras, A.; Gonzalez-Elipe, A.R.; Palmero, A. Perspectives on oblique angle deposition of thin films: From fundamentals to devices. Progr. Mater. Sci. 2016, 76, 59–153. [Google Scholar] [CrossRef]
- Climent-Font, A.; Pásztia, F.; García, G.; Fernández-Jiménez, M.T.; Agulló, F. First measurements with the Madrid 5 MV tandem accelerator. Nucl. Instr. Meth. Phys. Res. B 2004, 219-220, 400–404. [Google Scholar] [CrossRef]
- Mayer, M. SIMNRA User’s Guide 7.02; Max-Planck-Institut für Plasmaphysik: Garching, Germany, 2019. [Google Scholar]
- Nečas, D.; Klapetek, P. Gwyddion: An open-source software for SPM data analysis. Cent. Eur. J. Phys. 2012, 10, 181–188. [Google Scholar] [CrossRef]
- Ponomar, M.; Krasnyuk, E.; Butylskii, D.; Nikonenko, V.; Wang, Y.; Jiang, C.; Xu, T.; Pismenskaya, N. Sessile drop method: Critical analysis and optimization for measuring the contact angle of an ion-exchange membrane surface. Membranes 2022, 12, 765. [Google Scholar] [CrossRef]
- Laurenti, M.; Cauda, V. Porous zinc oxide thin films: Synthesis approaches and applications. Coatings 2018, 8, 67. [Google Scholar] [CrossRef]
- Bairagi, S.; Järrendahl, K.; Eriksson, F.; Hultman, L.; Birch, J.; Hsiao, C.-L. Glancing angle deposition and growth mechanism of inclined AlN nanostructures using reactive magnetron sputtering. Coatings 2020, 10, 768. [Google Scholar] [CrossRef]
- Nieuwenhuizen, J.M.; Haanstra, H.B. Microfractography of thin films. Philips Tech. Rev. 1966, 27, 1966. [Google Scholar]
- Tait, R.N.; Smy, T.; Brett, M.J. Modelling and characterization of columnar growth in evaporated films. Thin Solid Film. 1993, 226, 196–201. [Google Scholar] [CrossRef]
- Taschuk, M.T.; Hawkeye, M.M.; Brett, M.J. Glancing angle deposition. In Handbook of Deposition Technologies for Films and Coatings, 3rd ed.; Martin, P.M., Ed.; Elsevier: Oxford, UK, 2010; pp. 621–651. [Google Scholar]
- Lichter, S.; Chen, J. Model for columnar microstructure of thin solid films. Phys. Rev. Lett. 1986, 56, 1396. [Google Scholar] [CrossRef]
- Wenzel, R.N. Surface roughness and contact angle. J. Phys. Chem. 1949, 53, 1466–1467. [Google Scholar] [CrossRef]
- Masdosaité, R.; Jurkeviičiūtė, A.; Račkauskas, S. Superhydrophobic ZnO nanowires: Wettability mechanisms and functional applications. Cryst. Growth Des. 2021, 21, 4765–4779. [Google Scholar] [CrossRef]
- Yuste, M.; Escobar-Galindo, R.; Caretti, I.; Torres, R.; Sánchez, O. Influence of the oxygen partial pressure and post-deposition annealing on the structure and optical properties of ZnO films grown by dc magnetron sputtering at room temperature. J. Phys. D Appl. Phys. 2012, 45, 025303. [Google Scholar] [CrossRef]
- Ramadan, R.; Dadgostar, S.; Manso-Silván, M.; Pérez-Casero, R.; Hernández-Vélez, M.; Jiménez, I.; Sánchez, O. Silver-enriched ZnO:Ag thin films deposited by magnetron co-sputtering: Post annealing effects on structural and physical properties. Mater. Sci. Eng. B 2022, 276, 115558. [Google Scholar] [CrossRef]
- Shohany, B.G.; Zak, A.K. Doped ZnO nanostructures with selected elements-structural, morphology and optical properties: A review. Ceram. Int. 2020, 46, 5507–5520. [Google Scholar] [CrossRef]
- Yuste, M.; Escobar-Galindo, R.; Benito, N.; Palacio, C.; Martínez, O.; Albella, J.M.; Sánchez, O. Effect of the incorporation of titanium on the optical properties of ZnO thin films: From doping to mixed oxide formation. Coatings 2019, 9, 180. [Google Scholar] [CrossRef]
- Ma, Y.; Chen, C.T.; Meigs, G.; Randall, K.; Sette, F. High-resolution K-shell photoabsorption measurements of simple molecules. Phys. Rev. A 1991, 44, 1848. [Google Scholar] [CrossRef]
- Guest, R.J.; Hernnäs, B.; Bennich, P.; Björneholm, O.; Nilsson, A.; Palmer, R.E.; Mårtensson, N. Orientation of a molecular precursor: A NEXAFS study of O2/Ag (110). Surf. Sci. 1992, 278, 239–245. [Google Scholar] [CrossRef]
- Bukhtiyarov, V.I.; Hävecker, M.; Kaichev, V.V.; Knop-Geriche, A.; Mayer, R.W.; Schlögl, R. Atomic oxygen species on silver: Photoelectron spectroscopy and x-ray absorption studies. Phys. Rev. B 2003, 67, 235422. [Google Scholar] [CrossRef]
- Yang, X.M.; Zhong, Z.W.; Diallo, E.M.; Wang, Z.H.; Yue, W.S. Silicon wafer wettability and aging behaviors: Impact on gold thin-film morphology. Mater. Sci. Semicond. Process. 2014, 26, 25–32. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).