Comparison of Medium-Pressure UV/Peracetic Acid to Remove Three Typical Refractory Contaminants of Textile Wastewater
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Experimental Section
2.3. Analysis Methods
3. Results and Discussions
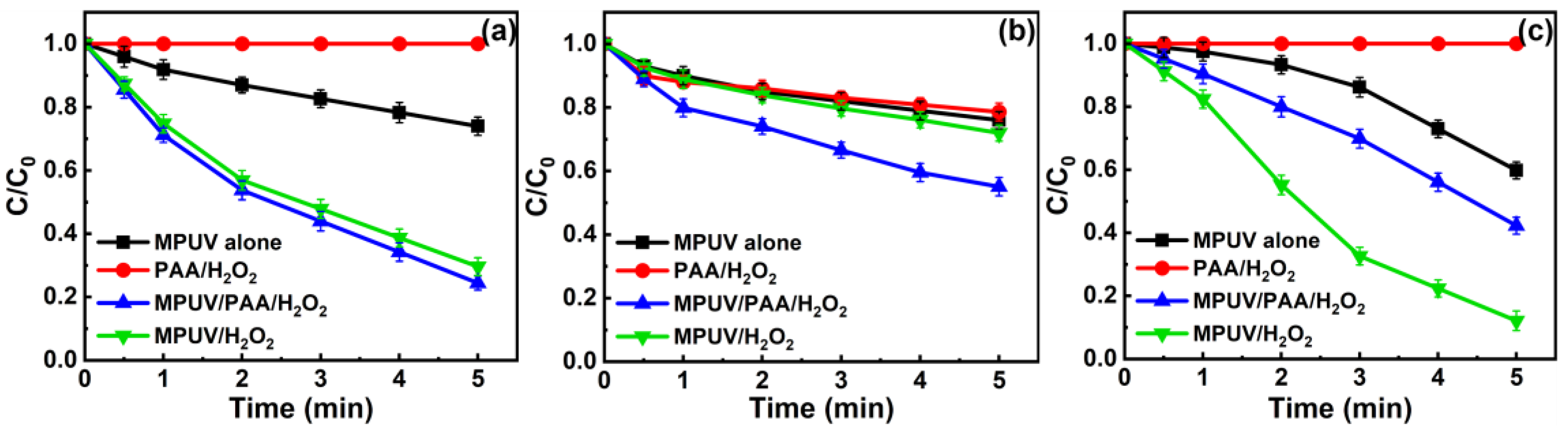
3.1. Removal Efficiency Assessment for Different Systems
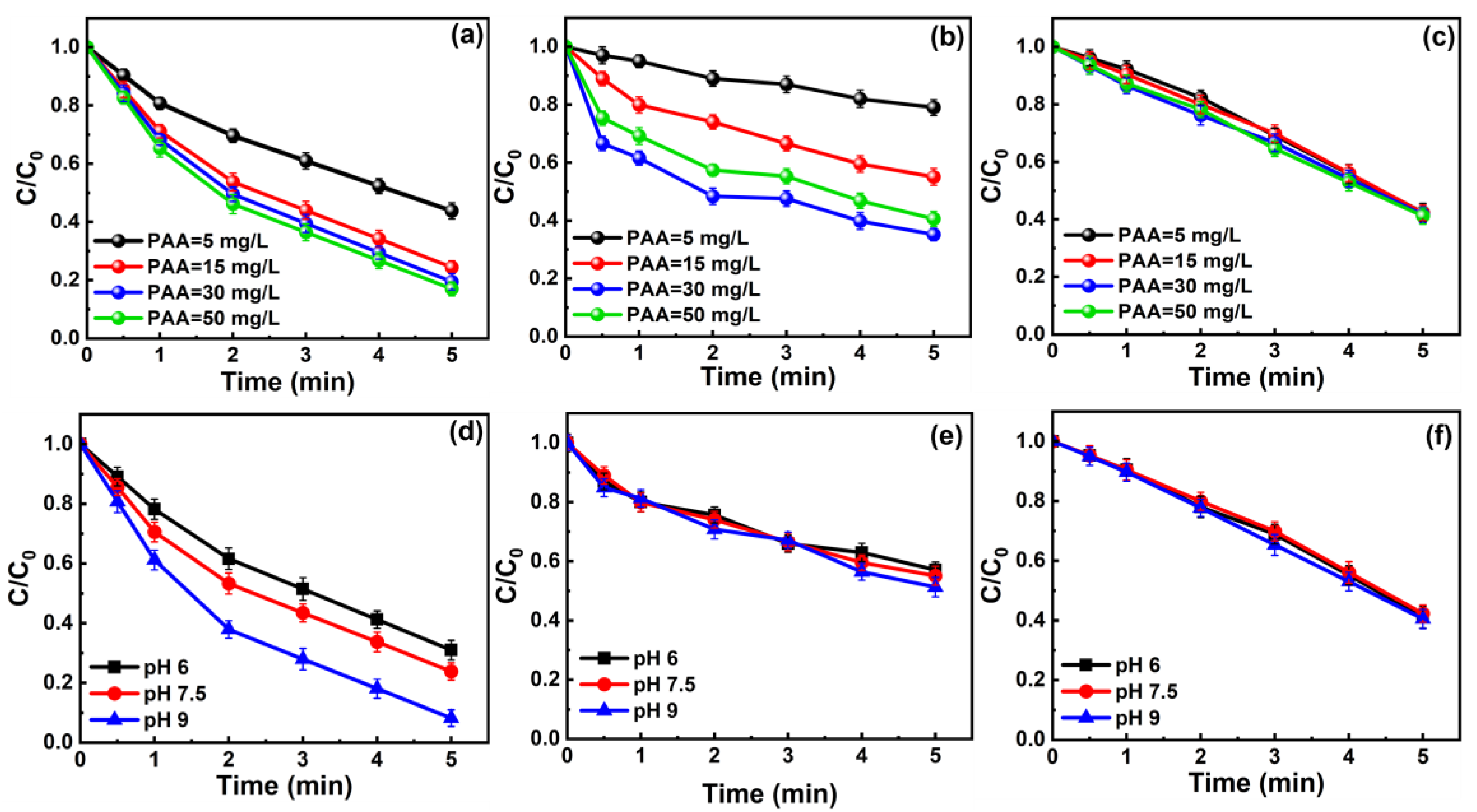
3.2. Effects of PAA Dosage and pH on Pollutants’ Removal
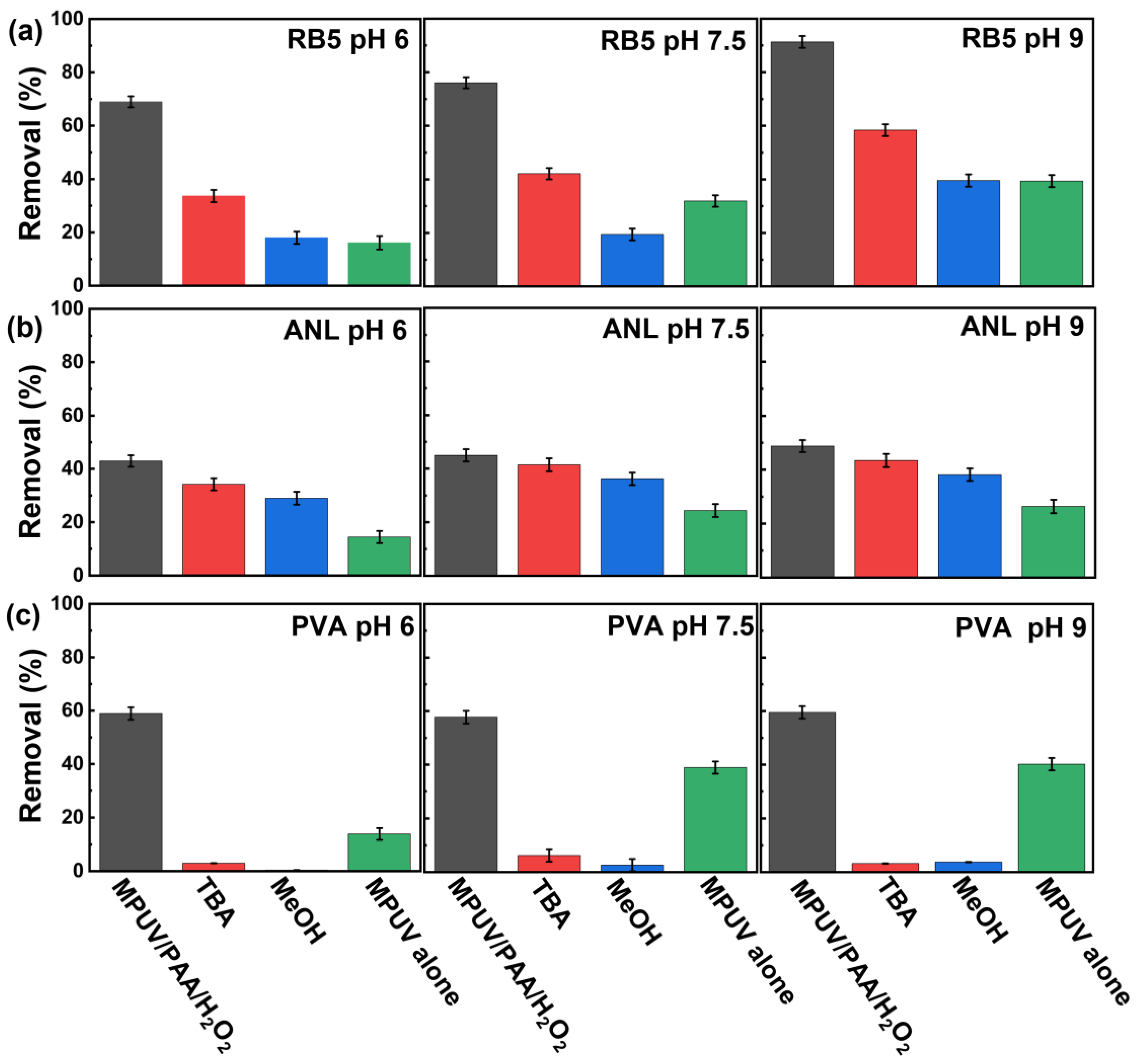
3.3. Radicals’ Contribution in the MPUV/PAA/H2O2 System
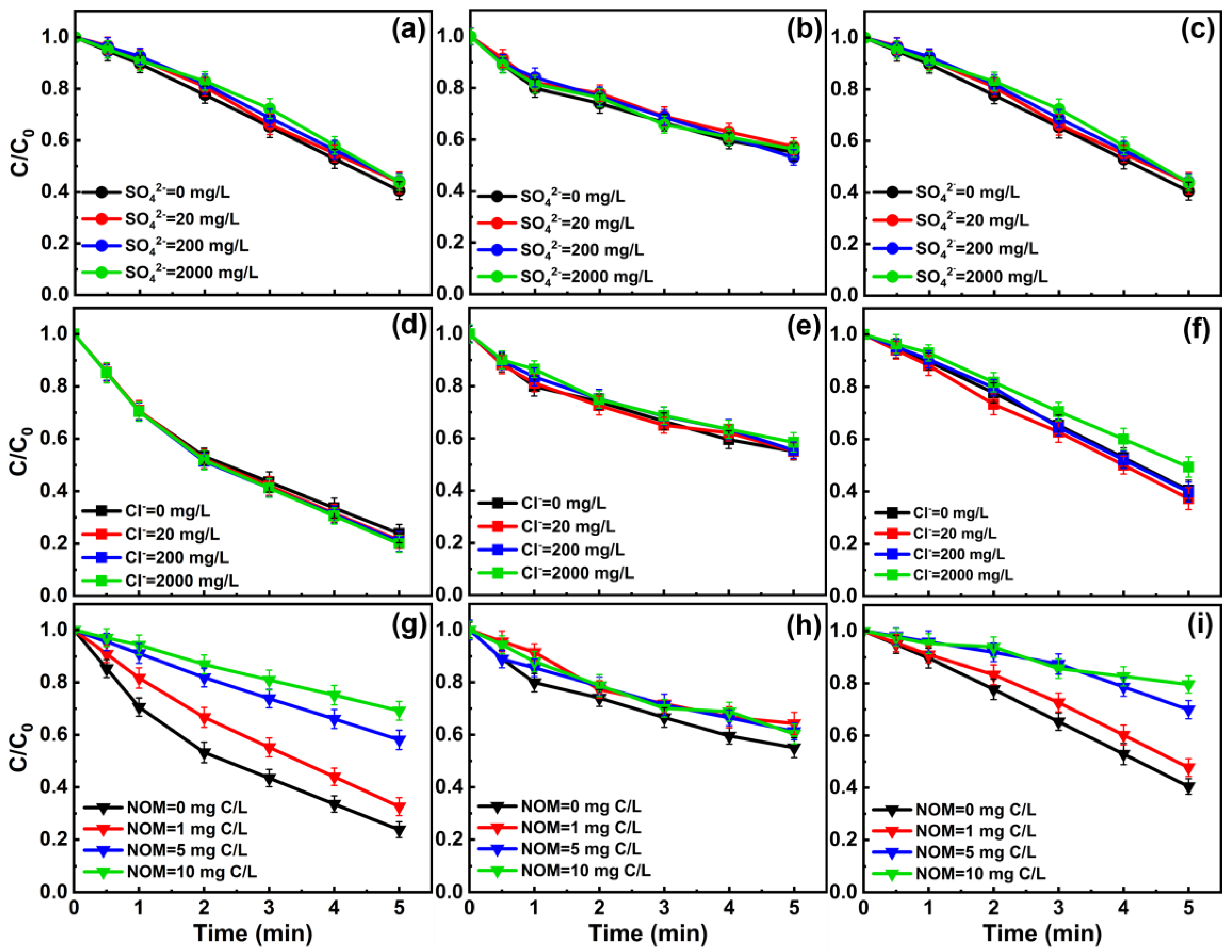
3.4. Effect of Coexisting Substances on MPUV/PAA Degradation of Target Pollutants
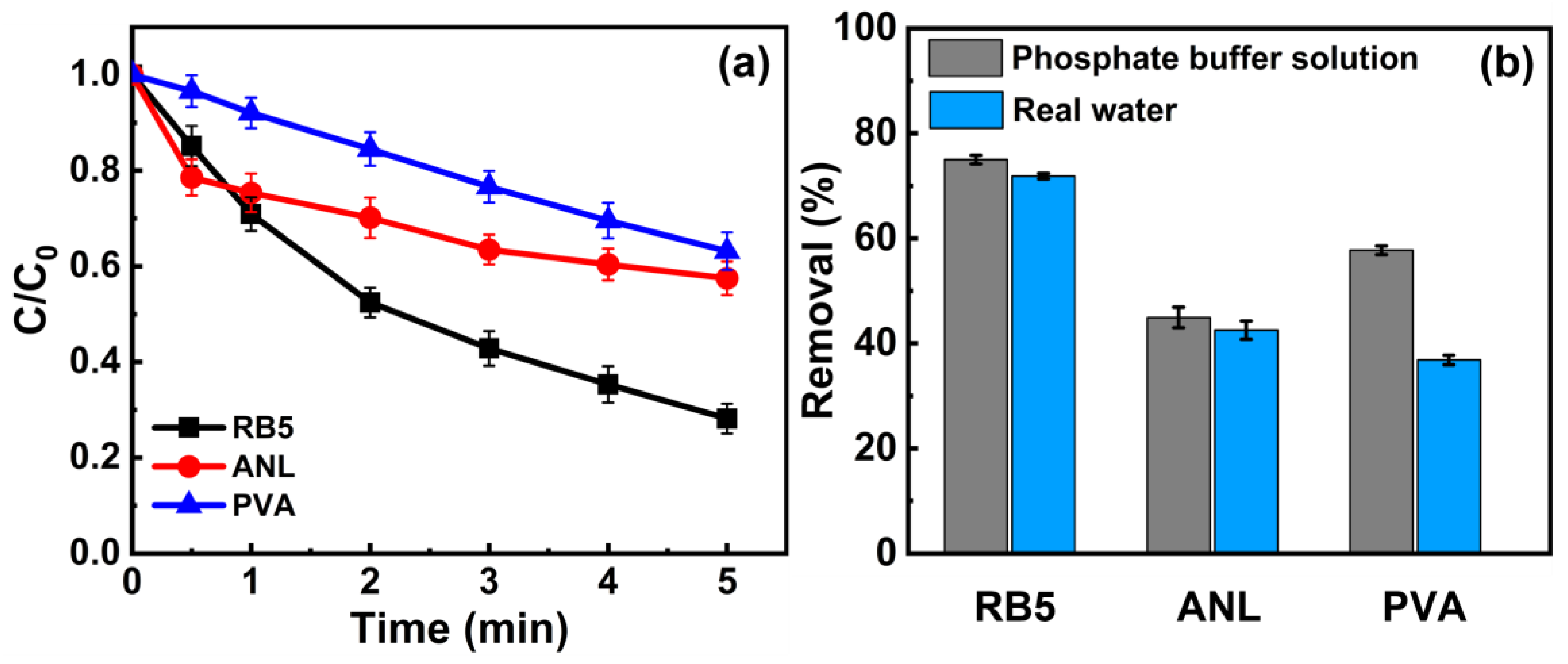
3.5. Degradation Performance of MPUV/PAA in Real Water Bodies
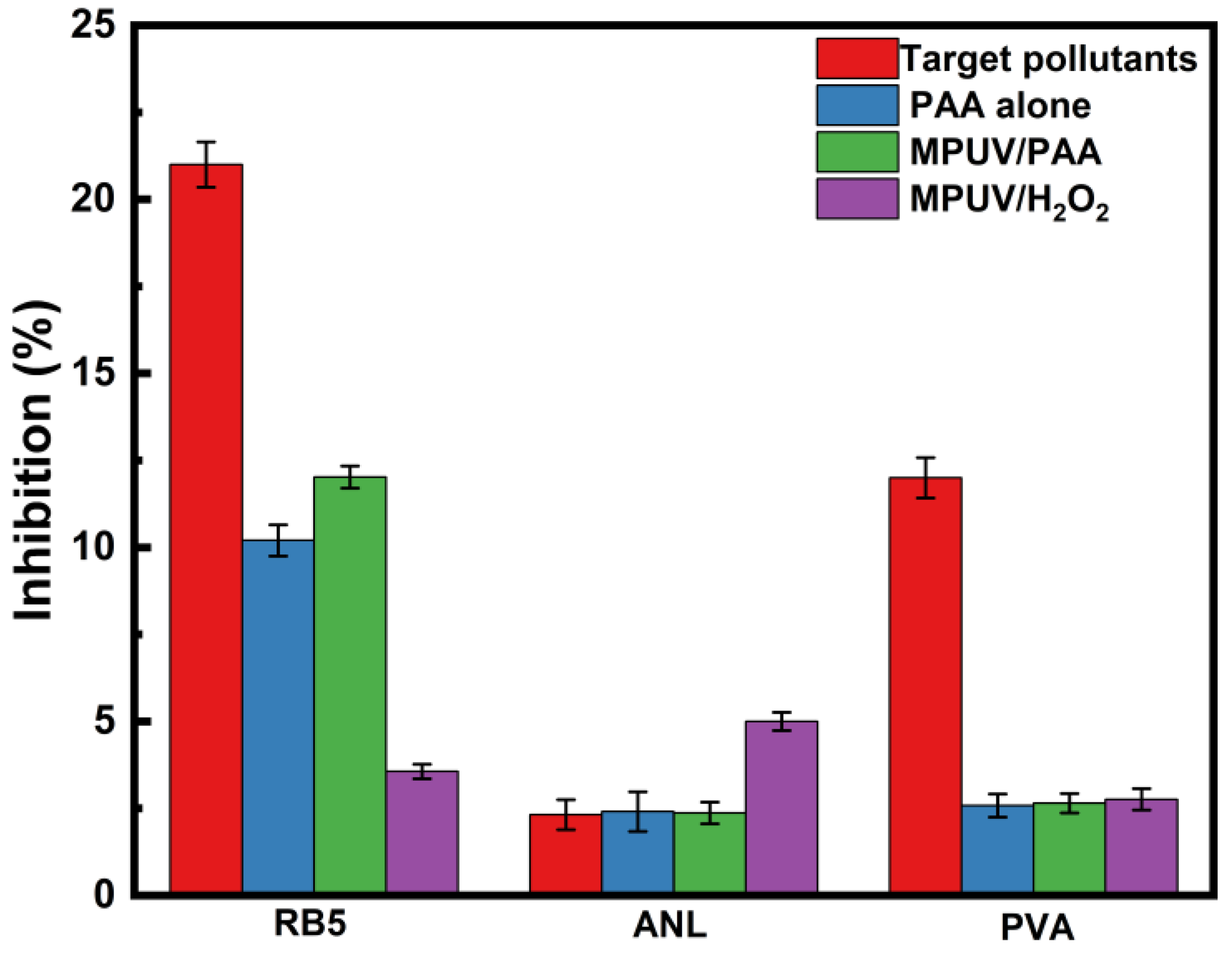
3.6. Acute Toxicity Evaluation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Méndez-Martínez, A.J.; Dávila-Jiménez, M.M.; Ornelas-Dávila, O.; Elizalde-González, M.P.; Arroyo-Abad, U.; Sirés, I.; Brillas, E. Electrochemical reduction and oxidation pathways for reactive black 5 dye using nickel electrodes in divided and undivided cells. Electrochim. Acta 2012, 59, 140–149. [Google Scholar] [CrossRef]
- Zhang, Y.; Teng, J.; Zhang, X. Research progress on printing and dyeing wastewater treatment technology. Guangdong Chem. Ind. 2010, 37, 217–218. [Google Scholar]
- Kishor, R.; Purchase, D.; Saratale, G.D.; Ferreira, L.F.R.; Bilal, M.; Chandra, R.; Bharagava, R.N. Ecotoxicological and health concerns of persistent coloring pollutants of textile industry wastewater and treatment approaches for environmental safety. J. Environ. Chem. Eng. 2021, 9, 105012. [Google Scholar] [CrossRef]
- Ren, S.; Liu, D.; Chen, Y.; An, S.; Zhao, Y.; Zhang, Y. Anionic channel membrane encircled by SO3H-polyamide 6 particles for removal of anionic dyes. J. Membr. Sci. 2019, 570, 34–43. [Google Scholar] [CrossRef]
- Rafatullah, M.; Sulaiman, O.; Hashim, R.; Ahmad, A. Adsorption of methylene blue on low-cost adsorbents: A review. J. Hazard. Mater. 2010, 177, 70–80. [Google Scholar] [CrossRef]
- Zhu, M.-X.; Lee, L.; Wang, H.-H.; Wang, Z. Removal of an anionic dye by adsorption/precipitation processes using alkaline white mud. J. Hazard. Mater. 2007, 149, 735–741. [Google Scholar] [CrossRef]
- Ibrahim, S.M.; Badawy, A.A.; Essawy, H.A. Improvement of dyes removal from aqueous solution by Nanosized cobalt ferrite treated with humic acid during coprecipitation. J. Nanostruct. Chem. 2019, 9, 281–298. [Google Scholar] [CrossRef]
- Lau, Y.; Wong, Y.; Teng, T.; Morad, N.; Rafatullah, M.; Ong, S.-A. Degradation of cationic and anionic dyes in coagulation–flocculation process using bi-functionalized silica hybrid with aluminum-ferric as auxiliary agent. RSC Adv. 2015, 5, 34206–34215. [Google Scholar] [CrossRef]
- Moghaddam, S.S.; Moghaddam, M.A.; Arami, M. Coagulation/flocculation process for dye removal using sludge from water treatment plant: Optimization through response surface methodology. J. Hazard. Mater. 2010, 175, 651–657. [Google Scholar] [CrossRef]
- Javaid, R.; Qazi, U.Y. Catalytic oxidation process for the degradation of synthetic dyes: An overview. Int. J. Environ. Res. Public Health 2019, 16, 2066. [Google Scholar] [CrossRef]
- Zaharia, C.; Suteu, D.; Muresan, A.; Muresan, R.; Popescu, A. Textile wastewater treatment by homogenous oxidation with hydrogen peroxide. Environ. Eng. Manag. J. 2009, 8, 1359–1369. [Google Scholar] [CrossRef]
- Cheng, L.; Wei, M.; Huang, L.; Pan, F.; Xia, D.; Li, X.; Xu, A. Efficient H2O2 oxidation of organic dyes catalyzed by simple copper (II) ions in bicarbonate aqueous solution. Ind. Eng. Chem. Res. 2014, 53, 3478–3485. [Google Scholar] [CrossRef]
- Bhatia, D.; Sharma, N.R.; Singh, J.; Kanwar, R.S. Biological methods for textile dye removal from wastewater: A review. Crit. Rev. Environ. Sci. Technol. 2017, 47, 1836–1876. [Google Scholar] [CrossRef]
- Araghi, M.S.; Olya, M.E.; Marandi, R.; Siadat, S.D. Investigation of enhanced biological dye removal of colored wastewater in a lab-scale biological activated carbon process. Appl. Biol. Chem. 2016, 59, 463–470. [Google Scholar] [CrossRef]
- Amini, M.; Arami, M.; Mahmoodi, N.M.; Akbari, A. Dye removal from colored textile wastewater using acrylic grafted nanomembrane. Desalination 2011, 267, 107–113. [Google Scholar] [CrossRef]
- Nguyen, T.A.; Juang, R.-S. Treatment of waters and wastewaters containing sulfur dyes: A review. Chem. Eng. J. 2013, 219, 109–117. [Google Scholar] [CrossRef]
- Dasgupta, J.; Sikder, J.; Chakraborty, S.; Curcio, S.; Drioli, E. Remediation of textile effluents by membrane based treatment techniques: A state of the art review. J. Environ. Manag. 2015, 147, 55–72. [Google Scholar] [CrossRef]
- Man, X.; Ning, X.A.; Lu, X.; Liang, J.; Liu, D.; Lai, X. Degradation of polycyclic aromatic hydrocarbons from textile dyeing sludge by ultrasound/Fe~0/EDTA system. Acta Sci. Circumst. 2018, 38, 1049–1055. [Google Scholar]
- Anan, D.; Yanlei, S.; Wenjuan, C.; Peng, J.; Zhang, Y.; Jiang, Z. Ultrafiltration enhanced with activated carbon adsorption for efficient dye removal from aqueous solution. Chin. J. Chem. Eng. 2011, 19, 863–869. [Google Scholar]
- Lucas, M.S.; Peres, J.A. Decolorization of the azo dye Reactive Black 5 by Fenton and photo-Fenton oxidation. Dyes Pigments 2006, 71, 236–244. [Google Scholar] [CrossRef]
- Tehrani-Bagha, A.; Mahmoodi, N.M.; Menger, F. Degradation of a persistent organic dye from colored textile wastewater by ozonation. Desalination 2010, 260, 34–38. [Google Scholar] [CrossRef]
- Hou, L.; Wu, Q.; Gu, Q.; Zhou, Q.; Zhang, J. Community structure analysis and biodegradation potential of aniline-degrading bacteria in biofilters. Curr. Microbiol. 2018, 75, 918–924. [Google Scholar] [CrossRef]
- Miralles-Cuevas, S.; Oller, I.; Agüera, A.; Llorca, M.; Pérez, J.S.; Malato, S. Combination of nanofiltration and ozonation for the remediation of real municipal wastewater effluents: Acute and chronic toxicity assessment. J. Hazard. Mater. 2017, 323, 442–451. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Chen, L.; Wang, J. Degradation of PVA (polyvinyl alcohol) in wastewater by advanced oxidation processes. J. Adv. Oxid. Technol. 2017, 20, 20170018. [Google Scholar] [CrossRef]
- Cheng, C.; Li, H.D.; Wang, J.L.; Wang, H.L.; Yang, X.J. A review of measurement methods for peracetic acid (PAA). Front. Environ. Sci. Eng. 2020, 14, 87. [Google Scholar] [CrossRef]
- Ketel, D.H. Distribution and accumulation of thiophenes in plants and calli of different Tagetes species. J. Exp. Bot. 1987, 38, 322–330. [Google Scholar] [CrossRef]
- Ao, X.W.; Eloranta, J.; Huang, C.H.; Santoro, D.; Sun, W.J.; Lu, Z.D.; Li, C. Peracetic acid-based advanced oxidation processes for decontamination and disinfection of water: A review. Water Res. 2021, 188, 116479. [Google Scholar] [CrossRef]
- Sharma, S.; Mukhopadhyay, M.; Murthy, Z.V.P. UV/peroxyacetic acid mediated chlorophenol congener degradation. CLEAN–Soil Air Water 2014, 42, 276–283. [Google Scholar] [CrossRef]
- Cai, M.; Sun, P.; Zhang, L.; Huang, C.H. UV/peracetic acid for degradation of pharmaceuticals and reactive species evaluation. Environ. Sci. Technol. 2017, 51, 14217–14224. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Y.Q.; Fu, Y.S. Degradation kinetics and mechanism of diclofenac by UV/peracetic acid. RSC Adv. 2020, 10, 9907–9916. [Google Scholar] [CrossRef]
- Araujo, F.V.D.; Yokoyama, L.; Teixeira, L.A.C. Color removal in reactive dye solutions by UV/H2O2 oxidation. Quim. Nova 2006, 29, 11–14. [Google Scholar] [CrossRef]
- Xue, G.; Zheng, M.H.; Qian, Y.J.; Li, Q.; Gao, P.; Liu, Z.; Chen, H.; Li, X. Comparison of aniline removal by UV/CaO2 and UV/H2O2: Degradation kinetics and mechanism. Chemosphere 2020, 255, 126983. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.C.; Lee, L.T. Degradation of polyvinyl alcohol in aqueous solutions using UV/oxidant process. J. Ind. Eng. Chem. 2015, 21, 569–574. [Google Scholar] [CrossRef]
- Chen, S.; Cai, M.Q.; Liu, Y.Z.; Zhang, L.Q. Effects of water matrices on the degradation of naproxen by reactive radicals in the UV/peracetic acid process. Water Res. 2019, 150, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Xingzu, W.; Cheng, X.; Dezhi, S.; Qi, H. Biodecolorization and partial mineralization of reactive black 5 by a strain of Rhodopseudomonas palustris. J. Environ. Sci. 2008, 20, 1218–1225. [Google Scholar]
- Zhang, S.J.; Yu, H.Q. Radiation-induced degradation of polyvinyl alcohol in aqueous solutions. Water Res. 2004, 38, 309–316. [Google Scholar] [CrossRef]
- Xiao, J.; Wang, M.; Pang, Z.; Dai, L.; Lu, J.; Zou, J. Simultaneous spectrophotometric determination of peracetic acid and the coexistent hydrogen peroxide using potassium iodide as the indicator. Anal. Methods 2019, 11, 1930–1938. [Google Scholar] [CrossRef]
- Liu, B.; Guo, W.; Wang, H.; Si, Q.; Zhao, Q.; Luo, H.; Ren, N. Activation of peroxymonosulfate by cobalt-impregnated biochar for atrazine degradation: The pivotal roles of persistent free radicals and ecotoxicity assessment. J. Hazard. Mater. 2020, 398, 122768. [Google Scholar] [CrossRef]
- Su, C.C.; Pagaling, E.D.; Peralta, G.L.; Lu, M.-C. Comparison of Aniline Degradation by Fenton and Electro-Fenton Reactors Using Plate and Rod Electrodes. Environ. Prog. Sustain. Energy 2014, 33, 410–418. [Google Scholar] [CrossRef]
- Du, P.; Liu, W.; Cao, H.; Zhao, H.; Huang, C.-H. Oxidation of amino acids by peracetic acid: Reaction kinetics, pathways and theoretical calculations. Water Res. X 2018, 1, 100002. [Google Scholar] [CrossRef]
- Zhang, K.; Zhou, X.; Du, P.; Zhang, T.; Cai, M.; Sun, P.; Huang, C.-H. Oxidation of β-lactam antibiotics by peracetic acid: Reaction kinetics, product and pathway evaluation. Water Res. 2017, 123, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Huang, C.H. Reactivity of peracetic acid with organic compounds: A critical review. ACS ES&T Water 2020, 1, 15–33. [Google Scholar]
- Sun, P.; Zhang, T.; Mejia-Tickner, B.; Zhang, R.; Cai, M.; Huang, C.-H. Rapid Disinfection by Peracetic acid combined with UV Irradiation. Environ. Sci. Technol. Lett. 2018, 5, 400–404. [Google Scholar] [CrossRef]
- Hollman, J.; Dominic, J.A.; Achari, G. Degradation of pharmaceutical mixtures in aqueous solutions using UV/peracetic acid process: Kinetics, degradation pathways and comparison with UV/H2O2. Chemosphere 2020, 248, 125911. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.Q.; Huang, C.H. Modeling the Kinetics of UV/Peracetic acid Advanced Oxidation Process. Environ. Sci. Technol. 2020, 54, 7579–7590. [Google Scholar] [CrossRef]
- Hamad, D.; Mehrvar, M.; Dhib, R. Experimental study of polyvinyl alcohol degradation in aqueous solution by UV/H2O2 process. Polym. Degrad. Stab. 2014, 103, 75–82. [Google Scholar] [CrossRef]
- Liang, H.; Chen, Z.M.; Huang, D.; Zhao, Y.; Li, Z.Y. Impacts of aerosols on the chemistry of atmospheric trace gases: A case study of peroxides and HO2 radicals. Atmos. Chem. Phys. 2013, 13, 11259–11276. [Google Scholar] [CrossRef]
- Gao, J.; Luo, C.W.; Gan, L.; Wu, D.J.; Tan, F.X.; Cheng, X.X.; Zhou, W.W.; Wang, S.S.; Zhang, F.M.; Ma, J. A comparative study of UV/H2O2 and UV/PDS for the degradation of micro-pollutants: Kinetics and effect of water matrix. Environ. Sci. Pollut. Res. 2020, 27, 24531–24541. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, Y.; Fu, Y. Photochemical Degradation of triclosan by Ultraviolet-Peracetic acid. Technol. Water Treat. 2021, 47, 36. [Google Scholar]
- Joseph, C.G.; Taufiq-Yap, Y.H.; Krishnan, V. Ultrasonic assisted photolytic degradation of reactive black 5 (RB5) simulated wastewater. ASEAN J. Chem. Eng. 2017, 17, 37–50. [Google Scholar] [CrossRef]
- Kunz, A.; Mansilla, H.; Duran, N. A degradation and toxicity study of three textile reactive dyes by ozone. Environ. Technol. 2002, 23, 911–918. [Google Scholar] [CrossRef]
- Anipsitakis, G.P.; Dionysiou, D.D. Radical generation by the interaction of transition metals with common oxidants. Environ. Sci. Technol. 2004, 38, 3705–3712. [Google Scholar] [CrossRef] [PubMed]
- Grosjean, D.; Williams, E.L., II. Environmental persistence of organic compounds estimated from structure-reactivity and linear free-energy relationships. Unsaturated aliphatics. Atmos. Environ. Part A 1992, 26, 1395–1405. [Google Scholar] [CrossRef]
- Kim, J.; Du, P.; Liu, W.; Luo, C.; Zhao, H.; Huang, C.H. Cobalt/peracetic acid: Advanced oxidation of aromatic organic compounds by acetylperoxyl radicals. Environ. Sci. Technol. 2020, 54, 5268–5278. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Li, C.; Yang, K.; Hu, Y.; Xu, X. Sb(V)removal by different iron oxides from simulated textile-wastewater. Acta Sci. Circumst. 2022, 42, 96–107. [Google Scholar]
- Li, L.; Huang, J.; Hu, X.; Zhang, S.; Dai, Q.; Chai, H.; Gu, L. Activation of sodium percarbonate by vanadium for the degradation of aniline in water: Mechanism and identification of reactive species. Chemosphere 2019, 215, 647–656. [Google Scholar] [CrossRef]
- Liu, Y.L.; Li, C.; Lou, Z.M.; Zhou, C.C.; Yang, K.L.; Xu, X.H. Antimony removal from textile wastewater by combining PFS&PAC coagulation: Enhanced Sb(V) removal with presence of dispersive dye. Sep. Purif. Technol. 2021, 275, 119037. [Google Scholar]
- Luo, X.; Zheng, Z.; Greaves, J.; Cooper, W.J.; Song, W. Trimethoprim: Kinetic and mechanistic considerations in photochemical environmental fate and AOP treatment. Water Res. 2012, 46, 1327–1336. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Ma, J.; Jiang, J.; Liu, Y.; Song, Y.; Yang, Y.; Guan, Y.; Wu, D. Simulation and comparative study on the oxidation kinetics of atrazine by UV/H2O2, UV/HSO5− and UV/S2O82−. Water Res. 2015, 80, 99–108. [Google Scholar] [CrossRef]
- Li, N.; Qiao, X.; Uiu, Y.; Fei, Z. Oxidization of dissolved organic matters from different sources by hydroxyl radical. Environ. Chem. 2015, 34, 1246–1251. [Google Scholar]
- Yu, J.L.; Shu, S.H.; Wang, Q.F.; Gao, N.Y.; Zhu, Y.P. Evaluation of Fe2+/Peracetic acid to degrade three typical refractory pollutants of textile wastewater. Catalysts 2022, 12, 684. [Google Scholar] [CrossRef]
- Prasse, C.; Ford, B.; Nomura, D.K.; Sedlak, D.L. Unexpected transformation of dissolved phenols to toxic dicarbonyls by hydroxyl radicals and UV light. Proc. Natl. Acad. Sci. USA 2018, 115, 2311–2316. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Y.; Cao, Y.; Shu, S.; Zhu, P.; Wang, D.; Xu, H.; Cai, D. Comparison of Medium-Pressure UV/Peracetic Acid to Remove Three Typical Refractory Contaminants of Textile Wastewater. Processes 2023, 11, 1183. https://doi.org/10.3390/pr11041183
Zhu Y, Cao Y, Shu S, Zhu P, Wang D, Xu H, Cai D. Comparison of Medium-Pressure UV/Peracetic Acid to Remove Three Typical Refractory Contaminants of Textile Wastewater. Processes. 2023; 11(4):1183. https://doi.org/10.3390/pr11041183
Chicago/Turabian StyleZhu, Yanping, Yuxuan Cao, Shihu Shu, Pengjin Zhu, Dongfang Wang, He Xu, and Dongqing Cai. 2023. "Comparison of Medium-Pressure UV/Peracetic Acid to Remove Three Typical Refractory Contaminants of Textile Wastewater" Processes 11, no. 4: 1183. https://doi.org/10.3390/pr11041183
APA StyleZhu, Y., Cao, Y., Shu, S., Zhu, P., Wang, D., Xu, H., & Cai, D. (2023). Comparison of Medium-Pressure UV/Peracetic Acid to Remove Three Typical Refractory Contaminants of Textile Wastewater. Processes, 11(4), 1183. https://doi.org/10.3390/pr11041183





