Waste Biomass Based Carbon Aerogels Prepared by Hydrothermal-carbonization and Their Ethanol Cracking Performance for H2 Production
Abstract
1. Introduction
2. Experiments and Analysis Methods
2.1. Materials
2.2. Preparation of Hydrothermal Carbon Aerogel
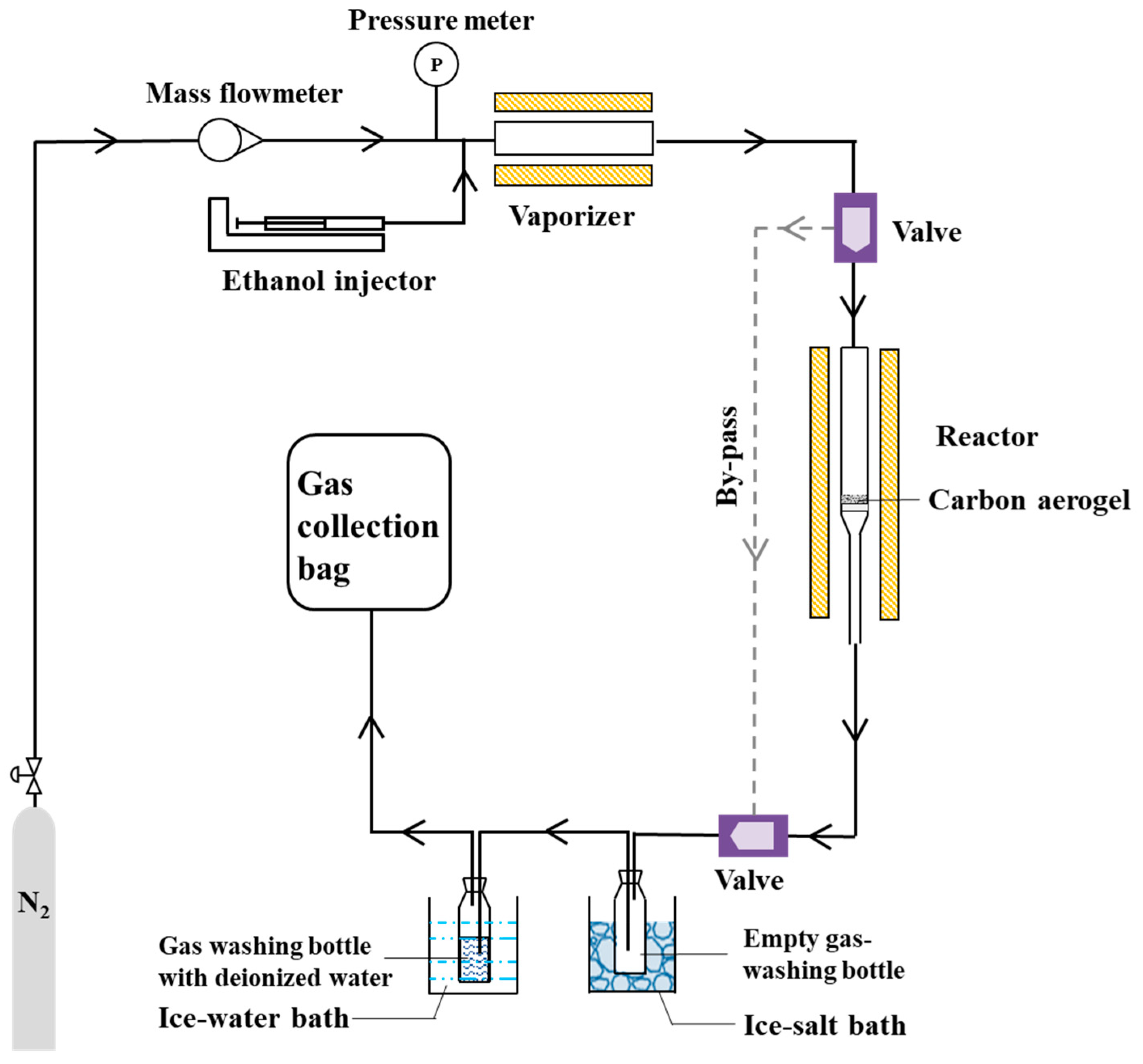
2.3. Evaluation of Ethanol Cracking Performances
2.4. Characterization of Carbon Aerogels and Gas Productions
3. Results and Discussion
3.1. Ethanol Cracking Performance of CAs Prepared from Different Waste Biomasses
3.2. Structures of CAs Prepared from Different Waste Biomasses
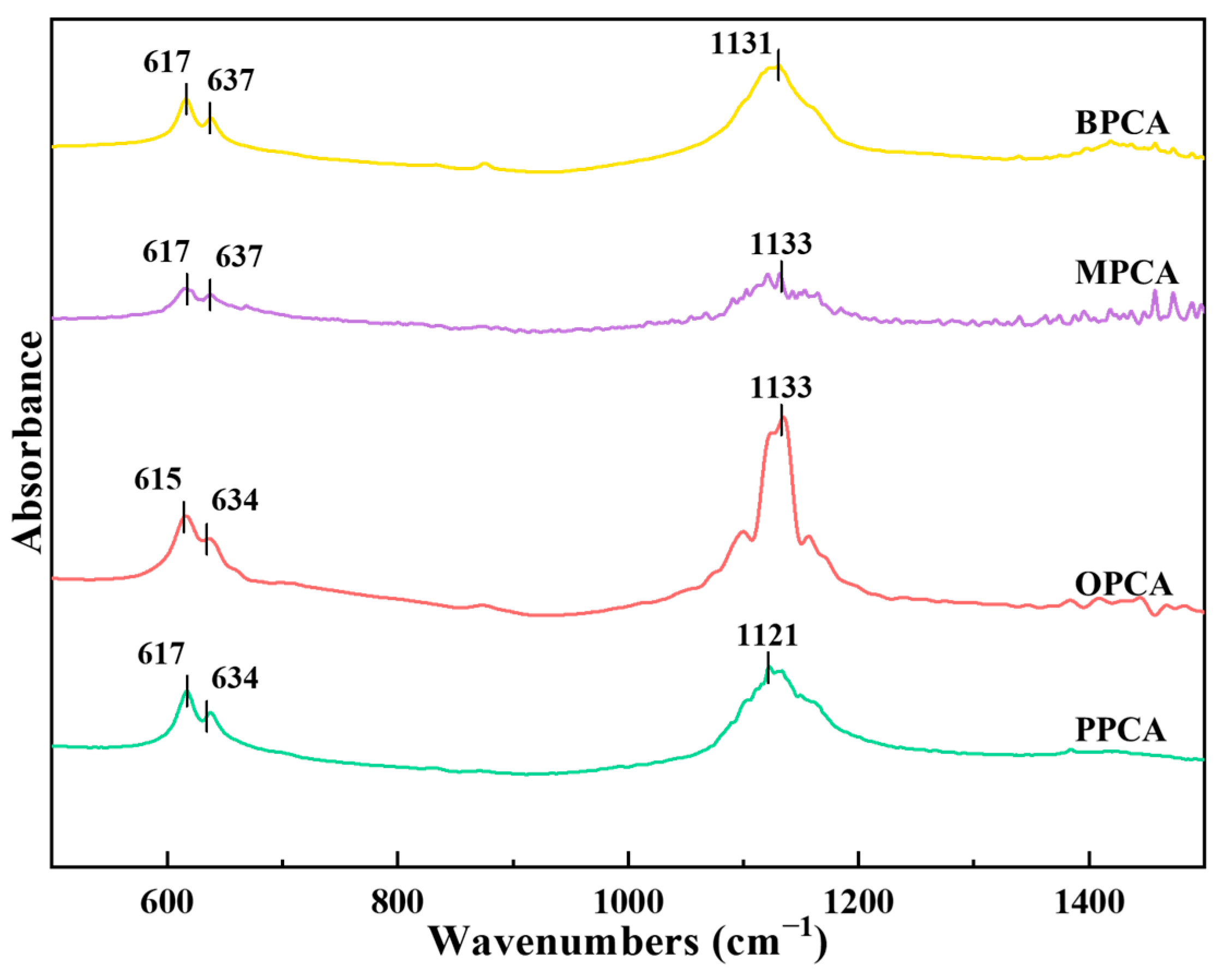
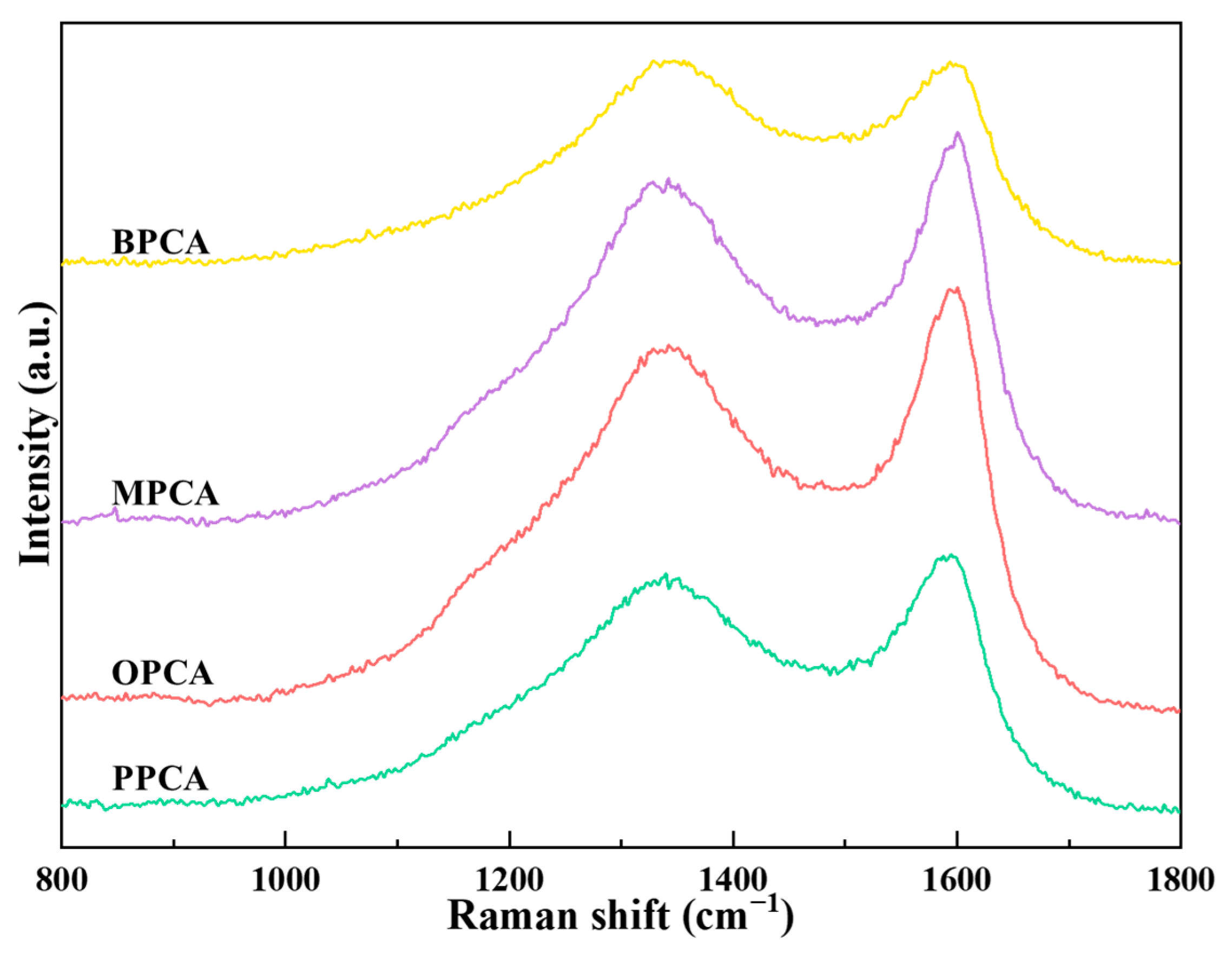
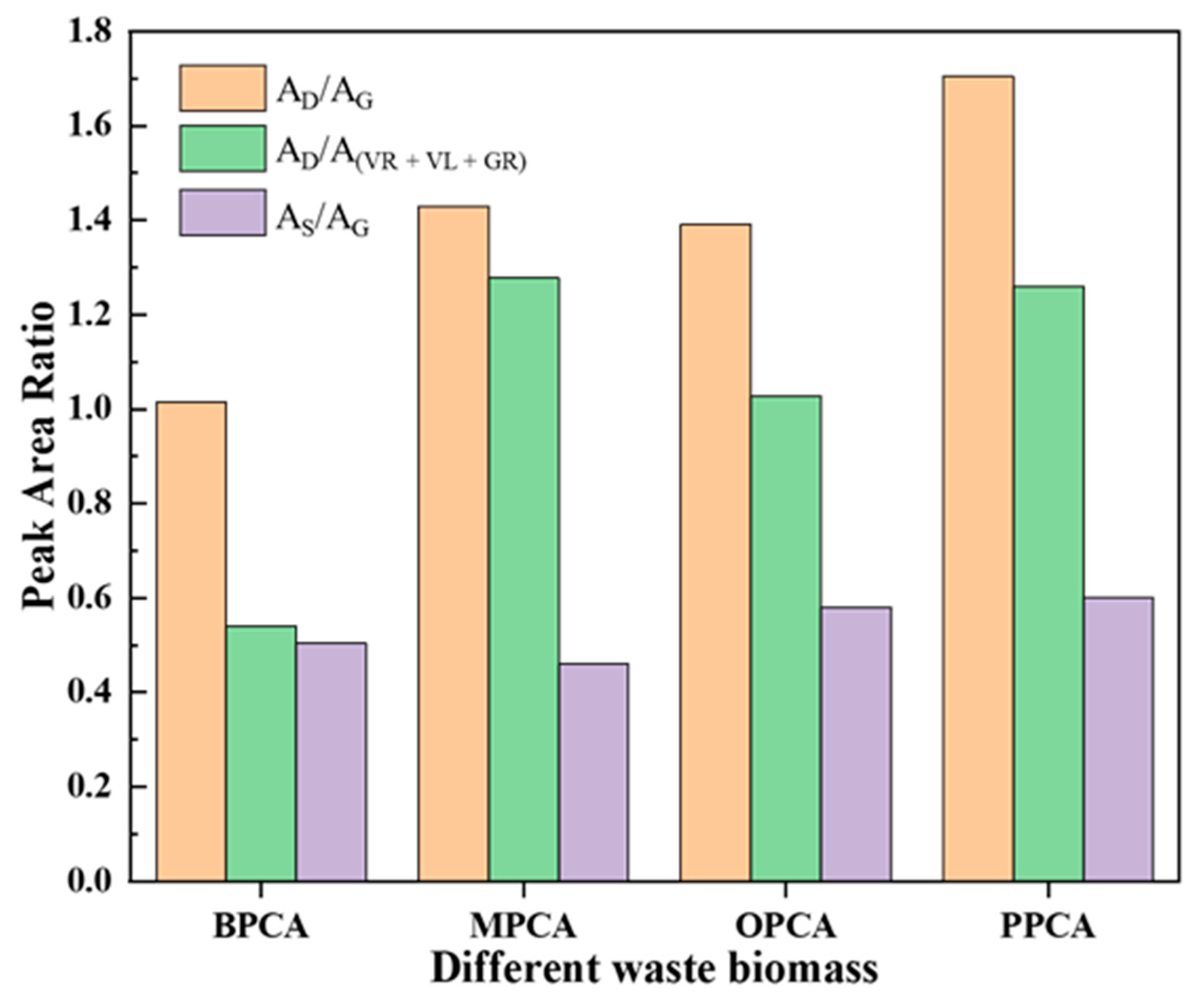
3.2.1. Chemical Structure of Different CAs Prepared from Different Waste Biomasses
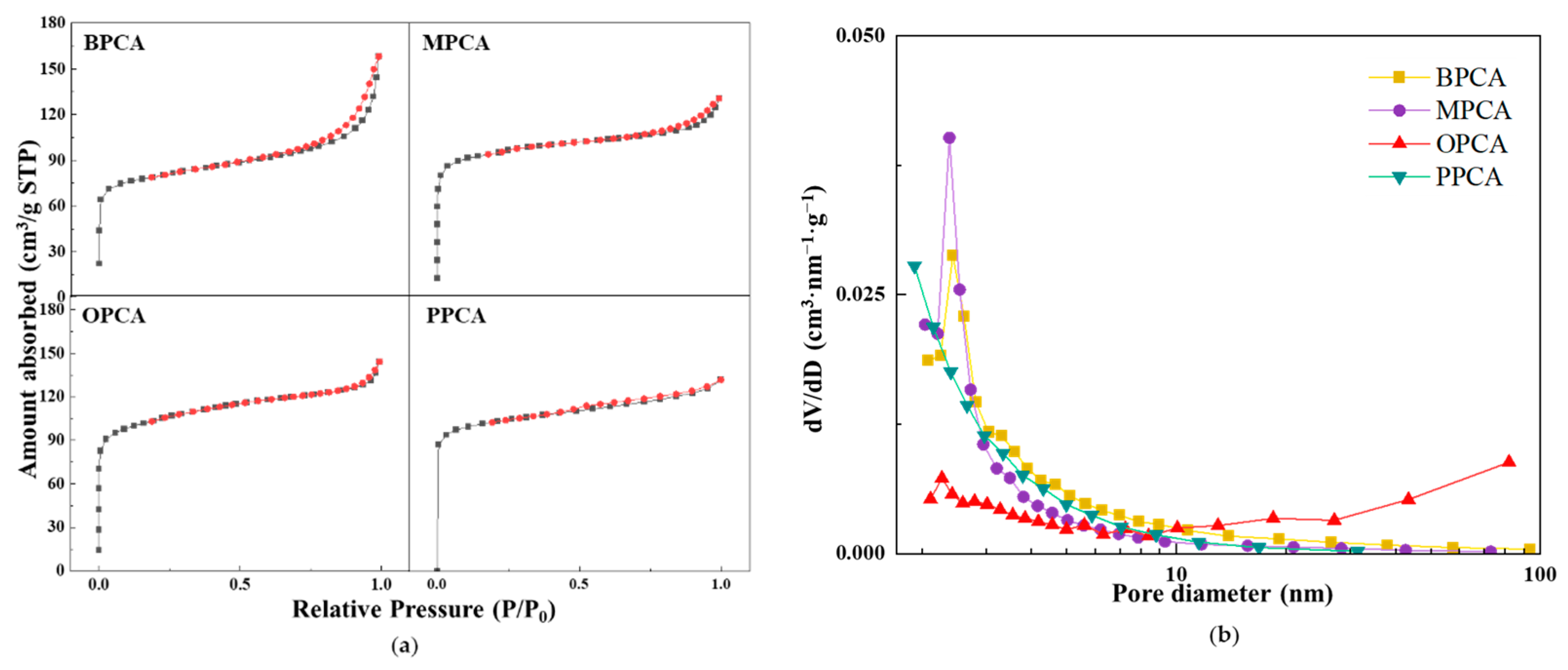
3.2.2. Pore Structures of Different CAs Prepared from Different Waste Biomasses
3.3. Inorganic Components of CAs Prepared from Different Waste Biomasses
3.4. Mechanism of Ethanol Cracking Performance for H2 Production with CAs Prepared from Different Waste Biomasses
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| A | Ash |
| AD | The peak area of the D band |
| AG | The peak area of the G band |
| A(VR + VL + GR) | The peak area of the VR, VL, and GR band |
| A(gases) | Amount of gases |
| A(N2) | Amount of N2 |
| AAEMs | Alkali metals and alkaline earth metals |
| BET | Beunauer–Emmett–Teller |
| BJH | Barret–Joyner–Halenda |
| BP | Banana peel |
| BPCA | Banana peel carbon aerogel |
| CA | Carbon aerogel |
| FC | Fixed carbon |
| FT-IR | Fourier transform infrared spectroscopy |
| IUPAC | International Union of Pure and Applied Chemistry |
| M | Moisture |
| MP | Mangosteen peel |
| MPCA | Mangosteen peel carbon aerogel |
| MSW | Municipal solid waste |
| The moles of H atoms | |
| The moles of H atoms in ethanol | |
| OP | Orange peel |
| OPCA | Orange peel carbon aerogel |
| PP | Pomelo peel |
| GC | Gas chromatography |
| PPCA | Pomelo peel carbon aerogel |
| VM | Volatile matters |
| XRF | X-ray fluorescence spectrometer |
References
- Beyene, H.D.; Werkneh, A.A.; Ambaye, T.G. Current updates on waste to energy (WtE) technologies: A review. Renew. Energy Focus 2018, 24, 1–11. [Google Scholar] [CrossRef]
- Rana, R.; Ganguly, R.; Gupta, A.K. Physico-chemical characterization of municipal solid waste from Tricity region of Northern India: A case study. J. Mater. Cycles Waste Manag. 2017, 20, 678–689. [Google Scholar] [CrossRef]
- Nanda, S.; Berruti, F. Municipal solid waste management and landfilling technologies: A review. Environ. Chem. Lett. 2020, 19, 1433–1456. [Google Scholar] [CrossRef]
- Uçkun Kiran, E.; Trzcinski, A.P.; Ng, W.J.; Liu, Y. Bioconversion of food waste to energy: A review. Fuel 2014, 134, 389–399. [Google Scholar] [CrossRef]
- Pradhan, S.; Abdelaal, A.H.; Mroue, K.; Al-Ansari, T.; Mackey, H.R.; McKay, G. Biochar from vegetable wastes: Agro-environmental characterization. Biochar 2020, 2, 439–453. [Google Scholar] [CrossRef]
- Lewoyehu, M. Comprehensive review on synthesis and application of activated carbon from agricultural residues for the remediation of venomous pollutants in wastewater. J. Anal. Appl. Pyrolysis 2021, 159, 105279. [Google Scholar] [CrossRef]
- Sakhiya, A.K.; Anand, A.; Kaushal, P. Production, activation, and applications of biochar in recent times. Biochar 2020, 2, 253–285. [Google Scholar] [CrossRef]
- Nizamuddin, S.; Baloch, H.A.; Griffin, G.; Mubarak, N.; Bhutto, A.W.; Abro, R.; Mazari, S.A.; Ali, B.S. An overview of effect of process parameters on hydrothermal carbonization of biomass. Renew. Sustain. Energy Rev. 2017, 73, 1289–1299. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Z.; Wang, X.; Li, N.; Tao, J.; Zheng, W.; Yan, B.; Cui, X.; Cheng, Z.; Chen, G. A Review on the Hydrothermal Treatment of Food Waste: Processing and Applications. Processes 2022, 10, 2439. [Google Scholar] [CrossRef]
- Shen, Y. A review on hydrothermal carbonization of biomass and plastic wastes to energy products. Biomass Bioenergy 2020, 134, 105479. [Google Scholar] [CrossRef]
- Cakan, R.D.; Titirici, M.-M.; Antonietti, M.; Cui, G.; Maier, J.; Hu, Y.-S. Hydrothermal carbon spheres containing silicon nanoparticles: Synthesis and lithium storage performance. Chem. Commun. 2008, 32, 3759–3761. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.H.; Cui, X.J.; Li, L.L.; Li, K.; Yu, B.; Antonietti, M.; Cölfen, H. From starch to metal/carbon hybrid nanostructures: Hydrothermal metal-catalyzed carbonization. Adv. Mater. 2004, 16, 1636–1640. [Google Scholar] [CrossRef]
- Titirici, M.-M.; Antonietti, M.; Baccile, N.J. Hydrothermal carbon from biomass: A comparison of the local structure from poly-to monosaccharides and pentoses/hexoses. Green Chem. 2008, 10, 1204–1212. [Google Scholar] [CrossRef]
- Titirici, M.M.; Thomas, A.; Yu, S.H.; Müller, J.O.; Antonietti, M. Back in the black: Hydrothermal carbonization of plant material as an efficient chemical process to treat the CO2 problem? New J. Chem. 2007, 31, 787–789. [Google Scholar] [CrossRef]
- Cao, L.; Zhang, C.; Chen, H.; Tsang, D.C.W.; Luo, G.; Zhang, S.; Chen, J. Hydrothermal liquefaction of agricultural and forestry wastes: State-of-the-art review and future prospects. Bioresour. Technol. 2017, 245 Pt A, 1184–1193. [Google Scholar] [CrossRef]
- Kong, L.; Li, G.; Zhang, B.; He, W.; Wang, H. Hydrogen Production from Biomass Wastes by Hydrothermal Gasification. Energy Source Part A 2008, 30, 1166–1178. [Google Scholar] [CrossRef]
- Zhu, L.; Wang, Y.; Wang, Y.; You, L.; Shen, X.; Li, S. An environmentally friendly carbon aerogels derived from waste pomelo peels for the removal of organic pollutants/oils. Microporous Mesoporous Mater. 2017, 241, 285–292. [Google Scholar] [CrossRef]
- Madusari, S.; Jamari, S.S.; Nordin, N.I.A.A.; Bindar, Y.; Prakoso, T.; Restiawaty, E.; Steven, S. Hybrid Hydrothermal Carbonization and Ultrasound Technology on Oil Palm Biomass for Hydrochar Production. ChemBioEng Rev. 2022, 10, 37–54. [Google Scholar] [CrossRef]
- Fu, L.; Zhu, J.; Huang, W.; Fang, J.; Sun, X.; Wang, X.; Liao, K. Preparation of Nano-Porous Carbon-Silica Composites and Its Adsorption Capacity to Volatile Organic Compounds. Processes 2020, 8, 372. [Google Scholar] [CrossRef]
- Gong, F.; Li, H.; Wang, W.; Huang, J.; Xia, D.; Liao, J.; Wu, M.; Papavassiliou, D.V. Scalable, eco-friendly and ultrafast solar steam generators based on one-step melamine-derived carbon sponges toward water purification. Nano Energy 2019, 58, 322–330. [Google Scholar] [CrossRef]
- Wang, W.; Li, K.; Song, G.; Zhou, M.; Tan, P. Activated Carbon Aerogel as an Electrode with High Specific Capacitance for Capacitive Deionization. Processes 2022, 10, 2330. [Google Scholar] [CrossRef]
- Yang, M.; Wang, P.; Li, Y.; Tang, S.; Lin, X.; Zhang, H.; Zhu, Z.; Chen, F. Graphene aerogel-based NiAl-LDH/g-C3N4 with ultratight sheet-sheet heterojunction for excellent visible-light photocatalytic activity of CO2 reduction. Appl. Catal. B-Environ. 2022, 306, 121065. [Google Scholar] [CrossRef]
- Yu, S.; Song, S.; Li, R.; Fang, B. The lightest solid meets the lightest gas: An overview of carbon aerogels and their composites for hydrogen related applications. Nanoscale 2020, 12, 19536–19556. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Liu, J.; Zhang, X.; Xie, W.; Sun, J.; Chang, K.; Kuo, J.; Xie, W.; Liu, C.; Sun, S.; et al. Co-combustion thermal conversion characteristics of textile dyeing sludge and pomelo peel using TGA and artificial neural networks. Appl. Energy 2018, 212, 786–795. [Google Scholar] [CrossRef]
- Dayarathna, S.G.A.R.M.; Karunarathna, B. Effect of Different Fruit Peel Powders as Natural Fertilizers on Growth of Okra (Abelmoschus esculentus L.). J. Agric. Sci.-Sri Lanka 2021, 16, 67–79. [Google Scholar] [CrossRef]
- Wei, Y.; Fakudze, S.; Zhang, Y.; Ma, R.; Shang, Q.; Chen, J.; Liu, C.; Chu, Q. Co-hydrothermal carbonization of pomelo peel and PVC for production of hydrochar pellets with enhanced fuel properties and dechlorination. Energy 2022, 239, 122350. [Google Scholar] [CrossRef]
- Pathak, P.D.; Mandavgane, S.A.; Kulkarni, B.D.J.C.S. Fruit peel waste: Characterization and its potential uses. Curr. Sci. India 2017, 113, 444–454. [Google Scholar] [CrossRef]
- Rattanahirun, S.; Khamwichit, A.; Dechapanya, W. Quality improvement of syngas from gasification process of palm kernel using NiO/CaO catalysts on ceramic supporter in coupled with biochar absorbent from agricultural residues. IOP Conf. Ser. Earth Environ. Sci. 2020, 463, 012131. [Google Scholar] [CrossRef]
- Ullah, H.; Lun, L.; Riaz, L.; Naseem, F.; Shahab, A.; Rashid, A. Physicochemical characteristics and thermal degradation behavior of dry and wet torrefied orange peel obtained by dry/wet torrefaction. Biomass Convers. Biorefin. 2021, 1–17. [Google Scholar] [CrossRef]
- Fatsikostas, A. Reaction network of steam reforming of ethanol over Ni-based catalysts. J. Catal. 2004, 225, 439–452. [Google Scholar] [CrossRef]
- Keiluweit, M.; Nico, P.S.; Johnson, M.G.; Kleber, M. Dynamic molecular structure of plant biomass-derived black carbon (biochar). Environ. Sci. Technol. 2010, 44, 1247–1253. [Google Scholar] [CrossRef] [PubMed]
- Ren, Q.; Zhang, J.; Hu, S.; Ma, S.; Huang, R.; Su, S.; Wang, Y.; Jiang, L.; Xu, J.; Xiang, J. Novel photothermal pyrolysis on waste tire to generate high-yield limonene. Fuel 2022, 329, 125482. [Google Scholar] [CrossRef]
- Cantrell, K.B.; Hunt, P.G.; Uchimiya, M.; Novak, J.M.; Ro, K.S. Impact of pyrolysis temperature and manure source on physicochemical characteristics of biochar. Bioresour. Technol. 2012, 107, 419–428. [Google Scholar] [CrossRef]
- Su, C.; Acik, M.; Takai, K.; Lu, J.; Hao, S.-J.; Zheng, Y.; Wu, P.; Bao, Q.; Enoki, T.; Chabal, Y.J.; et al. Probing the catalytic activity of porous graphene oxide and the origin of this behaviour. Nat. Commun. 2012, 3, 1298. [Google Scholar] [CrossRef] [PubMed]
- Thushari, I.; Babel, S.; Samart, C. Biodiesel production in an autoclave reactor using waste palm oil and coconut coir husk derived catalyst. Renew. Energy 2019, 134, 125–134. [Google Scholar] [CrossRef]
- Chen, Y.; Syed-Hassan, S.S.A.; Xiong, Z.; Li, Q.; Hu, X.; Xu, J.; Ren, Q.; Deng, Z.; Wang, X.; Su, S.; et al. Temporal and spatial evolution of biochar chemical structure during biomass pellet pyrolysis from the insights of micro-Raman spectroscopy. Fuel Process. Technol. 2021, 218, 106839. [Google Scholar] [CrossRef]
- Guizani, C.; Haddad, K.; Limousy, L.; Jeguirim, M. New insights on the structural evolution of biomass char upon pyrolysis as revealed by the Raman spectroscopy and elemental analysis. Carbon 2017, 119, 519–521. [Google Scholar] [CrossRef]
- Ren, Q.; Wu, Z.; Hu, S.; He, L.; Su, S.; Wang, Y.; Jiang, L.; Xiang, J. Sulfur self-doped char with high specific capacitance derived from waste tire: Effects of pyrolysis temperature. Sci. Total Environ. 2020, 741, 140193. [Google Scholar] [CrossRef]
- Li, X.; Hayashi, J.; Li, C. FT-Raman spectroscopic study of the evolution of char structure during the pyrolysis of a Victorian brown coal. Fuel 2006, 85, 1700–1707. [Google Scholar] [CrossRef]
- Ren, Q.; Hu, S.; Li, Q.; Zhang, J.; Han, H.; Su, S.; Wang, Y.; Jiang, L.; Xu, J.; Xiang, J. Treating waste tire to prepare high-yield sulfur-doped porous char via ZnCl2–KOH heat treatment method. J. Clean Prod. 2022, 372, 133672. [Google Scholar] [CrossRef]
- Liu, R.; Xi, X.; Xing, X.; Wu, D. A facile biomass based approach towards hierarchically porous nitrogen-doped carbon aerogels. RSC Adv. 2016, 6, 83613–83618. [Google Scholar] [CrossRef]
- Yang, J.; Fu, L.; Wu, F.; Chen, X.; Wu, C.; Wang, Q. Recent developments in activated carbon catalysts based on pore size regulation in the application of catalytic ozonation. Catalysts 2022, 12, 1085. [Google Scholar] [CrossRef]
- Long, J.; Song, H.; Jun, X.; Sheng, S.; Lun-Shi, S.; Kai, X.; Yao, Y. Release characteristics of alkali and alkaline earth metallic species during biomass pyrolysis and steam gasification process. Bioresour. Technol. 2012, 116, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Jiang, L.; Wang, Y.; Su, S.; Sun, L.; Xu, B.; He, L.; Xiang, J. Effects of inherent alkali and alkaline earth metallic species on biomass pyrolysis at different temperatures. Bioresour. Technol. 2015, 192, 23–30. [Google Scholar] [CrossRef]
- Wang, W.; Lemaire, R.; Bensakhria, A.; Luart, D. Review on the catalytic effects of alkali and alkaline earth metals (AAEMs) including sodium, potassium, calcium and magnesium on the pyrolysis of lignocellulosic biomass and on the co-pyrolysis of coal with biomass. J. Anal. Appl. Pyrolysis 2022, 163, 105479. [Google Scholar] [CrossRef]
- Xue, T.; Fakudze, S.; Ma, R.; Han, J.; Chu, Q.; Zhou, P.; Chen, J. Comparative study of pomelo-peel-derived hydrochar and torrefied poultry-litter on coal fuel blends: Combustibility, synergy factor, and ash analysis. Biofuel Bioprod. Bior. 2022, 16, 1240–1253. [Google Scholar] [CrossRef]
- Hu, B.; Wang, K.; Wu, L.; Yu, S.H.; Antonietti, M.; Titirici, M.M. Engineering carbon materials from the hydrothermal carbonization process of biomass. Adv Mater. 2010, 22, 813–828. [Google Scholar] [CrossRef]
- Shandilya, M.; Rai, R.; Singh, J. Review: Hydrothermal technology for smart materials. Adv. Appl. Ceram. 2016, 115, 354–376. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, F.; Zhu, H.; Zhou, L.; Zheng, X.; Li, X.; Chen, Z.; Wang, Y.; Zhang, D.; Pan, D. Preparation of nitrogen-doped biomass-derived carbon nanofibers/graphene aerogel as a binder-free electrode for high performance supercapacitors. Appl. Surf. Sci. 2017, 426, 99–106. [Google Scholar] [CrossRef]
- Norinaga, K.; Shoji, T.; Kudo, S.; Hayashi, J.-I. Detailed chemical kinetic modelling of vapour-phase cracking of multi-component molecular mixtures derived from the fast pyrolysis of cellulose. Fuel 2013, 103, 141–150. [Google Scholar] [CrossRef]
- Jankhah, S.; Abatzoglou, N.; Gitzhofer, F. Thermal and catalytic dry reforming and cracking of ethanol for hydrogen and carbon nanofilaments’ production. Int. J. Hydrogen Energy 2008, 33, 4769–4779. [Google Scholar] [CrossRef]


| Feedstock | Proximate Analysis (Dry Basis, wt%) | Ultimate Analysis (Dry Basis, wt%) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| M a | V b | FC c | A d | C | H | N | S | O e | |
| Banana peel | 1.26 | 64.95 | 21.46 | 12.34 | 42.51 | 5.10 | 1.69 | 0.02 | 37.08 |
| Mangosteen peel | 0.97 | 63.73 | 32.53 | 2.78 | 51.81 | 4.95 | 0.50 | 0.05 | 38.95 |
| Orange peel | 3.66 | 75.27 | 17.73 | 3.35 | 44.78 | 5.40 | 0.98 | 0.03 | 41.81 |
| Pomelo peel | 5.47 | 74.46 | 16.46 | 3.61 | 40.52 | 5.51 | 0.81 | 0.06 | 44.02 |
| Sample | Specific Surface Area (m2/g) | Pore Volume (cm3/g) | Average Pore Diameter (nm) |
|---|---|---|---|
| BPCA | 246.80 | 0.24 | 3.91 |
| MPCA | 294.94 | 0.20 | 2.72 |
| OPCA | 329.33 | 0.22 | 2.67 |
| PPCA | 309.84 | 0.19 | 2.50 |
| Sample | Ash Content (wt%) | Inorganic Element Content (wt%) | ||||
|---|---|---|---|---|---|---|
| Mg | Cl | K | Ca | Other | ||
| BPCA | 9.67 | 0.01 | 0.68 | 4.91 | 3.88 | 0.19 |
| MPCA | 3.86 | 0.13 | 0.07 | 2.77 | 0.65 | 0.23 |
| OPCA | 4.43 | 0.33 | - | 0.54 | 3.21 | 0.35 |
| PPCA | 9.42 | 0.17 | 0.10 | 6.42 | 2.32 | 0.41 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Hu, S.; Ding, Y.; Huang, R.; Ren, Q.; Su, S.; Wang, Y.; Jiang, L.; Xu, J.; Xiang, J. Waste Biomass Based Carbon Aerogels Prepared by Hydrothermal-carbonization and Their Ethanol Cracking Performance for H2 Production. Processes 2023, 11, 892. https://doi.org/10.3390/pr11030892
Zhang J, Hu S, Ding Y, Huang R, Ren Q, Su S, Wang Y, Jiang L, Xu J, Xiang J. Waste Biomass Based Carbon Aerogels Prepared by Hydrothermal-carbonization and Their Ethanol Cracking Performance for H2 Production. Processes. 2023; 11(3):892. https://doi.org/10.3390/pr11030892
Chicago/Turabian StyleZhang, Jialin, Song Hu, Yong Ding, Rui Huang, Qiangqiang Ren, Sheng Su, Yi Wang, Long Jiang, Jun Xu, and Jun Xiang. 2023. "Waste Biomass Based Carbon Aerogels Prepared by Hydrothermal-carbonization and Their Ethanol Cracking Performance for H2 Production" Processes 11, no. 3: 892. https://doi.org/10.3390/pr11030892
APA StyleZhang, J., Hu, S., Ding, Y., Huang, R., Ren, Q., Su, S., Wang, Y., Jiang, L., Xu, J., & Xiang, J. (2023). Waste Biomass Based Carbon Aerogels Prepared by Hydrothermal-carbonization and Their Ethanol Cracking Performance for H2 Production. Processes, 11(3), 892. https://doi.org/10.3390/pr11030892











