Ability of Deep Eutectic Solvent Modified Oat Straw for Cu(II), Zn(II), and Se(IV) Ions Removal
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Biomass Preparation
2.3. Biomass Modification
2.4. Material Characterization
2.5. Ultrasound and Water Stability Test of IOS
2.6. Adsorption Study
3. Results
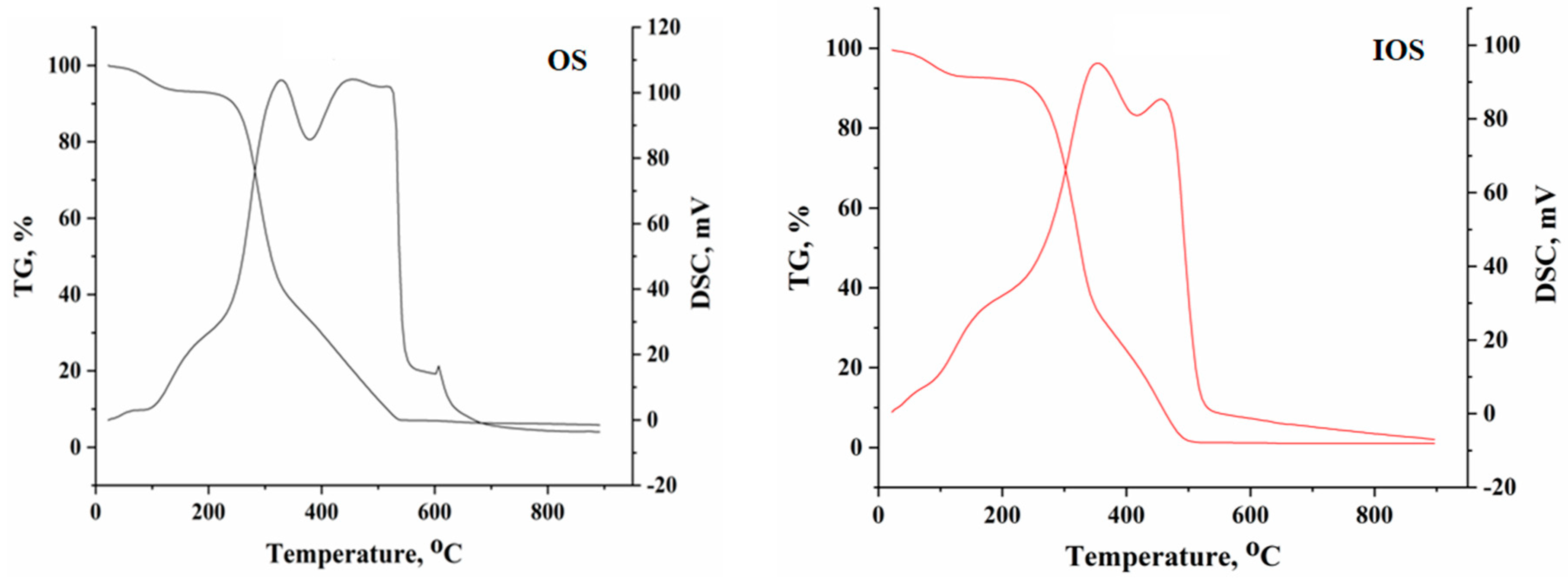
3.1. Material Characterization
3.2. Ultrasound and Water Stability of IOS
3.3. Batch Adsorption Tests
3.3.1. Preliminary Adsorption Test
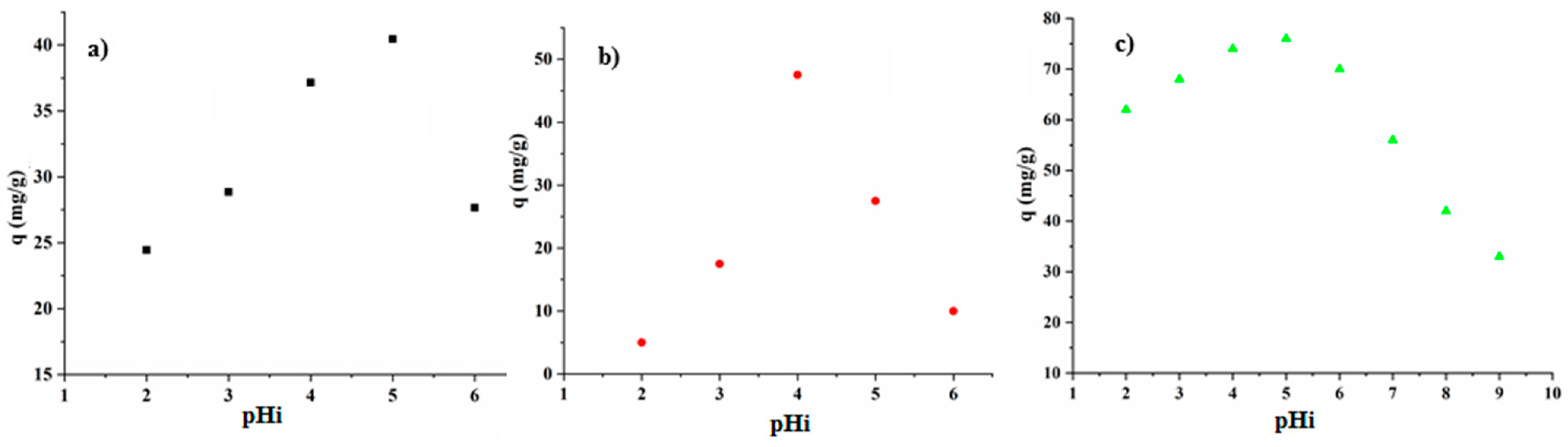
3.3.2. The Effect of Initial pH
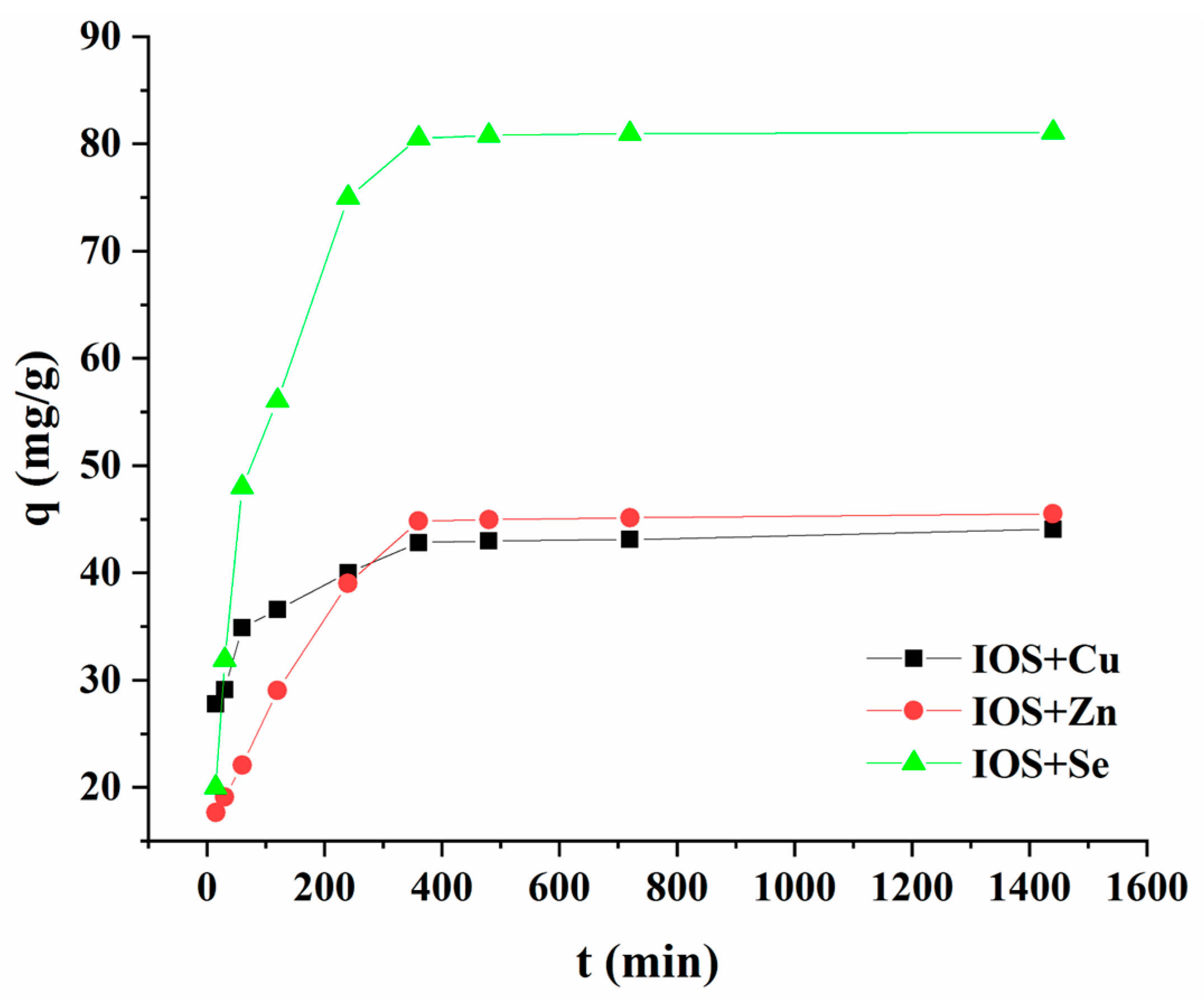
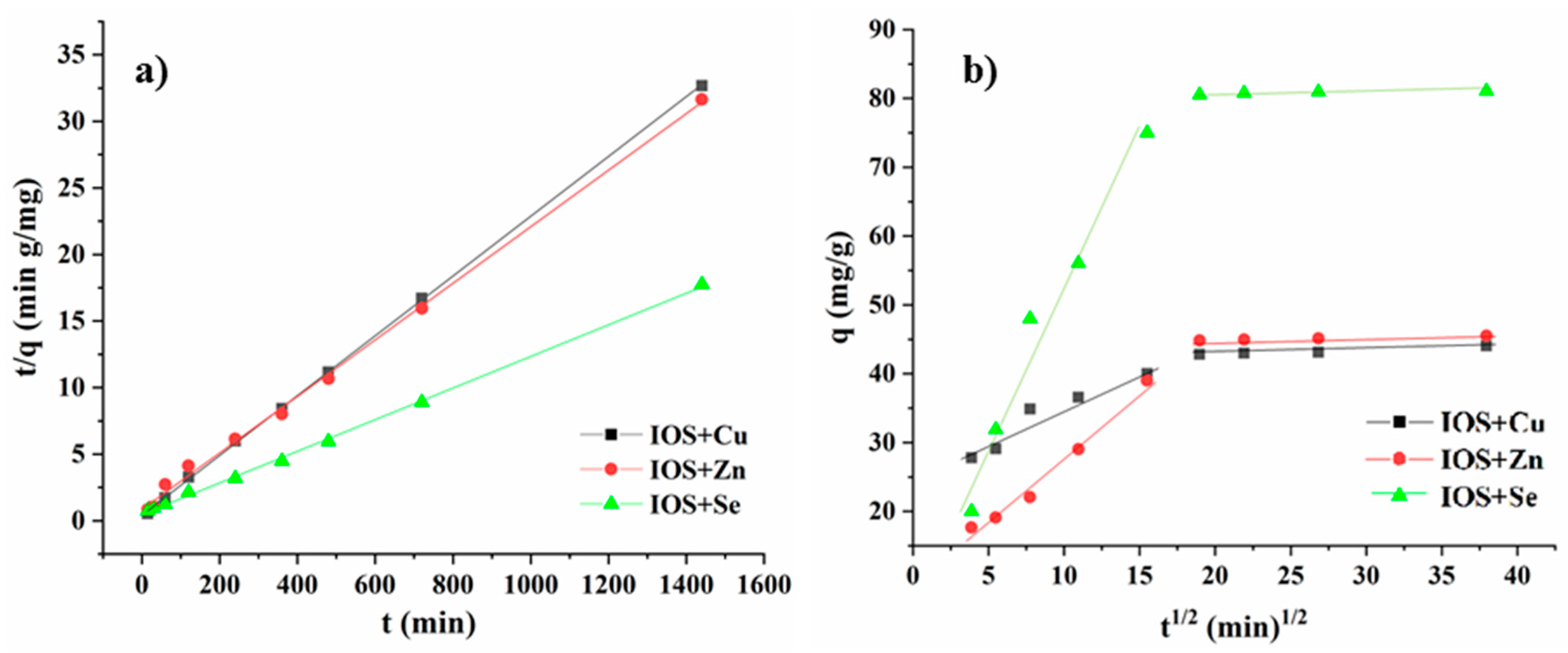
3.3.3. Effect of Contact Time and Kinetics Studies
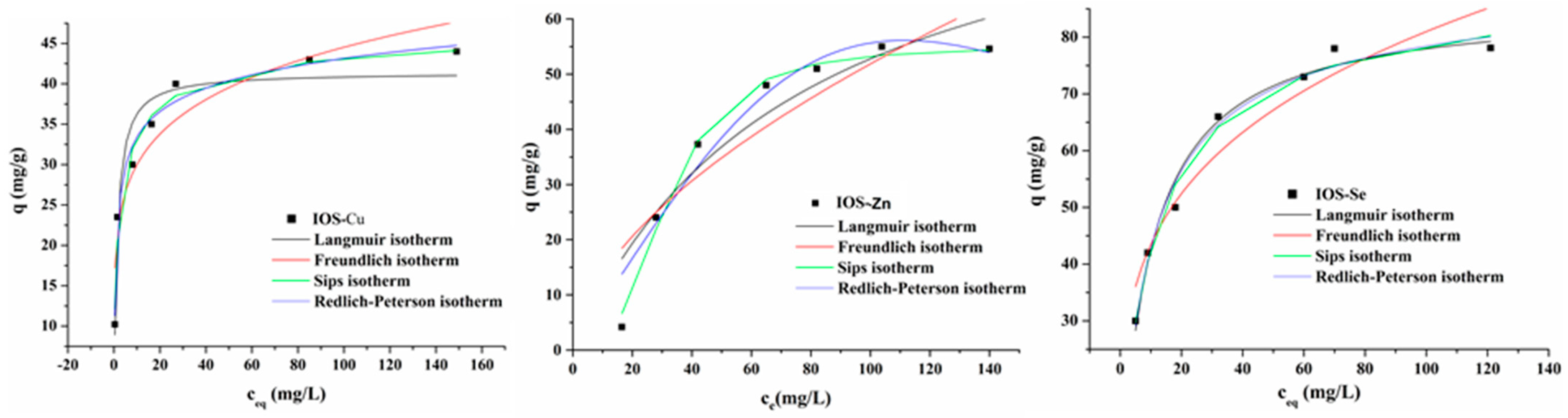
3.3.4. Isotherm Study
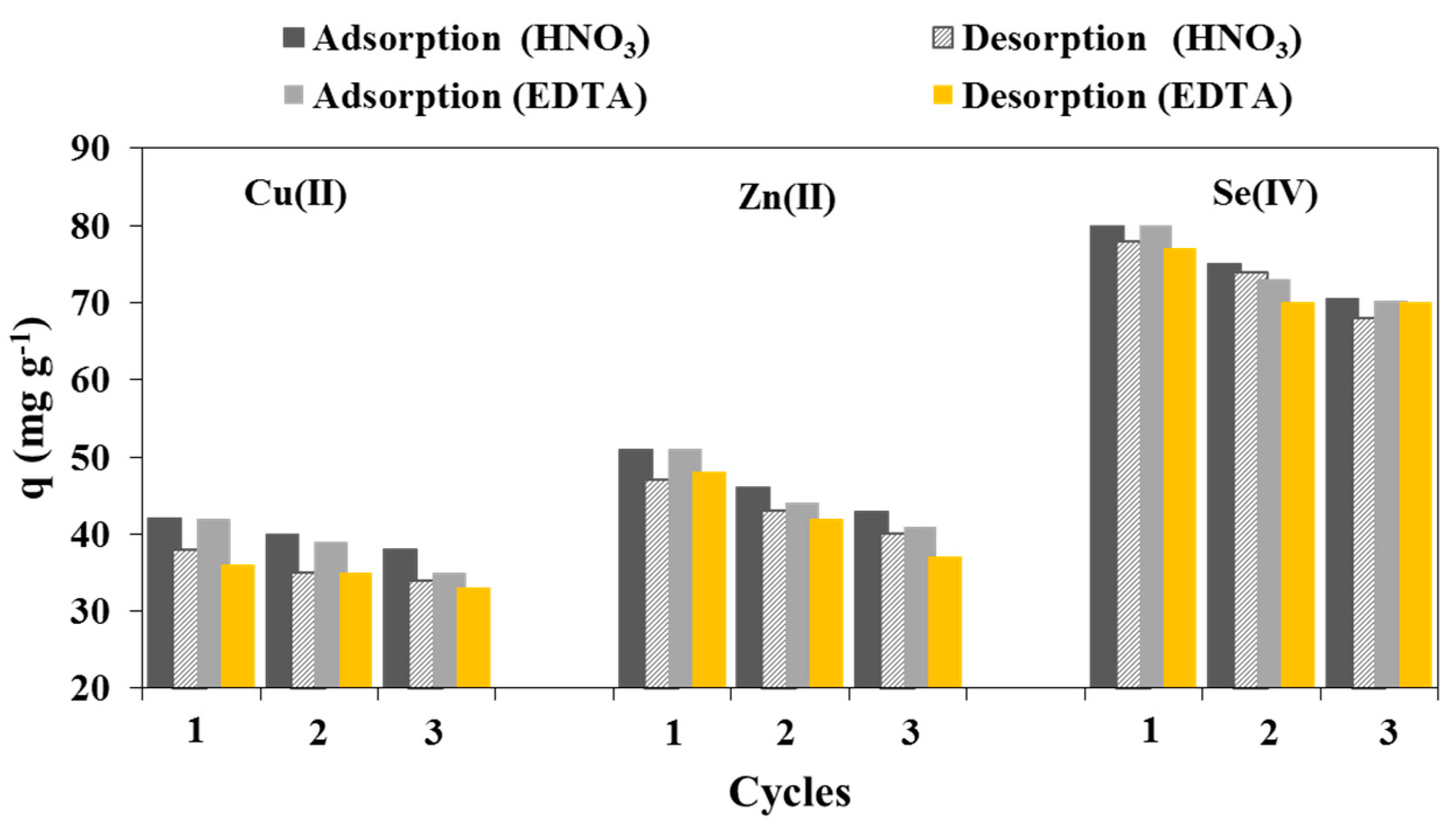
3.3.5. Desorption Study
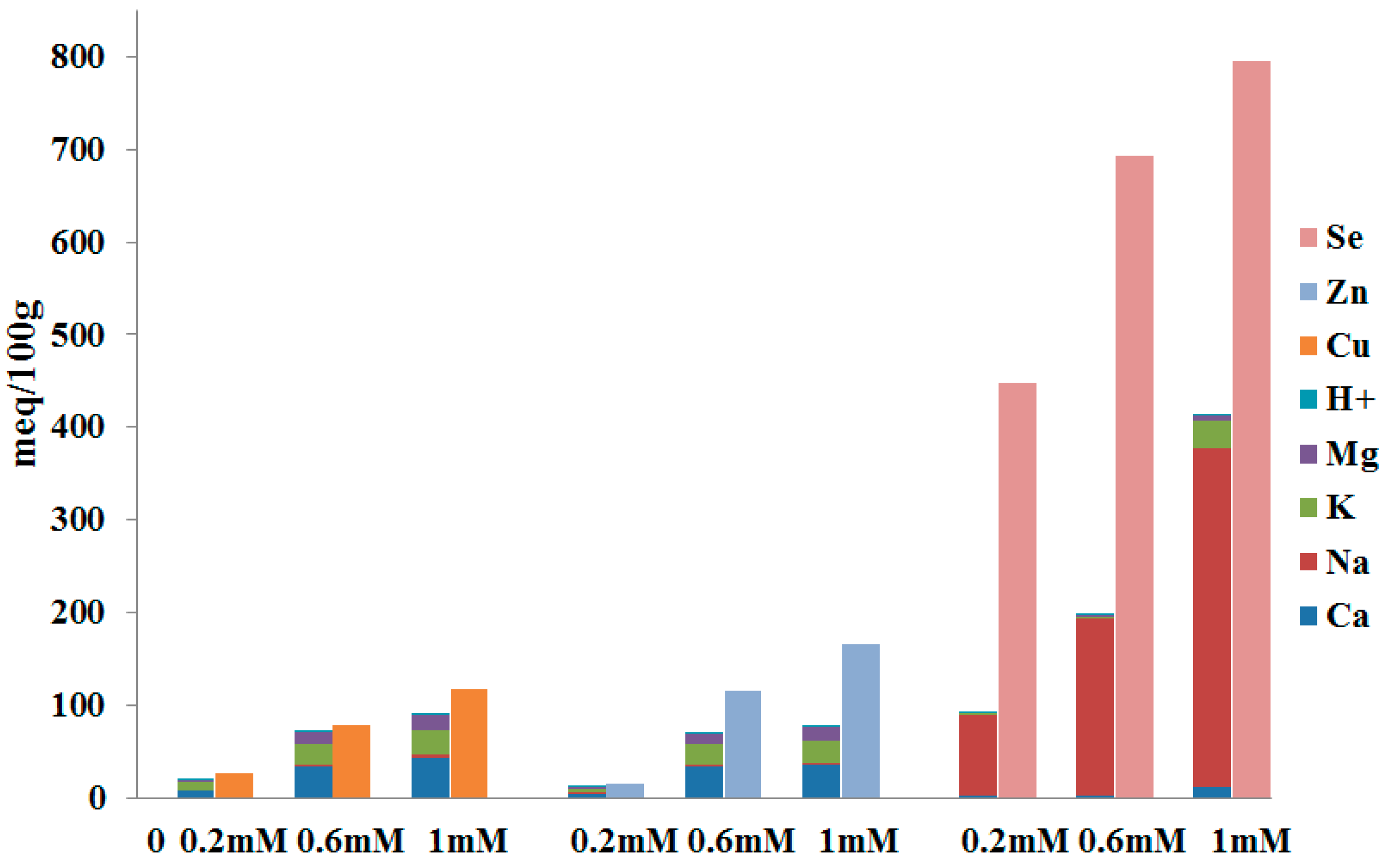
3.3.6. Ion-Exchange Mechanism
3.3.7. Real Effluent Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Simić, M.; Petrović, J.; Šoštarić, T.; Ercegović, M.; Milojković, J.; Lopičić, Z.; Kojić, M. A Mechanism Assessment and Differences of Cadmium Adsorption on Raw and Alkali-Modified Agricultural Waste. Processes 2022, 10, 1957. [Google Scholar] [CrossRef]
- Zhang, T.; Jiang, W.; Cao, Y.; Zhu, C.; Toukouki, S.; Yao, S. A facile one-pot synthesis of ionic liquid@porous organic frameworks for rapid high-capacity removal of heavy metal ions, pesticides and aflatoxin from two non-food bioactive products. Ind. Crop. Prod. 2022, 181, 114859. [Google Scholar] [CrossRef]
- Gollakota, R.K.A.; Munagapati, S.V.; Shu, M.C.; Wen, C.J. Adsorption of Cr (VI), and Pb (II) from aqueous solution by 1-Butyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide functionalized biomass Hazel Sterculia (Sterculia foetida L.). J. Mol. Liq. 2022, 350, 118534. [Google Scholar] [CrossRef]
- Fan, L.; Miao, J.; Yang, J.; Zhao, X.; Shi, W.; Xie, M.; Wang, X.; Chen, W.; An, X.; Luo, H.; et al. Invasive plant-crofton weed as adsorbent for effective removal of copper from aqueous solution. Environ. Technol. Innov. 2022, 26, 102280. [Google Scholar] [CrossRef]
- Aguilar, G.L.D.; Rodríguez Miranda, P.J.; Miller, A.X.M.; Astudillo, M.I.R.; Muñoz, E.A.J. Removal of Zn(II) in Synthetic Wastewater Using Agricultural Wastes. Metals 2020, 10, 1465. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, Q.; Zhao, H.; Dang, J.; Jin, R.; Zhao, W.; Li, Y. Wheat Straws and Corn Straws as Adsorbents for the Removal of Cr(VI) and Cr(III) from Aqueous Solution: Kinetics, Isotherm, and Mechanism. ACS Omega 2020, 5, 6003–6009. [Google Scholar] [CrossRef] [PubMed]
- Hatiya, A.N.; Reshad, S.A.; Negie, W.Z. Chemical Modification of Neem (Azadirachta indica) Biomass as Bioadsorbent for Removal of Pb2+ Ion from Aqueous Waste Water. Adsorpt. Sci. Technol. 2022, 2022, 7813513. [Google Scholar] [CrossRef]
- Zhang, Y.; Meng, Y.; Ma, L.; Ji, H.; Lu, X.; Pang, Z.; Dong, C. Production of biochar from lignocellulosic biomass with acidic deep eutectic solvent and its application as efficient adsorbent for Cr (VI). J. Clean. Prod. 2021, 324, 129270. [Google Scholar] [CrossRef]
- Xu, H.; Dong, C.; Wang, W.; Liu, Y.; Li, B.; Liu, F. Machine learning prediction of deep eutectic solvents pretreatment of lignocellulosic biomass. Ind. Crop. Prod. 2023, 196, 116431. [Google Scholar] [CrossRef]
- Jose, D.; Tawai, A.; Divakaran, D.; Bhattacharyya, D.; Venkatachalam, P.; Tantayotai, P.; Sriariyanun, M. Integration of deep eutectic solvent in biorefining process of lignocellulosic biomass valorization. Bioresour. Technol. Rep. 2023, 21, 101365. [Google Scholar] [CrossRef]
- Borrega, M.; Hinkka, V.; Hörhammer, H.; Kataja, K.; Kenttä, K.; Ketoja, A.J.; Palmgren, R.; Salo, M.; Sundqvist-Andberg, H.; Tanaka, A. Utilizing and Valorizing Oat and Barley Straw as an Alternative Source of Lignocellulosic Fibers. Materials 2022, 15, 7826. [Google Scholar] [CrossRef]
- Szufa, S.; Wielgosiński, G.; Piersa, P.; Czerwińska, J.; Dzikuć, M.; Adrian, L.; Lewandowska, W.; Marczak, M. Torrefaction of Straw from Oats and Maize for Use as a Fuel and Additive to Organic Fertilizers—TGA Analysis, Kinetics as Products for Agricultural Purposes. Energies 2020, 13, 2064. [Google Scholar] [CrossRef]
- Onyenwoke, C.; Tabil, G.L.; Dumonceaux, T.; Cree, D.; Mupondwa, E.; Adapa, P.; Karunakaran, C. Investigation of Steam Explosion Pretreatment of Sawdust and Oat Straw to Improve Their Quality as Biofuel Pellets. Energies 2022, 15, 7168. [Google Scholar] [CrossRef]
- Kosiorek, M.; Wyszkowski, M. Content of macronutrients in oat (Avena sativa L.) after remediation of soil polluted with cobalt. Environ. Monit. Assess. 2019, 191, 389. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.Q.; Hou, D.X.; Li, N.; Zong, H.M. Ionic liquids from renewable biomaterials: Synthesis, characterization and application in the pretreatment of biomass. Green Chem. 2012, 14, 304–307. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, J.W.; Yang, J.Y.; Li, M.L.; Peng, F.; Bian, J. Efficient fractionation of woody biomass hemicelluloses using cholinium amino acids-based deep eutectic solvents and their aqueous mixtures. Bioresour. Technol. 2022, 354, 127139. [Google Scholar] [CrossRef] [PubMed]
- Milonjić, S.K.; Ruvarac, A.L.; Šušić, M.V. The heat of immersion of natural magnetite in aqueous solutions. Thermochim. Acta 1975, 11, 261–266. [Google Scholar] [CrossRef]
- Breitbach, M.; Bathen, D. Influence of ultrasound on adsorption processes. Ultrason. Sonochemistry 2001, 8, 277–283. [Google Scholar] [CrossRef]
- Li, Q.; Li, R.; Ma, Z.; Zhang, W.; Sarkar, B.; Sun, X.; Bolan, N. Efficient removal of antimonate from water by yttrium-based metal-organic framework: Adsorbent stability and adsorption mechanism investigation. Colloids Surf. A Physicochem. Eng. Asp. 2022, 633, 127877. [Google Scholar] [CrossRef]
- Lai, P.; Zhou, H.; Niu, Z.; Li, L.; Zhu, W.; Dai, L. Deep eutectic solvent-mediated preparation of solvothermal carbon with rich carboxyl and phenol groups from crop straw for high-efficient uranium adsorption. Chem. Eng. J. 2023, 457, 141255. [Google Scholar] [CrossRef]
- Babicka, M.; Woźniak, M.; Bartkowiak, M.; Peplińska, B.; Waliszewska, H.; Zborowska, M.; Borysiak, S.; Ratajczak, I. Miscanthus and Sorghum as sustainable biomass sources for nanocellulose production. Ind. Crop. Prod. 2022, 186, 115177. [Google Scholar] [CrossRef]
- Lawal, I.A.; Chetty, D.; Akpotu, S.O. Moodley, Sorption of Congo red and reactive blue on biomass and activated carbon derived from biomass modified by ionic liquid. Environ. Nanotechnol. Monit. Manag. 2017, 8, 83–91. [Google Scholar] [CrossRef]
- Dehkhoda, S.; Bagher, M.; Heydari, M. Extraction of carboxylated nanocellulose from oat husk: Characterization, surface modification and in vitro evaluation of indomethacin drug release. Int. J. Biol. Macromol. 2022, 212, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Ying, D.; Hlaing, M.M.; Lerisson, J.; Pitts, K.; Cheng, L.; Sanguansri, L.; Augustin, M.A. Physical properties and FTIR analysis of rice-oat flour and maize-oat flour based extruded food products containing olive pomace. Int. Food Res. J. 2017, 100, 665–673. [Google Scholar] [CrossRef]
- Kubovský, I.; Kačíková, D.; Kačík, F. Structural Changes of Oak Wood Main Components Caused by Thermal Modification. Polymers 2020, 12, 485. [Google Scholar] [CrossRef]
- Petrović, J.; Ercegović, M.; Simić, M.; Kalderis, D.; Koprivica, M.; Milojović, J.; Radulović, D. Novel Mg-doped pyro-hydrochars as methylene blue adsorbents: Adsorption behavior and mechanism. J. Mol. Liq. 2023, 376, 121424. [Google Scholar] [CrossRef]
- Ghaly, A.E.; Ergüdenler, A.; Al Taweel, A.M. Determination of the kinetic parameters of oat straw using thermogravimetric analysis. Biomass Bioenergy 1993, 5, 457–465. [Google Scholar] [CrossRef]
- Mlonka-Mędrala, A.; Evangelopoulos, P.; Sieradzka, M.; Zajemska, M.; Magdziarz, A. Pyrolysis of agricultural waste biomass towards production of gas fuel and high-quality char: Experimental and numerical investigations. Fuel 2021, 296, 120611. [Google Scholar] [CrossRef]
- Mukhambet, Y.; Shah, D.; Tatkeyeva, G.; Sarbassov, Y. Slow pyrolysis of flax straw biomass produced in Kazakhstan: Characterization of enhanced tar and high-quality biochar. Fuel 2022, 324, 124676. [Google Scholar] [CrossRef]
- Nicole Labbé, N.; Kline, M.L.; Moens, L.; Kim, K.; Kim, C.P.; Hayes, G.D. Activation of lignocellulosic biomass by ionic liquid for biorefinery fractionation. Bioresour. Technol. 2012, 104, 701–707. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Maraver, A.; Salvachúa, D.D.; Martínez, M.J.; Diaz, F.L.; Zamorano, M. Analysis of the relation between the cellulose, hemicellulose and lignin content and the thermal behavior of residual biomass from olive trees. Waste Manag. 2013, 33, 2245–2249. [Google Scholar] [CrossRef] [PubMed]
- Bi, H.; Ni, Z.; Tian, J.; Wang, C.; Jiang, C.; Zhou, W.; Bao, L.; Sun, H.; Lin, Q. The effect of biomass addition on pyrolysis characteristics and gas emission of coal gangue by multi-component reaction model and TG-FTIR-MS. Sci. Total Environ. 2021, 798, 149290. [Google Scholar] [CrossRef]
- Jevtić, S.; Arčon, I.; Rečnik, A.; Babić, B.; Mazaj, M.; Pavlović, J.; Matijašević, D.; Nikšić, M.; Rajić, N. The iron(III)-modified natural zeolitic tuff as an adsorbent and carrier for selenium oxyanions. Microporous Mesoporous Mater. 2014, 197, 92–100. [Google Scholar] [CrossRef]
- Petrović, J.; Stojanović, M.; Milojković, J.; Petrović, M.; Šoštarić, T.; Laušević, M.; Mihajlović, L.M. Alkali modified hydrochar of grape pomace as a perspective adsorbent of Pb2+ from aqueous solution. J. Environ. Manag. 2016, 182, 292–300. [Google Scholar] [CrossRef]
- Lagergren, S. Zur theorie der sogenannten adsorption gelöster stoffe. K. Sven. Vetenskapsakad. Handl. 1898, 24, 1–39. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process. Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Weber, W.; Morris, J. Kinetics of adsorption on carbon from solution. J. Sanit. Eng. Div. 1963, 89, 31–60. [Google Scholar] [CrossRef]
- Marjanović, V.; Radmila Marković, R.; Steharnik, M.; Dimitrijević, S.; Marinković, D.A.; Perić-Grujić, A.; Đolic, M. Lignin Microspheres Modified with Magnetite Nanoparticles as a Selenate Highly Porous Adsorbent. Int. J. Mol. Sci. 2022, 23, 13872. [Google Scholar] [CrossRef] [PubMed]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Webber, T.W.; Chakkravorti, R.K. Pore and solid diffusion models for fixed-bed adsorbers. AlChE J. 1974, 20, 228–238. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Freundlich, H.M.F. Über die adsorption in lösungen. Z. Phys. Chem. 1906, 57A, 385–470. [Google Scholar] [CrossRef]
- Sips, R. On the structure of a catalyst surface. J. Chem. Phys. 1948, 16, 490–495. [Google Scholar] [CrossRef]
- Redlich, O.; Peterson, D. A useful adsorption isotherm. J. Phys. Chem. 1959, 63, 1024–1027. [Google Scholar] [CrossRef]
- Putra, P.W.; Kamari, A.; Yusoff, S.N.M.; Ishak, C.F.; Mohamed, A.; Hashim, N.; Isa, I.M. Biosorption of Cu(II), Pb(II) and Zn(II) Ions from Aqueous Solutions Using Selected Waste Materials: Adsorption and Characterisation Studies. J. Encapsulation Adsorpt. Sci. 2014, 4, 25–35. [Google Scholar] [CrossRef]
- Petrović, M.; Šoštarić, T.; Stojanović, M.; Petrović, J.; Mihajlović, M.; Ćosović, A.; Stanković, S. Mechanism of adsorption of Cu2+ and Zn2+ on the corn silk (Zea mays L.). Ecol. Eng. 2017, 99, 83–90. [Google Scholar] [CrossRef]
- Velazquez-Jimenez, H.L.; Pavlick, A.; Rangel-Mendez, J.R. Chemical characterization of raw and treated agave bagasse and its potential as adsorbent of metal cations from water. Ind. Crop. Prod. 2013, 43, 200–206. [Google Scholar] [CrossRef]
- Khakpour, H.; Younesi, H.; Mohammadhosseini, M. Two-stage biosorption of selenium from aqueous solution using dried biomass of the baker’s yeast Saccharomyces cerevisiae. J. Environ. Chem. Eng. 2014, 2, 532–542. [Google Scholar] [CrossRef]
- Tuzen, M.; Sarı, A. Biosorption of selenium from aqueous solution by green algae (Cladophora hutchinsiae) biomass: Equilibrium, thermodynamic and kinetic studies. Chem. Eng. J. 2010, 158, 200–206. [Google Scholar] [CrossRef]



| Adsorbent IOS | Cu | Zn | Se |
|---|---|---|---|
| qeq, exp [mg/g] | 42.16 ± 0.51 | 34.5 ± 1.25 | 79.90 ± 2.10 |
| Pseudo-First-Order Model | |||
| qeq [mg/g] | 41.49 ± 0.84 | 40.13 ± 0.99 | 86.73 ± 1.85 |
| k1 [1/min] | 8.95 ± 0.25 | 23.20 ± 0.36 | 50.35 ± 0.69 |
| R2 | 0.8757 ± 0.08 | 0.8073 ± 0.05 | 0.9974 ± 0.008 |
| Pseudo-Second-Order Model | |||
| qeq [mg/g] | 44.52 ± 0.52 | 47.07 ± 1.02 | 83.47 ± 0.99 |
| k2 [g/mg min−1] | 0.0545 ± 0.004 | 0.0251 ± 0.001 | 0.0254 ± 0.0015 |
| R2 | 0.9998 ± 0.0001 | 0.9978 ± 0.002 | 0.9988 ± 0.0001 |
| Weber–Morris diffusion Model | |||
| Kid1 [mg/g min−1/2] | 1.0662 ± 0.032 | 1.8833 ± 0.22 | 4.5293 ± 0.85 |
| C1 [mg/g] | 24.3892 ± 0.014 | 8.9812 ± 0.84 | 6.7541 ± 1.23 |
| R2 | 0.9255 ± 0.022 | 0.9830 ± 0.023 | 0.9669 ± 0.036 |
| Kid2 [mg/g min−1/2] | 0.0658 ± 0.001 | 0.0355 ± 0.001 | 0.0253 ± 0.008 |
| C2 [mg/g] | 41.5664 ± 1.25 | 44.1516 ± 0.98 | 80.1554 ± 0.97 |
| R2 | 0.9619 ± 0.015 | 0.9995 ± 0.00 | 0.8798 ± 0.053 |
| Models | Parameters | Cu | Zn | Se |
|---|---|---|---|---|
| Langmuir | qm (mg/g) | 41.41 ± 2.15 | 99.12 ± 5.28 | 85.89 ± 1.98 |
| KL (L/mg) | 0.70 ± 0.02 | 0.01 ± 0.001 | 0.09 ± 0.01 | |
| R2 | 0.9347 ± 0.0021 | 0.8890 ± 0.0013 | 0.9813 ± 0.0028 | |
| RL | 0.010 ± 0.001 | 0.42 ± 0.02 | 0.066 ± 0.001 | |
| χ2 | 8.27 ± 0.08 | 4.79 ± 0.04 | 8.13 ± 0.02 | |
| Freundlich | KF (mg/g)(L/mg)1/n | 20.21 ± 1.18 | 3.68 ± 0.45 | 23.33 ± 1.26 |
| 1/n | 5.83 ± 1.02 | 1.74 ± 0.15 | 3.71 ± 0.78 | |
| R2 | 0.8959 ± 0.0127 | 0.8203 ± 0.0098 | 0.9281 ± 0.0104 | |
| χ2 | 18.48 ± 1.89 | 7.77 ± 0.98 | 31.33 ± 2.45 | |
| Sips | qm (mg/g) | 48.21 ± 1.98 | 55.06 ± 2.18 | 87.85 ± 4.24 |
| KS (L/mg) | 0.58 ± 0.09 | 0.00003 ± 0.00001 | 0.12 ± 0.01 | |
| ns | 0.58 ± 0.08 | 0.98 ± 0.11 | 0.86 ± 0.09 | |
| R2 | 0.9818 ± 0.14 | 0.9931 ± 0.11 | 0.9838 ± 0.12 | |
| χ2 | 1.20 ± 0.23 | 3.73 ± 0.89 | 8.81 ± 1.12 | |
| Redlich–Peterson | KRP (L/g) | 49.67 ± 1.78 | 0.83 ± 0.05 | 9.30 ± 0.78 |
| aRP (L/mg) | 1.68 ± 0.21 | 0.0005 ± 0.00001 | 0.12 ± 0.01 | |
| β | 0.91 ± 0.11 | 0.51 ± 0.08 | 0.96 ± 0.15 | |
| R2 | 0.9771 ± 0.0213 | 0.9471 ± 0.0199 | 0.9821 ± 0.0214 | |
| χ2 | 5.82 ± 0.45 | 28.60 ± 1.15 | 9.70 ± 0.98 |
| Adsorbent | qm (mg/g) | Reference | ||
|---|---|---|---|---|
| Cu(II) | Zn(II) | Se(IV) | ||
| Plant-crofton weed | 33.87 | [4] | ||
| Eggshell | 34.48 | 35.71 | [45] | |
| Sugarcane bagasse | 3.65 | 40.00 | [45] | |
| Corn silk (Zea mays L.) | 15.35 | 13.98 | [46] | |
| Coffee pulp | 13.53 | [5] | ||
| NaOH modified Agave bagasse | 20.24 | [47] | ||
| S. cerevisiae biomass | 39.00 | [48] | ||
| Green algae | 74.90 | [49] | ||
| Iron(III)-modified zeolitic Fe-CLI | 21.60 | [33] | ||
| Modified lignin microspheres | 69.90 | [38] | ||
| IOS | 48.21 | 55.06 | 87.85 | This study |
| OS | 13.76 | 16.85 | 14.25 | This study |
| Cu(mg/L) | Zn(mg/L) | Pb(mg/L) | Cd(mg/L) | Ni(mg/L) | Fe(mg/L) | |
|---|---|---|---|---|---|---|
| IWW | 6.62 | 6.05 | 4.7 | 0.16 | 0.4 | 0.94 |
| IWW-IOS | 3.54 | 2.12 | 3.3 | 0.06 | 0.1 | 0.45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dimitrijević, J.; Jevtić, S.; Marinković, A.; Simić, M.; Koprivica, M.; Petrović, J. Ability of Deep Eutectic Solvent Modified Oat Straw for Cu(II), Zn(II), and Se(IV) Ions Removal. Processes 2023, 11, 1308. https://doi.org/10.3390/pr11051308
Dimitrijević J, Jevtić S, Marinković A, Simić M, Koprivica M, Petrović J. Ability of Deep Eutectic Solvent Modified Oat Straw for Cu(II), Zn(II), and Se(IV) Ions Removal. Processes. 2023; 11(5):1308. https://doi.org/10.3390/pr11051308
Chicago/Turabian StyleDimitrijević, Jelena, Sanja Jevtić, Aleksandar Marinković, Marija Simić, Marija Koprivica, and Jelena Petrović. 2023. "Ability of Deep Eutectic Solvent Modified Oat Straw for Cu(II), Zn(II), and Se(IV) Ions Removal" Processes 11, no. 5: 1308. https://doi.org/10.3390/pr11051308
APA StyleDimitrijević, J., Jevtić, S., Marinković, A., Simić, M., Koprivica, M., & Petrović, J. (2023). Ability of Deep Eutectic Solvent Modified Oat Straw for Cu(II), Zn(II), and Se(IV) Ions Removal. Processes, 11(5), 1308. https://doi.org/10.3390/pr11051308









