Storage Stability of Spray- and Freeze-Dried Chitosan-Based Pickering Emulsions Containing Roasted Coffee Oil: Color Evaluation, Lipid Oxidation, and Volatile Compounds
Abstract
1. Introduction
2. Materials and Methods
2.1. Microencapsulation of Roasted Coffee Oil
2.1.1. Synthesis of Chitosan Nanoparticles
2.1.2. Preparation of Pickering Emulsions
2.1.3. Microencapsulation by Spray- and Freeze-Drying
2.2. Storage Conditions
2.3. Water Sorption Isotherms
2.4. Color Parameters
2.5. Peroxide Value
2.6. Conjugated Dienes
2.7. Volatile Compounds
2.8. Statistical Analysis
3. Results and Discussion
3.1. Water Sorption Isotherms
3.2. Color Parameters
3.3. Peroxide Value
3.4. Conjugated Dienes
3.5. Volatile Compounds
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Reddy, M.N.P.; Ishwarya, S.P.; Anandharamakrishnan, C. Nanoencapsulation of roasted coffee bean oil in whey protein wall system through nanospray drying. J. Food Process. Preserv. 2019, 43, e13893. [Google Scholar] [CrossRef]
- Ogrodowska, D.; Tańska, M.; Brandt, W. The Influence of Drying Process Conditions on the Physical Properties, Bioactive Compounds and Stability of Encapsulated Pumpkin Seed Oil. Food Bioprocess Technol. 2017, 10, 1265–1280. [Google Scholar] [CrossRef]
- Oliveira, A.L.; Cruz, P.M.; Eberlin, M.N.; Cabral, F.A. Brazilian roasted coffee oil obtained by mechanical expelling: Compositional analysis by GC-MS. Food Sci. Technol. 2005, 25, 677–682. [Google Scholar] [CrossRef]
- Frascareli, E.C.; Silva, V.M.; Tonon, R.V.; Hubinger, M.D. Effect of process conditions on the microencapsulation of coffee oil by spray drying. Food Bioprod. Process. 2012, 90, 413–424. [Google Scholar] [CrossRef]
- Atarian, M.; Rajaei, A.; Tabatabaei, M.; Mohsenifar, A.; Bodaghi, H. Formulation of Pickering sunflower oil-in-water emulsion stabilized by chitosan-stearic acid nanogel and studying its oxidative stability. Carbohyd. Polym. 2019, 210, 47–55. [Google Scholar] [CrossRef]
- Yang, Y.; Fang, Z.; Chen, X.; Zhang, W.; Xie, Y.; Chen, Y.; Liu, Z.; Yuan, W. An Overview of Pickering Emulsions: Solid-Particle Materials, Classification, Morphology, and Applications. Front. Pharmacol. 2017, 8, 287. [Google Scholar] [CrossRef]
- Garg, U.; Chauhan, S.; Nagaich, U.; Jain, N. Current Advances in Chitosan Nanoparticles Based Drug Delivery and Targeting. Pharm. Bull. 2019, 9, 195–204. [Google Scholar] [CrossRef]
- Rizeq, B.R.; Younes, N.N.; Rasool, K.; Nasrallah, G.K. Synthesis, Bioapplications, and Toxicity Evaluation of Chitosan-Based Nanoparticles. Int. J. Mol. Sci. 2019, 20, 5776. [Google Scholar] [CrossRef]
- Ribeiro, E.F.; Barros-Alexandrino, T.T.; Assis, O.B.G.; Cruz-Jr, A.; Quiles, A.; Hernando, I.; Nicoletti, V.R. Chitosan and crosslinked chitosan nanoparticles: Synthesis, characterization and their role as Pickering emulsifiers. Carbohyd. Polym. 2020, 250, 116878. [Google Scholar] [CrossRef]
- Ribeiro, E.F.; Borreani, J.; Moraga, G.; Nicoletti, V.R.; Quiles, A.; Hernando, I. Digestibility and Bioaccessibility of Pickering Emulsions of Roasted Coffee Oil Stabilized by Chitosan and Chitosan-Sodium Tripolyphosphate Nanoparticles. Food Biophys. 2020, 15, 196–205. [Google Scholar] [CrossRef]
- Ribeiro, E.F.; Polachini, T.C.; Alvim, I.D.; Quiles, A.; Hernando, I.; Nicoletti, V.R. Microencapsulation of roasted coffee oil Pickering emulsions using spray- and freeze-drying: Physical, structural and in vitro bioaccessibility studies. Int. J. Food Sci. Technol. 2022, 57, 145–153. [Google Scholar] [CrossRef]
- Rodriguez, E.S.; Julio, L.M.; Henning, C.; Diehl, B.W.K.; Tomás, M.C.; Ixtaina, V.Y. Effect of natural antioxidants on the physicochemical properties and stability of freeze-dried microencapsulated chia seed oil. J. Sci. Food Agric. 2019, 99, 1682–1690. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, M.L.F.F.; Roos, Y.H.; Ribeiro, A.P.B.; Nicoletti, V.R. Effects of maltodextrin content in double-layer emulsion for production and storage of spray-dried carotenoid-rich microcapsules. Food Bioprod. Process. 2020, 124, 208–221. [Google Scholar] [CrossRef]
- Labuza, T. Creation of Moisture Sorption Isotherms for Hygroscopic Materials. Sorption Isotherm Methods; American Society of Heating, Refrigerating and Air Conditioning Engineers: Washingtion, DC, USA, 1963. [Google Scholar]
- AOAC. Official Methods of Analysis of Association of Official Analytical Chemists; Association of Official Analytical Chemists: Washington, DC, USA, 2010. [Google Scholar]
- Bastıoğlu, A.Z.; Koç, M.; Ertekin, F.K. Moisture sorption isotherm of microencapsulated extra virgin olive oil by spray drying. J. Food Meas. Charact. 2017, 11, 1295–1305. [Google Scholar] [CrossRef]
- Partanen, R.; Raula, J.; Seppänen, R.; Buchert, J.; Kauppinen, E.; Forssell, P. Effect of Relative Humidity on Oxidation of Flaxseed Oil in Spray Dried Whey Protein Emulsions. J. Agric. Food Chem. 2008, 56, 5717–5722. [Google Scholar] [CrossRef]
- Anese, M.; De Pilli, T.; Massini, R.; Lerici, C.R. Oxidative stability of the lipid fraction in roasted coffee. Ital. J. Food Sci. 2002, 12, 457–462. [Google Scholar]
- International Dairy Federation; IDF-Square Vergote 41: Brussels, Belgium, 1991.
- Satué-Gracia, M.T.; Frankel, E.N.; Rangavajhyala, N.; German, J.B. Lactoferrin in Infant Formulas: Effect on Oxidation. J. Agric. Food Chem. 2000, 48, 4984–4990. [Google Scholar] [CrossRef]
- Drusch, S.; Schwarz, K. Microencapsulation properties of two different types of n-octenylsuccinate-derivatised starch. Eur. Food Res. Technol. 2006, 222, 155–164. [Google Scholar] [CrossRef]
- Chan, H.W.-S.; Levett, G. Autoxidation of methyl linoleate. Separation and analysis of isomeric mixtures of methyl linoleate hydroperoxides and methyl hydroxylinoleates. Lipids 1997, 12, 99–104. [Google Scholar] [CrossRef]
- Freiberger, E.B.; Kaufmann, K.C.; Bona, E.; de Araújo, P.H.H.; Sayer, C.; Leimann, F.V.; Gonçalves, O.H. Encapsulation of roasted coffee oil in biocompatible nanoparticles. LWT-Food Sci. Technol. 2015, 64, 381–389. [Google Scholar] [CrossRef]
- Van Den Dool, H.; Kratz, P.D.J. A generalization of the retention index system including linear temperature programmed gas-liquid partition chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.W.; Liu, X.; Wong, W.S.E.; Liu, S.Q. Effects of sucrose monopalmitate (P90), Tween 80 and modified starch on coffee aroma retention and release in coffee oil-based emulsions. Food Hydrcolloid. 2017, 66, 128–135. [Google Scholar] [CrossRef]
- Hurtado-Benavides, A.; Dorado, A.D.; Sánchez-Camargo, A.P. Study of the fatty acid profile and the aroma composition of oilobtained from roasted Colombian coffee beans by supercritical fluidextraction. J. Supercrit. Fluid. 2016, 113, 44–52. [Google Scholar] [CrossRef]
- Cincotta, F.; Tripodi, G.; Merlino, M.; Verzera, A.; Condurso, C. Variety and shelf-life of coffee packaged in capsules. LWT-Food Sci. Technol. 2020, 118, 108718. [Google Scholar] [CrossRef]
- Acree, T.; Arn, H. Flavornet and Human Odor Space. Available online: http://www.flavornet.org/flavornet.html (accessed on 9 October 2016).
- Brunauer, S.; Deming, L.S.; Deming, W.E.; Teller, E. On a Theory of the van der Waals Adsorption of Gases. J. Am. Chem. Soc. 1940, 62, 1723–1732. [Google Scholar] [CrossRef]
- Polachini, T.C.; Betiol, L.F.L.; Lopes-Filho, J.F.; Telis-Romero, J. Water adsorption isotherms and thermodynamic properties of cassava bagasse. Thermochim. Acta 2016, 632, 79–85. [Google Scholar] [CrossRef]
- Lewicki, P.P. The applicability of the GAB model to food water sorption isotherms. Int. J. Food Sci. Technol. 1997, 32, 553–557. [Google Scholar] [CrossRef]
- Koc, B.; Isleroglu, H.; Turker, I. Sorption behavior and storage stability of microencapsulated transglutaminase by ultrasonic spray–freeze–drying. Dry. Technol. 2022, 40, 337–351. [Google Scholar] [CrossRef]
- Wibowo, S.; Vervoort, L.; Tomic, J.; Santiago, J.S.; Lemmens, L.; Panozzo, A.; Grauwet, T.; Hendrickx, M.; Van Loey, A. Colour and carotenoid changes of pasteurised orange juice during storage. Food Chem. 2015, 171, 330–340. [Google Scholar] [CrossRef]
- Menin, A.; Zanoni, F.; Vakarelova, M.; Chignola, R.; Donà, G.; Rizzi, C.; Mainente, F.; Zoccatelli, G. Effects of microencapsulation by ionic gelation on the oxidative stability of flaxseed oil. Food Chem. 2018, 269, 293–299. [Google Scholar] [CrossRef]
- Ferreira, C.D.; Conceição, E.J.L.; Machado, B.A.S.; Hermes, V.S.; Rios, A.O.; Druzian, J.I.; Nunes, I.L. Physicochemical Characterization and Oxidative Stability of Microencapsulated Crude Palm Oil by Spray Drying. Food Bioprocess Technol. 2016, 9, 124–136. [Google Scholar] [CrossRef]
- Hoyos-Leyva, J.D.; Bello-Perez, L.A.; Agama-Acevedo, J.E.; Alvarez-Ramirez, J.; Jaramillo-Echeverry, L.M. Characterization of spray drying microencapsulation of almond oil into taro starch spherical aggregates. LWT-Food Sci. Technol. 2019, 101, 526–533. [Google Scholar] [CrossRef]
- Chang, H.W.; Tan, T.B.; Tan, P.Y.; Abas, F.; Lai, O.M.; Wang, Y.; Wang, Y.; Nehdi, I.A.; Tan, C.P. Microencapsulation of fish oil using thiol-modified β-lactoglobulin fibrils/chitosan complex: A study on the storage stability and in vitro release. Food Hydrocolloid. 2018, 80, 186–194. [Google Scholar] [CrossRef]
- Talón, E.; Vargas, M.; Chiralt, A.; González-Martínez, C. Antioxidant starch-based films with encapsulated eugenol. Application to sunflower oil preservation. LWT-Food Sci. Technol. 2019, 113, 108290. [Google Scholar] [CrossRef]
- Kenari, R.E.; Amiri, Z.R.; Motamedzadegan, A.; Milani, J.M.; Farmani, J.; Farahmandfar, R. Optimization of Iranian golpar (Heracleum persicum) extract encapsulation using sage (Salvia macrosiphon) seed gum: Chitosan as a wall materials and its effect on the shelf life of soybean oil during storage. J. Food Meas. Charact. 2020, 14, 2828–2839. [Google Scholar] [CrossRef]
- Moser, P.; Nicoletti, V.R.; Drusch, S.; Brückner-Gühmann, M. Functional properties of chickpea protein-pectin interfacial complex in buriti oil emulsions and spray dried microcapsules. Food Hydrocoll. 2020, 107, 105929. [Google Scholar] [CrossRef]
- Akiyama, M.; Murukami, K.; Ikeda, M.; Iwatsuki, K.; Kokubo, S.; Wada, A.; Tokuno, K.; Onishi, M.; Iwabuchi and Tanaka, K. Characterization of Flavor Compounds Released During Grinding of Roasted Robusta Coffee Beans. Food Sci. Technol. Res. 2005, 11, 298–307. [Google Scholar] [CrossRef]
- Ferreira, M.V.; Cappato, L.P.; Silva, R.; Rocha, R.S.; Guimarães, J.T.; Balthazar, C.F.; Esmerino, E.A.; Freitas, M.Q.; Rodrigues, F.N.; Granato, D.; et al. Ohmic heating for processing of whey-raspberry flavored beverage. Food Chem. 2019, 297, 125018. [Google Scholar] [CrossRef]
- Agnoletti, B.Z.; Folli, G.S.; Pereira, L.L.; Pinheiro, P.F.; Guarçoni, R.C.; Oliveira, E.C.S.; Filgueiras, P.R. Multivariate calibration applied to study of volatile predictors of arabica coffee quality. Food Chem. 2022, 367, 130679. [Google Scholar] [CrossRef]
- Anwar, S.H.; Kunz, B. The influence of drying methods on the stabilization of fish oil microcapsules: Comparison of spray granulation, spray drying, and freeze drying. J. Food Eng. 2011, 105, 367–378. [Google Scholar] [CrossRef]
 ,
,  ) and freeze-dried (
) and freeze-dried ( ,
,  ) samples. Lines represent the fitted GAB model.
) samples. Lines represent the fitted GAB model.
 ,
,  ) and freeze-dried (
) and freeze-dried ( ,
,  ) samples. Lines represent the fitted GAB model.
) samples. Lines represent the fitted GAB model.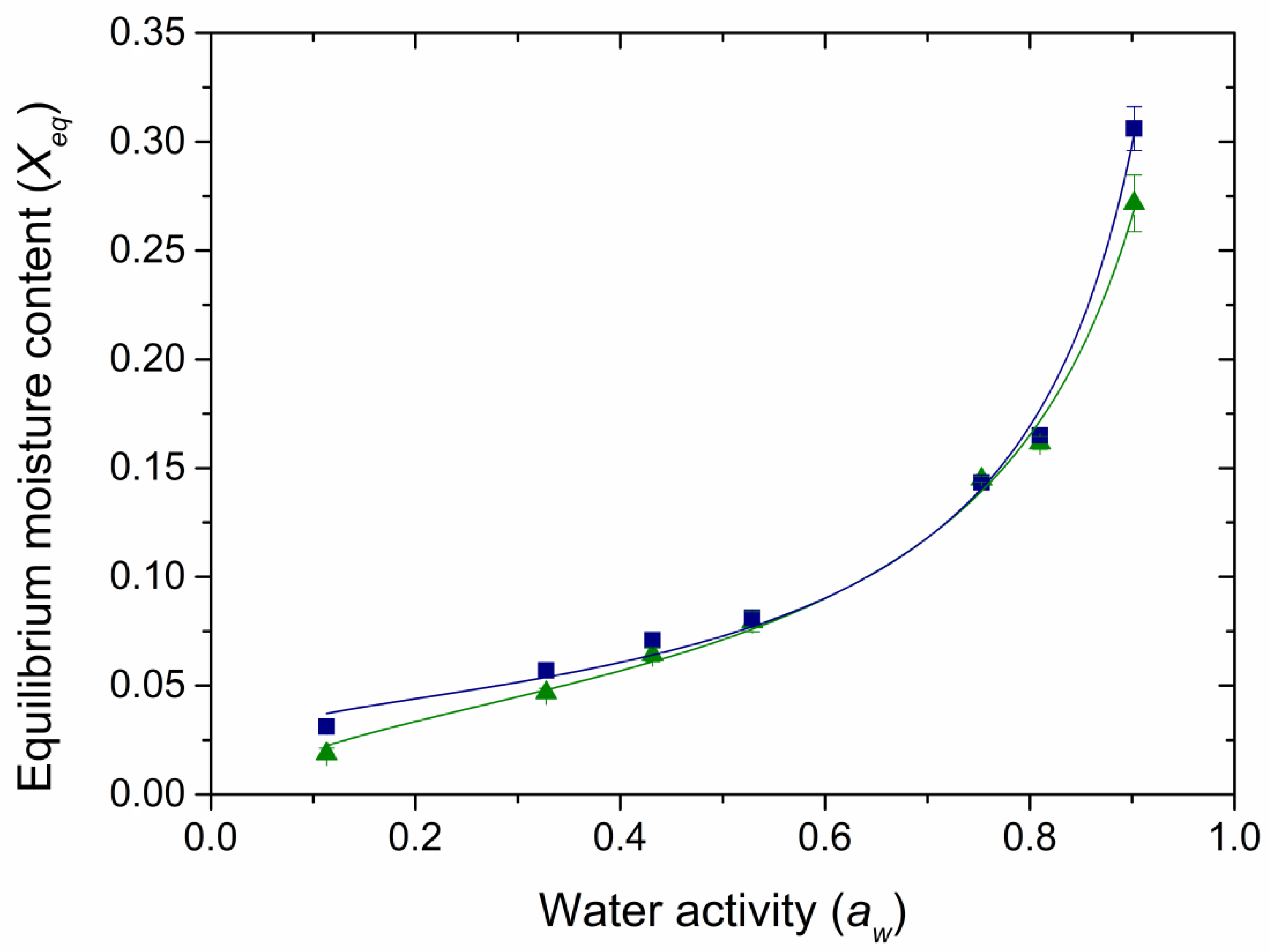
 ), freeze-dried (
), freeze-dried ( ), and non-encapsulated RCO (
), and non-encapsulated RCO ( ) over 30 days of storage at 25 °C.
) over 30 days of storage at 25 °C.
 ), freeze-dried (
), freeze-dried ( ), and non-encapsulated RCO (
), and non-encapsulated RCO ( ) over 30 days of storage at 25 °C.
) over 30 days of storage at 25 °C.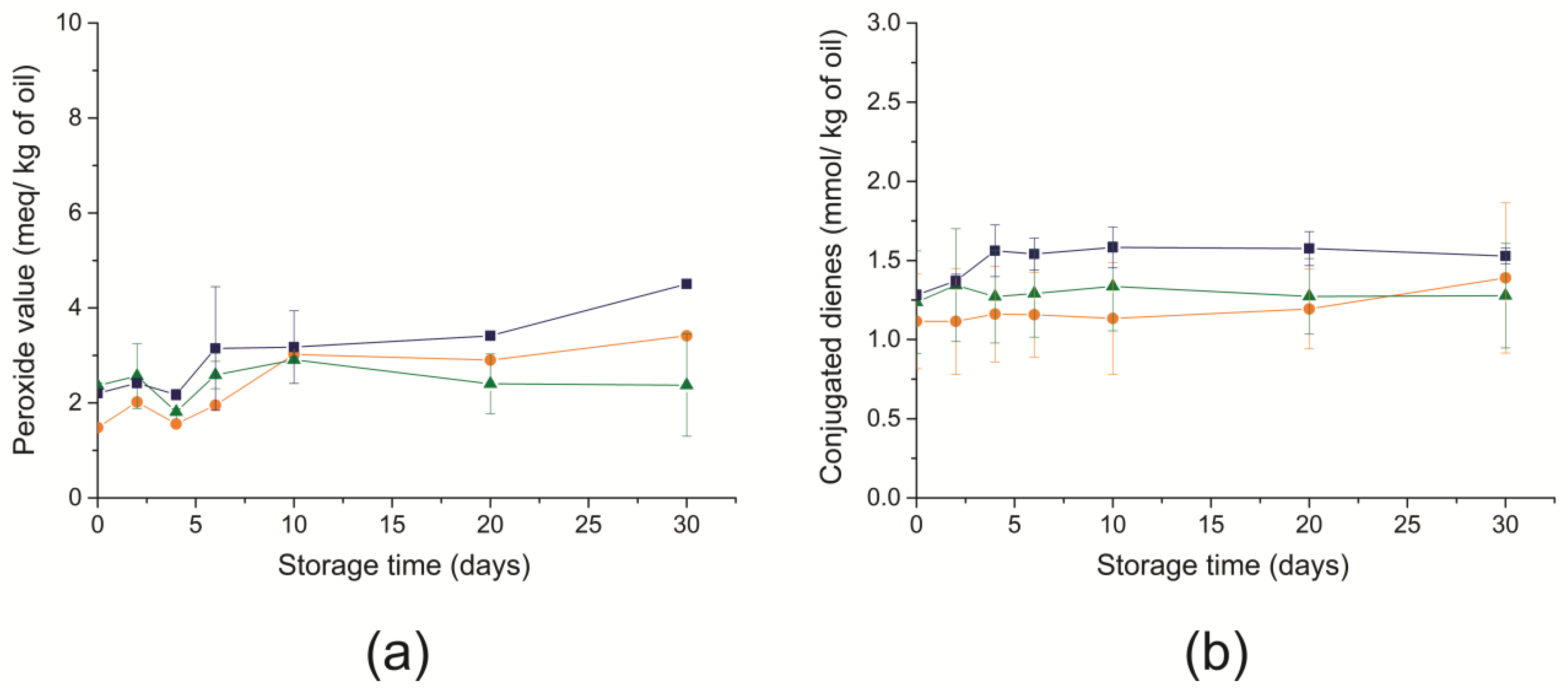
 ), day 10 (
), day 10 ( ), day 20 (
), day 20 ( ), and day 30 (
), and day 30 ( ).
).
 ), day 10 (
), day 10 ( ), day 20 (
), day 20 ( ), and day 30 (
), and day 30 ( ).
).
 ), freeze-dried (
), freeze-dried ( ), and non-encapsulated RCO (
), and non-encapsulated RCO ( ) on the peak areas of specific compounds.
) on the peak areas of specific compounds.
 ), freeze-dried (
), freeze-dried ( ), and non-encapsulated RCO (
), and non-encapsulated RCO ( ) on the peak areas of specific compounds.
) on the peak areas of specific compounds.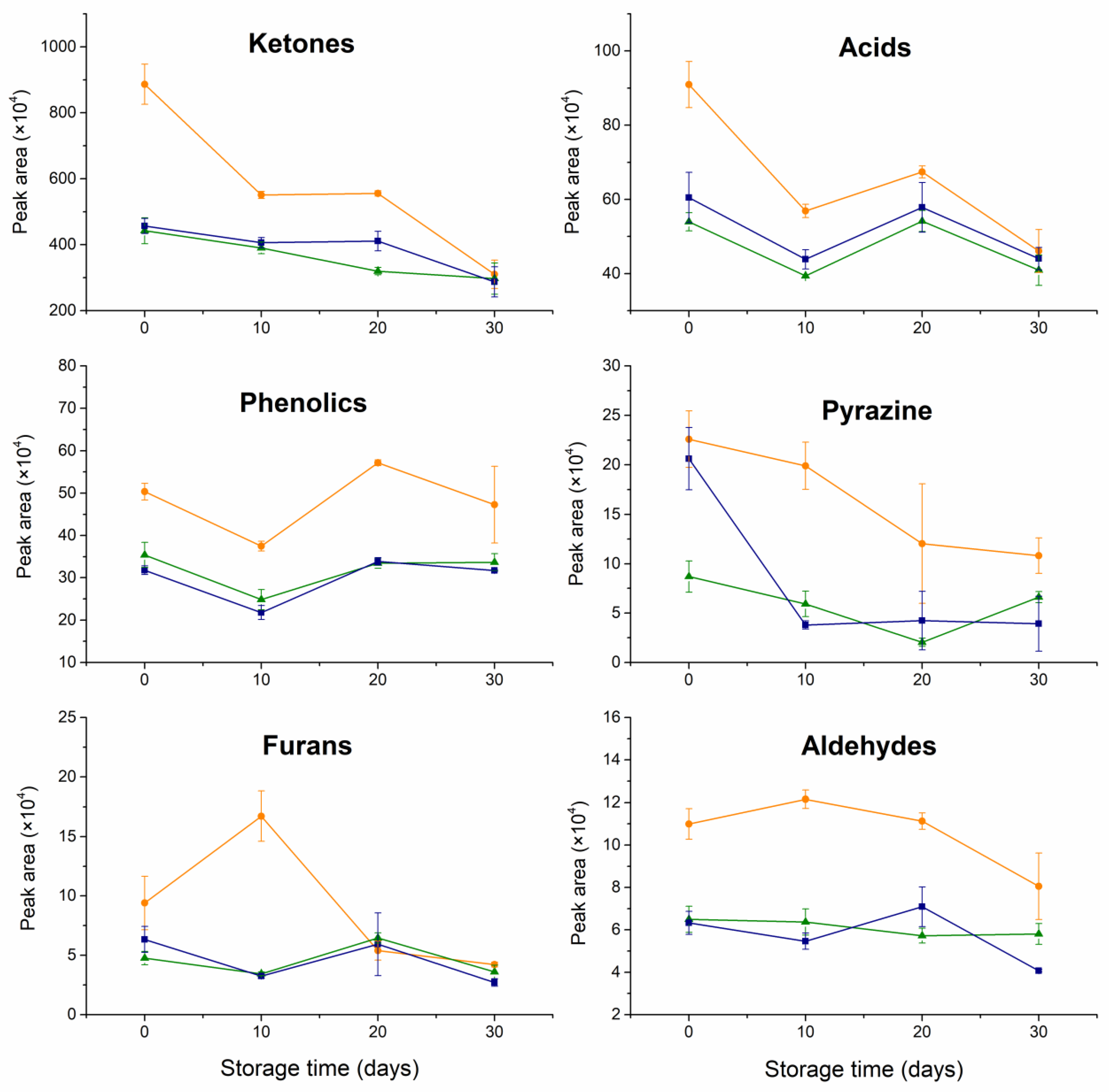
| Parameters | Samples | |
|---|---|---|
| Spray-Dried | Freeze-Dried | |
| Xm (g water/g dry matter) | 0.044 ± 0.003 a | 0.038 ± 0.001 b |
| C | 7.04 ± 1.08 b | 29.82 ± 4.34 a |
| k | 0.931 ± 0.019 b | 0.969 ± 0.009 a |
| >0.991 | >0.990 | |
| RMSE | <0.0064 | <0.0072 |
| Samples | Days | Color Parameters | |||
|---|---|---|---|---|---|
| a | b | L | ΔE | ||
| RCO | 0 | 0.02 ± <0.01 e | 0.11 ± 0.09 d | 0.09 ± <0.01 d | - |
| 2 | 0.44 ± 0.01 de | 0.13 ± 0.08 cd | 0.16 ± 0.04 cd | 0.45 ± <0.01 d | |
| 4 | 1.66 ± 0.46 bcd | 0.42 ± 0.09 bcd | 0.40 ± 0.10 bc | 1.71 ± 0.44 bcd | |
| 6 | 1.56 ± 0.71 cd | 0.38 ± 0.21 bcd | 0.38 ± 0.21 bc | 1.61 ± 0.77 cd | |
| 10 | 4.21 ± 0.38 a | 1.15 ± 0.09 a | 0.85 ± 0.09 a | 4.39 ± 0.35 a | |
| 20 | 2.60 ± 0.61 bc | 0.65 ± 0.13 bc | 0.58 ± 0.12 ab | 2.69 ±0.63 bc | |
| 30 | 2.87 ± 1.07 ab | 0.72 ± 0.39 ab | 0.57 ± 0.18 ab | 2.97 ± 1.12 b | |
| Spray-dried | 0 | 0.94 ± 0.05 c | 12.43 ± 0.40 ab | 86.54 ± 0.31 ab | - |
| 2 | 0.89 ± 0.04 c | 11.63 ± 0.08 c | 85.45 ± 0.05 c | 1.35 ± 0.03 a | |
| 4 | 0.87 ± 0.01 bc | 11.71 ± 0.03 c | 85.52 ± 0.04 c | 1.25 ± 0.05 ab | |
| 6 | 0.95 ± 0.02 bc | 12.35 ± 0.16 b | 86.78 ± 0.08 a | 0.29 ± 0.02 d | |
| 10 | 0.99 ± 0.01 b | 12.36 ± 0.13 b | 86.63 ± 0.07 ab | 0.17 ± 0.04 d | |
| 20 | 1.15 ± 0.02 a | 12.91 ± 0.06 a | 86.32 ± 0.16 b | 0.59 ± 0.12 c | |
| 30 | 1.17 ± 0.02 a | 12.65 ± 0.23 ab | 85.49 ± 0.13 c | 1.11 ± 0.09 b | |
| Freeze-dried | 0 | 2.9 ± 0.01 b | 21.63 ± 0.04 a | 77.19 ± 0.04 ab | - |
| 2 | 2.75 ± 0.08 c | 20.51 ± 0.52 c | 76.84 ± 0.29 c | 1.18 ± 0.58 b | |
| 4 | 2.90 ± 0.01 b | 21.28 ± 0.04 ab | 77.02 ± 0.07 bc | 0.39 ± 0.06 c | |
| 6 | 2.93 ± 0.01 b | 21.03 ± 0.13 abc | 76.69 ± 0.04 cd | 0.78 ± 0.10 bc | |
| 10 | 2.95 ± 0.04 b | 20.88 ± 0.10 bc | 76.91 ± 0.08 bc | 0.81 ± 0.12 bc | |
| 20 | 2.86 ± 0.01 b | 19.25 ± 0.02 d | 77.49 ± 0.05 a | 2.40 ± 0.02 a | |
| 30 | 3.19 ± 0.01 a | 20.81 ± 0.01 bc | 76.38 ± 0.09 d | 1.19 ± 0.07 b | |
| Volatile Compounds | Retention Index (RI) | GC-FID Peak Area (×104) | Aroma Description | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| t = 0 | t = 10 | t = 20 | t = 30 | ||||||||||||
| Experimental | Literature a | Coffee Oil | SD | FD | Coffee Oil | SD | FD | Coffee Oil | SD | FD | Coffee Oil | SD | FD | ||
| Pyridine | - | 1194 2 | 13.4 ± 4.8 a | 1.1 ± 0.3 b | 0.3 ± 0.1 c | 18.3 ± 1.8 a | 0.5 ± 0.1 c | 0.8 ± 0.1 b | 7.2 ± 0.2 a | 1.4 ± 0.3 b | 1.3 ± 0.2 b | 7.4 ± 0.2 a | 0.8 ± 0.2 b | 0.6 ± 0.0 b | Roasted 3 |
| Pyrazine | |||||||||||||||
| Pyrazine | - | - | 4.5 ± 0.5 a | 0.5 ± 0.4 c | 2.4 ± 1.4 b | 1.9 ± 0.8 a | 0.4 ± 0.1 b | 0.5 ± 0.2 b | 1.5 ± 0.4 a | 0.4 ± 0.0 b | 0.3 ± 0.1 b | 0.5 ± 0.1 a | 0.1 ± 0.1 b | 0.2 ± 0.0 b | Rancid peanuts 3 |
| 2,5-Dimethylpyrazine | 1314 | 1329 2 | 15.4 ± 1.2 a | 2.8 ± 0.9 b | 1.4 ± 0.4 b | 17.0 ± 0.9 a | 1.2 ± 0.3 b | 0.6 ± 0.1 c | 7.8 ± 3.7 a | 1.5 ± 0.3 b | 2.3 ± 1.7 b | 10.2 ± 1.3 a | 1.9 ± 0.3 b | 3.6 ± 2.5 b | Nuts 3 |
| 2,6-Dimethylpyrazine | 1337 | 1335 2 | 0.1 ± 0.1 b | 0.5 ± 0.2 a | 0.7 ± 0.1 a | 0.0 ± 0.0 b | 0.1 ± 0.0 a | 0.0 ± 0.0 b | 0.0 ± 0.0 c | 0.1 ± 0.0 b | 0.3 ± 0.0 a | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 0.0 ± 0.0 a | Roasted 3 |
| 2-Ethyl-5-methylpyrazine | 1432 | 1428 2 | 2.6 ± 0.1 | 4.8 ± 0.1 | 16.1 ± 1.2 | 1.0 ± 0.7 c | 4.2 ± 0.8 a | 2.7 ± 0.2 b | 2.6 ± 2.0 a | 0.1 ± 0.1 b | 1.4 ± 1.2 ab | 0.1 ± 0.0 b | 4.6 ± 0.1 a | 0.1 ± 0.1 b | Nuts, peanut 2 |
| Aldehyde | |||||||||||||||
| Pyrrole-2-carboxaldehyde | 2040 | 2017 2 | 9.8 ± 0.6 a | 6.2 ± 0.6 b | 5.6 ± 0.4 b | 9.0 ± 0.2 a | 5.8 ± 0.6 b | 5.0 ± 0.4 b | 10.5 ± 0.4 a | 5.3 ± 0.3 b | 6.3 ± 0.8 b | 8.1 ± 1.6 a | 5.6 ± 0.4 b | 4.1 ± 0.1 c | Roasted, smoky 2 |
| Nonanal | 1397 | - | 1.2 ± 0.1 a | 0.3 ± 0.0 c | 0.8 ± 0.2 b | 3.2 ± 0.2 a | 0.6 ± 0.0 b | 0.5 ± 0.0 b | 0.6 ± 0.0 b | 0.4 ±0.0 c | 0.8 ± 0.1 a | 0.0 ± 0.0 b | 0.2 ± 0.1 a | 0.0 ± 0.0 b | - |
| Furans | |||||||||||||||
| 2-Furancarboxaldehyde | 1467 | 1465 2 | 1.6 ± 0.1 a | 1.8 ± 0.1 a | 1.6 ± 0.0 a | 1.2 ± 0.1 a | 0.8 ± 0.0 b | 0.4 ± 0.0 c | 0.8 ± 0.0 b | 4.3 ± 0.3 a | 3.9 ± 1.8 a | 2.4 ± 0.1 a | 0.9 ± 0.1 b | 0.7 ± 0.1 b | Caramellic, cinnamon, almond 2 |
| Furfural | 1484 | 1485 1 | 4.8 ± 2.1 a | 0.0 ± 0.0 c | 0.2 ± 0.0 b | 13.4 ± 0.9 a | 0.2 ± 0.1 b | 0.2 ± 0.0 b | 1.8 ± 0.5 a | 0.4 ± 0.0 b | 0.5 ± 0.1 b | 0.0 ± 0.0 a | 0.2 ± 0.2 a | 0.1 ± 0.1 a | Caramellic, woody 1 |
| 2-Furanmethhyl acetate | 1538 | 1539 2 | 0.2 ± 0.0 b | 0.3 ± 0.1 b | 1.4 ± 0.3 a | 0.2 ± 0.0 b | 0.4 ± 0.1 a | 0.4 ± 0.1 a | 1.0 ± 0.2 a | 0.1 ± 0.0 c | 0.2 ± 0.0 b | 0.2 ± 0.0 b | 0.8 ± 0.3 a | 0.0 ± 0.0 b | Roasted nut, floral 2 |
| Furan-3-methanol | 1677 | 1673 2 | 2.8 ± 0.1 a | 2.6 ± 0.4 a | 3.2 ± 0.3 a | 1.8 ± 0.0 c | 2.0 ± 0.0 b | 2.3 ± 0.0 a | 1.8 ± 0.0 a | 1.6 ± 0.1 b | 1.3 ± 0.7 ab | 1.6 ± 0.1 b | 1.7 ± 0.0 b | 1.9 ± 0.1 a | Carmellic 3 |
| Ketones | |||||||||||||||
| 4-Cyclopentene-1,3-dione | 1574 | 1573 | 64.5 ± 2.8 a | 40.8 ± 2.7 b | 44.1 ± 4.0 b | 19.6 ± 0.6 a | 14.0 ± 0.6 b | 19.3 ± 1.5 a | 24.7 ± 2.1 a | 10.2 ± 3.3 c | 19.8 ± 2.1 b | 5.0 ± 0.6 c | 7.2 ± 0.3 b | 13.6 ± 1.3 a | - |
| 1,2-Cyclopentanedione | 1748 | 1742 | 123.9 ± 5.3 c | 196.0 ± 13.9 b | 226.5 ± 6.7 a | 67.6 ± 1.8 c | 172.2 ± 4.8 b | 207.0 ± 8.5 a | 59.7 ± 2.4 c | 159.3 ± 0.3 b | 210.2 ± 15.4 a | 48.9 ± 8.8 b | 112 ± 39.7 a | 116.8 ± 29.3 a | - |
| 2-Hydroxy-3-methyl-2-cyclopenten-1-one | 1855 | 1857 1 | 5.0 ± 0.2 a | 4.1 ± 0.9 b | 5.0 ± 1.1 a | 4.2 ± 0.1 a | 3.4 ± 0.3 b | 2.9 ± 0.1 b | 4.2 ± 0.1 a | 2.4 ± 0.2 b | 3.1 ± 0.1 a | 2.7 ± 0.3 a | 2.6 ± 0.0 a | 2.8 ± 0.1 a | Caramellic 1 |
| 3-Ethyl-2-hydroxy-2-cyclopenten-1-one | 1920 | 1909 1 | 646.3 ± 50.0 a | 152.4 ± 14.6 b | 139.7 ± 3.2 b | 422.0 ± 7.5 a | 144.7 ± 7.5 b | 130.5 ± 3.8 c | 411.8 ± 2.0 a | 92.9 ± 5.5 c | 124.5 ± 1.7 b | 207.2 ± 25.5 a | 119.0 ± 4.4 b | 103.3 ± 14.5 b | Caramellic, smoky 1 |
| 3-Hydroxy-2-methyl-4-pyrone | 1965 | 1955 2 | 13.4 ± 0.9 b | 16.3 ± 1.4 a | 16.6 ± 2.9 a | 12.1 ± 0.2 c | 16.5 ± 1.3 a | 14.1 ± 0.6 b | 15.2 ± 0.4 c | 17.5 ± 0.8 b | 20.1 ± 0.3 a | 11.3 ± 1.5 b | 17.4 ± 0.4 a | 16.8 ± 0.1 a | Caramel |
| 2-Pentadecanone | 2143 | 2031 | 33.5 ± 1.7 a | 32.9 ± 5.8 a | 24.7 ± 2.2 b | 25.2 ± 0.5 c | 39.8 ± 3.2 a | 32.3 ± 0.8 b | 40.0 ± 1.0 a | 37.4 ± 0.9 b | 33.3 ± 4.9 ab | 35.0 ± 6.2 ab | 39.1 ± 2.6 a | 34.2 ± 0.6 b | Waxy 2 |
| Acids | |||||||||||||||
| 3-Methyl-2-butenoic acid | 1772 | 1777 2 | 33.4 ± 0.2 a | 22.1 ± 0.9 b | 24.8 ± 3.8 b | 16.4 ± 0.6 b | 21.8 ± 0.1 a | 21.1 ± 1.9 a | 23.3 ± 1.1 b | 33.4 ± 1.9 a | 31.4 ± 1.1 a | 13.4 ± 1.5 b | 23.3 ± 0.1 a | 23.3 ± 1.4 a | Dairy, fermented 3 |
| Caproic acid | 1844 | 1829 | 88.6 ± 3.5 b | 102.4 ± 3.5 a | 95.1 ± 11.5 ab | 69.6 ± 2.2 b | 83.5 ± 4.0 a | 78.0 ± 1.7 a | 61.8 ± 0.6 b | 58.2 ± 2.8 c | 63.8 ± 0.6 a | 37.7 ± 4.9 b | 66.9 ± 0.4 a | 67.7 ± 1.2 a | Goat-like odor |
| Acetic acid | 1451 | 1458 1 | 4.3 ± 0.2 | 4.7 ± 0.5 | 5.6 ± 0.5 | 0.8 ± 0.1 a | 0.2 ± 0.0 b | 0.2 ± 0.0 b | 1.2 ± 0.0 b | 7.8 ± 0.1 a | 6.6 ± 4.5 a | 0.6 ± 0.1 c | 5.1 ± 3.6 a | 1.9 ± 0.2 b | Acidic, pungent 1 |
| Butanoic acid | 1630 | 1632 1 | 29.3 ± 1.1 a | 22.8 ± 0.7 b | 25.1 ± 1.6 b | 16.5 ± 0.3 a | 13.6 ± 0.1 c | 15.5 ± 0.2 b | 15.9 ± 0.1 a | 8.7 ± 0.8 c | 14.8 ± 0.2 b | 6.5 ± 0.7 c | 9.4 ± 0.2 b | 13.1 ± 0.8 a | Sharp, buttery 1 |
| Benzoic acid | 2443 | 2443 2 | 23.9 ± 4.7 a | 4.3 ± 0.4 b | 4.9 ± 0.8 b | 23.1 ± 0.2 a | 3.7 ± 0.2 c | 7.1 ± 0.5 b | 27.0 ± 0.4 a | 4.2 ± 0.0 b | 5.0 ± 0.9 b | 25.6 ± 3.5 a | 3.1 ± 0.1 c | 5.7 ± 0.7 b | - |
| Palmitic acid | 2900 | 2919 | 0.9 ± 0.1 a | 0.4 ± 0.0 b | 0.4 ± 0.0 b | 3.0 ± 0.2 a | 0.3 ± 0.0 c | 0.9 ± 0.0 b | 2.4 ± 0.0 a | 0.3 ± 0.0 b | 0.4 ± 0.0 b | 1.8 ± 0.0 a | 1.9 ± 0.1 a | 0.7 ± 0.1 b | - |
| Phenolics | |||||||||||||||
| 4-Ethyl-2-methoxy-phenol | 2048 | 2044 2 | 20.7 ± 0.7 a | 17.3 ± 1.2 b | 15.3 ± 0.6 c | 8.3 ± 0.4 a | 6.7 ± 0.9 b | 5.3 ± 0.7 c | 22.3 ± 0.3 a | 15.9 ± 0.8 b | 16.9 ± 0.7 b | 17.2 ± 3.0 a | 15.5 ± 0.6 a | 15.6 ± 0.0 a | Spicy; smoky 1 |
| 4-Methoxyphenol | 2098 | 2099 2 | 17.1 ± 0.3 a | 10.3 ± 0.7 b | 8.8 ± 0.4 c | 16.1 ± 0.5 a | 9.4 ± 0.7 b | 8.3 ± 0.8 c | 19.4 ± 0.1 a | 9.1 ± 0.3 b | 9.4 ± 0.0 b | 15.8 ± 3.1 a | 9.7 ± 0.6 b | 8.8 ± 0.2 b | - |
| 2-Methoxy-4-vinylphenol | 2215 | 2219 2 | 12.6 ± 1.0 a | 7.8 ± 1.0 b | 7.7 ± 0.0 b | 13.2 ± 0.3 a | 8.8 ± 0.8 b | 8.2 ± 0.2 b | 15.5 ± 0.3 a | 8.4 ± 0.1 b | 7.6 ± 0.1 c | 14.3 ± 2.9 a | 8.6 ± 0.8 b | 7.3 ± 0.0 b | Spicy, peanut 2 |
| Total | 1330.6 ± 83.4 a | 735.2 ± 9.8 b | 757.8 ± 32.8 b | 956.0 ± 24.3 a | 639.5 ± 0.6 c | 668.6 ± 25.1 b | 980.8 ± 0.1 a | 566.8 ± 12.5 c | 669.0 ± 6.7 b | 654.1 ± 81.4 a | 529.7 ± 57.6 b | 530.9 ± 48.7 b | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribeiro, E.F.; Polachini, T.C.; Locali-Pereira, A.R.; Janzantti, N.S.; Quiles, A.; Hernando, I.; Nicoletti, V.R. Storage Stability of Spray- and Freeze-Dried Chitosan-Based Pickering Emulsions Containing Roasted Coffee Oil: Color Evaluation, Lipid Oxidation, and Volatile Compounds. Processes 2023, 11, 1048. https://doi.org/10.3390/pr11041048
Ribeiro EF, Polachini TC, Locali-Pereira AR, Janzantti NS, Quiles A, Hernando I, Nicoletti VR. Storage Stability of Spray- and Freeze-Dried Chitosan-Based Pickering Emulsions Containing Roasted Coffee Oil: Color Evaluation, Lipid Oxidation, and Volatile Compounds. Processes. 2023; 11(4):1048. https://doi.org/10.3390/pr11041048
Chicago/Turabian StyleRibeiro, Elisa Franco, Tiago Carregari Polachini, Adilson Roberto Locali-Pereira, Natália Soares Janzantti, Amparo Quiles, Isabel Hernando, and Vânia Regina Nicoletti. 2023. "Storage Stability of Spray- and Freeze-Dried Chitosan-Based Pickering Emulsions Containing Roasted Coffee Oil: Color Evaluation, Lipid Oxidation, and Volatile Compounds" Processes 11, no. 4: 1048. https://doi.org/10.3390/pr11041048
APA StyleRibeiro, E. F., Polachini, T. C., Locali-Pereira, A. R., Janzantti, N. S., Quiles, A., Hernando, I., & Nicoletti, V. R. (2023). Storage Stability of Spray- and Freeze-Dried Chitosan-Based Pickering Emulsions Containing Roasted Coffee Oil: Color Evaluation, Lipid Oxidation, and Volatile Compounds. Processes, 11(4), 1048. https://doi.org/10.3390/pr11041048






