Abstract
An extension of the equilibrium stage model to improve its applicability to reactive distillation is presented. The significant aeration of the liquid holdup on trays leads to the amount of clear liquid present being significantly less than the volume available. Tray hydraulic correlations are incorporated by leveraging the existing inside-out algorithm to rigorously calculate the liquid holdup on distillation trays, turning this parameter into an additional model output and eliminating the need to estimate this parameter beforehand. Application of this extended model shows that the aeration of the liquid holdup cannot be neglected for systems where the reaction kinetics limit the reactive productivity, and leads to column designs where additional reactive trays are needed to provide adequate reactive capacity. The workflow of this model provides a more robust path to obtaining reactive distillation column and tray designs that comply with liquid holdup requirements and tray hydraulic limitations.
1. Introduction
Reactive distillation (RD) is one of the most prominent examples of process intensification (PI) [1], a design philosophy driven by the need and desire to reduce the carbon footprint and increase the safety and profitability of the chemical processing industry. RD integrates the unit operations of reaction and separation into a single unit, potentially simplifying process flowsheets and reducing capital and operational expenditure [2,3]. By continuously removing one of the products from the reacting phase, an equilibrium limited reversible reaction can be partially or fully driven to completion (Le Chatelier’s principle). This in turn reduces or even removes the need to recycle in a process. RD can also be used to solve difficult separation problems, in which the reaction converts a component in the mixture which would otherwise be difficult to separate due to a low volatility difference, e.g., as the result of an azeotrope. Application of RD is not always technically feasible or economically desirable, however, as overlapping temperature and pressure conditions for reaction and separation (related to reaction kinetics, chemical equilibrium and physical equilibrium) are necessary for the integrated process option to outperform the conventional process design with dedicated units and optimized conditions for reaction and separation [4].
From a reaction engineering perspective, the liquid holdup is one of the key design parameters for accommodating reaction kinetics in an RD column. The liquid serves as the reacting phase (homogeneously catalyzed and uncatalyzed systems) or as the source of reactants through contacting with a solid catalyst (heterogeneously catalyzed systems). While a distillation column is strictly speaking a suboptimal choice for providing large liquid holdups (as this runs contrary to design constraints such as limited pressure drop), the combination of in situ reaction and separation has the potential to save costs to such a degree that an RD column becomes a preferable reactive vessel over a dedicated liquid phase reactor in some cases. For a model to be able to identify such opportunities, the modelling of the liquid holdup needs to be taken into account with sufficient accuracy to include its effect on the reaction and separation capacity of the RD column, while also adhering to the limits imposed by the hydraulics of the column internals to maintain separation efficiency. Specialized high liquid holdup tray design was one of the key features of the development of the Eastman methyl acetate process [5], which is the biggest success story of reactive distillation. It is surprising that since then, little has been published on further development of reactive tray design for high liquid holdup applications. This may be due to the esterification and etherification reactions that were investigated being good candidates for heterogeneous catalysis via reactive packings.
The modelling of RD is performed using the equilibrium (EQ) or non-equilibrium (NEQ) stage models. These models are covered extensively by Taylor and Krishna [2]. The NEQ stage model directly obtains the liquid holdup from tray hydraulic relations and is already available in commercial software, for instance in Aspen Plus. This model is more fundamentally sound in the description of mass transfer processes and their effects on chemical reactions, but also brings with it the requirement of providing additional parameters for mass transfer coefficients, interfacial areas and, when the Maxwell-Stefan theory is used, additional diffusion coefficients. While correlations for certain internals are available, the development and/or publishing of these correlations tends to lag behind the availability of new internals. Consequently, the majority of (initial) RD column design studies still rely on the EQ stage model. The addition of tray hydraulic relations to the EQ model has been explored before to more accurately describe the pressure profile throughout the RD column, thereby evaluating the reaction kinetics, chemical equilibrium and vapor–liquid equilibrium at their correct pressure and temperature [6]. The weir height used to evaluate the hydraulics of the trays are also used to calculate the liquid holdup calculation in this work. This can be contrasted to the workflow of commercial flow sheeting software implementations of the EQ model (e.g., Aspen Plus), where the liquid holdup is specified as a constant value per stage to be estimated separately by the user, which may happen independently of considering the tray weir height, diameter and other tray parameters. Liquid holdup (for defining a tray reaction rate) is often estimated by taking the geometric volume available on a tray (bubbling area x weir height) [6,7,8,9]. However, correlations used in conventional distillation predict the liquid fraction of the froth (clear liquid height) to be only 10–40% of the weir height under typical distillation conditions, depending on tray type, design and vapor and liquid traffic on the trays [10]. Basing the liquid holdup estimate on the geometrical volume on the tray has two clear shortcomings: (1) the amount of liquid holdup is overestimated as there is a significant volumetric vapor fraction in the froth and this may lead to column designs that cannot produce the desired conversion capacity when built, (2) the effect of the nonuniform distribution of the liquid holdup that sets in on a uniform set of reactive trays on the performance of the column cannot be assumed to be trivial. Not including this feature in an RD model may at best lead to missing an opportunity for process optimization and at worst result in an underperforming RD column. Adding this consideration by external means to the EQ model will require iterative calculation procedures as the bubbling area will depend on the calculated column profiles (through the vapor velocity) which in turn depend on the liquid holdup, for which you need to know a bubbling area to make an estimate. This has been achieved, for instance, by coupling CFD tools to Aspen Plus [11]. However, such an approach can be expected to be prohibitive for optimization due to the computational load.
In this work, we therefore propose a generalized extension of the EQ model to include the prediction of stage liquid holdups from tray hydraulic correlations for computation of the stage reaction rates. The tray type and dimensions replace the liquid holdup as model input, and the non-uniformity of the liquid holdup is accounted for by resolving the tray hydraulics on each tray using the local compositions, temperatures, and flow rates. The liquid holdup then becomes an additional output of the model. This formulation enables the evaluation of the hydraulic stability criteria of the tray design without the need for an additional iterative loop to match the liquid holdup input of the conventional distillation model to the calculated liquid holdup from the post-processing of converged simulations. In the following sections of this article, we first provide the equations that are added to the conventional (reactive) distillation model to rigorously calculate the liquid holdup, together with the structure of the inside-out algorithm that incorporates these holdup equations. Ideally, the improved predictive capabilities of this model would be shown by application to an experimental data set. A literature survey, however, did not yield any suitable data sets for which a liquid phase reaction was performed in an RD column where trays were used as reactive internals and the dimensions of these trays were also reported. Instead, the results of applying this model to the column design of Luyben [12] are shown to showcase how this method applies to the evaluation of the column and tray design.
2. Model Formulation
The steady state equilibrium (referring to the phase equilibrium only) stage model used in this work follows the same formulation as that of Naphtali and Sandholm [13]. This formulation uses algebraic mass balances, heat balances, vapor–liquid equilibrium relations and implicit molar summation relations to describe the exchange of mass and energy at each stage, collectively known as the MESH equations (Equations (1)–(3)). A schematic overview of this model with the conventional method of including reaction kinetics is shown in Figure 1a.
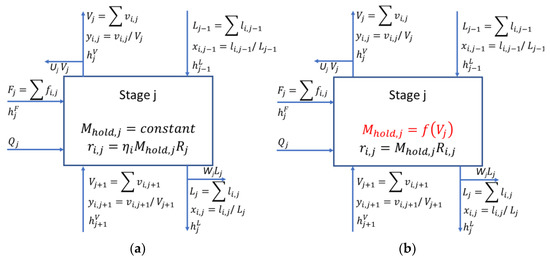
Figure 1.
Schematic overviews of the equilibrium stage model (a) and the proposed extension (b) for reactive distillation. The holdup function in (b) is the simplified form derived from Equation (4).
Here index i refers to the component (1 to NC) and j to the stage number (1 to Nstages, top to bottom), vi,j and li,j are the vapor and liquid component flowrates (mol/s), Vj and Lj are the total vapor and liquid flowrates (mol/s), Fj is the feed flow rate (mol/s), Ki,j is the vapor–liquid partitioning coefficient, hj is the mixture enthalpy (J/mol), is heat duty (J/s), Uj and Wj are the vapor and liquid side-draw factors, is the liquid holdup (m3) and is the overall component reaction rate (mol/m3 s). The heat of the reaction is accounted for implicitly in Equation (3) by considering the component enthalpies from their reference state. Note that this formulation does not use the molar summation (S) equations as the molar fractions are eliminated by using component molar flow rates.
The holdup term appearing in Equation (1) is normally assigned a constant value for each equilibrium stage in commercial flow sheeting software. Here, we introduce a methodology for calculating the holdup rigorously as a function of the tray geometry, temperature, flow rates and compositions (Figure 1b):
where is the set of tray dimensions that is relevant for the used holdup correlation e.g., weir height, weir length, bubbling area etc., is the temperature (K), is the pressure (Pa), x and y are the liquid and vapor mole fractions.
The exact form of Equation (4) depends on the type of internal considered. Using sieve trays as an example, the tray liquid holdup can be determined as the product of clear liquid height and bubbling area:
where is the tray bubbling area (m2) and is the clear liquid height (m). The clear liquid height takes into account the aeration of the froth held by the weir and the liquid height flowing over the weir using the Francis weir equation;
where is the weir height (m), is a Francis weir coefficient, is the liquid load over the weir (m3/s) and is the weir length (m). The effective froth density accounts for the aeration of the froth. Several empirical models are available for sieve trays, notably Colwell’s [14] and Agrawal, Bennett and Cook’s (ABC) [15] methods, both containing an inverse relation between and the superficial gas velocity . The ABC method is given as:
where and are correlation specific coefficients. is the vapor velocity through the bubbling area (m/s) and , are vapor and liquid densities, respectively, (kg/m3). Equations (5)–(8) indicate the dependencies of Equation (4) on the tray geometry (through Ab, hw and Lw), stage temperature (through and , depending on the thermodynamic models used), liquid flow rate (through ) and the vapor flow rate (through ). Incorporating such a liquid holdup model directly may cause the mass balance Equation (1) to become excessively non-linear, making convergence difficult. To alleviate this, the inside-out method [16] is applied to use a simplified form of Equation (4) for each equilibrium stage when solving the MESH equations. The froth density , via , is the term that varies most strongly between iterations of the solution of the MESH equations. Therefore, the functional form of Equation (9) is chosen to serve as the ‘simple’ correlation to be used when solving the MESH equations and assuming a fixed tray geometry with and finetuned for each iteration.
This reduction in parameters is supported by a sensitivity analysis shown in Appendix A.
Parameter aj will be such that is of the same order of magnitude as the weir height. Parameter bj contains the sensitivity of the clear liquid height to the vapor flow rate and at the same time absorbs the smaller effects of temperature, L/V and the fluid densities between iterations. The sign of bj will be positive for trays (inverse relationship of clear liquid height and vapor flow rate) and negative for packings (direct relationship between clear liquid height and vapor flow rate) [17].
The use of Equation (9) as the liquid holdup relation makes the proposed model flexible in accepting user-specified correlations for clear liquid height, without having to provide specific analytical derivatives for a solution by the Newton–Raphson method. Stage specific values for the constants are obtained by perturbation of in the outer loop of the inside-out method. A schematic overview of this extended equilibrium stage model is given in Figure 2. The simplified models for the K-values and excess enthalpy are also shown and are part of the original implementations of the inside-out method. The solution of the MESH equations in the inner loop is achieved with the Newton–Raphson method once the sum of squares of the function vector becomes less than the tolerance (Nstages × 10−10):
where is the sum of the squared residuals of the entire vector of equations for the column. Convergence of the outer loop is achieved by direct substitution of the simple correlation parameters until the relative change in the holdup and K-values between iterations falls below the tolerance (1 × 10−8):
where is the sum of the squared residuals of the stage holdups and vapor–liquid distribution coefficients. The residuals are calculated over the current (k+1) values, obtained by applying the rigorous correlations with the latest converged T, L, V, x, y profiles from the converged inner loop, and the previous (k) values, obtained from the simple correlations with the set of constants that went into the preceding inner loop.
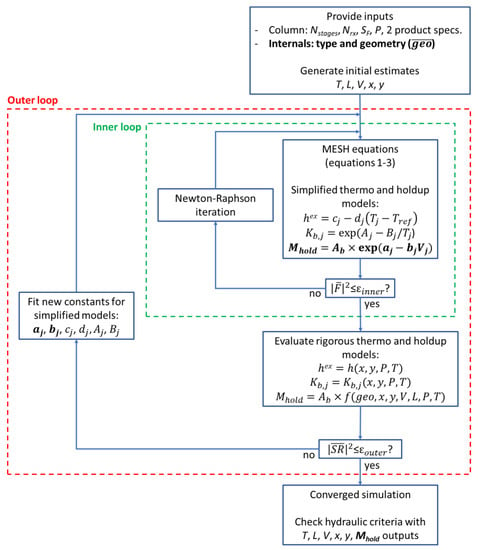
Figure 2.
Overview of the inside-out algorithm with the proposed liquid holdup model.
The described equilibrium stage model, together with the relevant physical property methods, was implemented in MATLAB as a custom standalone RD column model. The MESH equations of the inner loop are solved with a custom implementation of the Newton–Raphson method for nonlinear equations.
3. Results and Discussion
The proposed model inherently deals with some of the assumptions that are made regarding the liquid holdup on trays and provides a straightforward path to evaluating realistic tray designs as well. Luyben formulated a column design for the generic quaternary liquid phase reaction and investigated the effects of various parameters, including the liquid holdup [12]. This case will be used in this work to show the differences in several design aspects of the column and trays when using both models. This reference case consists of an RD column with five stripping, five rectifying and nine reactive stages. The liquid phase reaction kinetics on a reactive tray are described by Equation (12):
where is the tray liquid holdup in mol, henceforth obtained by multiplying by the molar liquid density.
The physiochemical and column design parameters for this case are given in Table 1.

Table 1.
Physiochemical and design parameters of the reference case, parameter values adapted from Luyben [12]. *Added in this work for the tray hydraulic correlations.
Luyben arrives at a value of 1000 mol for the tray liquid holdup, based on a column diameter of 0.8 m and an assumed (clear) liquid height of 0.12 m, together with a liquid density of 800 kg/m3 and a molecular weight of 50 g/mol for each of the compounds:
It is unclear how the downcomer area is considered in this case, however, the clear liquid in the downcomer backup (the height of the froth in the downcomer) will be at least twice the tray clear liquid height and the downcomer area is typically around 10% of the tray area (per downcomer, so 20% in total). Taking the tray holdup to be the product of the column cross sectional area and clear liquid height is then a reasonable approximation in this context. The following calculations have been performed with this same assumption, as the feature of interest here is the inclusion of the froth density in the liquid holdup model and not so much the extent to which the downcomer backup is modelled.
Assuming the clear liquid height to be equal to the weir height of the tray significantly overestimates the amount of unaerated liquid present on trays. The difference between this assumption and the clear liquid height calculated by pressure-drop correlations, taking into account the aeration of the liquid, for sieve trays is shown in Figure 3a. The reflux ratio required to maintain the 95% product purity for the reference case increases from 2.65 to 2.78 based on our calculations, as seen in Figure 3b. This increase is necessary to compensate the lower clear liquid height predicted by the rigorous liquid holdup model. The intrinsic kinetics (equivalent to roughly 0.9 h residence time in a CSTR to approach 95% of chemical equilibrium) are sufficiently fast to enable the designed column to meet the required productivity with a limited reflux ration increase, despite the lower than anticipated liquid holdup.
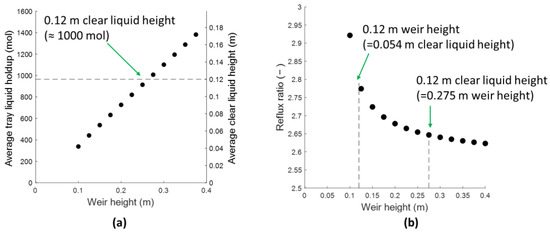
Figure 3.
Average liquid holdup and corresponding average clear liquid height (a) and reflux ratio (b) as function of the weir height to obtain 95% top and bottom purities of C and D using sieve trays for Luyben’s column. AG/An = 0.10, each downcomer area = 10% of column cross section.
It is anticipated that more significant differences in column design will arise when the kinetics become slower. To test this, the number of reactive stages at the minimized reflux ratio was determined with and without considering the froth density (keeping all other parameters at their original values). This was evaluated at slower kinetics relative to the reference case, shown in Figure 4. Unlike for conventional distillation (where the minimal efflux ratio occurs at an infinite number of distillation stages), for RD the reflux ratio reaches a minimum at a finite number of reactive stages, since the total consumption of either reactant before reaching the feed stage of the other reactant is detrimental to the conversion process. This has also been shown for this column by Luyben [12]. These results were obtained by fixing the number of rectifying and stripping stages (five each and also a total condenser and reboiler) and varying the number of reactive stages until the minimum in the reflux ratio to obtain 95% product purity was obtained. It is seen in Figure 4 that as the relative reaction rate decreases, the difference in the number of reactive stages at this minimized reflux becomes larger. For a forward reaction rate (kf) of 20% of that for the base case (kf,0), at an unchanged equilibrium constant, more than twice the number of reactive stages would be required for the liquid holdup to be considered properly (according to our methodology), indicating the importance of accounting for the hydraulics, and resulting gas and liquid holdup in that case. The total (reactive) liquid holdup converges towards the same value for both models at kf/kf,0 = 0.2, indicating that the column design here is strongly sensitive to the amount of liquid holdup available, whereas the ratio of the two liquid holdups is a factor 2 for the base case kinetics, shown in Figure 4c. This indicates that the productivity was indeed not limited by the kinetics at the reference case values but is becoming limited towards kf/kf,0 = 0.2. It can be concluded that it is important to consider the froth density of the liquid holdup, especially at slower kinetics.
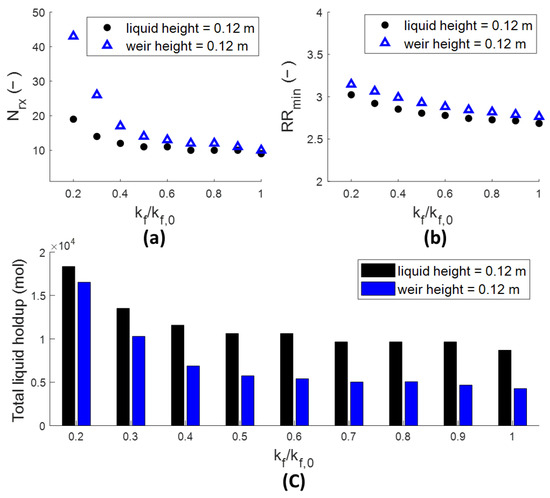
Figure 4.
Number of reactive stages (a), minimized reflux ratio (b) and summed total liquid holdup over the reactive section (c) for both models when designing the RD column at minimized reflux. hw = 0.12 m. Kinetics correspond to 0.9–4.6 h of residence time in a CSTR to approach 95% of chemical equilibrium. Feed points are kept at the first and last reactive stages.
Determining the optimal number of (reactive) stages and reflux would require the use of a cost function and an iterative optimization loop built around the presented model, which is outside the scope of this discussion. However, the differences shown for the number of reactive stages at the minimized reflux ratio give a first indication of how optimized column designs diverge between the two models towards slower kinetics.
3.1. Feasibility of Tray Designs
Another aspect of the tray design is considering whether stable operation can be achieved from a hydraulics perspective. For this model system, achieving the assumed liquid height of 0.12 m (on average throughout the reactive section) would require a weir height of 0.275 m on sieve trays (see Figure 3b). This weir height is higher than normally encountered in distillation and will require a tray spacing that is also higher than the typically used 400–600 mm [10] to accommodate the tray hydraulics. The non-reactive trays have no liquid holdup requirements and can easily be designed at a weir height that satisfy the entrainment and weeping criteria for stable operation. Figure 5 shows the criteria for stable tray operation i.e., the downcomer backup, fractional entrainment and weeping for this column (calculated using available literature correlations [10,18,19], see Appendix B). When the clear liquid height is increased on a given tray, both the downcomers connecting it to the previous and the following trays need to be increased in height to accommodate the increase in downcomer backup, as dictated by the pressure balance around the downcomers (see Appendix B). Hence, an increase in clear liquid height on a tray is paid for twice in the height of the column shell. The fractional weeping will always exceed the limit of 0.1 [20] for this column when the weir height is higher than 0.1 m, as seen in Figure 5b. The weeping can be counteracted somewhat by increasing the vapor pressure drop through altering the tray design i.e., lowering the tray open area, reducing the hole size, or increasing the tray thickness. Alternatively, a different tray type i.e., valve or bubble-cap can be considered. Nonetheless, the pressure-drop contributions associated with these tray designs will still lead to the downcomer backup to exceed the typical 400–600 mm tray spacing known from conventional distillation when trying to provide 0.12 m of clear liquid height. This evaluation of the tray hydraulics shows that assigning an estimated value to the liquid holdup opens up the possibility of arriving at a tray design that is not possible from a hydraulics perspective when applying typical tray design values from conventional distillation. From a workflow point of view, it would make sense then that the RD model uses the dimensions of the internals directly as an input to calculate a corresponding liquid holdup rather than requiring an estimated value for the holdup. This avoids the need for an external iteration loop to match a tray design to an initial holdup input.
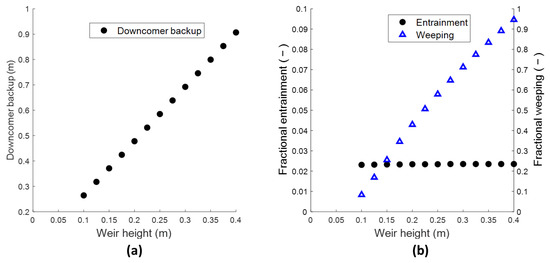
Figure 5.
Limiting hydraulic phenomena using sieve trays for Luyben’s column at varying weir heights; downcomer backup (a), fractional entrainment and weeping (b). Values reported are the highest encountered in the reactive section. TS = 600 mm, each downcomer area = 10% of column cross section. AG/An = 0.10 *, dt = 3.4 mm *, dh = 13 mm *. * Based on normal values for conventional distillation trays [21]. The desired product specifications cannot be achieved in the RD column using weir heights below 0.095 m without adding additional reactive trays.
3.2. Algorithm Features
The choice to adopt the inside-out algorithm was made to include tray hydraulic correlations in the solution of the MESH equations. Here, we show how the liquid holdup calculations influence the solution procedure with calculation examples and briefly discuss the reasoning for this choice compared to existing implementations using an equation-oriented approach [6]. The MESH equations for steady-state distillation modelling form a set of nonlinear algebraic equations that are typically solved by Newton–Raphson methods. In the inside-out algorithm, this is applicable for the inner loop, where the outer loop convergence can be achieved with direct substitution of the new values, although Newton–Raphson methods are also applicable if the system has highly nonlinear properties. Table 2 summarizes the convergence characteristics of several test cases to show how the solution procedure is affected by the incremental addition of nonlinear properties. The inner loop iterations are the solution of the MESH equations, the outer loop iterations (given by the number of values for the inner loop iterations) resolve the rigorous physical property and holdup models.

Table 2.
Convergence characteristics of several test cases. All cases are for Luyben’s column with real component properties for cases 3 and 4. T-dependent properties are taken as ideal component properties of the methyl acetate system (A = methanol, B = acetic acid, C = methyl acetate, D = water), Antoine and enthalpy (Cp, ) parameters and correlation coefficients were retrieved from the Aspen Plus databank. The number of values given for the iterations represents the number of outer loop iterations.
Comparing these four cases, it can be concluded that the majority of the nonlinearity comes from including T-dependent component properties, which describe the vapor–liquid equilibrium and enthalpy. The rigorous holdup calculations add a few outer loop iterations; however, the converged results from previous inner loops are already close to the final result, as evidenced by the low number of subsequent inner loop iterations for case 2.
Newton–Raphson methods require the derivatives of the MESH equations to the temperature, flow rates and compositions. These derivatives can be obtained either numerically or analytically, with analytical derivatives being preferred for better convergence and computational speed (see Appendix C), especially in the context of optimization where many designs of the same RD column are evaluated. Analytical derivatives for tray holdup correlations are cumbersome to obtain; we give an example of such a derivative in Appendix C. The established inside-out algorithm in this work only samples the rigorous holdup correlations in the outside loop and does not require the derivatives of these correlations. The simplified form of the holdup correlation that is used while solving the MESH equations has a straightforward derivative that maintains its shape regardless of the holdup correlation used (provided the main dependence is the vapor velocity). In light of these considerations, we make the following recommendations; if the target application is the design/optimization of a single case, then the equation-oriented approach is the most straightforward to implement with a manageable penalty of calculation time. For a general simulation tool that handles multiple holdup correlations and is to be used for design/optimization of multiple cases, the presented inside-out structure has a higher upfront development time cost but lower calculation times afterwards. As it stands, this model accounts for the liquid holdup on the bubbling area of the reactive trays, but does not yet account for the liquid holdup in the downcomer. The model therefore gives a conservative estimate of the reactive capacity on the trays. A further improvement could be made by modelling the downcomer as a plug flow reactor using a n-CSTR in series approach. This would involve adding n-repetitions of the mass and energy balances (Equations (1) and (3), ignoring the vapor terms) in between the sets of tray equations. Such an approach would require the availability of a correlation that gives the (de)aeration of the froth flowing through the downcomer. This extension has only been tested for the equilibrium stage model. The presented algorithm should be applicable to the non-equilibrium stage model, however, the convergence behavior for such a combination needs to be investigated.
4. Conclusions
In this work, we present an extension of the equilibrium stage model to include rigorous calculation of the tray liquid holdup. The inside-out method, which was originally formulated for rapid and stable thermodynamic calculations in distillation models, was utilized to incorporate holdup calculations in a generalized manner. This formulation replaces the stage independent holdup/residence time as a necessary model input with the tray geometry and turns the holdup into an additional model output. This extension leads to a more realistic and more accurate model description of the liquid and gas holdups and consequently to a more reliable description of the reactant conversion and product purities as a function of the number of (reactive) stages and reflux ratio. The clear liquid height that is calculated with this newly developed model is significantly lower than the clear liquid height under the assumption that it equals the weir height. Using the realistic clear liquid height assures proper representation of the RD column performance, avoiding overprediction, such as too high conversion or product quality, which could be predicted under the assumption that the clear liquid height is equal to the weir height. The difference between the design methods is most significant when the reaction kinetics are slow and limit the reactive productivity. In those cases, it can be the difference between a performing or underperforming design. For cases where the reaction kinetics are less limiting, a change in reflux ratio may be sufficient to remediate the lower-than-expected conversion level, provided that this higher reflux ratio can be met within constraints of the original column design. Additionally, in contrast to the methodology, assuming that the clear liquid height is equal to the weir height, incorporation of the rigorous tray holdup calculation directly shows whether the obtained liquid holdup (as required for the intended conversion and product purity) leads to a feasible tray design and fulfils weeping and flooding criteria. The developed model thus provides a more robust workflow towards obtaining a realistic RD column design, where the actual, stage dependent, liquid holdup is taken into account for the tray reaction rate and tray hydraulics.
Author Contributions
Writing—original draft preparation, L.N.; writing—review and editing, L.v.d.H., S.O., A.t.K., G.B. and S.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded through project ReaDi for which funding was provided by Nouryon and Nobian Industrial Chemicals B.V. (formerly AkzoNobel Chemicals International B.V.)
Data Availability Statement
The data presented in this study are available on request from the first author.
Conflicts of Interest
The authors declare no conflict of interest.
List of Symbols
| Ab | Bubbling area (m2) |
| Ah | Total hole area (ft2) |
| aj | Clear liquid height coefficient |
| Aj | Kb model coefficient |
| AG/An | Tray open area as fraction of net area (−) |
| bj | Clear liquid height coefficient |
| Bj | Kb model coefficient |
| C4, C5 | Froth density model coefficients (−) |
| Cl | Francis weir coefficient (−) |
| Csbf | Capacity factor for entrainment flooding |
| cj | Excess enthalpy coefficient (J/mol) |
| Dc | Column diameter (m) |
| dh | Hole diameter (mm) |
| dj | Excess enthalpy coefficient (J/mol K) |
| dt | Tray thickness (mm) |
| E | Equilibrium relation for VLE (−) |
| EA,f | Forward activation energy (kJ/mol) |
| EA,b | Backward activation energy (kJ/mol) |
| Function norm of the equation vector (−) | |
| F | Feed rate (mol/s) |
| FLV | Flow factor (−) |
| Frh | Hole Froude number (−) |
| fflood | Fraction of flooding velocity (−) |
| f | Partial molar feed rate (mol/s) |
| ∆rH | Enthalpy of reaction (kJ/mol) |
| ∆vapH | Enthalpy of vaporization (kJ/mol) |
| h | Enthalpy (kJ/mol) |
| hex | Excess enthalpy (kJ/mol) |
| hc | Clear liquid height (m) |
| hda | Downcomer apron pressure drop (m) |
| hdc | Height of downcomer (m) |
| hdt | Dry tray pressure drop (m) |
| hhg | Clear liquid height from hydraulic gradient (m) |
| how | Clear liquid height over weir (m) |
| ht | Tray pressure drop (m) |
| hw | Weir height (m) |
| Ki,j | Vapor–liquid distribution coefficient for component i at stage j (−) |
| KEQ | Chemical equilibrium constant (−) |
| K | Vapor–liquid distribution coefficient (−) |
| KS | Flow parameter for Equation (7) |
| k0,b | Backward pre-exponential factor (mol/molHoldup s) |
| k0,f | Forward pre-exponential factor (mol/molHoldup s) |
| kb | Backward reaction rate constant (mol/molHoldup s) |
| kf | Forward reaction rate constant (mol/molHoldup s) |
| L | Total liquid flow rate (mol/s) |
| LW | Weir length (m) |
| l | Component liquid flow rate (mol/s) |
| M | Mass balance (mol/s) |
| Mhold | Liquid holdup on volumetric basis (m3) |
| Liquid holdup on molar basis (mol) | |
| Mw | Molar weight (g/mol) |
| Nc | Number of components (−) |
| Nstages | Number of stages (−) |
| Nrx | Number of reactive stages (−) |
| P | Pressure (Pa) |
| Q | Heat duty (J/s) |
| QL | Volumetric liquid flow (m3/s) |
| R | Gas constant (J/mol K) |
| Ri,j | Overall reaction rate of component i at stage j (mol/m3 s) |
| Sum of squares of the correlation residuals (−) | |
| T | Temperature (K) |
| TS | Tray spacing (mm) |
| Uj | Vapor side-draw factor (−) |
| Ua | Superficial vapor velocity (m/s) |
| Unf | Flooding velocity of vapor (m/s) |
| uh | Vapor velocity through hole (m/s) |
| V | Total vapor flow rate (mol/s) |
| v | Component vapor flow rate (mol/s) |
| W | Weep rate (m3/s) |
| Wj | Liquid side-draw factor (−) |
| xi | Liquid mole fraction of component i (−) |
| yi | Vapor mole fraction of component i (−) |
Greek Letters
| α | Relative volatility (-) |
| σ | Surface tension (mN/m) |
| µ | Viscosity (cP) |
| ρ | Density (kg/m3) |
| ϕe | Effective froth density (-) |
Abbreviations
| ABC | Agrawal, Bennett and Cook |
| CSTR | Continuously stirred tank reactor |
| MESH | Mass, equilibrium, summation and heat balances describing a separation stage |
| RD | Reactive distillation |
Superscripts and Subscripts
| * | Intrinsic rate constant |
| i | Component index |
| j | Stage index |
| k | Iteration index |
| L | Liquid phase |
| m | Molar basis |
| ref | At reference point |
| tot | Total of all components |
| V | Vapor phase |
Appendix A. Holdup Model Sensitivity to Physical Parameters
The sensitivity of the rigorous holdup model to realistic ranges of the relevant parameters is shown in Figure A1. Out of the four parameters; T, , V and V/L, the vapor rate V is concluded to be the most influential parameter. The temperature affects the vapor density through the ideal gas law. A higher temperature results in a lower vapor density. The fluid density difference term in Equation (8) decreases in magnitude by a fractional power, whereas the vapor velocity increases linearly with vapor density. The net effect is that the froth density, and thereby the clear liquid height, decreases with increasing T. A higher liquid density ρL positively affects the clear liquid height, as the density difference term in Equation (8) decreases. The flow ratio L/V, assuming a fixed V, affects the height over weir component of the Francis weir equation. A larger liquid flow rate leads to an increased liquid height over the weir, which results in the higher clear liquid height. The vapor rate V has an inverse relationship with the clear liquid height. The froth density decreases exponentially with vapor velocity as given in Equations (7) and (8).
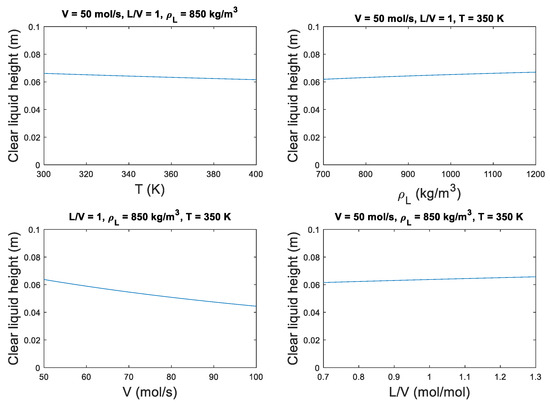
Figure A1.
Effect of various parameters on clear liquid height on a sieve tray. Ab = 0.8 m, hw = 0.1 m, P = 1 bar, Mw = 50 g/mol.
Appendix B. Tray Hydraulics
The region of stable tray operation is encapsulated by the detrimental hydraulic phenomena that all lead to a significant decrease in tray efficiency; excessive entrainment (spray entrainment flooding), excessive weeping and downcomer flooding. Froth entrainment flooding is uncommon and disregarded in this work [10]. Additionally, predicting the liquid residence time and pressure drop (for downcomer backup) of the frothy liquid pool held by the weir involves modelling the effective froth density on the trays.
Appendix B.1. Entrainment
It is rather common practice, at least for conventional distillation, to choose the column diameter such that entrainment by flooding is prevented within acceptable limits while keeping the diameter as small as possible for economic reasons. Typically, this approaches 80–90% of flooding velocity, Unf, of the vapor phase [10]. The most used correlation for predicting flooding velocity is that of Fair [18], applicable to sieve, valve and bubble-cap trays:
The capacity factor, Csbf, is a measure of the tray’s ability to deal with vapor and liquid flow, which is dependent on the available tray spacing and the flow parameter:
Application of this correlation, however, dictates that the weir height does not exceed 15% of the tray spacing for estimating the tray capacity parameter [10]. To account for weir heights exceeding this limit in this work, we introduce a correction to the tray spacing where the overspill of liquid height is subtracted from the tray spacing to arrive at an ‘effective tray spacing’ to use in Equation (A3).
Additionally, entrainment rates are advised to be kept below 0.1 mol of liquid/mol of vapor to limit loss of efficiency due to back mixing of liquid enriched in less volatile components [21]. The various tray types have different rates of entrainment due to differences in how the vapor passes through the dispersing unit but are nonetheless always dependent on the flow factor for the tray.
Appendix B.2. Weeping
Weeping as a phenomenon can be encountered on sieve and valve trays, while it is mostly avoided on bubble-cap trays. The most often used correlations to predict weeping are based around the Froude number of the tray disperser unit (holes for sieve trays, valve slots for valve trays). In this work, the Lockett and Banik correlation is used [19]:
where the hole Froude number is defined as:
Tray hole size and open area are designed such that weeping is kept below 10% of the liquid flow rates [20].
Appendix B.3. Downcomer Flooding
Downcomer backup flooding as a phenomenon can be encountered on all tray types and is a consequence of the frothy liquid backup due to the sum of the pressure drops across the tray exceeding the available tray spacing, causing the backup in the downcomer to reach the tray above, causing flooding of this tray. This is tied to the hole velocity of the vapor phase (dry pressure drop), depth of the frothy liquid pool on the tray and the velocity of the liquid under the downcomer clearance, where each contributing pressure drop component is counted in the equivalent height of the liquid [10]:
The tray pressure drop ht belongs to the tray feeding liquid into the downcomer and is the sum of the dry tray pressure drop, pressure drop from the liquid pool held by the weir and pressure drops from the liquid height over the weir and hydraulic gradient:
An overview of the liquid height components is also given in Figure A1. For a set of reactive trays this means that designing for increased weir height to increase liquid holdup leads to a double increase in downcomer height per tray (from the clear liquid height on the tray belonging to the downcomer and the liquid height on the tray above).
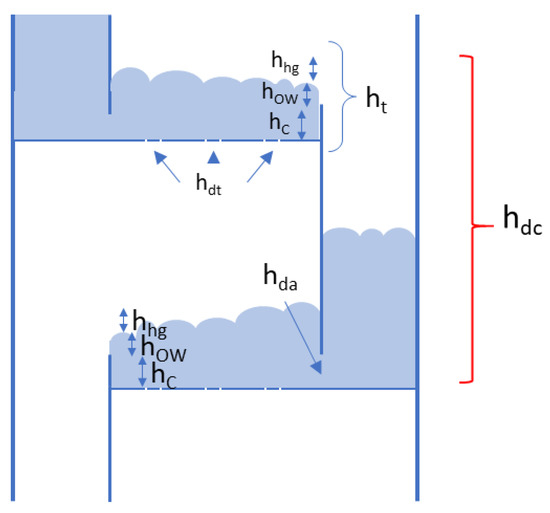
Figure A2.
Overview of the pressure drop/liquid height components that determine the downcomer backup.
Appendix B.4. Effective Froth Density
The frothy liquid pool held on the trays is significantly aerated by the passing vapor stream. Since liquid residence time, or holdup, is a key parameter to design for adequate reaction performance, obtaining a good estimate of the effective froth density on the trays is important in RD column design. Several empirical froth density models are available in the open literature, notably Colwell’s method and the correlation by Bennett, Agrawal and Cook [14,15]. Both models incorporate an inverse relation between the gas velocity and the froth density. In this work, the correlation by Bennett, Agrawal and Cook is used.
The froth density is given by:
where
The clear liquid height is the sum of the liquid held by the weir and the liquid crest over the weir:
where

Table A1.
Froth density and clear liquid height model coefficients [15].
Table A1.
Froth density and clear liquid height model coefficients [15].
| Coefficient | Sieve tray |
|---|---|
| C1 | 0.03272 |
| C2 | 0.02865 |
| C3 | 137.8 |
| C4 | 12.55 |
| C5 | 0.91 |
Little is published about operating with high weir heights/deep liquid pools on trays. As such, this presents a significant uncertainty when designing high holdup trays for homogeneous reactive distillation. Furthermore, the underlying data sets for entrainment, weeping and flooding only contain experimentally measured points with trays up to 0.1 m weirs and mostly air/water as fluid mixture. A single study using up to 0.9 m weirs [22] reports data that suggests that the known weeping prediction methods overestimate weeping at weir heights outside of the typical ranges. By necessity, extrapolating the tray design rules to high weir heights needs to be approached with due diligence.
Appendix C. Algorithm Considerations
Appendix C.1. Comparison of Numerical and Analytical Derivatives
The MESH equations for steady state distillation modelling form a set of nonlinear algebraic equations that are solved by Newton–Raphson methods. Such a numerical procedure relies on the Jacobian of the equation vector with respect to the temperatures and molar flow rates, which can be obtained either numerically (by perturbation) or analytically (by prior derivation). With a central difference scheme, a single numerical determination of the Jacobian would require approximately 2 × Nstages × (2 × NC + 1) vector function evaluations, compared to a single matrix function evaluation for analytical derivatives. In either case, subsequent iterations may make use of Broyden updates, circumventing the need for additional function evaluations, at the cost of some convergence properties.
Table A2 shows the number of inner loop iterations (i.e., to solve the MESH equations) and the corresponding number of evaluations of the set of MESH equations when using numerical and analytical derivatives. When moving towards real systems where enthalpies and relative volatilities are temperature dependent, several outer loop iterations are also needed. Additional system features such as nonidealities will further increase the number of inner and outer loop iterations.

Table A2.
Comparison of the number of function evaluations in the solution of the MESH equations for the Luyben case with Nstages = 21. T-dependent properties are taken for the methyl acetate system (methanol+acetic acid ↔ methyl acetate+water) using the ideal gas law. Number of values given for the iterations corresponds to the number of outer loop iterations.
Table A2.
Comparison of the number of function evaluations in the solution of the MESH equations for the Luyben case with Nstages = 21. T-dependent properties are taken for the methyl acetate system (methanol+acetic acid ↔ methyl acetate+water) using the ideal gas law. Number of values given for the iterations corresponds to the number of outer loop iterations.
| Constant Relative Volatility, Enthalpy | T Dependent Relative Volatility, Enthalpy | |
|---|---|---|
| Numerical derivatives | ||
| Inner loop iterations | 12 | 15, 13, 7, 5, 3 |
| Mesh evaluations | 4492 | 14,223 |
| Time to solve (s) | 6.5 | 23.4 |
| Analytical derivatives | ||
| Iterations | 12 | 32, 15, 8, 8, 4 |
| Mesh evaluations | 17 | 79 |
| Time to solve (s) | 0.3 | 0.9 |
Appendix C.2. Example of Analytical Derivative for Tray Liquid Holdup Correlation
The liquid holdup correlation for sieve trays (Equation (6)) is dependent on the molar vapor flow rate through the vapor velocity Ua and the vapor density ρV (given by Equation (A15). The analytical partial derivative of Equation (6) with respect to the molar vapor flow rate of component 1 is given by Equation (A14).
where the vapor density is defined as:
References
- Harmsen, G.J. Reactive Distillation: The Front-Runner of Industrial Process Intensification: A Full Review of Commercial Applications, Research, Scale-up, Design and Operation. Chem. Eng. Process. Process Intensif. 2007, 46, 774–780. [Google Scholar] [CrossRef]
- Taylor, R.; Krishna, R. Modelling Reactive Distillation. Chem. Eng. Sci. 2000, 55, 5183–5229. [Google Scholar] [CrossRef]
- Malone, M.F.; Doherty, M.F. Reactive Distillation. Ind. Eng. Chem. Res. 2000, 39, 3953–3957. [Google Scholar] [CrossRef]
- Shah, M.; Kiss, A.; Zondervan, E.; Haan, A. A Systematic Framework for the Feasibility and Technical Evaluation of Reactive Distillation Processes. Chem. Eng. Process. 2012, 60, 55–64. [Google Scholar] [CrossRef]
- Agreda, V.H.; Partin, L.R.; Heise, W.H. High-Purity Methyl Acetate via Reactive Distillation. Chem. Eng. Prog. 1990, 86, 40–46. [Google Scholar]
- Li, K.; Luo, Y.; Yuan, X. Equation-Oriented Optimization of Reaction Distillation Column Considering Tray Hydraulics. Sep. Purif. Technol. 2022, 295, 121229. [Google Scholar] [CrossRef]
- Jimoh, M.; Garcia, H.A.; Wozny, G.; Bock, H.; Gutsche, B. Transesterification of Methyl Myristate in a Continuous Reactive Distillation Column: Simulation and Experiment. Lipid Fett. 1999, 101, 50–56. [Google Scholar] [CrossRef]
- Singh, D.; Gupta, R.K.; Kumar, V. Simulation of a Plant Scale Reactive Distillation Column for Esterification of Acetic Acid. Comput. Chem. Eng. 2015, 73, 70–81. [Google Scholar] [CrossRef]
- Ciric, A.R.; Gu, D. Synthesis of Nonequilibrium Reactive Distillation Processes by MINLP Optimization. AIChE J. 1994, 40, 1479–1487. [Google Scholar] [CrossRef]
- Kister, H.Z.; Matthias, P.M.; Steinmeyer, D.E.; Penney, W.R.; Monical, V.S.; Fair, J.R. Equipment for Distillation, gas absorption, phase dispersion, and phase separation. In Perry’s Chemical Engineers’ Handbook; McGraw-Hill Education: New York, NY, USA, 2019. [Google Scholar]
- May-Vázquez, M.M.; Rodríguez-Ángeles, M.A.; Gómez-Castro, F.I.; Uribe-Ramírez, A.R. Hydrodynamic Feasibility of the Production of Biodiesel Fuel in a High-Pressure Reactive Distillation Column. Chem. Eng. Process Process Intensif. 2017, 112, 31–37. [Google Scholar] [CrossRef]
- Luyben, W.L.; Yu, C.C. Reactive Distillation Design and Control; Wiley: Hoboken, NJ, USA, 2009; ISBN 9780470377796. [Google Scholar]
- Naphtali, L.M.; Sandholm, D.P. Multicomponent Separation Calculations by Linearization. AIChE J. 1971, 17, 148–153. [Google Scholar] [CrossRef]
- Colwell, C.J. Clear Liquid Height and Froth Density on Sieve Trays. Ind. Eng. Chem. Process Des. Dev. 1981, 20, 298–307. [Google Scholar] [CrossRef]
- Bennett, D.L.; Agrawal, R.; Cook, P.J. New Pressure Drop Correlation for Sieve Tray Distillation Columns. AIChE J. 1983, 29, 434–442. [Google Scholar] [CrossRef]
- Boston, J.F. Inside-Out algorithms for multicomponent separation process calculations. In Computer Applications to Chemical Engineering; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 1980; Volume 124, pp. 135–151. ISBN 9780841205499. [Google Scholar]
- Taiwo, E.A.; Fasesan, S.O. Model for Dynamic Liquid Hold-up in a Packed Distillation Column. Ind. Eng. Chem. Res. 2004, 43, 197–202. [Google Scholar] [CrossRef]
- Fair, J.R. How to Predict Sieve Tray Entrainment and Flooding. Petro. Chem. Eng. 1961, 33, 45–52. [Google Scholar]
- Lockett, M.J.; Banik, S. Weeping from Sieve Trays. Ind. Eng. Chem. Process. Des. Dev. 1986, 25, 561–569. [Google Scholar] [CrossRef]
- Seader, J.D.; Henley, E.J.; Roper, D.K. Separation Process Principles, 3rd ed.; John Wiley Incorporated: Hoboken, NJ, USA, 2010; ISBN 9781118139622. [Google Scholar]
- Ludwig, E.E. (Ed.) Part 3 Mechanical Designs for Tray Performance. In Applied Process Design for Chemical & Petrochemical Plants; Gulf Professional Publishing: Oxford, UK, 1997; Volume 2, pp. 122–229. ISBN 1874-8635. [Google Scholar]
- Haug, H.F. Stability of Sieve Trays with High Overflow Weirs. Chem. Eng. Sci. 1976, 31, 295–307. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).