A Facile Hydrothermal Synthesis of S-VO2-Cellulose Nanocomposite for Photocatalytic Degradation of Methylene Blue Dye
Abstract
1. Introduction
| Methods of Textile Pollutant Treatment | Advantages | Disadvantages |
|---|---|---|
| Coagulation or flocculation | Economically convenient | Excess sludge production |
| Biological treatment | Low costs | Low bio decomposition of dyes |
| Adsorption | Low cost of preparation methods and uses | Poor capacity for organic pollutants, and adsorbents demand disposal |
| Ion exchange | High capacity for dyes | High costs |
| Oxidation | Effective decolonization | Generation of sludge |
| Membrane process | Low pressure | Insufficient quality of the treated pollutants |
2. Experimental Methods
2.1. Materials
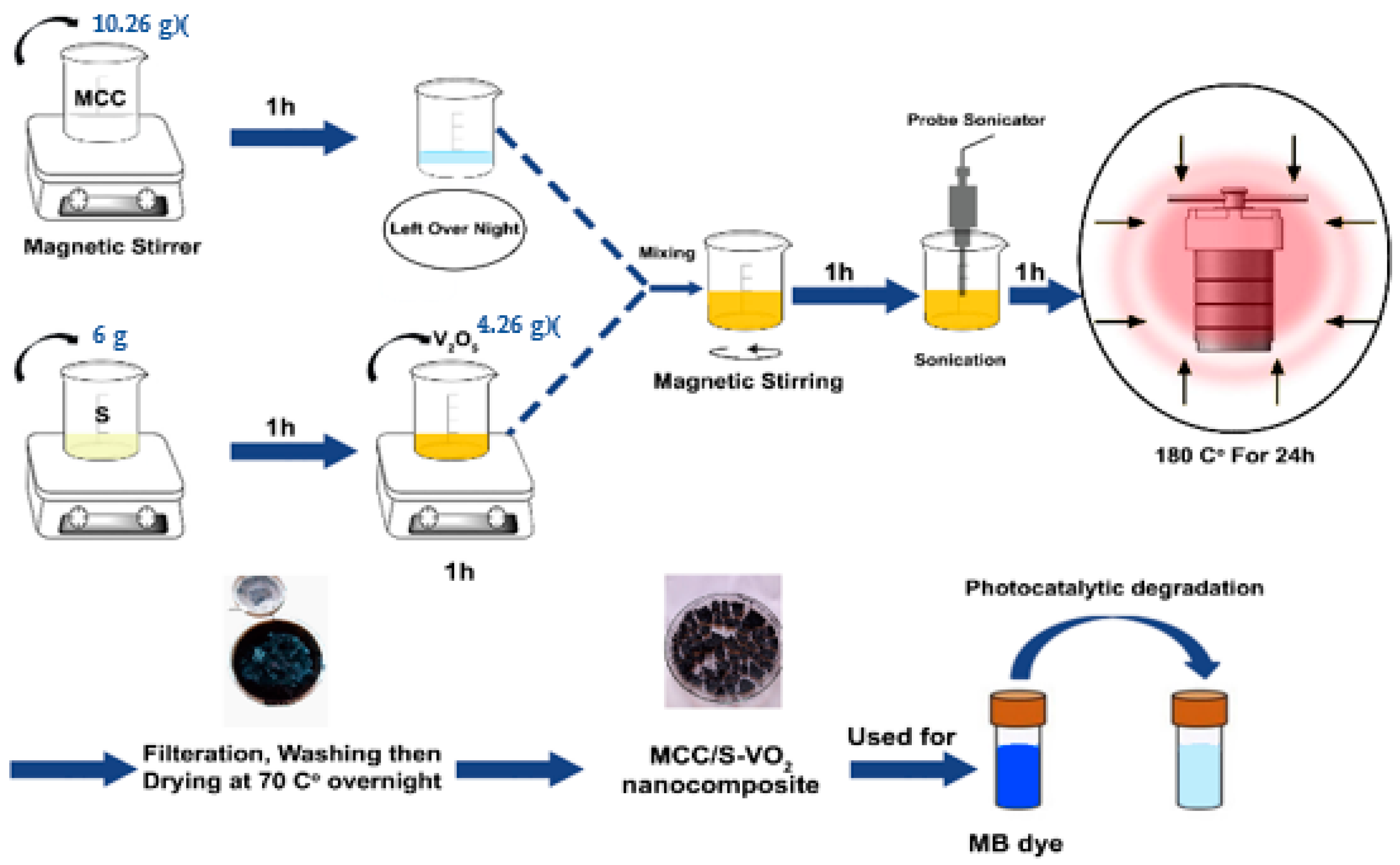
2.2. Synthesis of the Products
2.2.1. Synthesis of Microcrystalline Cellulose (MCC)
2.2.2. Synthesis of S-VO2 and MCC/S-VO2 Nanocomposite
2.3. Photocatalytic Degradation Experiments
2.4. Instruments
3. Results and Discussion
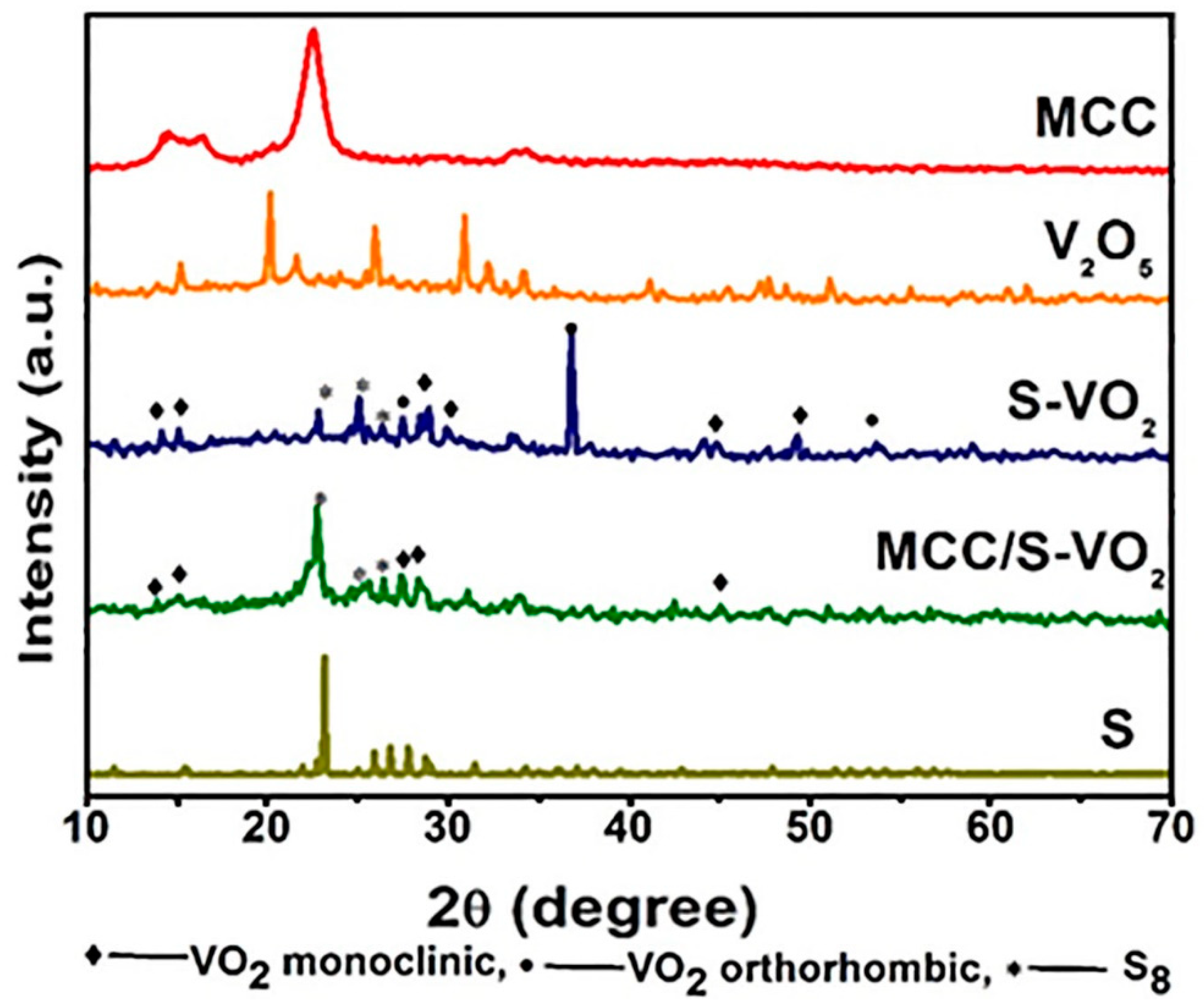
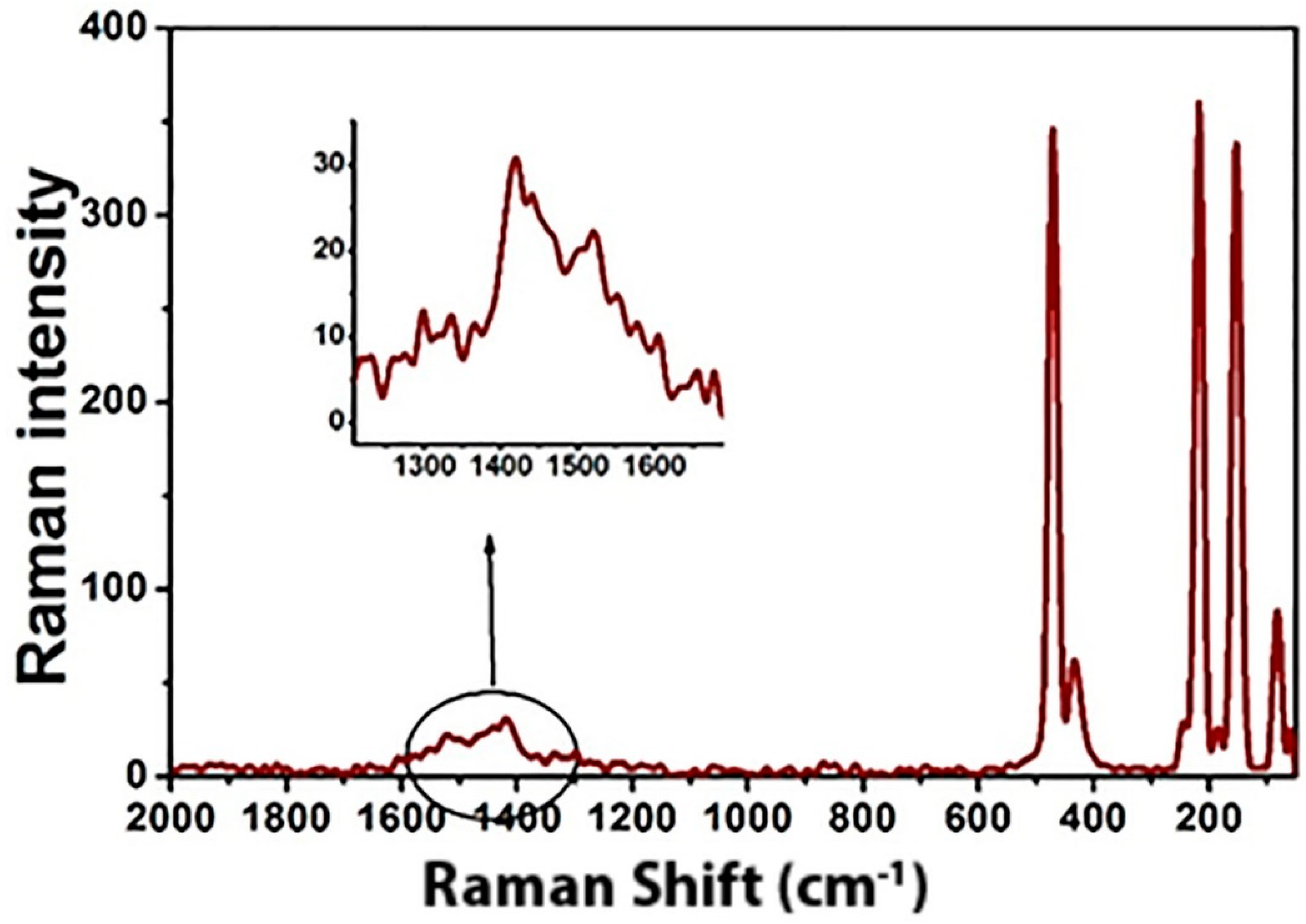
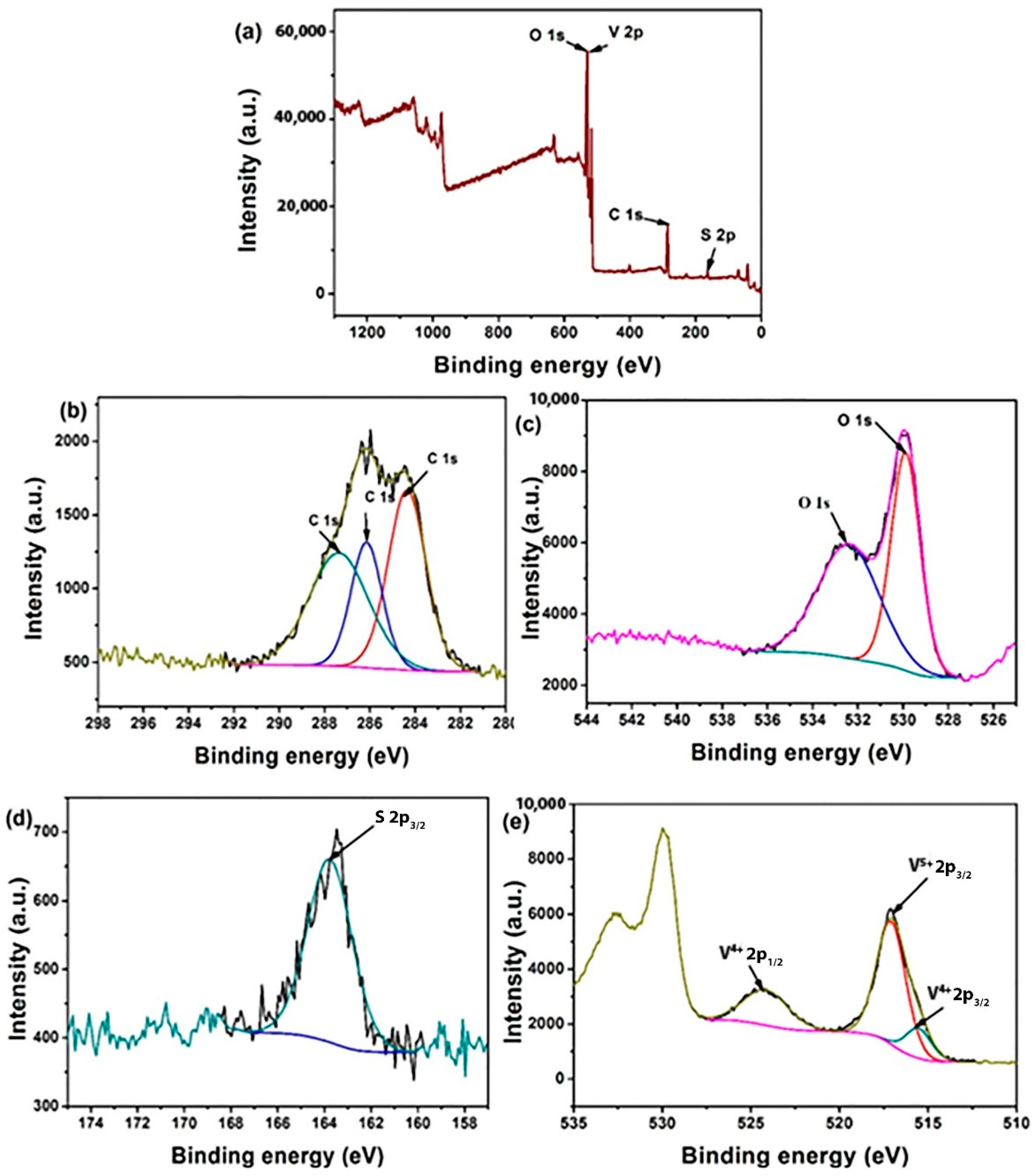
3.1. Characterization of the As-Prepared Products
3.2. Photocatalytic Activity
3.3. Active Species Scavenging
3.4. Proposed MB Photodegradation Mechanism
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- El-Feky, H.H.; Behiry, M.S.; Amin, A.S.; Nassar, M.Y. Facile Fabrication of Nano-sized SiO2 by an Improved Sol–Gel Route: As an Adsorbent for Enhanced Removal of Cd(II) and Pb(II) Ions. J. Inorg. Organomet. Polym. Mater. 2022, 32, 1129–1141. [Google Scholar] [CrossRef]
- Jahin, H.S.; Kandil, M.I.; Nassar, M.Y. Facile auto-combustion synthesis of calcium aluminate nanoparticles for efficient removal of Ni(II) and As(III) ions from wastewater. Environ. Technol. 2022, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, K.; Khaledyan, E.; Mansourpanah, Y. Novel modified magnetic mesopouros silica for rapid and efficient removal of methylene blue dye from aqueous media. J. Iran. Chem. Soc. 2020, 8, 159–170. [Google Scholar]
- Chen, C.-Y.; Hsu, L.-J. Kinetic study of self-assembly of Ni (II)-doped TiO2 nanocatalysts for the photodegradation of azo pollutants. RSC Adv. 2015, 5, 88266–88271. [Google Scholar] [CrossRef]
- Aljohani, M.M.; Masoud, E.M.; Mohamed, N.M.; Nassar, M.Y. Cobalt aluminate/carbon nanocomposite via an auto-combustion method: An efficient photocatalyst for photocatalytic degradation of organic dyes from aqueous media. Int. J. Environ. Anal. Chem. 2021, 1–21. [Google Scholar] [CrossRef]
- Fresno, F.; Portela, R.; Suárez, S.; Coronado, J.M. Photocatalytic materials: Recent achievements and near future trends. J. Mater. Chem. A 2014, 2, 2863–2884. [Google Scholar] [CrossRef]
- Huang, J.; Cao, Y.; Liu, Z.; Deng, Z.; Wang, W. Application of titanate nanoflowers for dye removal: A comparative study with titanate nanotubes and nanowires. Chem. Eng. J. 2012, 191, 38–44. [Google Scholar] [CrossRef]
- Mahmoud, H.R.; Ibrahim, S.M.; El-Molla, S.A. Textile dye removal from aqueous solutions using cheap MgO nanomaterials: Adsorption kinetics, isotherm studies and thermodynamics. Adv. Powder Technol. 2016, 27, 223–231. [Google Scholar] [CrossRef]
- Nassar, M.Y.; Ahmed, I.S. Template-free hydrothermal derived cobalt oxide nanopowders: Synthesis, characterization, and removal of organic dyes. Mater. Res. Bull. 2012, 47, 2638–2645. [Google Scholar] [CrossRef]
- Nassar, M.Y.; Ali, E.I.; Zakaria, E.S. Tunable auto-combustion preparation of TiO2 nanostructures as efficient adsorbents for the removal of an anionic textile dye. RSC Adv. 2017, 7, 8034–8050. [Google Scholar] [CrossRef]
- Nassar, M.Y.; Khatab, M. Cobalt ferrite nanoparticles via a template-free hydrothermal route as an efficient nano-adsorbent for potential textile dye removal. RSC Adv. 2016, 6, 79688–79705. [Google Scholar] [CrossRef]
- Raizada, P.; Singh, P.; Kumar, A.; Pare, B.; Jonnalagadda, S.B. Zero valent iron-brick grain nanocomposite for enhanced solar-Fenton removal of malachite green. Sep. Purif. Technol. 2014, 133, 429–437. [Google Scholar] [CrossRef]
- Parvizi, E.; Tayebee, R.; Koushki, E.; Abdizadeh, M.F.; Maleki, B.; Audebert, P.; Galmiche, L. Photocatalytic efficacy of supported tetrazine on MgZnO nanoparticles for the heterogeneous photodegradation of methylene blue and ciprofloxacin. RSC Adv. 2019, 9, 23818–23831. [Google Scholar] [CrossRef]
- Yu, J.; Trapalis, C.; Zhang, P.; Li, G.; Yu, H. Environmental Photocatalysis 2013. Int. J. Photoenergy 2013, 2013, 1–3. [Google Scholar] [CrossRef]
- Nassar, M.Y.; Aly, H.M.; Abdelrahman, E.A.; Moustafa, M.E. Synthesis, characterization, and biological activity of some novel Schiff bases and their Co(II) and Ni(II) complexes: A new route for Co3O4 and NiO nanoparticles for photocatalytic degradation of methylene blue dye. J. Mol. Struct. 2017, 1143, 462–471. [Google Scholar] [CrossRef]
- Zhu, H.; Jiang, R.; Fu, Y.; Guan, Y.; Yao, J.; Xiao, L.; Zeng, G. Effective photocatalytic decolorization of methyl orange utilizing TiO2/ZnO/chitosan nanocomposite films under simulated solar irradiation. Desalination 2012, 286, 41–48. [Google Scholar] [CrossRef]
- Pathania, D.; Kumari, M.; Gupta, V.K. Fabrication of ZnS–cellulose nanocomposite for drug delivery, antibacterial and photocatalytic activity. Mater. Des. 2015, 87, 1056–1064. [Google Scholar] [CrossRef]
- Zhang, G.; Wang, S.; Zhao, S.; Fu, L.; Chen, G.; Yang, F. Oxidative degradation of azo dye by hydrogen peroxide electrogenerated in situ on anthraquinonemonosulphonate/polypyrrole composite cathode with heterogeneous CuO/γ-Al2O3 catalyst. Appl. Catal. B Environ. 2011, 106, 370–378. [Google Scholar] [CrossRef]
- Saleh, K.A.; Sadiq, Y.K. Synthesis and characterization of chrome (VI) ion/iron oxide/chitosan composite for oxidation of methylene blue by photo-fenton reaction. Chem. Methodol. 2023, 7, 112–122. [Google Scholar]
- Kalam, A.; Al-Sehemi, A.G.; Assiri, M.; Du, G.; Ahmad, T.; Ahmad, I.; Pannipara, M. Modified solvothermal synthesis of cobalt ferrite (CoFe2O4) magnetic nanoparticles photocatalysts for degradation of methylene blue with H2O2/visible light. Results Phys. 2018, 8, 1046–1053. [Google Scholar] [CrossRef]
- Darder, M.; Aranda, P.; Ruiz-Hitzky, E. Bionanocomposites: A New Concept of Ecological, Bioinspired, and Functional Hybrid Materials. Adv. Mater. 2007, 19, 1309–1319. [Google Scholar] [CrossRef]
- Ghani, M.; Khodkavandi, S.; Jafari, Z.; Ghamari kargar, P.; Maleki, B.; Tabari, H.F. Synthesis of cellulose nanofibers-based ImSalophen@ Fe3O4 as a green sorbent for magnetic solid-phase extraction of chlorophenols followed by quantification via high-performance liquid chromatography-ultraviolet detection. Microchem. J. 2023, 187, 108368. [Google Scholar] [CrossRef]
- Maleki, B.; Sandaroos, R.; Naderi, S.; Peiman, S. A crowned manganese-based Schiff complex supported on nanocellulose as an efficient and sustainable heterogeneous catalyst for the oxidation of benzyl alcohols. J. Organomet. Chem. 2023, 990. [Google Scholar] [CrossRef]
- Sandaroos, R.; Maleki, B.; Naderi, S.; Peiman, S. Efficient synthesis of sulfones and sulfoxides from sulfides by cobalt-based Schiff complex supported on nanocellulose as catalyst and Oxone as the terminal oxidant. Inorg. Chem. Commun. 2023, 148, 110294. [Google Scholar] [CrossRef]
- Mohamad Haafiz, M.K.; Eichhorn, S.J.; Hassan, A.; Jawaid, M. Isolation and characterization of microcrystalline cellulose from oil palm biomass residue. Carbohydr. Polym. 2013, 93, 628–634. [Google Scholar] [CrossRef]
- Tang, A.M.; Hu, T.T.; Su, X. Fabrication of Microcrystalline Cellulose/CdS Nanocomposites and their Photocatalytic Properties. Adv. Mater. Res. 2013, 634, 2475–2480. [Google Scholar] [CrossRef]
- Manorama, S.; Basak, P.; Singh, S. Anti-Microbial Polymer Nanocomposites. In Nanocomposite Particles for Bio-Applications; Jenny Stanford Publishing: New York, NY, USA, 2011; pp. 249–264. [Google Scholar]
- Dong, Y.-Y.; Zhu, Y.-H.; Ma, M.-G.; Liu, Q.; He, W.-Q. Synthesis and characterization of Ag@ AgCl-reinforced cellulose composites with enhanced antibacterial and photocatalytic degradation properties. Sci. Rep. 2021, 11, 3366. [Google Scholar] [CrossRef]
- Saeed, R.M.Y.; Bano, Z.; Sun, J.; Wang, F.; Ullah, N.; Wang, Q. CuS-functionalized cellulose based aerogel as biocatalyst for removal of organic dye. J. Appl. Polym. Sci. 2018, 136, 47404. [Google Scholar] [CrossRef]
- Zeng, J.; Liu, S.; Cai, J.; Zhang, L. TiO2 Immobilized in Cellulose Matrix for Photocatalytic Degradation of Phenol under Weak UV Light Irradiation. J. Phys. Chem. C 2010, 114, 7806–7811. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Z. Synthesis and field emission property of VO2 nanorods with a body-centered-cubic structure. Phys. E Low-Dimens. Syst. Nanostructures 2009, 41, 548–551. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Z.; Zhu, Y.; Li, Z.; Vajtai, R.; Ci, L.; Ajayan, P.M. Nanostructured VO2 Photocatalysts for Hydrogen Production. ACS Nano 2008, 2, 1492–1496. [Google Scholar] [CrossRef]
- Salamati, M.; Kamyabjou, G.; Mohamadi, M.; Taghizade, K.; Kowsari, E. Preparation of TiO2@W-VO2 thermochromic thin film for the application of energy efficient smart windows and energy modeling studies of the produced glass. Constr. Build. Mater. 2019, 218, 477–482. [Google Scholar] [CrossRef]
- Lee, S.J.; Choi, D.S.; Kang, S.H.; Yang, W.S.; Nahm, S.; Han, S.H.; Kim, T. VO2/WO3-based hybrid smart windows with thermochromic and electrochromic properties. ACS Sustain. Chem. Eng. 2019, 7, 7111–7117. [Google Scholar] [CrossRef]
- Cen, Y.; Li, S.; Zhou, Y.; Cai, X.; Wang, X.; Xiang, Q.; Hu, B.; Yu, D.; Liu, Y.; Chen, C. Ultrathin VO2(B) Nanosheets as Cathode Material for High-Performance Hybrid Magnesium-Lithium Ion Batteries. J. Electrochem. Soc. 2019, 166, A1660–A1667. [Google Scholar] [CrossRef]
- Ajeya, K.V.; Sadhasivam, T.; Kurkuri, M.D.; Kang, U.-I.; Park, I.-S.; Park, W.-S.; Kim, S.-C.; Jung, H.-Y. Recovery of spent VOSO4 using an organic ligand for vanadium redox flow battery applications. J. Hazard. Mater. 2020, 399, 123047. [Google Scholar] [CrossRef]
- Zhang, Q.; Lu, Y. Change in crystallization mechanism of Sb film by doping VO2 for ultra-retention and high-speed phase change memory. Cryst. Growth Des. 2019, 19, 3477–3483. [Google Scholar] [CrossRef]
- Meenu, P.K.; Dehiya, B.S. One step hydrothermal synthesis of nanostructured VO2(B) for photocatalytic application. J. Nanosci. Technol. 2019, 5, 584–586. [Google Scholar]
- Solis-Casados, D.A.; Escobar-Alarcon, L.; Infantes-Molina, A.; Klimova, T.; Serrato-García, L.; Rodríguez-Castellón, E.; Hernandez-Lopez, S.; Dorazco-Gonzalez, A. Synthesis and characterization of Ag-modified V2O5 photocatalytic materials. J. Chem. 2017, 2017, 5849103. [Google Scholar] [CrossRef]
- Gurulakshmi, M.; Selvaraj, M.; Selvamani, A.; Vijayan, P.; Rekha, N.S.; Shanthi, K. Enhanced visible-light photocatalytic activity of V2O5/S-TiO2 nanocomposites. Appl. Catal. A Gen. 2012, 449, 31–46. [Google Scholar] [CrossRef]
- Bolokang, A.; Motaung, D.E.; Arendse, C.; Muller, T. Morphology and structural development of reduced anatase-TiO2 by pure Ti powder upon annealing and nitridation: Synthesis of TiOx and TiOxNy powders. Mater. Charact. 2015, 100, 41–49. [Google Scholar] [CrossRef]
- Zhang, J.; Fu, W.; Xi, J.; He, H.; Zhao, S.; Lu, H.; Ji, Z. N-doped rutile TiO2 nano-rods show tunable photocatalytic selectivity. J. Alloys Compd. 2013, 575, 40–47. [Google Scholar] [CrossRef]
- Wu, T.; Niu, P.; Yang, Y.; Yin, L.; Tan, J.; Zhu, H.; Irvine, J.T.S.; Wang, L.; Liu, G.; Cheng, H. Homogeneous Doping of Substitutional Nitrogen/Carbon in TiO2 Plates for Visible Light Photocatalytic Water Oxidation. Adv. Funct. Mater. 2019, 29, 1901943. [Google Scholar] [CrossRef]
- Kerli, S.; Alver, Ü.; Eskalen, H.; Uruş, S.; Soğuksu, A.K. Structural and Morphological Properties of Boron Doped V2O5 Thin Films: Highly Efficient Photocatalytic Degradation of Methyl Blue. Russ. J. Appl. Chem. 2019, 92, 304–309. [Google Scholar] [CrossRef]
- Vorontsov, A.V.; Valdés, H. Insights into the visible light photocatalytic activity of S-doped hydrated TiO2. Int. J. Hydrogen Energy 2019, 44, 17963–17973. [Google Scholar] [CrossRef]
- Huang, F.; Yan, A.; Zhao, H. Influences of Doping on Photocatalytic Properties of TiO2 Photocatalyst. In Semiconductor Photocatalysis: Materials, Mechanisms and Applications; InTech: Rijeka, Croatia, 2016; pp. 31–80. [Google Scholar] [CrossRef]
- Xu, Q.; Wang, X.; Dong, X.; Ma, C.; Zhang, X.; Ma, H. Improved Visible Light Photocatalytic Activity for TiO2Nanomaterials by Codoping with Zinc and Sulfur. J. Nanomater. 2015, 16, 7. [Google Scholar] [CrossRef]
- Wawrzkiewicz, M.; Hubicki, Z. Anion exchange resins as effective sorbents for removal of acid, reactive, and direct dyes from textile wastewaters. In Ion Exchange—Studies and Applications; IntechOpen: London, UK, 2015; pp. 37–72. [Google Scholar]
- Wawrzkiewicz, M.; Hubicki, Z. Remazol Black B removal from aqueous solutions and wastewater using weakly basic anion exchange resins. Open Chem. 2011, 9, 867–876. [Google Scholar] [CrossRef]
- Chuayjuljit, S.; Su-Uthai, S.; Charuchinda, S. Poly(vinyl chloride) film filled with microcrystalline cellulose prepared from cotton fabric waste: Properties and biodegradability study. Waste Manag. Res. 2010, 28, 109–117. [Google Scholar] [CrossRef]
- Naraginti, S.; Li, Y.; Wu, Y.; Zhang, C.; Upreti, A.R. Mechanistic study of visible light driven photocatalytic degradation of EDC 17α-ethinyl estradiol and azo dye Acid Black-52: Phytotoxicity assessment of intermediates. RSC Adv. 2016, 6, 87246–87257. [Google Scholar] [CrossRef]
- Fu, Y.; Chen, H.; Sun, X.; Wang, X. Combination of cobalt ferrite and graphene: High-performance and recyclable visible-light photocatalysis. Appl. Catal. B Environ. 2012, 111, 280–287. [Google Scholar] [CrossRef]
- Liu, T.; Wang, L.; Lu, X.; Fan, J.; Cai, X.; Gao, B.; Miao, R.; Wang, J.; Lv, Y. Comparative study of the photocatalytic performance for the degradation of different dyes by ZnIn2S4: Adsorption, active species, and pathways. RSC Adv. 2017, 7, 12292–12300. [Google Scholar] [CrossRef]
- Sèbe, G.; Ham-Pichavant, F.; Ibarboure, E.; Koffi, A.L.C.; Tingaut, P. Supramolecular Structure Characterization of Cellulose II Nanowhiskers Produced by Acid Hydrolysis of Cellulose I Substrates. Biomacromolecules 2012, 13, 570–578. [Google Scholar] [CrossRef]
- Liu, L.; Yao, T.; Tan, X.; Liu, Q.; Wang, Z.; Shen, D.; Sun, Z.; Wei, S.; Xie, Y. Room-Temperature Intercalation-Deintercalation Strategy Towards VO2 (B) Single Layers with Atomic Thickness. Small 2012, 8, 3752–3756. [Google Scholar] [CrossRef]
- Jenkins, R.; Snyder, R.L. Introduction to X-ray Powder Diffractometry; John Wiley & Sons, Inc.: New York, NY, USA, 1996. [Google Scholar]
- Refat, N.M.; Nassar, M.Y.; Sadeek, S.A. A controllable one-pot hydrothermal synthesis of spherical cobalt ferrite nanoparticles: Synthesis, characterization, and optical properties. RSC Adv. 2022, 12, 25081–25095. [Google Scholar] [CrossRef]
- Elanthikkal, S.; Gopalakrishnapanicker, U.; Varghese, S.; Guthrie, J.T. Cellulose microfibres produced from banana plant wastes: Isolation and characterization. Carbohydr. Polym. 2010, 80, 852–859. [Google Scholar] [CrossRef]
- Wei, Y.; Ryu, C.-W.; Kim, K.-B. Improvement in electrochemical performance of V2O5 by Cu doping. J. Power Sources 2007, 165, 386–392. [Google Scholar] [CrossRef]
- Wu, X.; Wu, Z.; Ji, C.; Feng, H.; Ma, X.; Su, Y.; Zhou, Y.; Wang, J.; Jiang, Y. Influence of infrared optical properties by transformation of the crystal structure in Al-doped vanadium dioxide films. Opt. Mater. Express 2016, 6, 3500–3506. [Google Scholar] [CrossRef]
- Ji, H.; Liu, D.; Cheng, H.; Zhang, C.; Yang, L.; Ren, D. Infrared thermochromic properties of monoclinic VO2 nanopowders using a malic acid-assisted hydrothermal method for adaptive camouflage. RSC Adv. 2017, 7, 5189–5194. [Google Scholar] [CrossRef]
- Liu, P.; Gardner, J.M.; Kloo, L. Solution processable, cross-linked sulfur polymers as solid electrolytes in dye-sensitized solar cells. Chem. Commun. 2015, 51, 14660–14662. [Google Scholar] [CrossRef]
- Adar, F. Characterizing modified celluloses using Raman spectroscopy. Spectroscopy 2016, 31, 22–27. [Google Scholar]
- Kim, H.; Youn, J.R.; Song, Y.S. Eco-friendly flame retardant nanocrystalline cellulose prepared via silylation. Nanotechnology 2018, 29, 455702. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, X.; Zhang, Y.; Abas, A.; Zhao, X.; Yang, Z.; Su, Q.; Lan, W.; Xie, E. Lightweight, interconnected VO2 nanoflowers hydrothermally grown on 3D graphene networks for wide-voltage-window supercapacitors. RSC Adv. 2017, 7, 35558–35564. [Google Scholar] [CrossRef]
- Li, Y.; Shen, S.; Wang, C.; Peng, X.; Yuan, S. The effect of difference in chemical composition between cellulose and lignin on carbon based solid acids applied for cellulose hydrolysis. Cellulose 2018, 25, 1851–1863. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, P.; Jiang, F.; Tian, J.; Cui, H.; Yang, J. Vanadium sulfide sub-microspheres: A new near-infrared-driven photocatalyst. J. Colloid Interface Sci. 2017, 498, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Silversmit, G.; Depla, D.; Poelman, H.; Marin, G.B.; De Gryse, R. Determination of the V2p XPS binding energies for different vanadium oxidation states (V5+ to V0+). J. Electron Spectrosc. Relat. Phenom. 2004, 135, 167–175. [Google Scholar] [CrossRef]
- Owolabi, A.F.; Haafiz, M.M.; Hossain, S.; Hussin, M.H.; Fazita, M.N. Influence of alkaline hydrogen peroxide pre-hydrolysis on the isolation of microcrystalline cellulose from oil palm fronds. Int. J. Biol. Macromol. 2017, 95, 1228–1234. [Google Scholar] [CrossRef]
- Kian, L.K.; Jawaid, M.; Ariffin, H.; Alothman, O.Y. Isolation and characterization of microcrystalline cellulose from roselle fibers. Int. J. Biol. Macromol. 2017, 103, 931–940. [Google Scholar] [CrossRef] [PubMed]
- Murugan, A.V.; Quintin, M.; Delville, M.H.; Campet, G.; Vijayamohanan, K. Entrapment of poly (3, 4-ethylenedioxythiophene) between VS2 layers to form a new organic–inorganic intercalative nanocomposite. J. Mater. Chem. 2005, 15, 902–909. [Google Scholar] [CrossRef]
- Liu, L.; Cao, F.; Yao, T.; Xu, Y.; Zhou, M.; Qu, B.; Pan, B.; Wu, C.; Wei, S.; Xie, Y. New-phase VO2 micro/nanostructures: Investigation of phase transformation and magnetic property. New J. Chem. 2012, 36, 619–625. [Google Scholar] [CrossRef]
- Štengl, V.; Grygar, T.M. The simplest way to iodine-doped anatase for photocatalysts activated by visible light. Int. J. Photoenergy 2011, 2011, 685935. [Google Scholar] [CrossRef]
- Li, S.; Lin, Y.-H.; Zhang, B.-P.; Wang, Y.; Nan, C.-W. Controlled Fabrication of BiFeO3 Uniform Microcrystals and Their Magnetic and Photocatalytic Behaviors. J. Phys. Chem. C 2010, 114, 2903–2908. [Google Scholar] [CrossRef]
- Sun, J.; Li, X.; Zhao, Q.; Ke, J.; Zhang, D. Novel V2O5/BiVO4/TiO2 nanocomposites with high visible-light-induced photocatalytic activity for the degradation of toluene. J. Phys. Chem. C 2014, 118, 10113–10121. [Google Scholar] [CrossRef]
- Sheha, E.; Kamar, E. Structural characteristic of vanadium(V) oxide/sulfur composite cathode for magnesium battery applications. Mater. Sci. 2019, 37, 570–576. [Google Scholar] [CrossRef]
- Basu, R.; Ghosh, S.; Bera, S.; Das, A.; Dhara, S. Phase-pure VO2 nanoporous structure for binder-free supercapacitor performances. Sci. Rep. 2019, 9, 4621. [Google Scholar] [CrossRef]
- Mohamed, M.M.; Bayoumy, W.A.; Goher, M.E.; Abdo, M.H.; El-Ashkar, T.Y. Optimization of α-Fe2O3@ Fe3O4 incorporated N-TiO2 as super effective photocatalysts under visible light irradiation. Appl. Surf. Sci. 2017, 412, 668–682. [Google Scholar] [CrossRef]
- Virkutyte, J.; Jegatheesan, V.; Varma, R.S. Visible light activated TiO2/microcrystalline cellulose nanocatalyst to destroy organic contaminants in water. Bioresour. Technol. 2012, 113, 288–293. [Google Scholar] [CrossRef]
- Wei, G.; Zuo, H.-F.; Guo, Y.-R.; Pan, Q.-J. Synthesis of ZnO with Enhanced Photocatalytic Activity: A Novel Approach Using Nanocellulose. Bioresources 2016, 11, 6244–6253. [Google Scholar] [CrossRef]
- Zhu, C.; Zhang, X.; Zhang, Y.; Li, Y.; Wang, P.; Jia, Y.; Liu, J. Ultrasonic-Assisted Synthesis of CdS/Microcrystalline Cellulose Nanocomposites with Enhanced Visible-Light-Driven Photocatalytic Degradation of MB and the Corresponding Mechanism Study. Front. Chem. 2022, 10. [Google Scholar] [CrossRef]
- de Campos, E.A.; de Campos, S.D.; Roos, A.A.; de Souza, B.V.C.; Schneider, J.M.; Uliana, M.B.; Oliveira, R.C. Titanium dioxide dispersed on cellulose acetate and its application in methylene blue photodegradation. Polym. Polym. Compos. 2013, 21, 423–430. [Google Scholar] [CrossRef]
- Zang, Y.; Farnood, R. Effect of Hydrogen Peroxide on the Photocatalytic Degradation of Methyl tert-butyl Ether. Top. Catal. 2006, 37, 91–96. [Google Scholar] [CrossRef]
- Butler, M.A.; Ginley, D.S. Prediction of Flatband Potentials at Semiconductor-Electrolyte Interfaces from Atomic Electronegativities. J. Electrochem. Soc. 1978, 125, 228–232. [Google Scholar] [CrossRef]
- Xu, Y.; Schoonen, M.A. The absolute energy positions of conduction and valence bands of selected semiconducting minerals. Am. Miner. 2000, 85, 543–556. [Google Scholar] [CrossRef]


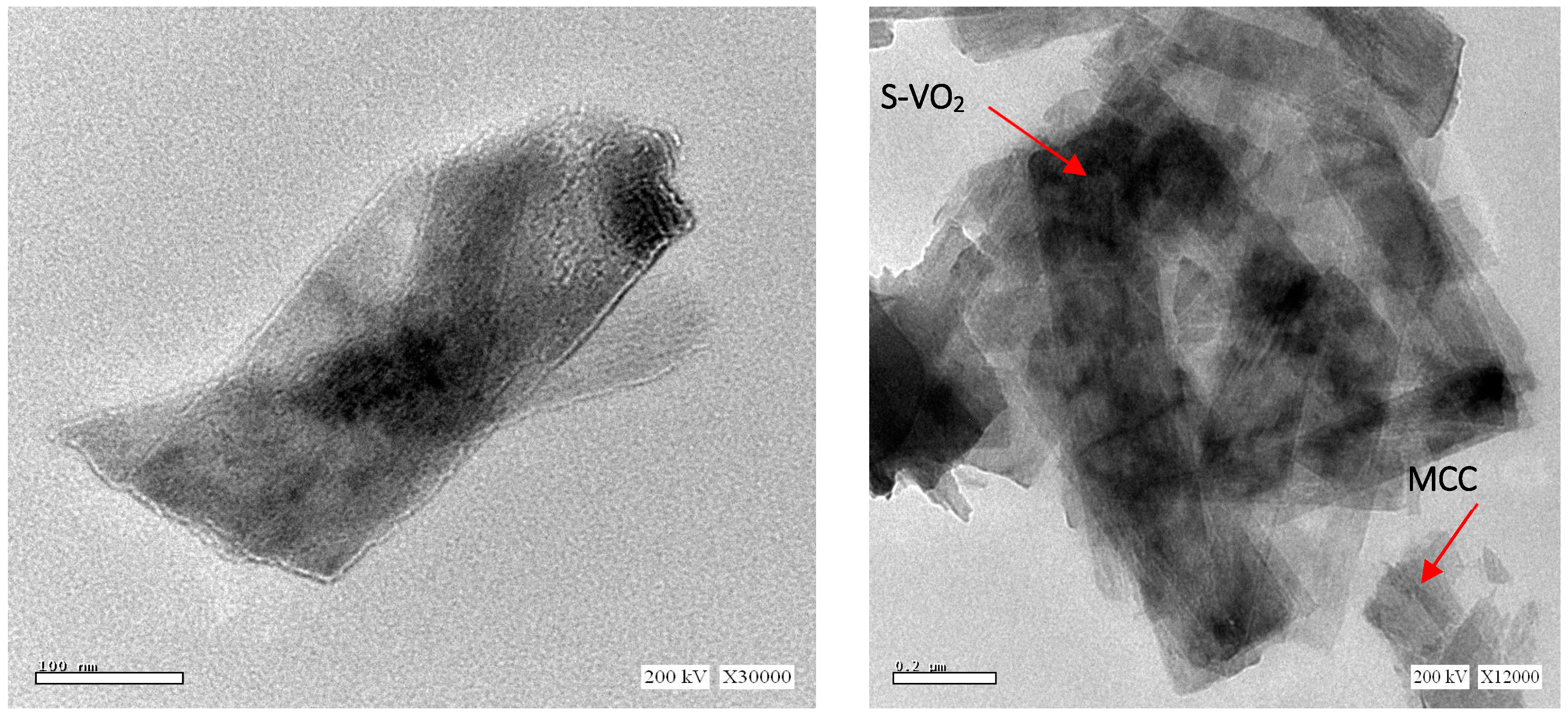
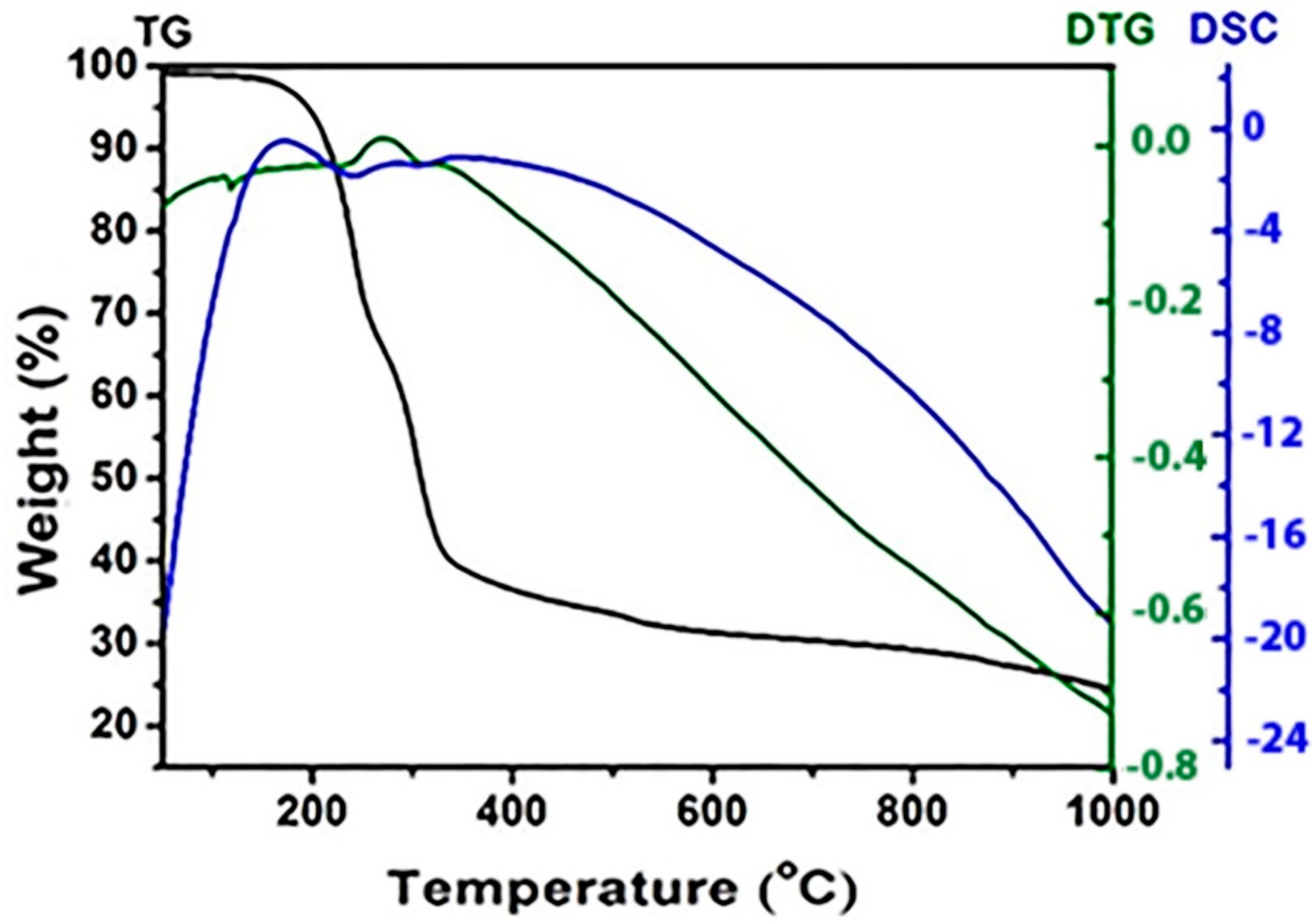
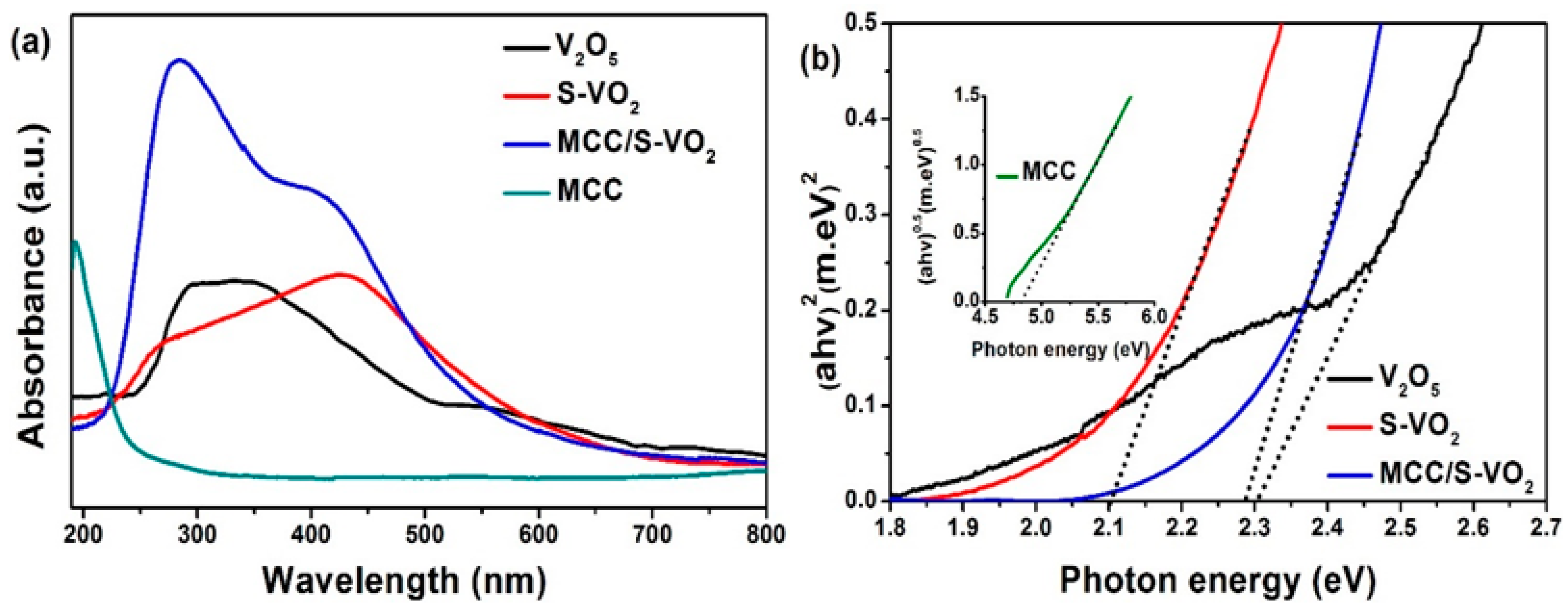
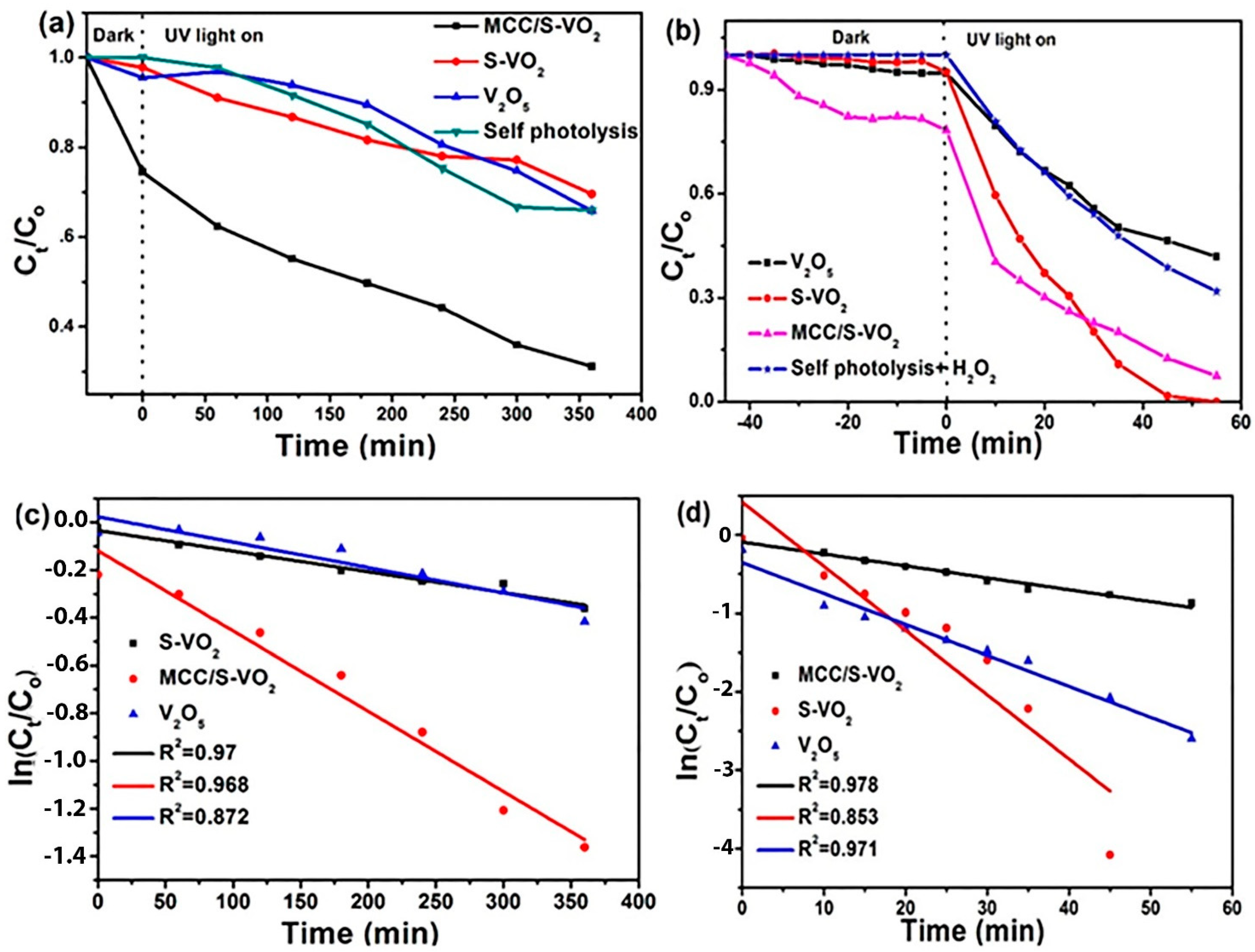

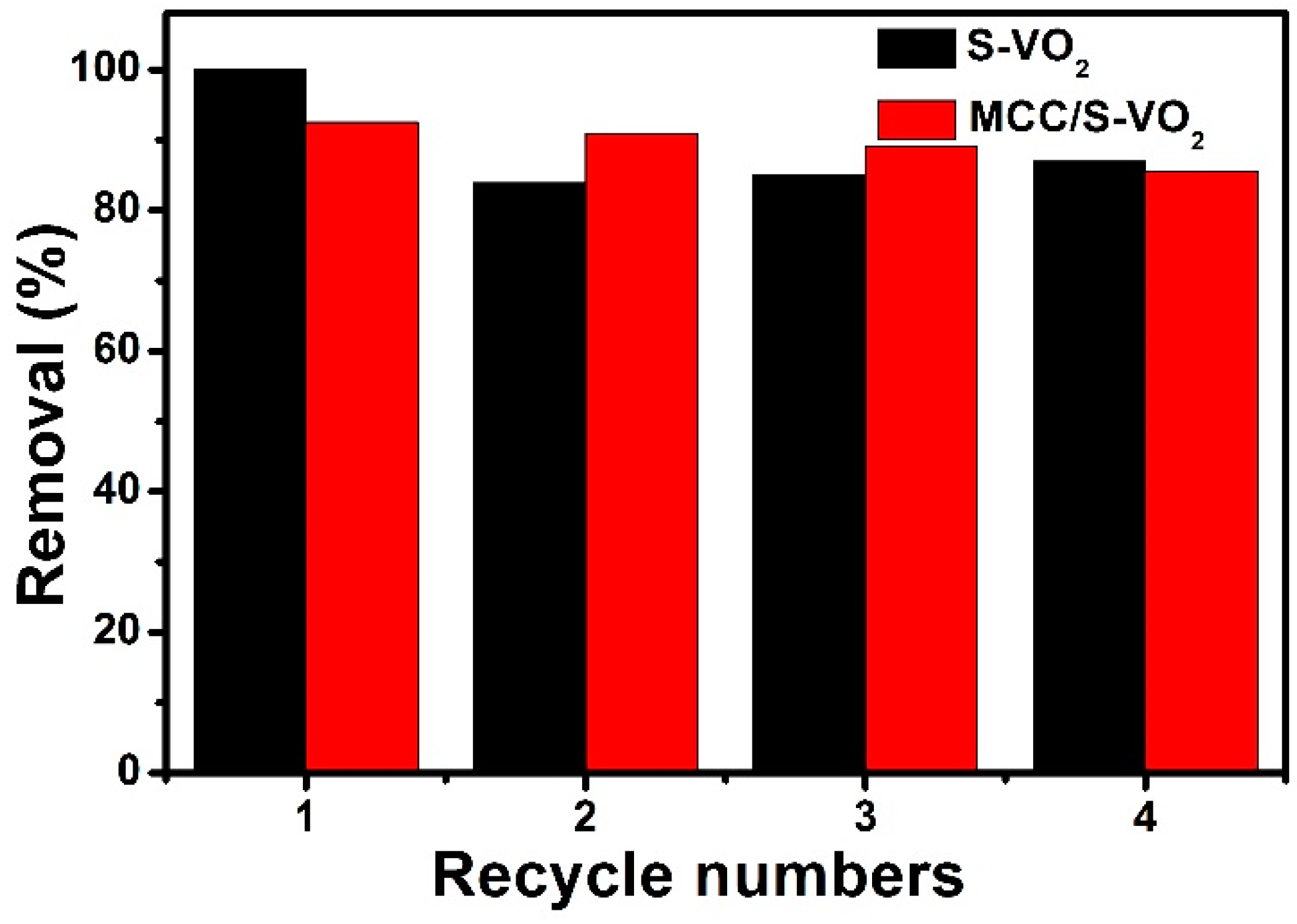

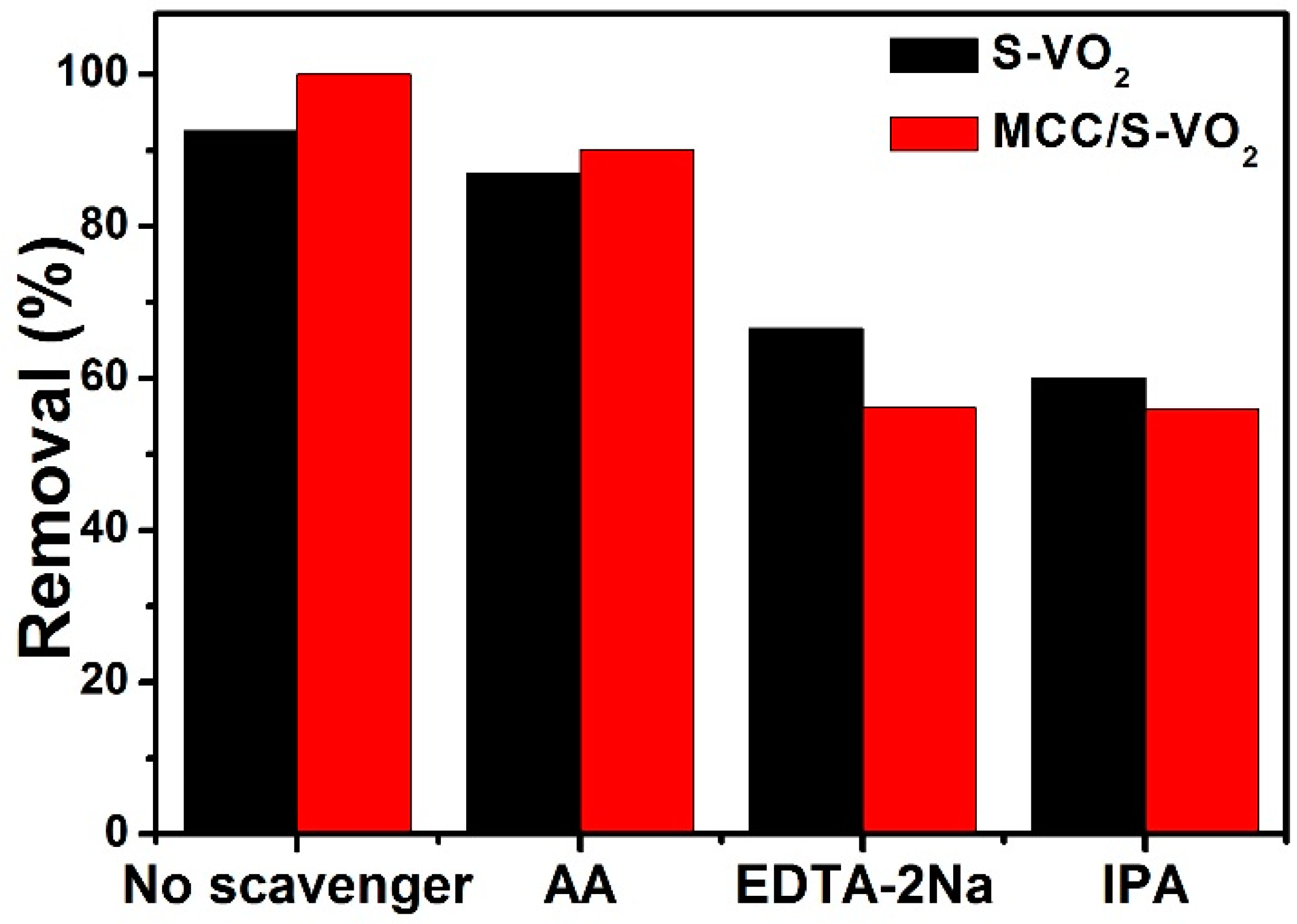
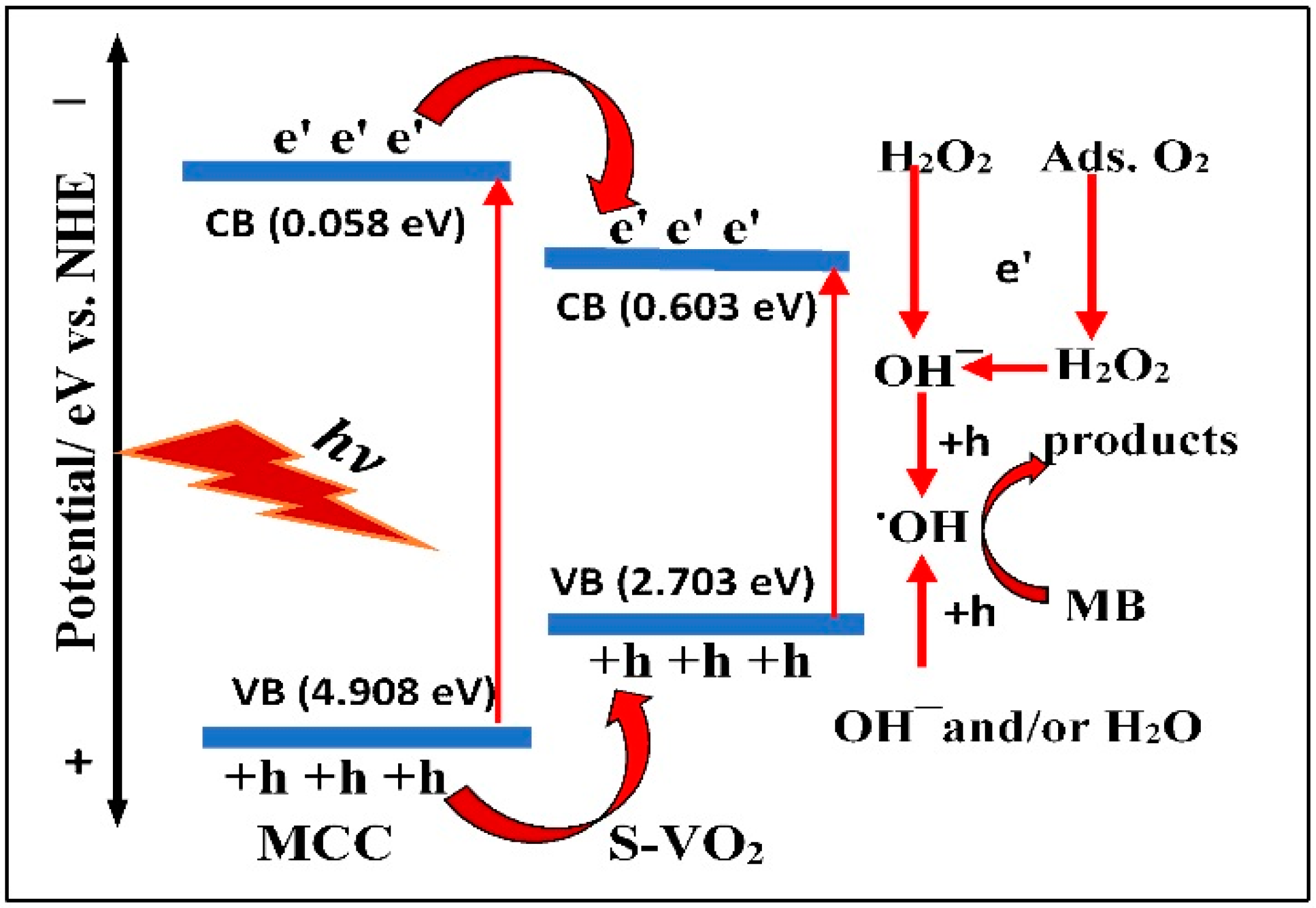
| Samples | With H2O2 | Without H2O2 | ||
|---|---|---|---|---|
| Kobs (min−1) | R2 | Kobs (min−1) | R2 | |
| S-VO2 | 0.0819 | 0.853 | 0.0008 | 0.97 |
| MCC/S-VO2 | 0.039 | 0.971 | 0.0034 | 0.97 |
| Photocatalyst Name | Catalyst Amount (mg) | MB Conc. (mg L−1) | Degradation Rate (%) | Irradiation Time (min) | Irradiation Light | Reference |
|---|---|---|---|---|---|---|
| S-VO2 | 20 | 20 | 100 | 55 | UV irradiation | Present study |
| Ag@AgCl-reinforced cellulose | 15 | 20 | 100 | 60 | Visible light | [28] |
| MCC/S-VO2 | 20 | 20 | 92.9 | 55 | UV irradiation | Present study |
| TiO2/MCC | 30 | 100 | 90 | 240 | UV irradiation | [79] |
| ZnO/MCC | 100 | 15 | 89.8 | 120 | UV irradiation | [80] |
| CdS/MCC | 50 | 10 | 81.5 | 60 | Visible light | [81] |
| CuS-functionalized CBA | 25 | 20 | 79.7 | 60 | Visible light | [29] |
| Cellulose acetate/TiO2 | 100 | 0.4 | 70 | 90 | Visible light | [82] |
| Samples | (eV) | Eg (eV) | ECB (eV) | EVB (eV) |
|---|---|---|---|---|
| MCC | 6.983 | 4.85 | 0.058 | 4.908 |
| S-VO2 | 6.153 | 2.10 | 0.603 | 2.703 |
| MCC/S-VO2 | 6.554 | 2.29 | 0.910 | 1.380 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nassar, M.Y.; NourEldien, M.S.; Ibrahim, I.M.; Aly, H.M. A Facile Hydrothermal Synthesis of S-VO2-Cellulose Nanocomposite for Photocatalytic Degradation of Methylene Blue Dye. Processes 2023, 11, 1322. https://doi.org/10.3390/pr11051322
Nassar MY, NourEldien MS, Ibrahim IM, Aly HM. A Facile Hydrothermal Synthesis of S-VO2-Cellulose Nanocomposite for Photocatalytic Degradation of Methylene Blue Dye. Processes. 2023; 11(5):1322. https://doi.org/10.3390/pr11051322
Chicago/Turabian StyleNassar, Mostafa Y., Mona S. NourEldien, Islam M. Ibrahim, and Hisham M. Aly. 2023. "A Facile Hydrothermal Synthesis of S-VO2-Cellulose Nanocomposite for Photocatalytic Degradation of Methylene Blue Dye" Processes 11, no. 5: 1322. https://doi.org/10.3390/pr11051322
APA StyleNassar, M. Y., NourEldien, M. S., Ibrahim, I. M., & Aly, H. M. (2023). A Facile Hydrothermal Synthesis of S-VO2-Cellulose Nanocomposite for Photocatalytic Degradation of Methylene Blue Dye. Processes, 11(5), 1322. https://doi.org/10.3390/pr11051322






