Abstract
Chemical investigations of L. stoechas subsp. luisieri and L. pedunculata essential oils were analyzed by GC-MS, and the antimicrobial activity was performed against bacteria and fungi isolated from food sources. The cytotoxicity of the essential oil was performed in NHDF cells using the MTT method. According to the results, the main compounds of L. stoechas subsp. luisieri essential oil were trans-α-necrodyl acetate (40.2%), lavandulyl acetate (11%), and trans-α-necrodol (10.4%), while fenchone (50.5%) and camphor (30.0%) in L. pedunculata essential oil. The antifungal activity of essential oils was confirmed with MIC values ranging from 1.2 to 18.7 µL/mL; for bacteria, it ranged from 4.7 to 149.3 µL/mL. Both the Lavandula species tested showed low or equal MIC and MBC/MFC values for L. stoechas subsp. luisieri essential oil, revealing greater efficacy in antimicrobial activity. The L. stoechas subsp. luisieri essential oil revealed cytotoxic effects (30 ± 2% of cell viability) in NHDF cells at all concentrations tested.
1. Introduction
Aromatic and medicinal plants have been intensively studied due to the biological potential of essential oils (EOs) and other extracts. Dispersed around the Mediterranean area, Lavandula species belong to the Lamiaceae (=Labiatae) family. Around 41 species are recognized in this genus [1]. Due to the geographical position and climate, this area represents a biodiversity hotspot, allowing the development of several endemic plant species. However, climate change and the high risk of fires are endangering Mediterranean species. Lavandula stoechas subsp. luisieri (Rozeira) Rozeira and L. pedunculata (Mill.) Cav. are perennial shrubs and endemic to the Iberian Peninsula. In Portugal, these plants are important resources for beekeeper activities and the extraction of their essential oils as a value-added product in poor agricultural regions. Both species produce valuable secondary metabolites, namely terpenes in their EOs, and phenolic compounds with great biological properties. Some ethnobotanical investigations reveal the uses of these species for healing indigestion, heartburn, headaches, blood circulation, and also act as a sedative, antidermatitis, asthma, and decongestive nasal bronchitis [1,2,3]. The chemical compounds of their EOs are mainly oxygenated monoterpenes (33–87%), followed by monoterpene hydrocarbons (0.1–17%), oxygenated sesquiterpenes (0.3–12%), and sesquiterpene hydrocarbons (0.5–10%) [4]. L. stoechas subsp. luisieri deserves particular interest due to the composition of their EO that contains unique compounds: the necrodane derivatives, such as trans-α-necrodol and trans-α-necrodyl acetate [5,6,7]. The main compounds reported in L. pedunculata EO are fenchone, camphor, and 1,8-cineole [8,9]. The morphological aspects of both species and their respective main compounds are presented in Figure 1.
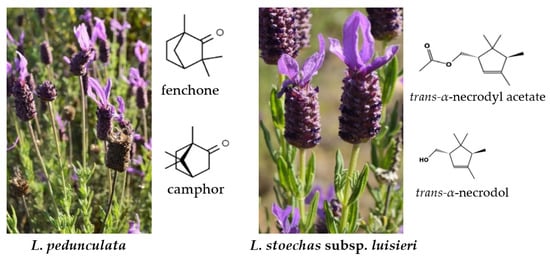
Figure 1.
Morphological traits of Lavandula pedunculata and L. stoechas subsp. luisieri and its main compounds. The chemical structures were downloaded from Chemical Structure Search–ChemSpider (https://www.chemspider.com/StructureSearch.aspx, accessed on 23 March 2023).
Essential oils are promising antimicrobial products, both against human and animal pathogenic microorganisms and for food spoilage control. The complex mixture of chemical components in the EO makes this natural product an effective agent in the resistance of microorganisms, contrary to what occurs in common antibiotics, which are constituted by a single compound [10]. The remarkable efficacy of essential oils is not related to the presence of their main compounds but often to the synergistic effect among varied constituents [11]. Specific reports have demonstrated the antimicrobial action of Lavandula EOs and their main compounds, such as camphor and 1,8-cineole, which agree that the activity of the EOs is not due to merely one compound [8,12,13,14]. Despite several studies on the antimicrobial action of the EOs, the mechanism of action in microorganisms is not evident among investigators. For example, some studies have reported greater susceptibility of Gram-positive than Gram-negative bacteria. Gram-negative bacteria have a complex cell envelope that includes an outer membrane linked to the inner peptidoglycan layer via lipoproteins, and due to this structure, this kind of bacteria is more resistant. On the other hand, other researchers do not find antibacterial differences between both bacteria types [15]. Furthermore, the mode of action may depend on the chemical profile and their components ratio [16]. The mechanism of action of EOs may involve different events in the cell’s outer membrane and within the cytoplasm. Among the different mechanisms, we enhance: the disintegration of the bacterial outer membrane; the alteration of the fatty acid composition; increase the membrane fluidity, resulting in leakage out of metabolites and ions; the interference with glucose uptake; and the inhibition of enzyme activity [15,17]. Concerning the major compounds found in each species, L. pedunculata EO is rich in fenchone and camphor [8,18]. Fenchone is a bicyclic monoterpene ketone, with a structure and odor like camphor, which is also a monoterpenoid ketone. Both compounds revealed high biological properties, such as antibacterial and antifungal activities [14,19]. However, other minor compounds may influence antimicrobial activity, such as α-pinene [20], limonene [19], and linalool [13]. Regarding the L. stoechas subsp. luisieri EO, the main compounds are irregular monoterpenoids with cyclopentenic structures, namely necrodane derivatives, such as trans-α-necrodol and trans-α-necrodyl acetate [5,7]. Eisner and Meinwald discovered these compounds in the defensive secretion of Necrodes surinamensis [21], and recently, these compounds were also discovered in Evolvulus alsinoides L. essential oils [22]. To understand the potential of these compounds, Zuzarte et al. (2012) studied two chemically distinct profiles of L. stoechas subsp. luisieri EO, one with high amounts of necrodanes and one with low amounts of these compounds. The great antifungal activity was revealed in EO with high necrodane compounds [5]. Despite the great antimicrobial activity, the cytotoxicity of the EOs in human cells must be considered to avoid toxic effects on the organism [23]. The cytotoxicity studies of the EOs of L. pedunculata and L. stoechas subsp. lusieri are sparse, and for the L. stoechas subsp. luisieri EO, only two studies report no cytotoxicity effects at small concentrations (<3.2 mg/mL and <0.08 µL/mL) in human skin fibroblasts [24] and in mouse macrophage cell line (RAW 264.7) [5]. Additionally, for the L. pedunculata, aqueous and hydroalcoholic extracts were reported in two studies with no cytotoxic effects revealed against the porcine liver (PLP2) and human keratinocytes (HaCat) cell lines [25,26]. On the other hand, some studies have reported the anticancer potential of EOs and other extracts. Regarding cancer cells, anti-proliferative effects were observed in L. pedunculata extracts against breast adenocarcinoma (MCF-7), cervical carcinoma (HeLa), lung cancer (NCI-H460), and hepatocellular carcinoma (HepG2) [26]. Moreover, L. stoechas subsp. lusieri showed cytotoxic effects against HepG2 [24]. This study aims to contribute to disseminating the chemical variation of the EO of two important types of endemism of the Iberian Peninsula, L. stoechas subsp. Luisieri, and L. pedunculata, and to contribute to a broad spectrum of microorganisms, some of which are reported for the first time in the antimicrobial activity of these species. The cytotoxicity of L. stoechas subsp. luisieri EO against normal human cells was also performed.
2. Materials and Methods
2.1. Plant Collection and Essential Oils
Flowering L. stoechas subsp. luisieri and L. pedunculata were collected in Serra da Malcata (558 m, 40°12′06.741″ N; 7°06′22.085″ W), Portugal. A replica of each plant was deposited in the herbarium of the Biology Laboratory of IPCB-ESA (Polytechnic Institute of Castelo Branco—Agrarian School). The voucher numbers are ESACBMLS08 and ESACBMLP01, for L. stoechas subsp. luisieri and L. pedunculata, respectively. The EO from the fresh aerial parts was obtained by hydrodistillation for 2 h in a Clevenger-type apparatus according to the procedure described in the European Pharmacopoeia [27].
2.2. GC-MS Analysis
The volatile profiles of the EOs were obtained in triplicate by gas chromatography coupled with mass spectrometry (GC/MS SCION-SQ 456 GC, Bruker Corporation, Massachusetts, United States of America). The separation was achieved on an HP-5MS capillary column (30 m × 0.25 mm id × 0.25 µm film thickness, Agilent J&W, Folsom, CA, USA). Helium was the carrier gas used with a flow rate of 1 mL/min. The EO samples were injected with a volume of 1 µL, using a split ratio of 1:100, and analyzed using electron impact ionization mass spectrometry (EI-MS) at 70 eV. The compounds were identified in scan mode with the positive polarity of ions 20–300 m/z with a time of 250.0 ms. The initial oven temperature was programmed to 45 °C, gradually increasing 3 °C/min to 175 °C and 300 °C with a heating rate of 15 °C/min, and maintaining this temperature for 10 min. The transfer line and the ion source were programmed at 250 °C and 220 °C, respectively. The identification of the compounds was based on the retention index (RI) compared with the RI given by the MS library (NIST 17 version 2.3) and with RI calculated from the n-alkane series standards (C7–C18 and C19–C30) that were injected under the same chromatographic column and chromatographic conditions. The relative amount of each compound was expressed as a percentage of the relative peak area of the compound, relative to the total area of the peaks identified in the samples.
2.3. Microorganism Cultures
Nine fungal cultures were earlier isolated from fruits of Arbutus unedo L. [28] in the Microbiology Laboratory of IPCB-ESA, and these cultures were identified by molecular approach in the Micoteca da Universidade do Minho (MUM). These fungi were identified as Alternaria section Alternaria (ESA.M.11), Penicillium simile (ESA.M.13), Aspergillus tubingensis (ESA.M.38), Aspergillus niger (ESA.M.45), Meyerozyma guilliermondii (ESA.M.47), Penicillium crustosum (ESA.M.48), Penicillium glabrum (ESA.M.54), Aureobasidium sp. (ESA.M.57), and Hanseniaspora sp. (ESA.M.99). Two ATCC reference strains, Saccharomyces cerevisiae ATCC 9763 and Aspergillus brasiliensis ATCC 16404, and a clinical isolate of Candida albicans ESALD/2016 were also tested. The reference cultures were acquired in ielab® (Alicante, Spain). Each culture was transferred to a potato dextrose agar (PDA, HiMedia Chemicals, Nagpur, India) medium, at 25 ± 2 °C during 48 h for yeasts and 4 to 5 days for molds (until the spore formation) and used for analysis after three subcultures. For molds, the spore suspensions were prepared according to Domingues et al. (2021) [6]. For yeasts, a suspension was prepared in 0.85% (w/v) NaCl (Applichem Panreac, Darmstadt, Germany) to match the turbidity of the 1.0 McFarland standard (bioMérieux, Lyon, France), representing approximately 3.0 × 107 yeasts/mL.
Nine bacterial cultures were used (Table 1), six Gram-negative bacteria, such as Aeromonas hydrophila, Burkholderia sp., Chromobacterium violaceum, Pseudomonas aeruginosa, Salmonella sp., and Serratia marcescens, and three Gram-positive bacteria such as Bacillus cereus, Listeria monocytogenes, and coagulase-positive Staphylococcus. Pseudomonas aeruginosa ATCC 27853 was used as reference strain. Bacterial cultures were obtained by growing the bacterial cultures for 18–24 h at 37 °C in tryptone soya yeast extract agar (TSA-YE, prepared with TSA (Oxoid, Chester, UK) and YE (Biokar, Beauvais, France)). The exception was B. cereus culture, which was grown for 15 h. B. cereus growth conditions were used to obtain non-sporulated cultures.

Table 1.
Identification and characterization of bacterial cultures.
2.4. Microdilution Method for MIC and MFC/MBC Determination
For bacterial cultures, a suspension was prepared in 0.85% (w/v) NaCl (Applichem Panreac, Darmstadt, Germany) to match the turbidity of the 0.5 McFarland standard (bioMérieux, Lyon, France), representing about 1.5 × 108 cells/mL. For molds and yeasts, the EOs were diluted in a potato dextrose broth (PDB, VWR Chemicals Prolabo, PA, USA) medium supplemented with 0.8% (v/v) of tween 80 (VWR Chemicals Prolabo, PA, USA). For the bacteria, the EOs were diluted in a Müeller–Hinton broth (MHB, Oxoid, Chester, UK) medium supplemented with 0.8% (v/v) of tween 80 (VWR Chemicals Prolabo, PA, USA). The MIC of the EOs was fulfilled according to the Clinical and Laboratory Standards Institute, CLSI (2002) method, with some modifications. Each microplate well was completed with 150 µL in total volume. The test wells were completed with 140 µL of EO/medium and 10 µL of inoculum. Negative control wells were completed with 150 µL of EO/medium, and the positive control wells were completed with 140 µL of medium and 10 µL of inoculum. A culture medium control was also made. The microplates were incubated under the optimum conditions for fungal and bacterial cultures under humid air. After incubation, all microplate wells were inoculated in PDA (HiMedia Chemicals, Nagpur, India) and nutritive agar (NA, Oxoid, Chester, UK) plates for fungi (MFC) and bacteria (MBC), respectively. For MFC and MBC determinations, 10 µL loops were used. The MFC/MBC values matched the lowest EO concentration, of which no growth was observed after incubation. Afterward, 30 µL of resazurin (VWR Chemicals Prolabo, PA, USA) was added to each microplate well. Then, the microplates were incubated for 2 h for bacteria and fungi until the positive control changed color. According to Tulio et al. (2006) and The et al. (2017), the results were visually assessed by comparing the color of the inoculated wells with the color of the positive and negative control wells [29,30]. The MIC value matched the lowest EO concentration in which the color was similar to the negative control. All experiments were performed in triplicate and repeated whenever the results of each triplicate did not agree.
2.5. Cell Viability
The evaluation of cell viability was performed using the MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay, as described by Santos et al. [31]. The cytotoxicity of the L. stoechas subsp. luisieri EO was determined after 24 h of incubation. Normal human dermal fibroblasts (NHDF) cell lines were maintained in a Roswell Park Memorial Institute (RPMI-1640) culture medium supplemented with 10% fetal bovine serum (FBS), 1% antibiotic/antimycotic mixture, 0.02 M of L-glutamine, 0.01 M of HEPES, and 0.001 M of sodium pyruvate. The cells were incubated at 37 °C in an air incubator in a 5% CO2-humidified atmosphere. They were seeded in 96-well plates (5 × 103 cells/well), which, after reaching confluence, were exposed to the samples dissolved in a RPMI-1640 culture medium. Supplemented RPMI-1640 culture medium was added to the negative control wells. At the end of incubation, the medium in the wells was removed and replaced by the MTT solution (0.5 mg/mL) and incubated again at 37 °C for 3 h. Afterward, the MTT solution was removed and formazan crystals were dissolved in 0.5% DMSO; the absorbances were recorded using a microplate reader at 570 nm.
3. Results
3.1. Chemical Profile of the Essential Oils
The constituents of the EOs of both species are listed in Table 2, according to their elution order, in an HP-5MS column.

Table 2.
Identification and relative amounts of compounds of L. stoechas subsp. luisieri (LSL) and L. pedunculata (LP) essential oils.
Thirty-three compounds were identified in samples and four unidentified compounds (NI C, D, E, and F) were observed in L. stoechas subsp. luisieri EO, which are always present in the EO of this species as observed from other lab work (data unpublished). Good identification of chemical compounds was obtained with 88.8% in L. stoechas subsp. luisieri and 98.7% in L. pedunculata. The L. stoechas subsp. luisieri and L. pedunculata EOs are characterized by high amounts of oxygenated monoterpenes (81.2% and 85.5%, respectively). The main compounds in the L. stoechas subsp. luisieri EOs were trans-α-necrodyl acetate (40.2%), lavandulyl acetate (11%), and trans-α-necrodol (10.4%). Significant amounts of linalool (5.6%), fenchone (3.6%), and 1,8-cineole (3.2%) were also found. As it has been described, the L. stoechas subsp. luisieri EO is singularly characterized by the presence of irregular oxygenated monoterpenes called necrodanes, which are absent in the remaining Lavandula species [4]. These compounds becoming L. stoechas subsp. luisieri as an interesting biological value and could be a chemotaxonomic marker of this species. In our work, compounds such as trans-α-necrodol, cis-α-necrodol, trans-α-necrodyl acetate, and cis-α-necrodyl acetate were identified by GC-MS. According to the geographic distribution and chemical studies of EO, L. stoechas subsp. luisieri is only reported in the Iberian Peninsula. The major compounds reported in the L. stoechas subsp. luisieri EO are camphor (1.1–74%), trans-α-necrodyl acetate (1.8–48%), fenchone (0.1–22%), and 1,8-cineole (1.3–21%) in plants from Portugal and Spain [5,6,18,33,34,35,36,37,38,39,40,41]. The chemical variability of EOs appears to be common between Lavandula populations; Zuzarte et al. (2012) noticed significant variations in the chemical composition of EO among plants from central and southern Portugal. From the central region, the essential oil was characterized by trans-α-necrodyl acetate (17%), trans-α-necrodol (7%), and 1,8-cineol (6%), contrasting to the southern plants, whereby 1,8-cineole (34%) and fenchone (18%) were the main compounds. Although necrodane compounds were not present as major compounds, the following compounds were reported at low concentrations: trans-α-necrodyl acetate (3.2%) and trans-α-necrodol (4.5%) [5]. According to this study, it would seem that abiotic factors have a crucial influence on chemical compound production [42].
Regarding L. pedunculata EO, the main compounds were fenchone (50.5%), camphor (30%), and α-pinene (7%). This species is widely distributed in the Mediterranean region and is abundant throughout Portugal [1]. The main compounds found in L. pedunculata EO are corroborated by other chemical studies; however, according to research conducted in these geographical regions, different chemotypes in this species have been found. Zuzarte et al. (2009) revealed considerable differences in the major compound between geographical origins, 1,8-cineol (34%) in northern region plants, fenchone (45%) in central region plants, and the chemotype camphor/1,8-cineol (34%/25%) in central–north region plants [8]. Another chemotype, camphor/fenchone (42%/37%), was reported in southern plants [9,18]. As previously described, identifying chemotypes in the L. pedunculata EO demonstrates the strong influence of extrinsic factors in producing chemical compounds [4,43].
3.2. Antifungal Activity of the Essential Oils
The minimum inhibitory concentration (MIC) and minimum fungicidal concentration (MFC) of the EOs of both Lavandula species (Table 3) were evaluated against nine cultures isolated from Arbutus unedo L. fruits, C. albicans (clinical isolate), and two reference cultures (A. brasiliensis ATCC 16404 and S. cerevisiae ATCC 9763). Concerning the antifungal activity of L. stoechas subsp. Luisieri, the scientific information is sparse. MIC and MFC values varied from 1.2 to 74.7 µL/mL. Generally, the concentration to inhibit a microorganism is lower than the concentration of lethality. However, the MIC value was the same in some microorganisms as in the MFC. Other studies have also verified this behavior, which reveals the fungicidal effects of these EOs [5,8,40]. The most sensible cultures to the action of EOs were Aureobasidium sp., Hanseniaspora sp., and S. cerevisiae ATCC 9763 for L. stoechas subsp. luisieri essential oil, with 1.2 µL/mL and 2.3 µL/mL MIC and MFC values, respectively. On the other hand, the most resistant microorganisms were Alternaria sp. section Alternaria, A. brasiliensis ATCC 16404, and P. glabrum. For these cultures, it was impossible to determine the MFC value for both species; the maximum concentration tests revealed the growth of the microorganisms. Özcan et al. (2018) also revealed the strong resistance of A. alternaria to the action of L. stoechas EO [44]. The L. stoechas subsp. luisieri EO revealed greater effectiveness than L. pedunculata due to the MIC or MFC values always being inferior or equal. Baptista et al. (2015) also reported the higher effectiveness of L. stoechas subsp. luisieri EO compared to the L. pedunculata. These authors evaluated the EOs of both species against A. niger, C. albicans, and S. cerevisiae, reporting higher MIC values (15.5 µg/mL, >100 µg/mL, and 31 µg/mL, respectively) [45]. In spite of the different analytical methods, Zuzarte et al. (2009) reported lower MIC and MFC values of L. pedunculata EO against C. albicans (2.5 and 5 µL/mL, respectively), but higher MFC values were revealed against A. niger (≥20 µL/mL) [8]. Regarding L. stoechas subsp. luisieri EO, Zuzarte et al. (2012) tested the antifungal activity against A. niger ATCC 16404 with a very low MIC value (0.32 µL/mL) but a high MFC value (20 µL/mL) [5].

Table 3.
Minimum inhibitory concentration (MIC) and minimum fungicidal concentration (MFC) of L. stoechas subsp. luisieri (LSL) and L. pedunculata (LP) essential oils.
3.3. Antibacterial Activity of Essential Oils
The Lavandula sp. EOs were also tested against several bacterial cultures, most potentially pathogenic. The antibacterial activity demonstrated similar behavior (Table 4). This means the L. stoechas subsp. luisieri showed a greater effectiveness against most microorganisms compared to L. pedunculata EO.

Table 4.
Minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of L. stoechas subsp. luisieri (LSL) and L. pedunculata (LP) essential oils.
The exception was against Salmonella sp., where the L. pedunculata EO showed a minimum bactericidal concentration (MBC) value (37.3 µL/mL) lower than L. stoechas subsp. luisieri (74.7 µL/mL). The greater antimicrobial activity of L. stoechas subsp. luisieri compared with other Lavandula sp. was also revealed in other studies [45,46]. This antimicrobial potential may be due to the presence of necrodane derivatives in their essential oil/non-polar extracts, which have been reported as compounds with high biological properties [18,35]. The most sensitive Gram-negative bacteria was C. violaceum for both EOs with MBC at 9.3 µL/mL. The most resistant Gram-negative bacteria was P. aeruginosa ATCC 27853 with the same MIC and MBC values (149.3 µL/mL) for both Lavandula EOs. The isolate P. aeruginosa SC-V-AP/2015 also demonstrated high resistance to the action of both EOs. However, the maximum concentration tested of L. pedunculata (149.3 µL/mL) did not show lethality against this culture. Some studies reveal the high resistance of P. aeruginosa to the action of L. stoechas subsp. luisieri and L. pedunculata [24,33,46,47]. Gram-negative bacteria are known for their strong resistance to antibacterial agents due to the external membrane surrounding the cell wall restricting the diffusion of hydrophobic compounds through the lipopolysaccharides [17].
Concerning Gram-positive bacteria, the most resistant was B. cereus, despite low MIC values of 4.7 and 9.3 µL/mL, for L. stoechas subsp. luisieri and L. pedunculata, respectively. The MBC of both EOs was higher than 149.3 µL/mL. This high MBC value must be caused by their ability to produce spores that are highly resistant to adverse conditions [48]. Other studies with high inhibitory concentrations reported this strong resistance of B. cereus [24,33]. Listeria monocytogenes showed a lower MBC value (18.7 µL/mL) to the action of L. stoechas subsp. luisieri EO. For L. stoechas EO of plants from the Morocco region, this culture showed a very low MIC value of 2.5 µL/mL [49]. Regarding coagulase-positive Staphylococcus, the MIC and MBC values were the same for both EOs (37.3 µL/mL). Pombal et al. also tested the antibacterial potential of L. stoechas subsp. luisieri EO against Staphylococcus aureus, where the MIC and MBC also had the same values [40].
3.4. Cytotoxicity of the Essential Oil
Due to the greater antimicrobial activity, the L. stoechas subsp. luisieri EO was selected for cytotoxicity evaluation. The NHDF cells were exposed to different concentrations of the EO (0.25 to 10 µL/mL) for 24 h. According to the results presented in Figure 2, the viability of the cells with the presence of L. stoechas subsp. luisieri EO was significantly reduced with mean values of around 30 ± 2%, even at a lower concentration of EO tested (0.25 µL/mL).
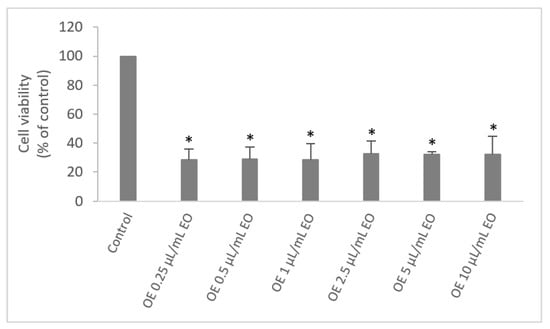
Figure 2.
Effect of Lavandula stoechas subsp. luisieri essential oil on NHDF cell viability (MTT assay). Results are expressed as a percentage of MTT reduction by control cells maintained in a culture medium. Each value represents the mean ± SD from triplicates (* p < 0.01 compared to control).
The cell viability did not differ statistically between different concentrations tested, which means that in these concentrations, the EO has a high potential of in vitro cytotoxicity in NHDF cells. As far as we know, the cytotoxicity of L. stoechas subsp. luisieri EO was only noticed by Zuzarte et al. (2012) [5]. The authors tested the cytotoxic effects of L. stoechas subsp. luisieri EO in a mouse macrophage cell line (RAW 264.7), and they demonstrated that the EO with higher percentages of necrodanes compounds (17% of trans-α-necrodyl acetate) had minor percentages of cell viability with values around 45% at 0.64 µL/mL. Only 0.08 µL/mL of EO did not affect the cell viability. However, we should not compare these values due to considerable differences, such as the kind of cell line, analytical methods, and the origin of plants/EO. We believe that the chemical profile of L. stoechas subsp. luisieri EO with high percentages of necrodanes compounds strongly contributes to this high cytotoxicity in fibroblast cells. On the other hand, cell lines have different behavior according to their origin; for example, the same study that used two mouse cell models, RAW 264.7 and fibroblasts, verified that fibroblasts cells were more resistant to the action of the same cytotoxic agents compared to macrophage cells [50]. Due to high cytotoxicity revealed by L. stoechas subsp. luisieri EO in NHDF cells, its direct application in topical uses is not recommended. However, new EO nanoencapsulation strategies could be considered as a way to reduce their toxicity, such as liposomes, emulsions, and biopolymeric nanoparticles [51].
4. Conclusions
In conclusion, our results reveal the great antimicrobial activity of the L. stoechas subsp. luisieri and L. pedunculata EOs against Gram-positive and Gram-negative bacteria, and also against yeasts and filamentous fungi. Comparing both species, the greater antimicrobial activity is attributed to the L. stoechas subsp. luisieri EO. According to these results, the EOs of both species are promising natural products to be used as antibacterial and antifungal agents against foodborne and potential pathogenic human and animal strains. Considering the microorganisms’ resistance to conventional antimicrobial agents, the use of these natural products could be applied in antimicrobial formulations. Regarding cytotoxicity, L. stoechas subsp. luisieri EO revealed low cell viability in NHDF cells. In order to explore the potential application by the food and pharmaceutical industries, more cytotoxic studies of the EOs are to be investigated.
Author Contributions
Conceptualization, J.D., F.D. and C.S.P.; methodology, J.D., M.G. and J.G.; validation, F.D. and C.S.P.; formal analysis, J.D.; investigation: J.D., M.G. and J.G.; resources, J.C.G. and C.S.P.; writing—original draft preparation, J.D.; writing—review and editing, F.D., J.C.G., J.G. and C.S.P.; visualization, J.D.; supervision, F.D., J.C.G. and C.S.P.; project administration, J.C.G. and C.S.P.; funding acquisition, J.C.G. and C.S.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partially supported by the Research Center for Natural Resources, Environment and Society (CERNAS-IPCB) (project UIDB/00681/2020) funding by the Portuguese Funding Agency for Science Research and Technology (FCT) and by the Programa Operacional EP-INTERREG V A Espanha-Portugal (POCTEProject), Projeto 0665_COOP4PAM_4_P “Cooperate to grow in the aromatic and medicinal plant sector”.
Data Availability Statement
The data used in this study are reported in the paper’s figures and tables.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Upson, T.M.; Andrews, S. The Genus Lavandula; The Royal Botanical Gardens: London, UK, 2004.
- Novais, M.H.; Santos, I.; Mendes, S.; Pinto-Gomes, C. Studies on Pharmaceutical Ethnobotany in Arrabida Natural Park (Portugal). J. Ethnopharmacol. 2004, 93, 183–195. [Google Scholar] [CrossRef]
- Neves, J.M.; Matos, C.; Moutinho, C.; Queiroz, G.; Gomes, L.R. Ethnopharmacological Notes about Ancient Uses of Medicinal Plants in Trás-Os-Montes (Northern of Portugal). J. Ethnopharmacol. 2009, 124, 270–283. [Google Scholar] [CrossRef] [PubMed]
- Domingues, J.; Delgado, F.; Gonçalves, J.C.; Zuzarte, M.; Duarte, A.P. Mediterranean Lavenders from Section Stoechas: An Undervalued Source of Secondary Metabolites with Pharmacological Potential. Metabolites 2023, 13, 337. [Google Scholar] [CrossRef] [PubMed]
- Zuzarte, M.; Gonçalves, M.J.; Cruz, M.T.; Cavaleiro, C.; Canhoto, J.; Vaz, S.; Pinto, E.; Salgueiro, L. Lavandula luisieri Essential Oil as a Source of Antifungal Drugs. Food Chem. 2012, 135, 1505–1510. [Google Scholar] [CrossRef]
- Domingues, J.; Delgado, F.; Gonçalves, J.C.; Santos Pintado, C. Essential Oils of Lavandula stoechas Subsp. luisieri as Antifungal Agent against Fungi from Strawberry Tree Fruit. J. Pharm. Pharmacol. 2021, 9, 98–106. [Google Scholar] [CrossRef]
- Zuzarte, M.; Sousa, C.; Cavaleiro, C.; Cruz, M.T.; Salgueiro, L. The Anti-Inflammatory Response of Lavandula luisieri and Lavandula pedunculata Essential Oils. Plants 2022, 11, 370. [Google Scholar] [CrossRef] [PubMed]
- Zuzarte, M.; Gonçalves, M.J.; Cavaleiro, C.; Dinis, A.M.; Canhoto, J.M.; Salgueiro, L.R. Chemical Composition and Antifungal Activity of the Essential Oils of Lavandula pedunculata (Miller) Cav. Chem. Biodivers. 2009, 6, 1283–1292. [Google Scholar] [CrossRef]
- Costa, P.; Gonçalves, S.; Valentão, P.; Andrade, P.B.; Almeida, C.; Nogueira, J.M.F.; Romano, A. Metabolic Profile and Biological Activities of Lavandula pedunculata Subsp. lusitanica (Chaytor) Franco: Studies on the Essential Oil and Polar Extracts. Food Chem. 2013, 141, 2501–2506. [Google Scholar] [CrossRef] [PubMed]
- Solórzano-Santos, F.; Miranda-Novales, M.G. Essential oils from aromatic herbs as antimicrobial agents. Curr. Opin. Biotechnol. 2012, 23, 136–141. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological Effects of Essential Oils-A Review. Food Chem. Toxicol. 2008, 1, 446–475. [Google Scholar] [CrossRef]
- Karaca, N.; Demirci, B.; Demirci, F. Evaluation of Lavandula stoechas L. Subsp. stoechas L., Mentha spicata L. Subsp. spicata L. Essential Oils and Their Main Components against Sinusitis Pathogens. Z. Für Nat. C 2018, 73, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Herman, A.; Tambor, K.; Herman, A. Linalool Affects the Antimicrobial Efficacy of Essential Oils. Curr. Microbiol. 2016, 72, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Vermaak, I.; Viljoen, A. Camphor—A Fumigant during the Black Death and a Coveted Fragrant Wood in Ancient Egypt and Babylon—A Review. Molecules 2013, 18, 5434–5454. [Google Scholar] [CrossRef]
- Angane, M.; Swift, S.; Huang, K.; Butts, C.A.; Quek, S.Y. Essential Oils and Their Major Components: An Updated Review on Antimicrobial Activities, Mechanism of Action and Their Potential Application in the Food Industry. Foods 2022, 11, 464. [Google Scholar] [CrossRef] [PubMed]
- Bora, H.; Kamle, M.; Mahato, D.K.; Tiwari, P.; Kumar, P. Citrus Essential Oils (CEOs) and Their Applications in Food: An Overview. Plants 2020, 9, 357. [Google Scholar] [CrossRef]
- Burt, S. Essential Oils: Their Antibacterial Properties and Potential Applications in Foods—A Review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Matos, F.; Miguel, M.G.; Duarte, J.; Venâncio, F.; Moiteiro, C.; Correia, A.I.D.; Figueiredo, A.C.; Barroso, J.; Pedro, L. Antioxidant Capacity of the Essential Oils from Lavandula luisieri, L. stoechas Subsp. lusitanica, L. stoechas Subsp. lusitanica x L. luisieri and L. viridis Grown in Algarve (Portugal). J. Essent. Oil Res. 2009, 21, 327–336. [Google Scholar] [CrossRef]
- Keskin, I.; Gunal, Y.; Ayla, S.; Kolbasi, B.; Sakul, A.; Kilic, U.; Gok, O.; Koroglu, K.; Ozbek, H. Effects of Foeniculum vulgare Essential Oil Compounds, Fenchone and Limonene, on Experimental Wound Healing. Biotech. Histochem. 2017, 92, 274–282. [Google Scholar] [CrossRef]
- Allenspach, M.; Steuer, C. α-Pinene: A never-ending story. Phytochemistry 2021, 190, 112857. [Google Scholar] [CrossRef]
- Eisner, T.; Meinwald, J. Defensive Spray Mechanism of A Silphid Beetle (Necrodes surinamensis). Psyche 1982, 89, 357–367. [Google Scholar] [CrossRef]
- Kashima, Y.; Miyazawa, M. Chemical Composition and Aroma Evaluation of Essential Oils from Evolvulus alsinoides L. Chem. Biodivers. 2014, 11, 396–407. [Google Scholar] [CrossRef] [PubMed]
- Baptista-Silva, S.; Borges, S.; Ramos, O.L.; Pintado, M.; Sarmento, B. The progress of essential oils as potential therapeutic agents: A review. J. Essent. Oil Res. 2020, 32, 279–295. [Google Scholar] [CrossRef]
- Nunes, R.; Pasko, P.; Tyszka-Czochara, M.; Szewczyk, A.; Szlosarczyk, M.; Carvalho, I.S. Antibacterial, Antioxidant and Anti-Proliferative Properties and Zinc Content of Five South Portugal Herbs. Pharm. Biol. 2017, 55, 114–123. [Google Scholar] [CrossRef]
- Pereira, F.; Baptista, R.; Ladeiras, D.; Madureira, A.M.; Teixeira, G.; Rosado, C.; Fernandes, A.S.; Ascensão, L.; Silva, C.O.; Reis, C.P.; et al. Production and Characterization of Nanoparticles Containing Methanol Extracts of Portuguese Lavenders. Meas. J. Int. Meas. Confed. 2015, 74, 170–177. [Google Scholar] [CrossRef]
- Lopes, C.L.; Pereira, E.; Soković, M.; Carvalho, A.M.; Barata, A.M.; Lopes, V.; Rocha, F.; Calhelha, R.C.; Barros, L.; Ferreira, I.C.F.R. Phenolic Composition and Bioactivity of Lavandula Pedunculata (Mill.) Cav. Samples from Different Geographical Origin. Molecules 2018, 23, 1037. [Google Scholar] [CrossRef] [PubMed]
- Council of Europe. European Pharmacopoeia, 9th ed.; Council of Europe: Strasbourg, France, 2016. [Google Scholar]
- Domingues, J.; Goulão, M.; Coelho, M.T.; Gonçalves, J.C.; Pintado, C.S. Different postharvest storage conditions of Arbutus unedo L. fruits, and their physicochemical and microbiological characterisation. Int. Food Res. J. 2022, 29, 32–41. [Google Scholar] [CrossRef]
- Tullio, V.; Nostro, A.; Mandras, N.; Dugo, P.; Banche, G.; Cannatelli, M.A.; Cuffini, A.M.; Alonzo, V.; Carlone, N.A. Antifungal activity of essential oils against filamentous fungi determined by broth microdilution and vapour contact methods. J. Appl. Microbiol. 2007, 102, 1544–1550. [Google Scholar] [CrossRef]
- Teh, C.H.; Nazni, W.A.; Lee, H.L.; Fairuz, A.; Tan, S.B.; Sofian-Azirun, M. In vitro antibacterial activity and physicochemical properties of a crude methanol extract of the larvae of the blow fly Lucilia cuprina. Med. Vet. Entomol. 2013, 27, 414–420. [Google Scholar] [CrossRef]
- Santos, E.S.; Luís, Â.; Gonçalves, J.; Rosado, T.; Pereira, L.; Gallardo, E.; Duarte, A.P. Julbernardia paniculata and Pterocarpus angolensis: From ethnobotanical surveys to phytochemical characterization and bioactivities evaluation. Molecules 2020, 25, 1828. [Google Scholar] [CrossRef]
- Babushok, V.I.; Linstrom, P.J.; Zenkevich, I.G. Retention Indices for Frequently Reported Compounds of Plant Essential Oils. J. Phys. Chem. Ref. Data 2011, 40, 043101. [Google Scholar] [CrossRef]
- Baldovini, N.; Lavoine-Hanneguelle, S.; Ferrando, G.; Dusart, G.; Lizzani-Cuvelier, L. Necrodane Monoterpenoids from Lavandula luisieri. Phytochemistry 2005, 66, 1651–1655. [Google Scholar] [CrossRef] [PubMed]
- Lavoine-Hanneguelle, S.; Casabianca, H. New Compounds from the Essential Oil and Absolute of Lavandula luisieri L. J. Essent. Oil Res. 2004, 16, 445–448. [Google Scholar] [CrossRef]
- González-Coloma, A.; Delgado, F.; Rodilla, J.M.; Silva, L.; Sanz, J.; Burillo, J. Chemical and Biological Profiles of Lavandula luisieri Essential Oils from Western Iberia Peninsula Populations. Biochem. Syst. Ecol. 2011, 39, 1–8. [Google Scholar] [CrossRef]
- Videira, R.; Castanheira, P.; Grãos, M.; Salgueiro, L.; Faro, C.; Cavaleiro, C. A Necrodane Monoterpenoid from Lavandula luisieri Essential Oil as a Cell-Permeable Inhibitor of BACE-1, the β-Secretase in Alzheimer’s Disease. Flavour Fragr. J. 2013, 28, 380–388. [Google Scholar] [CrossRef]
- Julio, L.F.; Martín, L.; Muñoz, R.; Mainar, A.M.; Urieta, J.S.; Sanz, J.; Burillo, J.; González-Coloma, A. Comparative Chemistry and Insect Antifeedant Effects of Conventional (Clevenger and Soxhlet) and Supercritical Extracts (CO2) of Two Lavandula luisieri Populations. Ind. Crops Prod. 2014, 58, 25–30. [Google Scholar] [CrossRef]
- Rufino, A.T.; Ferreira, I.; Judas, F.; Salgueiro, L.; Lopes, M.C.; Cavaleiro, C.; Mendes, A.F. Differential Effects of the Essential Oils of Lavandula luisieri and Eryngium duriaei Subsp. juresianum in Cell Models of Two Chronic Inflammatory Diseases. Pharm. Biol. 2015, 53, 1220–1230. [Google Scholar] [CrossRef]
- Arantes, S.; Candeias, F.; Lopes, O.; Lima, M.; Pereira, M.; Tinoco, T.; Cruz-Morais, J.; Martins, M.R. Pharmacological and Toxicological Studies of Essential Oil of Lavandula stoechas Subsp. luisieri. Planta Med. 2016, 82, 1266–1273. [Google Scholar] [CrossRef] [PubMed]
- Pombal, S.; Rodrigues, C.F.; Araújo, J.P.; Rocha, P.M.; Rodilla, J.M.; Diez, D.; Granja, Á.P.; Gomes, A.C.; Silva, L.A. Antibacterial and Antioxidant Activity of Portuguese Lavandula luisieri (Rozeira) Rivas-Martinez and Its Relation with Their Chemical Composition. Springerplus 2016, 5, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Dias, N.; Dias, M.C.; Cavaleiro, C.; Sousa, M.C.; Lima, N.; Machado, M. Oxygenated Monoterpenes-Rich Volatile Oils as Potential Antifungal Agents for Dermatophytes. Nat. Prod. Res. 2017, 31, 460–464. [Google Scholar] [CrossRef]
- Guitton, Y.; Nicolè, F.; Jullien, F.; Caissard, J.-C.; Saint-Marcoux, D.; Legendre, L.; Pasquier, B.; Moja, S. A comparative study of terpene composition in different clades of the genus Lavandula. Bot. Lett. 2018, 165, 494–505. [Google Scholar] [CrossRef]
- Vairinhos, J.; Miguel, M.G. Essential oils of spontaneous species of the genus Lavandula from Portugal: A brief review. Zeitschrift Naturforsch. 2020, 75, 233–245. [Google Scholar] [CrossRef]
- Özcan, M.M.; Starovic, M.; Aleksic, G.; Figueredo, G.; Juhaimi, F.A.; Chalchat, J.-C. Chemical Composition and Antifungal Activity of Lavender (Lavandula stoechas) Oil. Nat. Prod. Commun. 2018, 13, 1934578X1801300. [Google Scholar] [CrossRef]
- Baptista, R.; Madureira, A.M.; Jorge, R.; Adão, R.; Duarte, A.; Duarte, N.; Lopes, M.M.; Teixeira, G. Antioxidant and Antimycotic Activities of Two Native Lavandula Species from Portugal. Evid.-Based Complement. Altern. Med. 2015, 2015. [Google Scholar] [CrossRef]
- Teixeira, G.; Correia, A.I.; Vasconcelos, T.; Duarte, A.; Oliveira, N.; Madureira, A.M. Lavandula stoechas Subsp. luisieri and L. pedunculata: Comparative Antibacterial Activity. J. Phyther. Pharmacol. 2012, 1, 11–15. [Google Scholar]
- Soro, N.K.; Majdouli, K.; Khabbal, Y.; Zair, T. Chemical Composition and Antibacterial Activity of Lavandula Species L. dentata L., L. pedunculata Mill and Lavandula abrialis Essential Oils from Morocco against Food-Borne and Nosocomial Pathogens. Int. J. Innov. Appl. Stud. 2014, 7, 774–781. [Google Scholar]
- Huang, Y.; Flint, S.H.; Palmer, J.S. Bacillus cereus spores and toxins–The potential role of biofilms. Food Microbiol. 2020, 90, 103493. [Google Scholar] [CrossRef]
- Bouyahya, A.; Et-Touys, A.; Abrini, J.; Talbaoui, A.; Fellah, H.; Bakri, Y.; Dakka, N. Lavandula stoechas Essential Oil from Morocco as Novel Source of Antileishmanial, Antibacterial and Antioxidant Activities. Biocatal. Agric. Biotechnol. 2017, 12, 179–184. [Google Scholar] [CrossRef]
- Awaluddin, R.; Nugrahaningsih, D.A.A.; Solikhah, E.N.; Chabib, L. The Effect of Asiatic Acid and Metformin on The Viability Percentage of Mouse Macrophage Cell Lines RAW264. 7 and Mouse Fibroblast Cell Lines NIH3T3. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2020; p. 012021. [Google Scholar]
- Zuzarte, M.; Vitorino, C.; Salgueiro, L.; Girão, H. Plant Nanovesicles for Essential Oil Delivery. Pharmaceutics 2022, 14, 2581. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).