Enthalpic Determination of the Interaction of Modified Activated Carbons with Benzene and Hexane as Pure Solvents and Binary Mixtures
Abstract
1. Introduction
2. Materials and Methods
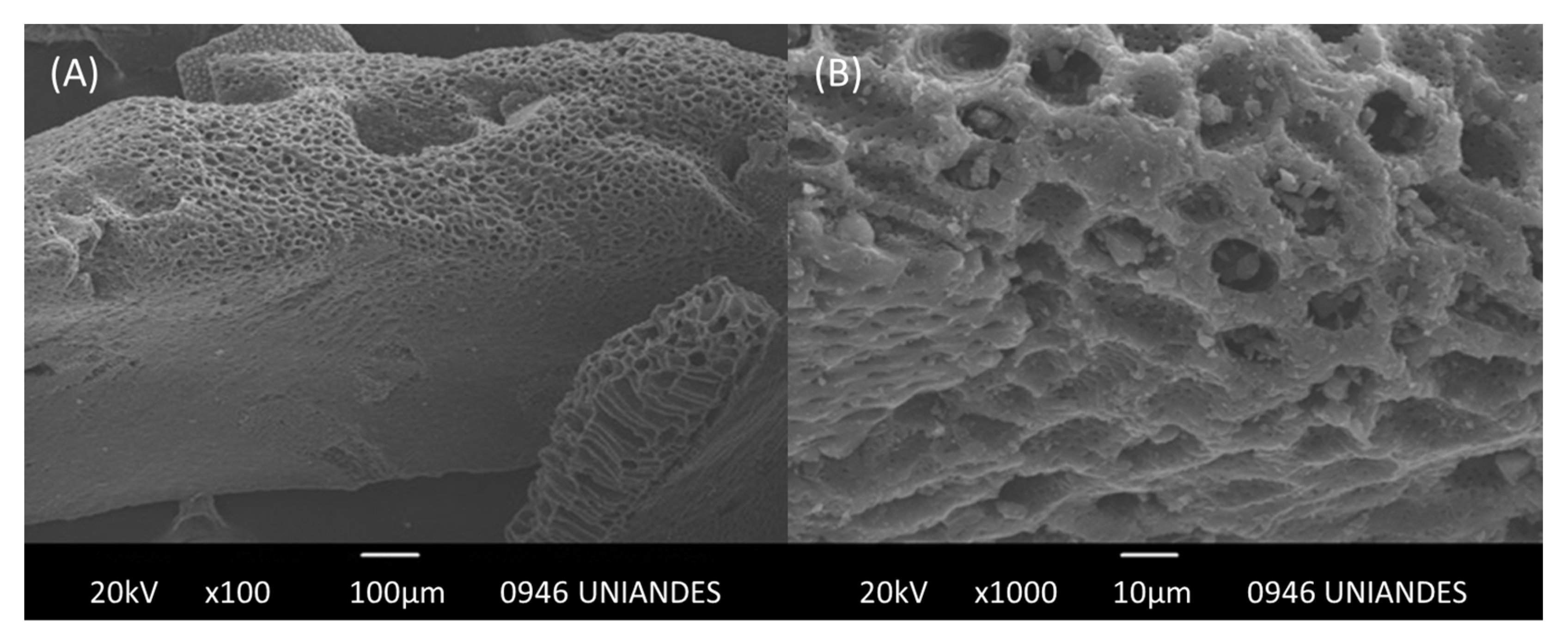
2.1. Activated Carbons
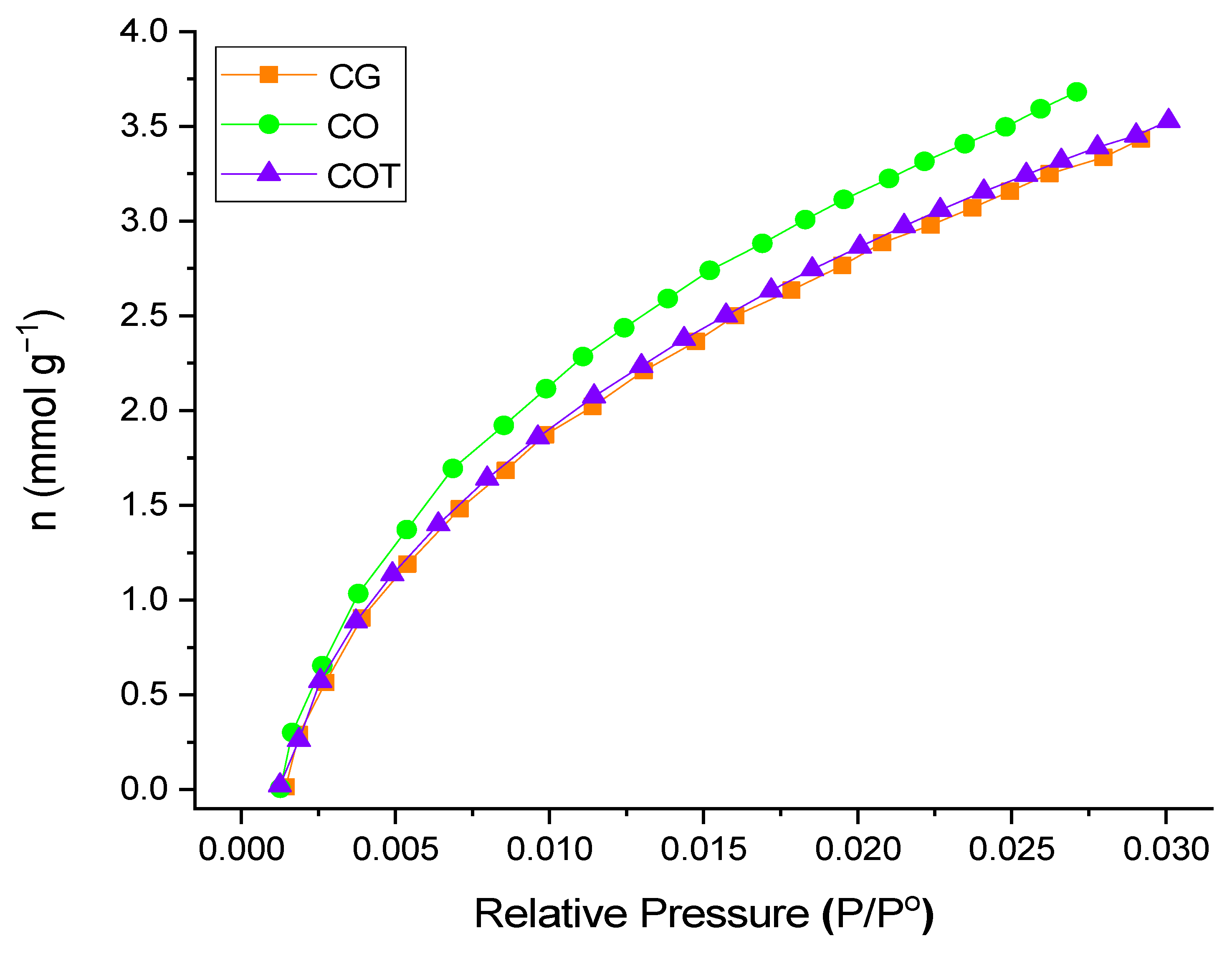
2.2. Determination of Surface Area
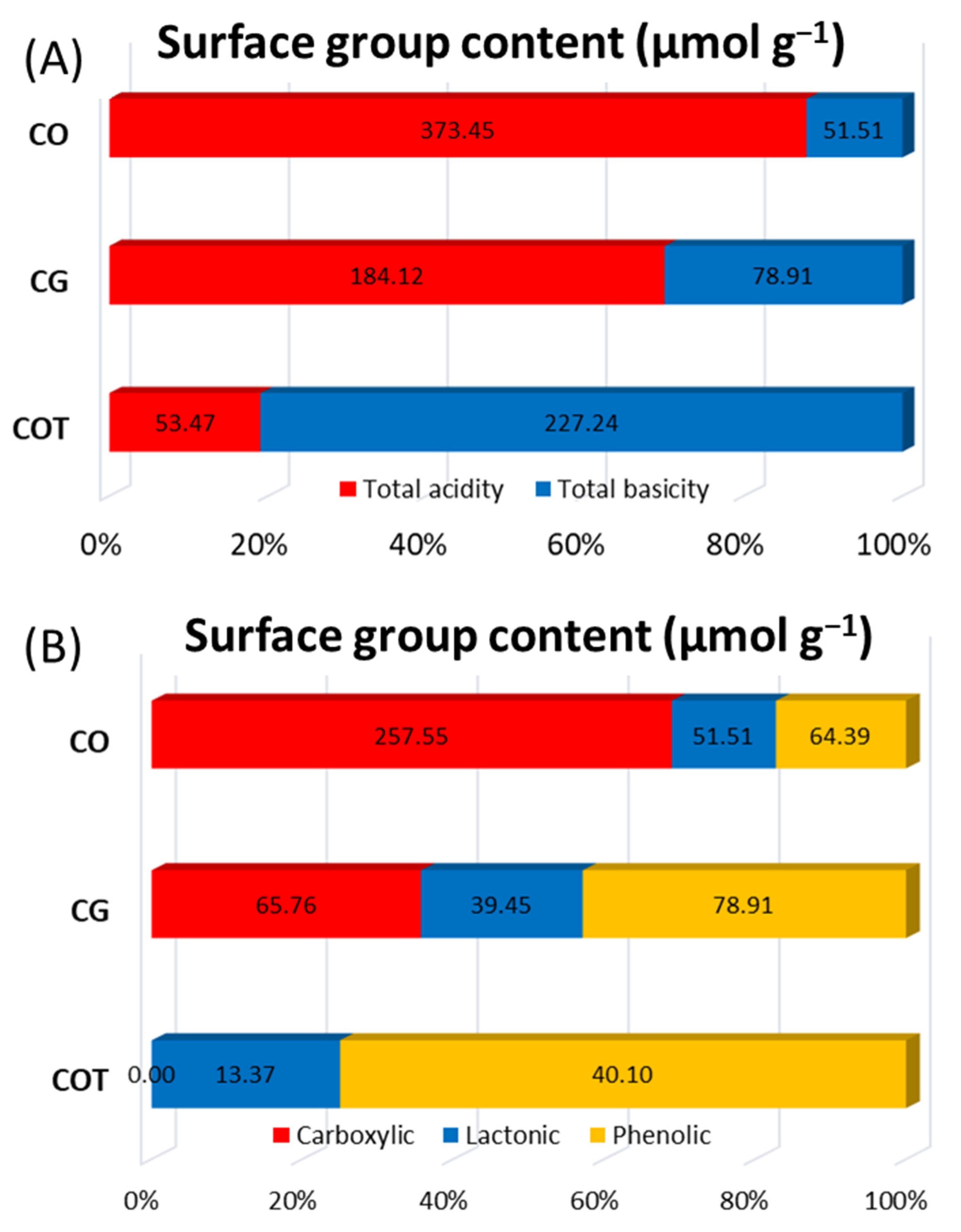
2.3. Determination of Total Acidity, Total Basicity, Carboxylic, Lactonic and Phenolic Groups
2.4. Determination of the Enthalpy of Immersion of Activated Carbons in Solvents and Mixtures
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Foo, K.Y.; Hameed, B.H. An overview of landfill leachate treatment via activated carbon adsorption process. J. Hazard. Mater. 2009, 171, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Friedlingstein, P.; O’Sullivan, M.; Jones, M.W.; Andrew, R.M.; Gregor, L.; Hauck, J.; Le Quéré, C.; Luijkx, I.T.; Olsen, A.; Peters, G.P.; et al. CO₂ and Greenhouse Gas Emissions. Our World Data 2020, 14, 3269–3340. [Google Scholar] [CrossRef]
- Cruz, O.F.; Campello-Gómez, I.; Casco, M.E.; Serafin, J.; Silvestre-Albero, J.; Martínez-Escandell, M.; Hotza, D.; Rambo, C.R. Enhanced CO₂ capture by cupuassu shell-derived activated carbon with high microporous volume. Carbon Lett. 2022, 1–9. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Huang, P.-J. Sono-assisted rapid dye removal by chromium-based metal organic frameworks derived from waste PET bottles: Characterization, kinetics and adsorption isotherms. J. Environ. Chem. Eng. 2021, 9, 106766. [Google Scholar] [CrossRef]
- Kafle, A.; Timilsina, A.; Gautam, A.; Adhikari, K.; Bhattarai, A.; Aryal, N. Phytoremediation: Mechanisms, plant selection and enhancement by natural and synthetic agents. Environ. Adv. 2022, 8, 100203. [Google Scholar] [CrossRef]
- Kwiatkowski, M.; Fierro, V.; Celzard, A. Confrontation of various adsorption models for assessing the porous structure of activated carbons. Adsorption 2019, 25, 1673–1682. [Google Scholar] [CrossRef]
- Wang, G.; Dou, B.; Zhang, Z.; Wang, J.; Liu, H.; Hao, Z. Adsorption of benzene, cyclohexane and hexane on ordered mesoporous carbon. J. Environ. Sci. 2015, 30, 65–73. [Google Scholar] [CrossRef]
- Rouquerol, J.; Rouquerol, F.; Llewellyn, P.; Maurin, G.; Sing, K.S.W. Adsorption by Powders and Porous Solids: Principles, Methodology and Applications, 2nd ed.; Academic Press: Cambridge, MA, USA, 2013. [Google Scholar]
- Giraldo, L.; Rodríguez-Estupiñán, P.; Moreno-Piraján, J.C. Calorimetry of Immersion in the Energetic Characterization of Porous Solids. In Calorimetry—Design, Theory and Applications in Porous Solids; Moreno-Piraján, J.C., Ed.; Intech Open Ltda: London, UK, 2018; pp. 35–53. [Google Scholar] [CrossRef]
- Boehm, H.P. Some aspects of the surface chemistry of carbon blacks and other carbons. Carbon N. Y. 1994, 32, 759–769. [Google Scholar] [CrossRef]
- Kim, Y.S.; Park, C.R. Chapter 13—Titration Method for the Identification of Surface Functional Groups. In Materials Science and Engineering of Carbon; Inagaki, M., Ed.; Tsinghua University Press Limited: Beijing, China, 2016; pp. 273–286. ISBN 9780128052563. [Google Scholar]
- Daud, W.M.A.W.; Houshamnd, A.H. Textural characteristics, surface chemistry and oxidation of activated carbon. J. Nat. Gas Chem. 2010, 19, 267–279. [Google Scholar] [CrossRef]
- Khaoula, H.; Imen, B.; Foued, M.; Jemni, A. Study of the adsorption interaction potential in a microporous solid. Int. J. Adv. Res. 2016, 4, 60–69. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, S.; Rout, K.R.; Chen, D. Development of polyethylenimine (PEI)-impregnated mesoporous carbon spheres for low-concentration CO₂ capture. Catal. Today 2021, 369, 69–76. [Google Scholar] [CrossRef]
- Sevilla, M.; Falco, C.; Titirici, M.-M.; Fuertes, A.B. High-performance CO₂ sorbents from algae. RSC Adv. 2012, 2, 12792–12797. [Google Scholar] [CrossRef]
- Wei, H.; Deng, S.; Hu, B.; Chen, Z.; Wang, B.; Huang, J.; Yu, G. Granular bamboo-derived activated carbon for high CO₂ adsorption: The dominant role of narrow micropores. Chemsuschem 2012, 5, 2354–2360. [Google Scholar] [CrossRef]
- Simmons, J.M.; Wu, H.; Zhou, W.; Yildirim, T. Carbon capture in metal–organic frameworks—A comparative study. Energy Environ. Sci. 2011, 4, 2177–2185. [Google Scholar] [CrossRef]
- Czakkel, O.; Nagy, B.; Dobos, G.; Fouquet, P.; Bahn, E.; László, K. Static and dynamic studies of hydrogen adsorption on nanoporous carbon gels. Int. J. Hydrogen Energy 2019, 44, 18169–18178. [Google Scholar] [CrossRef]
- Bader, N.; Zacharia, R.; Abdelmottaleb, O.; Cossement, D. How the activation process modifies the hydrogen storage behavior of biomass-derived activated carbons. J. Porous Mater. 2018, 25, 221–234. [Google Scholar] [CrossRef]
- Serafin, J.; Dziejarski, B.; Junior, O.F.C.; Sreńscek-Nazzal, J. Design of highly microporous activated carbons based on walnut shell biomass for H₂ and CO₂ storage. Carbon N. Y. 2023, 201, 633–647. [Google Scholar] [CrossRef]
- Yang, X.; Yi, H.; Tang, X.; Zhao, S.; Yang, Z.; Ma, Y.; Feng, T.; Cui, X. Behaviors and kinetics of toluene adsorption-desorption on activated carbons with varying pore structure. J. Environ. Sci. 2018, 67, 104–114. [Google Scholar] [CrossRef]
- Gallego, E.; Roca, F.J.; Perales, J.F.; Guardino, X. Experimental evaluation of VOC removal efficiency of a coconut shell activated carbon filter for indoor air quality enhancement. Build. Environ. 2013, 67, 14–25. [Google Scholar] [CrossRef]
- Li, L.; Liu, S.; Liu, J. Surface modification of coconut shell based activated carbon for the improvement of hydrophobic VOC removal. J. Hazard. Mater. 2011, 192, 683–690. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Prahas, D.; Kartika, Y.; Indraswati, N.; Ismadji, S. Activated carbon from jackfruit peel waste by H3PO4 chemical activation: Pore structure and surface chemistry characterization. Chem. Eng. J. 2008, 140, 32–42. [Google Scholar] [CrossRef]
- Phan, N.H.; Rio, S.; Faur, C.; Le Coq, L.; Le Cloirec, P.; Nguyen, T.H. Production of fibrous activated carbons from natural cellulose (jute, coconut) fibers for water treatment applications. Carbon N. Y. 2006, 44, 2569–2577. [Google Scholar] [CrossRef]
- Goncharuk, O.V. The heat of immersion of modified silica in polar and nonpolar liquids. J. Therm. Anal. Calorim. 2015, 120, 1365–1373. [Google Scholar] [CrossRef]
- Bandosz, T.J.; Ania, C.O. Chapter 4 Surface chemistry of activated carbons and its characterization. Interface Sci. Technol. 2006, 7, 159–229. [Google Scholar]
- Rashidi, N.A.; Yusup, S.; Borhan, A. Isotherm and Thermodynamic Analysis of Carbon Dioxide on Activated Carbon. Procedia Eng. 2016, 148, 630–637. [Google Scholar] [CrossRef]



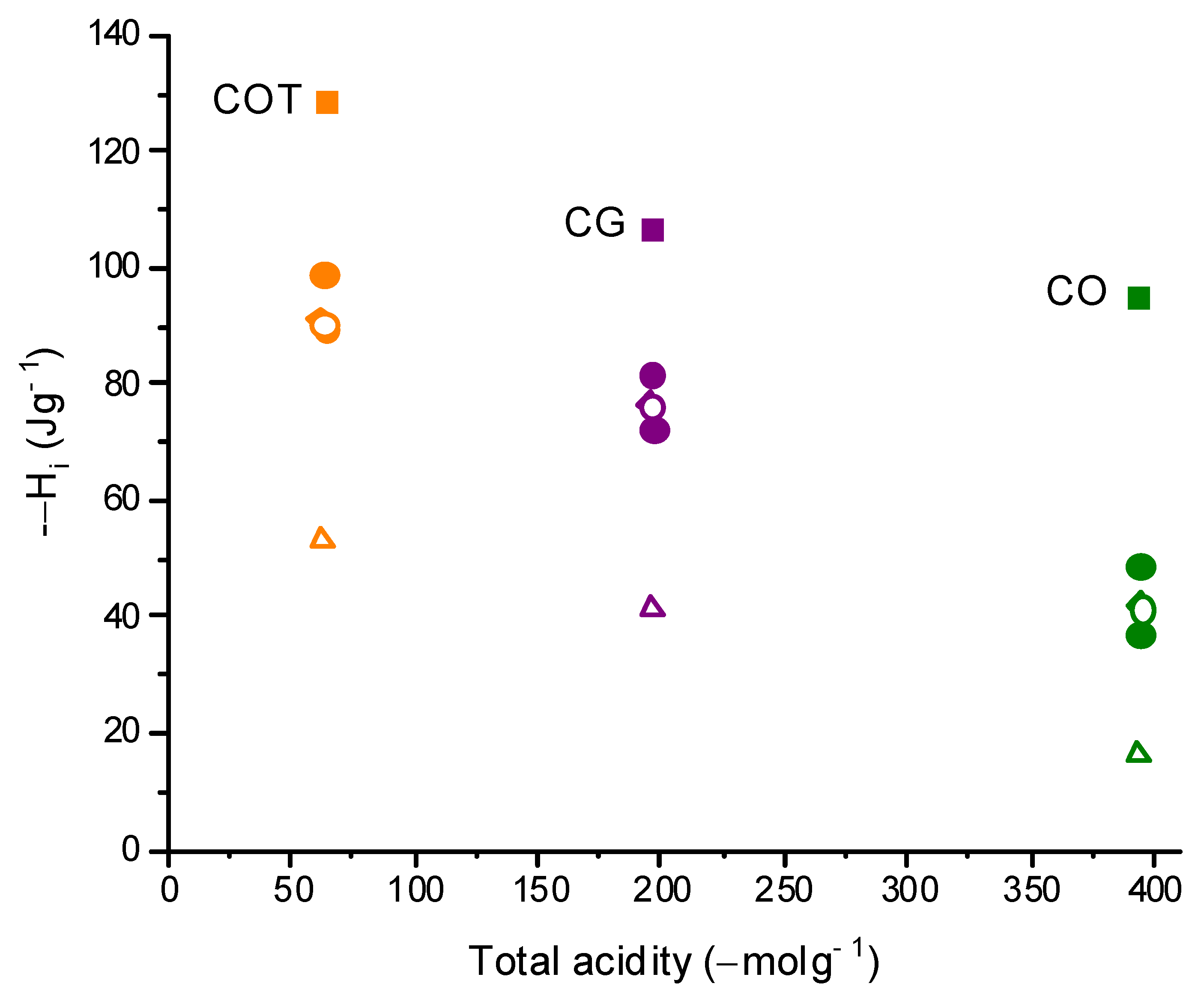
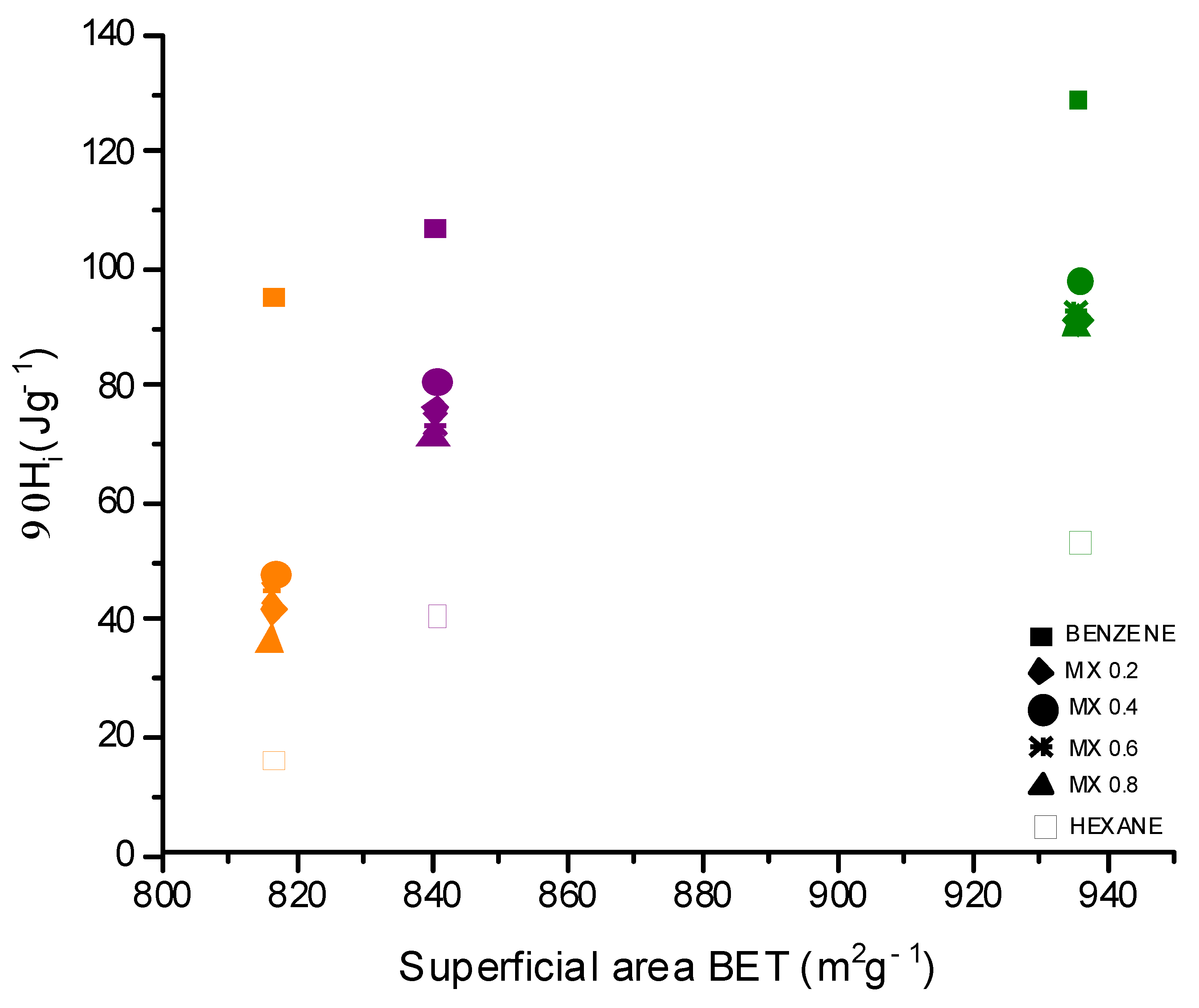
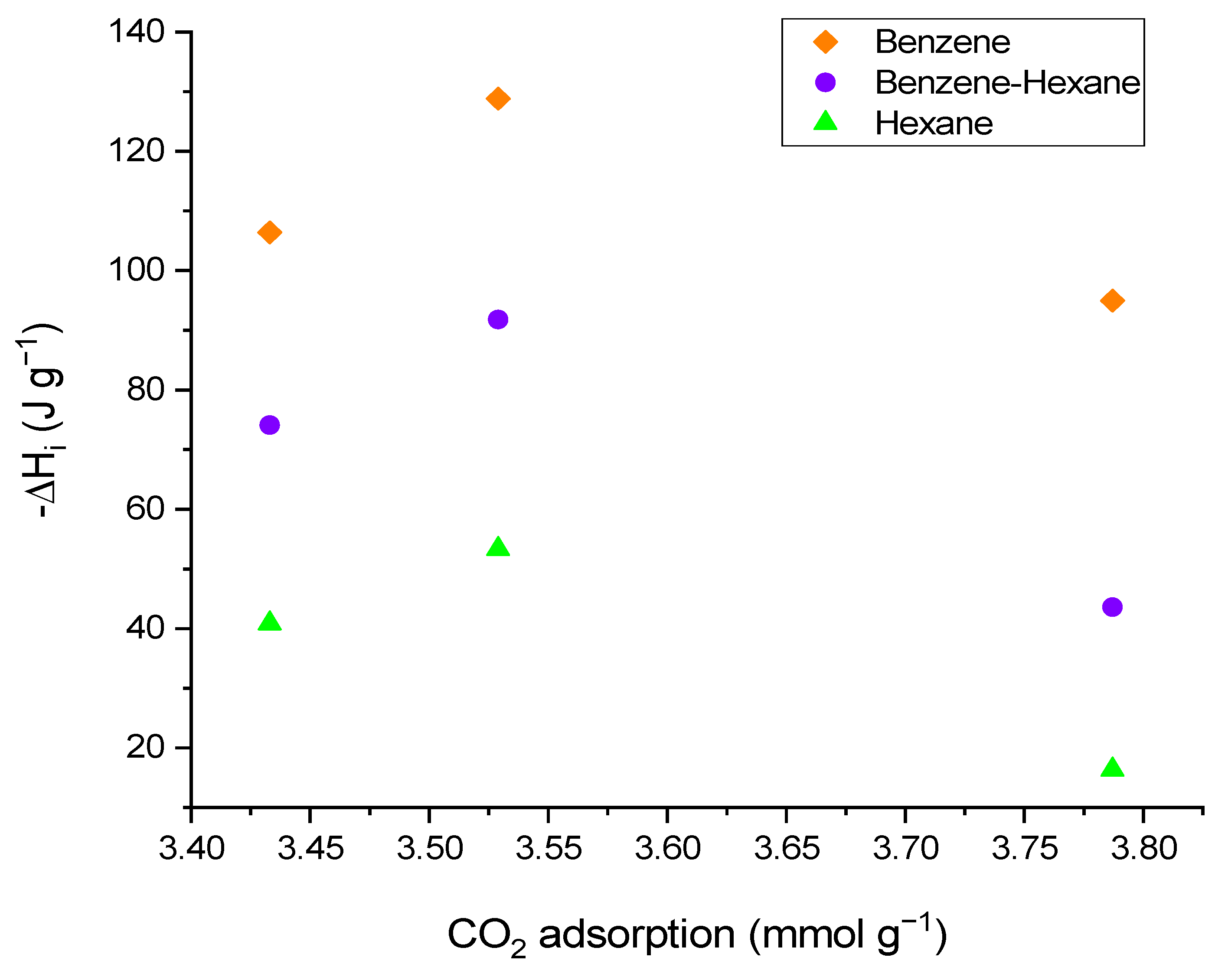
| Activated Carbon | N2 | CO2 | Adsorption CO2 | Acidity | Basicity | |||
|---|---|---|---|---|---|---|---|---|
| Area BET (m2/g) | V0 (cm3/g) | VMeso (cm3/g) | Vt (cm3/g) | Vn (cm3/g) | (mmolg−1) | (μmolg−1) | (μmolg−1) | |
| CG | 840 | 0.34 | 0.04 | 0.38 | 0.35 | 3.4 | 184.12 | 78.91 |
| CO | 816 | 0.32 | 0.05 | 0.37 | 0.38 | 3.8 | 373.45 | 51.51 |
| COT | 935 | 0.37 | 0.05 | 0.41 | 0.35 | 3.4 | 53.47 | 227.24 |
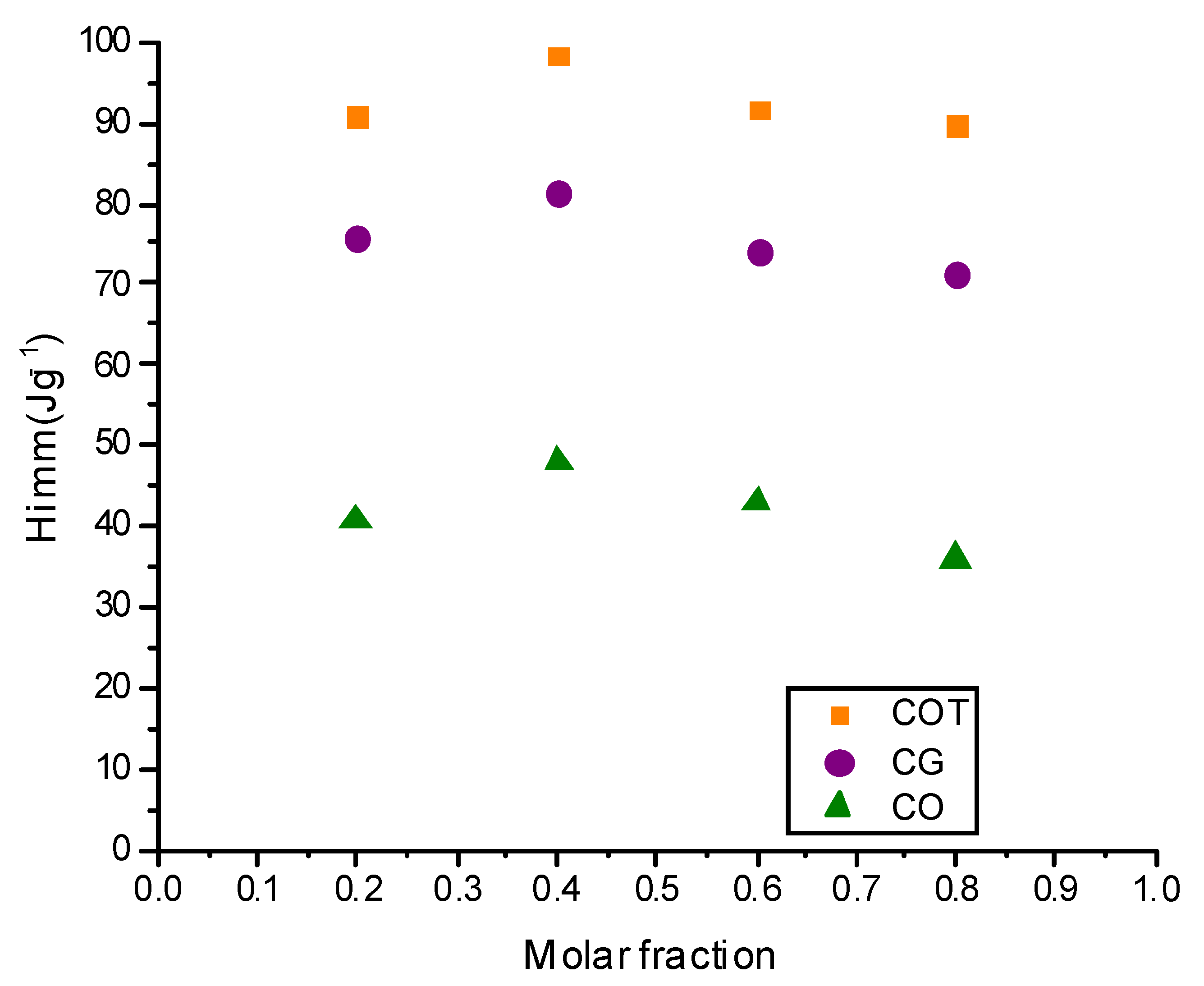
| Sample | −ΔHi C6H6 (Jg−1) | −ΔHi C6H14 (Jg−1) | −ΔHi 0.2 C6H6/ C6H14 (Jg−1) | −ΔHi 0.4 C6H6/ C6H14 (Jg−1) | −ΔHi 0.6 C6H6/ C6H14 (Jg−1) | −ΔHi 0.8 C6H6/ C6H14 (Jg−1) |
|---|---|---|---|---|---|---|
| CG | 106.40 | 40.87 | 75.87 | 81.22 | 74.10 | 71.35 |
| CO | 94.98 | 16.36 | 41.31 | 48.42 | 43.58 | 36.39 |
| COT | 128.80 | 53.35 | 90.70 | 98.37 | 91.81 | 89.54 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Monje, D.; Giraldo, L.; Serafin, J.; Moreno-Piraján, J.C. Enthalpic Determination of the Interaction of Modified Activated Carbons with Benzene and Hexane as Pure Solvents and Binary Mixtures. Processes 2023, 11, 1144. https://doi.org/10.3390/pr11041144
Hernández-Monje D, Giraldo L, Serafin J, Moreno-Piraján JC. Enthalpic Determination of the Interaction of Modified Activated Carbons with Benzene and Hexane as Pure Solvents and Binary Mixtures. Processes. 2023; 11(4):1144. https://doi.org/10.3390/pr11041144
Chicago/Turabian StyleHernández-Monje, Diana, Liliana Giraldo, Jarosław Serafin, and Juan Carlos Moreno-Piraján. 2023. "Enthalpic Determination of the Interaction of Modified Activated Carbons with Benzene and Hexane as Pure Solvents and Binary Mixtures" Processes 11, no. 4: 1144. https://doi.org/10.3390/pr11041144
APA StyleHernández-Monje, D., Giraldo, L., Serafin, J., & Moreno-Piraján, J. C. (2023). Enthalpic Determination of the Interaction of Modified Activated Carbons with Benzene and Hexane as Pure Solvents and Binary Mixtures. Processes, 11(4), 1144. https://doi.org/10.3390/pr11041144








