The Beneficial Effect of Salicornia herbacea Extract and Isorhamnetin-3-O-glucoside on Obesity
Abstract
1. Introduction
2. Materials and Methods
2.1. Salicornia herbacea Extraction and IR3G Isolation
2.2. Cells and Reagents
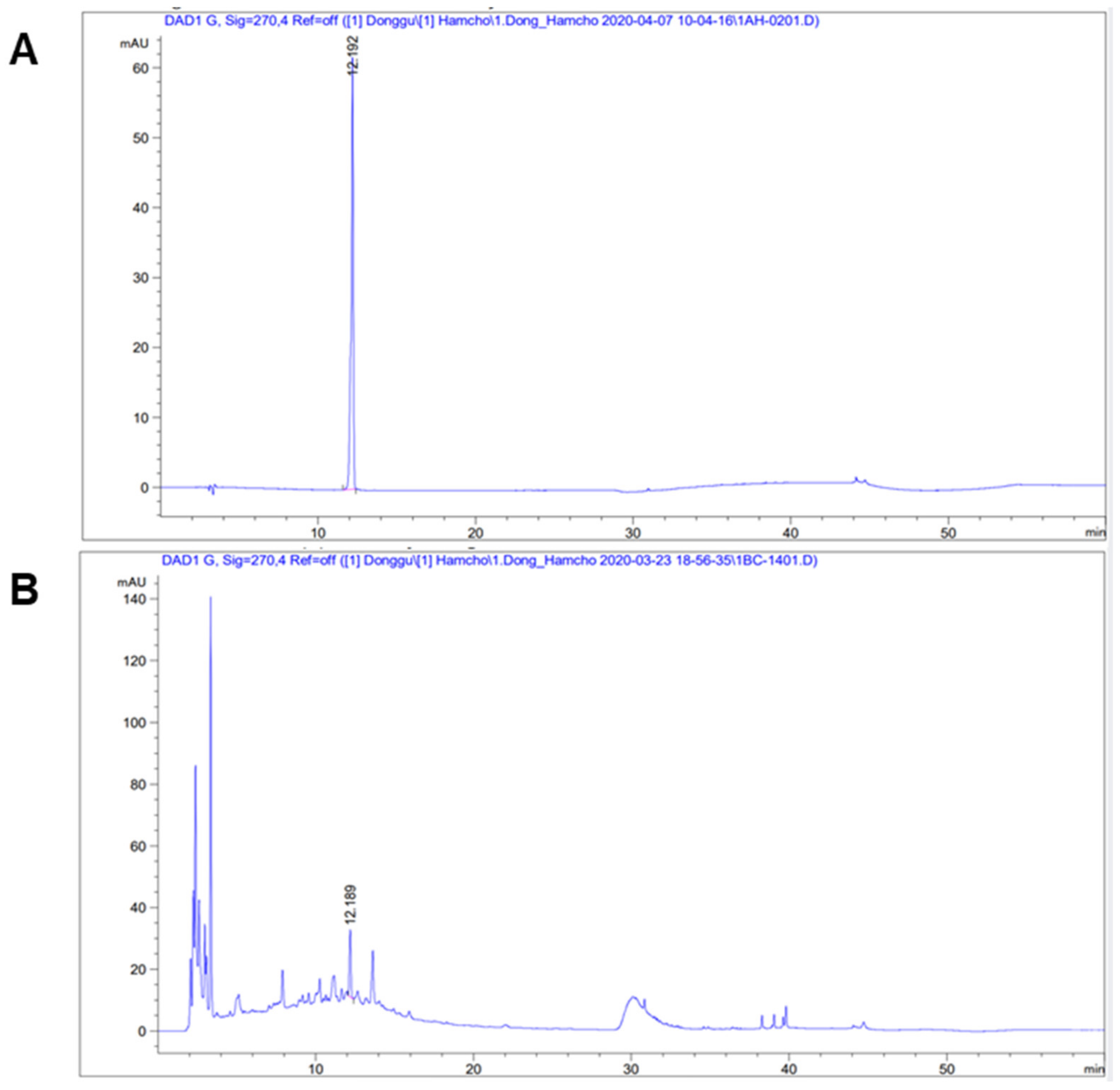
2.3. High-Performance Liquid Chromatography (HPLC)
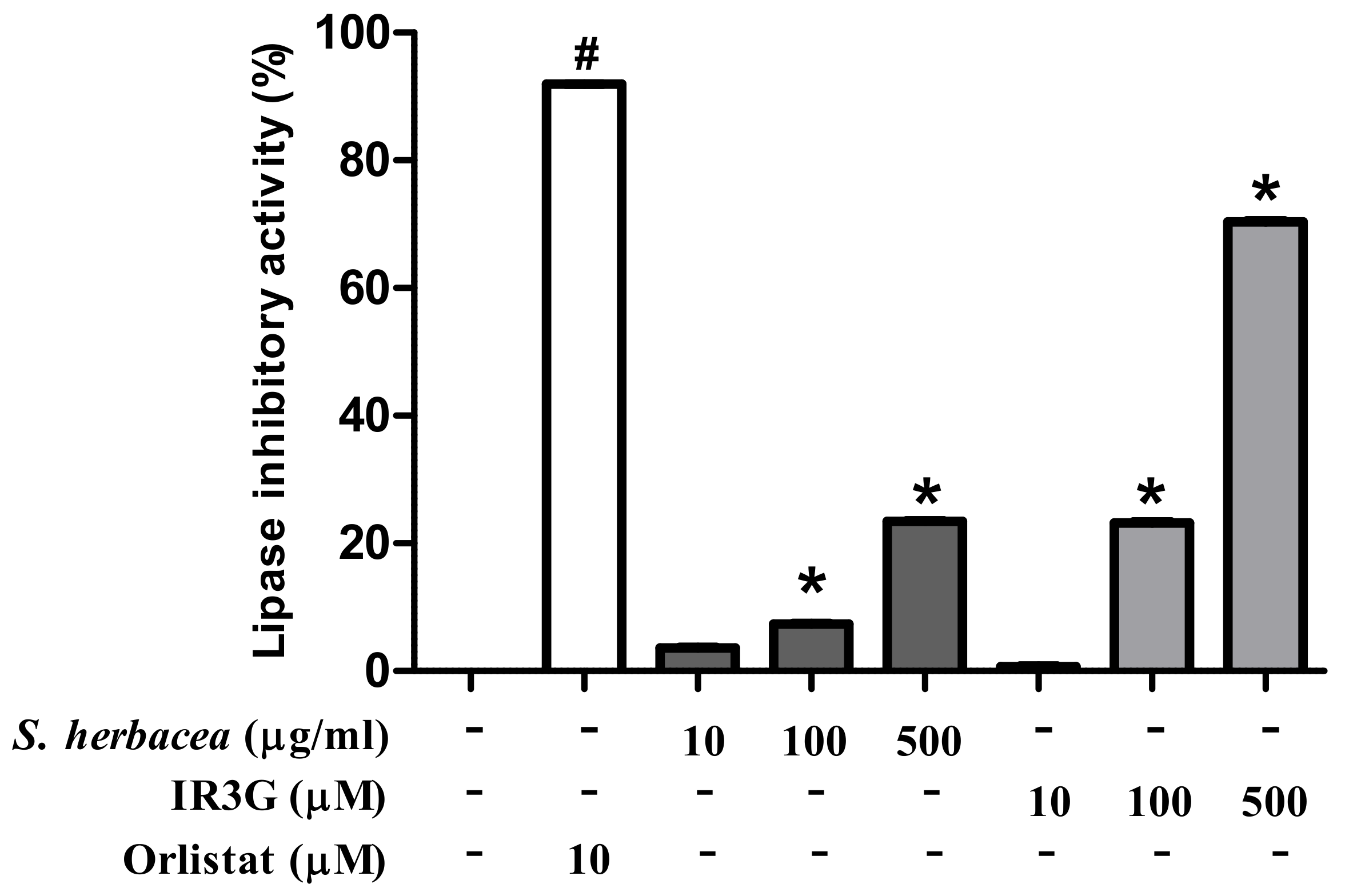
2.4. Lipase Inhibitory Activity
2.5. Cytotoxicity of S. herbacea Extract and IR3G in 3T3-L1 Preadipocytes
2.6. Adipocyte Differentiation
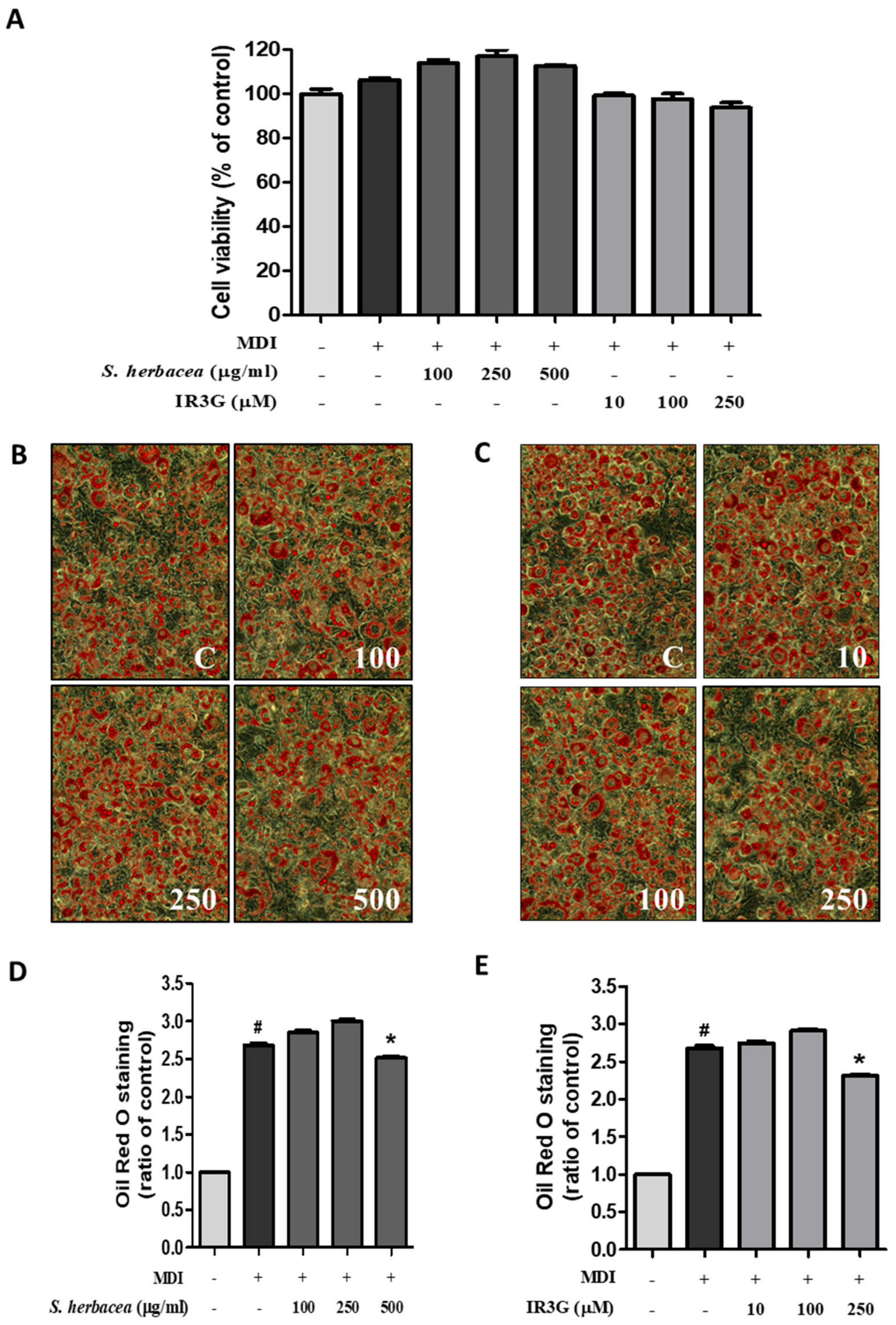
2.7. Intracellular Lipid Accumulation Staining (Oil Red O Staining)
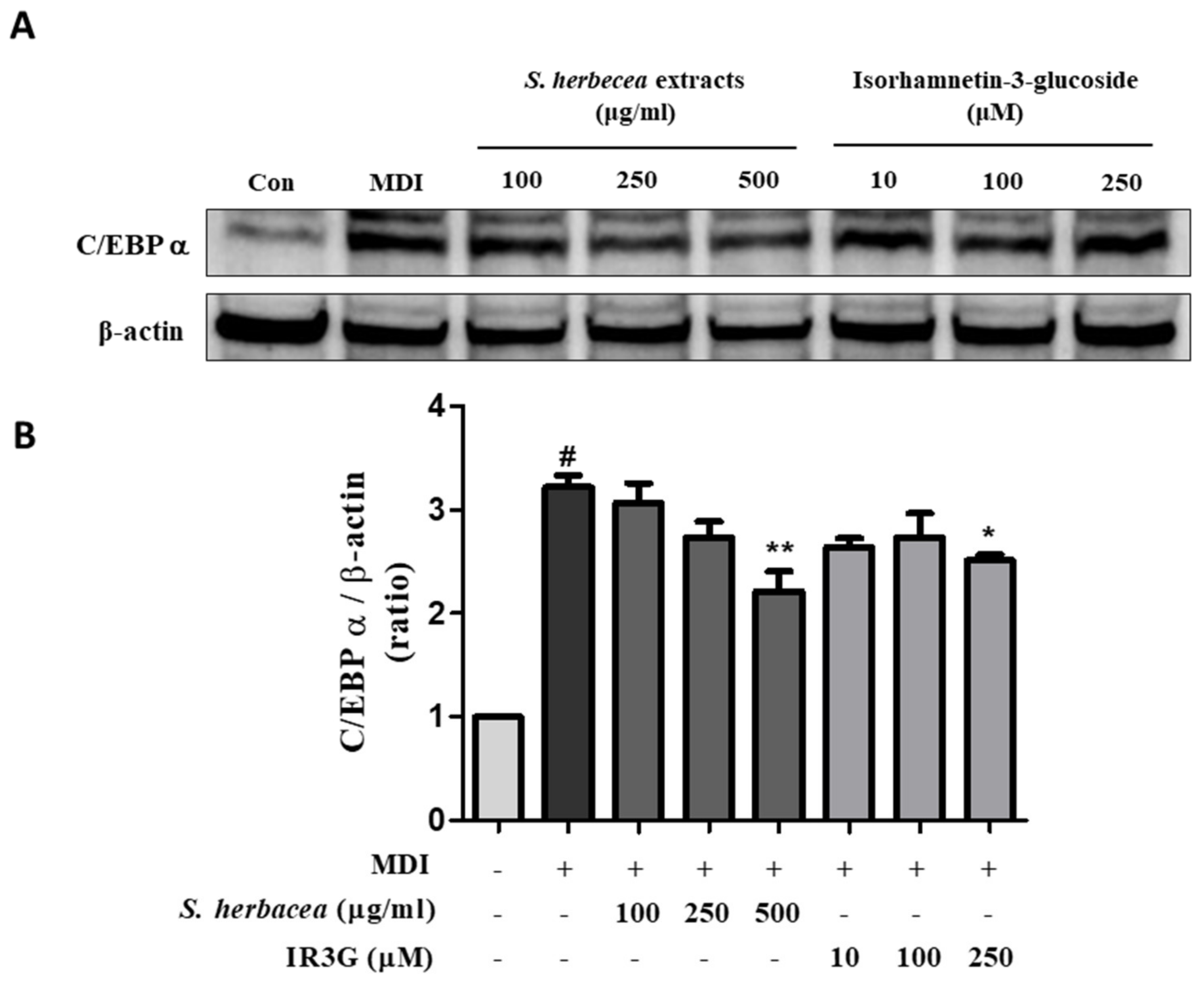
2.8. Immunoblotting
2.9. Experimental Animals and Their Care
2.10. Animal Experimental Design
2.11. Blood Biochemical Analysis
2.12. Statistical Analysis
3. Results
3.1. High-Performance Liquid Chromatography
3.2. Effects of S. herbacea and IR3G on Lipase Inhibition
3.3. Inhibitory Effects of S. herbacea and IR3G on Adipogenesis in Differentiated 3T3-L1 Preadipocytes
3.4. Effects of S. herbacea and IR3G on C/EBPα Expression in Differentiated 3T3-L1 Adipocytes
3.5. Effects of S. herbacea Extract and IR3G on Mouse Body Weight Gain, Feed Efficiency, and Epididymal Tissue Mass
3.6. Effects of S. herbacea Extract and IR3G on Leptin and Adiponectin
3.7. Effects of S. herbacea Extract and IR3G on Lipid Profiles
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chua, J.; Leibel, R. Obesity genes: Molecular and metabolic mechanisms. Diabetes Rev. 1997, 5, 2–7. [Google Scholar]
- Bray, M.S. Genomics, genes, and environmental interaction: The role of exercise. J. Appl. Physiol. 2000, 88, 788–792. [Google Scholar] [CrossRef] [PubMed]
- Kopelman, P.G. Obesity as a medical problem. Nature 2000, 404, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M. Multifactorial causation of obesity: Implications for prevention. Am. J. Clin. Nutr. 1998, 67, 563S–572S. [Google Scholar] [CrossRef]
- Wright, S.M.; Aronne, L.J. Causes of obesity. Abdom. Radiol. 2012, 37, 730–732. [Google Scholar] [CrossRef]
- Sui, Y.; Zhao, H.; Wong, V.; Brown, N.; Li, X.; Kwan, A.; Hui, H.; Ziea, E.; Chan, J. A systematic review on use of Chinese medicine and acupuncture for treatment of obesity. Obes. Rev. 2012, 13, 409–430. [Google Scholar] [CrossRef] [PubMed]
- Dandona, P.; Aljada, A.; Chaudhuri, A.; Mohanty, P.; Garg, R. Metabolic syndrome: A comprehensive perspective based on interactions between obesity, diabetes, and inflammation. Circulation 2005, 111, 1448–1454. [Google Scholar] [CrossRef]
- Zeller, M.H.; Reiter-Purtill, J.; Ramey, C. Negative peer perceptions of obese children in the classroom environment. Obesity 2008, 16, 755–762. [Google Scholar] [CrossRef]
- Lang, A.; Froelicher, E.S. Management of overweight and obesity in adults: Behavioral intervention for long-term weight loss and maintenance. Eur. J. Cardiovasc. Nurs. 2006, 5, 102–114. [Google Scholar] [CrossRef]
- Powell, A.; Apovian, C.; Aronne, L. New drug targets for the treatment of obesity. Clin. Pharmacol. Ther. 2011, 90, 40–51. [Google Scholar] [CrossRef]
- Padwal, R.S.; Majumdar, S.R. Drug treatments for obesity: Orlistat, sibutramine, and rimonabant. Lancet 2007, 369, 71–77. [Google Scholar] [CrossRef]
- Kang, J.G.; Park, C.Y. Anti-obesity drugs: A review about their effects and safety. Diabetes Metab. J. 2012, 36, 13–25. [Google Scholar] [CrossRef]
- Solas, M.; Milagro, F.I.; Martínez-Urbistondo, D.; Ramirez, M.J.; Martínez, J.A. Precision obesity treatments including pharmacogenetic and nutrigenetic approaches. Trends Pharmacol. Sci. 2016, 37, 575–593. [Google Scholar] [CrossRef] [PubMed]
- Gregoire, F.M.; Smas, C.M.; Sul, H.S. Understanding adipocyte differentiation. Physiol. Rev. 1998, 78, 783–809. [Google Scholar] [CrossRef] [PubMed]
- Rosen, E.D.; MacDougald, O.A. Adipocyte differentiation from the inside out. Nat. Rev. Mol. Cell Biol. 2006, 7, 885–896. [Google Scholar] [CrossRef] [PubMed]
- Darlington, G.J.; Ross, S.E.; MacDougald, O.A. The role of C/EBP genes in adipocyte differentiation. J. Biol. Chem. 1998, 273, 30057–30060. [Google Scholar] [CrossRef]
- Linhart, H.G.; Ishimura-Oka, K.; DeMayo, F.; Kibe, T.; Repka, D.; Poindexter, B.; Bick, R.J.; Darlington, G.J. C/EBPα is required for differentiation of white, but not brown, adipose tissue. Proc. Natl. Acad. Sci. USA 2001, 98, 12532–12537. [Google Scholar] [CrossRef]
- Rosen, E.D.; Sarraf, P.; Troy, A.E.; Bradwin, G.; Moore, K.; Milstone, D.S.; Spiegelman, B.M.; Mortensen, R.M. PPARγ is required for the differentiation of adipose tissue in vivo and in vitro. Mol. Cell 1999, 4, 611–617. [Google Scholar] [CrossRef]
- Wu, Z.; Rosen, E.D.; Brun, R.; Hauser, S.; Adelmant, G.; Troy, A.E.; McKeon, C.; Darlington, G.J.; Spiegelman, B.M. Cross-regulation of C/EBPα and PPARγ controls the transcriptional pathway of adipogenesis and insulin sensitivity. Mol. Cell 1999, 3, 151–158. [Google Scholar] [CrossRef]
- Rosen, E.D.; Hsu, C.H.; Wang, X.; Sakai, S.; Freeman, M.W.; Gonzalez, F.J.; Spiegelman, B.M. C/EBPα induces adipogenesis through PPARγ: A unified pathway. Genes Dev. 2002, 16, 22–26. [Google Scholar] [CrossRef]
- Lefterova, M.I.; Zhang, Y.; Steger, D.J.; Schupp, M.; Schug, J.; Cristancho, A.; Feng, D.; Zhuo, D.; Stoeckert, C.J.; Liu, X.S. PPARγ and C/EBP factors orchestrate adipocyte biology via adjacent binding on a genome-wide scale. Genes Dev. 2008, 22, 2941–2952. [Google Scholar] [CrossRef] [PubMed]
- Madsen, M.S.; Siersbæk, R.; Boergesen, M.; Nielsen, R.; Mandrup, S. Peroxisome proliferator-activated receptor γ and C/EBPα synergistically activate key metabolic adipocyte genes by assisted loading. Mol. Cell. Biol. 2014, 34, 939–954. [Google Scholar] [CrossRef] [PubMed]
- Camp, H.S.; Ren, D.; Leff, T. Adipogenesis and fat-cell function in obesity and diabetes. Trends Mol. Med. 2002, 8, 442–447. [Google Scholar] [CrossRef]
- Ahn, B.K.; Kim, R.; Choi, D.; Kim, Y.S. Effect of Salicornia bigelovii extract on the activities of whitening and anti-wrinkle. Appl. Chem. Eng. 2011, 22, 56–60. [Google Scholar]
- Choi, J. Korean herbs, flowers, and trees as traditional medicine. Hanmunhwa 2001, 1, 63–74. [Google Scholar]
- Rhee, M.H.; Park, H.J.; Cho, J.Y. Salicornia herbacea: Botanical, chemical and pharmacological review of halophyte marsh plant. J. Med. Plants Res 2009, 3, 548–555. [Google Scholar]
- SHIMIZU, K. Effects of salt treatments on the production and chemical composition of salt wort (Salicornia herbacea L.), rhodesgrass and alfalfa. Jpn. J. Trop. Agric. 2000, 44, 61–67. [Google Scholar]
- Jo, Y.C.; Ahn, J.H.; Chon, S.M.; Lee, K.S.; Bae, T.J.; Kang, D.S. Studies on pharmacological effects of glasswort (Salicornia herbacea L.). Korean J. Med. Crop Sci. 2002, 10, 93–99. [Google Scholar]
- Han, S.K. Antioxidant effect of fermented Salicornia herbacea L. liquid with EM (effective microorganism) on pork. Food Sci. Anim. Resour. 2004, 24, 298–302. [Google Scholar]
- Im, S.A.; Kim, G.W.; Lee, C.K. Immunomodulatory activity of Salicornia herbacea L. components. Nat. Prod. Sci. 2003, 9, 273–277. [Google Scholar]
- Lee, K.Y.; Lee, M.H.; Chang, I.Y.; Yoon, S.P.; Lim, D.Y.; Jeon, Y.J. Macrophage activation by polysaccharide fraction isolated from Salicornia herbacea. J. Ethnopharmacol. 2006, 103, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.T.; Jeong, Y.S.; An, B.J. Physiological activity of Salicornia herbacea and its application for cosmetic materials. Korea J. Herbol. 2002, 17, 51. [Google Scholar]
- Kim, K.S.; Park, S.H. Isolation and Identification of Antioxidant Flavonoids from Salicornia herbacea L. Appl. Biol. Chem. 2004, 47, 120–123. [Google Scholar]
- Kong, C.S.; Kim, J.A.; Qian, Z.J.; Kim, Y.A.; Im Lee, J.; Kim, S.K.; Nam, T.J.; Seo, Y. Protective effect of isorhamnetin 3-O-β-d-glucopyranoside from Salicornia herbacea against oxidation-induced cell damage. Food Chem. Toxicol. 2009, 47, 1914–1920. [Google Scholar] [CrossRef]
- Kim, Y.A.; Kong, C.S.; Um, Y.R.; Lim, S.Y.; Yea, S.S.; Seo, Y. Evaluation of Salicornia herbacea as a potential antioxidant and anti-inflammatory agent. J. Med. Food 2009, 12, 661–668. [Google Scholar] [CrossRef]
- Lee, Y.S.; Lee, S.; Lee, H.S.; Kim, B.K.; Ohuchi, K.; Shin, K.H. Inhibitory effects of isorhamnetin-3-O-β-D-glucoside from Salicornia herbacea on rat lens aldose reductase and sorbitol accumulation in streptozotocin-induced diabetic rat tissues. Biol. Pharm. Bull. 2005, 28, 916–918. [Google Scholar] [CrossRef]
- Park, J.Y.; Paje, L.A.; Kang, K.S.; Lee, S. Validation of an HPLC/UV-based method for Salicornia herbacea-derived isorhamnetin-3-O-glucoside and quercetin-3-O-glucoside quantification. J. Appl. Biol. Chem. 2021, 64, 285–290. [Google Scholar] [CrossRef]
- Lee, D.; Lee, J.H.; Kim, B.H.; Lee, S.; Kim, D.W.; Kang, K.S. Phytochemical combination (p-Synephrine, p-octopamine hydrochloride, and hispidulin) for improving obesity in obese mice induced by high-fat diet. Nutrients 2022, 14, 2164. [Google Scholar] [CrossRef] [PubMed]
- Moreno, D.A.; Ilic, N.; Poulev, A.; Brasaemle, D.L.; Fried, S.K.; Raskin, I. Inhibitory effects of grape seed extract on lipases. Nutrition 2003, 19, 876–879. [Google Scholar] [CrossRef]
- Kong, C.S.; Seo, Y. Antiadipogenic activity of isohamnetin 3-O-β-D-glucopyranoside from Salicornia herbacea. Immunopharmacol. Immunotoxicol. 2012, 34, 907–911. [Google Scholar] [CrossRef]
- MacDougald, O.A.; Hwang, C.S.; Fan, H.; Lane, M.D. Regulated expression of the obese gene product (leptin) in white adipose tissue and 3T3-L1 adipocytes. Proc. Natl. Acad. Sci. USA 1995, 92, 9034–9037. [Google Scholar] [CrossRef] [PubMed]
- Frederich, R.C.; Hamann, A.; Anderson, S.; Löllmann, B.; Lowell, B.B.; Flier, J.S. Leptin levels reflect body lipid content in mice: Evidence for diet-induced resistance to leptin action. Nat. Med. 1995, 1, 1311–1314. [Google Scholar] [CrossRef] [PubMed]
- Seo, M.; Lee, W.; Kim, S.; Kang, J.; Kang, J.; Kim, K.; Kim, B.; Kim, Y.; Kim, W.; Kim, E. Korean Society for the Study of obesity guideline for the Management of Obesity in Korea. J. Obes. Metab. Syndr. 2019, 28, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Lee, J.S. The reductive effects of oriental medicine on the body fat and abdominal obesity. J. Korean Med. Obes. Res. 2001, 1, 33–42. [Google Scholar]
- Mitchell, M.; Armstrong, D.; Robker, R.; Norman, R. Adipokines: Implications for female fertility and obesity. Reproduction 2005, 130, 583–597. [Google Scholar] [CrossRef]
- Maffei, Á.; Halaas, J.; Ravussin, E.; Pratley, R.; Lee, G.; Zhang, Y.; Fei, H.; Kim, S.; Lallone, R.; Ranganathan, S. Leptin levels in human and rodent: Measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nat. Med. 1995, 1, 1155–1161. [Google Scholar] [CrossRef]


| Time (min) | Mobile Phase A (%) | Mobile Phase B (%) |
|---|---|---|
| 0 | 83 | 17 |
| 10 | 70 | 30 |
| 25 | 70 | 30 |
| 30 | 20 | 80 |
| 35 | 0 | 100 |
| 40 | 0 | 100 |
| 50 | 83 | 17 |
| 55 | 83 | 17 |
| Gene Type | Contents | Leptin (µg/mL) | Adiponectin (ng/mL) |
|---|---|---|---|
| db/- | Saline | 0.2 ± 0.09 | 23.6 ± 6.93 |
| db/db | Saline | 3.7 ± 0.17 # | 16.7 ± 4.51 # |
| db/db | S. herbacea 50 mg/kg | 3.5 ± 0.15 | 15.4 ± 2.90 |
| db/db | S. herbacea 200 mg/kg | 3.3 ± 0.45 * | 18.1 ± 5.25 |
| db/db | IR3G 50 mg/kg | 3.5 ± 0.23 | 15.4 ± 4.75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.H.; Lee, S.; Park, J.Y.; Park, I.-H.; Kang, K.S.; Shin, M.-S. The Beneficial Effect of Salicornia herbacea Extract and Isorhamnetin-3-O-glucoside on Obesity. Processes 2023, 11, 977. https://doi.org/10.3390/pr11040977
Lee JH, Lee S, Park JY, Park I-H, Kang KS, Shin M-S. The Beneficial Effect of Salicornia herbacea Extract and Isorhamnetin-3-O-glucoside on Obesity. Processes. 2023; 11(4):977. https://doi.org/10.3390/pr11040977
Chicago/Turabian StyleLee, Ji Hwan, Sanghyun Lee, Jun Yeon Park, Il-Ho Park, Ki Sung Kang, and Myoung-Sook Shin. 2023. "The Beneficial Effect of Salicornia herbacea Extract and Isorhamnetin-3-O-glucoside on Obesity" Processes 11, no. 4: 977. https://doi.org/10.3390/pr11040977
APA StyleLee, J. H., Lee, S., Park, J. Y., Park, I.-H., Kang, K. S., & Shin, M.-S. (2023). The Beneficial Effect of Salicornia herbacea Extract and Isorhamnetin-3-O-glucoside on Obesity. Processes, 11(4), 977. https://doi.org/10.3390/pr11040977










