A Critical Evaluation of Recent Studies on Packed-Bed Bioreactors for Solid-State Fermentation
Abstract
1. Introduction
2. Traditional Designs for Packed-Bed Bioreactors
2.1. Will Jacketed Packed-Bed Bioreactors Be Useful at a Large Scale?
2.2. The Uniformity of Airflow through Packed-Bed Bioreactors Is Important
2.3. High Porosity and High Superficial Air Velocities Can Lead to Good Temperature Control
2.4. Intermittent Agitation of Packed-Bed Bioreactors Can Improve Product Uniformity
2.5. The Structured (Multi-Layered) Packed Bed Has Received a Revival of Interest
3. Packed-Bed Bioreactors with Novel Features
3.1. A Disposable Packed-Bed Bioreactor Is an Interesting New Design for Niche Applications
3.2. A Continuous Packed-Bed Bioreactor Might Find Niche Applications in Biorefineries
3.3. A Packed-Bed Bioreactor with a Helical Screw and Intermittent Trickling of Liquid
3.4. Several Designs Have Been Proposed with Air Blown into the Bed through Perforated Tubes
4. Mathematical Modeling of Packed-Bed Bioreactors
4.1. “Two-Phase” Models
4.2. Models Based on Computational Fluid Dynamics (CFD)
4.3. Mathematical Models as Tools for Estimating Solids–Air Heat and Mass Transfer Coefficients
4.4. Estimation of Other Parameters That Are Important in Heat and Mass Transfer Models
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mitchell, D.A.; Berovic, M.; Krieger, N. Solid-State Fermentation Bioreactors: Fundamentals of Design and Operation; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Mitchell, D.A.; de Lima Luz, L.F.; Krieger, N. Bioreactors for solid-state fermentation. In Comprehensive Biotechnology, 2nd ed.; Moo-Young, M., Ed.; Elsevier: Amsterdam, The Netherlands, 2011; Volume 2, pp. 347–360. [Google Scholar]
- Zanelato, A.I.; Shiota, V.M.; Gomes, E.; da Silva, R.; Thoméo, J.C. Endoglucanase production with the newly isolated Myceliophtora sp. I-1D3b in a packed bed solid state fermentor. Braz. J. Microbiol. 2012, 43, 1536–1544. [Google Scholar] [CrossRef] [PubMed]
- Casciatori, F.P.; Thoméo, J.C. Heat transfer in packed-beds of agricultural waste with low rates of air flow applicable to solid-state fermentation. Chem. Eng. Sci. 2018, 188, 97–111. [Google Scholar] [CrossRef]
- Ranjbar, S.; Hejazi, P. Modeling and validating Pseudomonas aeruginosa kinetic parameters based on simultaneous effect of bed temperature and moisture content using lignocellulosic substrate in packed-bed bioreactor. Food Bioprod. Process. 2019, 117, 51–63. [Google Scholar] [CrossRef]
- Perez, C.L.; Casciatori, F.P.; Thoméo, J.C. Strategies for scaling-up packed-bed bioreactors for solid-state fermentation: The case of cellulolytic enzymes production by a thermophilic fungus. Chem. Eng. J. 2019, 361, 1142–1151. [Google Scholar] [CrossRef]
- Cunha, L.P.; Casciatori, F.P.; Vincente, I.V.; Garcia, R.L.; Thoméo, J.C. Metarhizium anisopliae conidia production in packed-bed bioreactor using rice as substrate in successive cultivations. Process Biochem. 2020, 97, 104–111. [Google Scholar] [CrossRef]
- Chysirichote, T. Valorization of banana peel for citric acid production under solid state fermentation with Aspergillus niger. Chem. Biochem. Eng. Q. 2020, 34, 49–57. [Google Scholar] [CrossRef]
- Casciatori-Frassatto, P.A.; Casciatori, F.P.; Thoméo, J.C.; Gomes, E.; Boscolo, M.; da Silva, R. Fungal cellulases: Production by solid-state cultivation in packed-bed bioreactor using solid agro-industrial by-products as substrates and application for hydrolysis of sugarcane bagasse. Semin. Cienc. Agrar. 2020, 41, 2097–2116. [Google Scholar] [CrossRef]
- Pitol, L.O.; Biz, A.; Mallmann, E.; Krieger, N.; Mitchell, D.A. Production of pectinases by solid-state fermentation in a pilot-scale packed-bed bioreactor. Chem. Eng. J. 2016, 283, 1009–1018. [Google Scholar] [CrossRef]
- Finkler, A.T.J.; Weber, M.Z.; Fuchs, G.A.; Scholz, L.A.; de Lima Luz, L.F., Jr.; Krieger, N.; Mitchell, D.A.; Jorge, L.M.M. Estimation of heat and mass transfer coefficients in a pilot packed-bed solid-state fermentation bioreactor. Chem. Eng. J. 2021, 408, 127246. [Google Scholar] [CrossRef]
- Casciatori, F.P.; Bück, A.; Thoméo, J.C.; Tsotsas, E. Two-phase and two-dimensional model describing heat and water transfer during solid-state fermentation within a packed-bed bioreactor. Chem. Eng. J. 2016, 287, 103–116. [Google Scholar] [CrossRef]
- Mitchell, D.A.; Pandey, A.; Sangsurasak, P.; Krieger, N. Scale-up strategies for packed-bed bioreactors for solid-state fermentation. Process Biochem. 1999, 35, 167–178. [Google Scholar] [CrossRef]
- Roussos, S.; Raimbault, M.; Prebois, J.P.; Lonsane, B.K. Zymotis, a large scale solid state fermenter design and evaluation. Appl. Biochem. Biotechnol. 1993, 42, 37–52. [Google Scholar] [CrossRef]
- Hejazi, P.; Shojaosadati, S.A.; Hamidi-Esfahani, Z.; Vasheghani-Farahani, E. Solid State Fermentation in Modified Zymotis Packed Bed Bioreactor. U.S. Patent 2010/0203626A1, 13 April 2010. [Google Scholar]
- Finkler, A.T.J.; de Lima Luz, L.F., Jr.; Krieger, N.; Mitchell, D.A.; Jorge, L.M. A model-based strategy for scaling-up traditional packed-bed bioreactors for solid-state fermentation based on measurement of O2 uptake rates. Biochem. Eng. J. 2021, 166, 107854. [Google Scholar] [CrossRef]
- Karimi, A.; Shojaosadati, S.A.; Hejazi, P.; Vasheghani-Farahani, E.; Hashemi, M. Porosity changes during packed bed solid-state fermentation. J. Ind. Eng. Chem. 2014, 20, 4022–4027. [Google Scholar] [CrossRef]
- Piedrahíta-Aguirre, C.A.; Bastos, R.G.; Carvalho, A.L.; Monte Alegre, R. The influence of process parameters in production of lipopeptide iturin A using aerated packed bed bioreactors in solid-state fermentation. Bioprocess Biosyst. Eng. 2014, 37, 1569–1576. [Google Scholar] [CrossRef]
- Castro, A.M.; Castilho, L.R.; Freire, D.M.G. Performance of a fixed-bed solid-state fermentation bioreactor with forced aeration for the production of hydrolases by Aspergillus awamori. Biochem. Eng. J. 2015, 93, 303–308. [Google Scholar] [CrossRef]
- Melikoglu, M.; Lin, C.S.K.; Webb, C. Solid state fermentation of waste bread pieces by Aspergillus awamori: Analysing the effects of air flow rate on enzyme production in packed bed bioreactors. Food Bioprod. Process. 2015, 95, 63–75. [Google Scholar] [CrossRef]
- Tsotsas, E.; Schluender, E.U. On axial dispersion in packed beds with fluid flow. Chem. Eng. Process. 1988, 24, 15–31. [Google Scholar] [CrossRef]
- Tsotsas, E. On mass transfer, dispersion, and macroscopical flow maldistribution in packed tubes. Chem. Eng. Process. 1992, 31, 181–190. [Google Scholar] [CrossRef]
- Gómez-Ramos, G.A.; Castillo-Araiza, C.O.; Huerta-Ochoa, S.; Couder-García, M.; Prado-Barragán, A. Assessment of hydrodynamics in a novel bench-scale wall-cooled packed bioreactor under abiotic conditions. Chem. Eng. J. 2019, 375, 121945. [Google Scholar] [CrossRef]
- Pessoa, D.R.; Finkler, A.T.J.; Machado, A.V.L.; Luz, L.F.L., Jr.; Mitchell, D.A. Fluid dynamics simulation of a pilot-scale solid-state fermentation bioreactor. Chem. Eng. Trans. 2016, 49, 49–54. [Google Scholar] [CrossRef]
- Pessoa, D.R.; Finkler, A.T.J.; Machado, A.V.L.; Mitchell, D.A.; Luz, L.F.L., Jr. CFD simulation of a packed-bed solid-state fermentation bioreactor. Appl. Math. Model. 2019, 70, 439–458. [Google Scholar] [CrossRef]
- Van Breukelen, F.R.; Haemers, S.; Wijffels, R.H.; Rinzema, A. Bioreactor and substrate selection for solid-state cultivation of the malaria mosquito control agent Metarhizium anisopliae. Process Biochem. 2011, 46, 751–757. [Google Scholar] [CrossRef]
- Sala, A.; Barrena, R.; Sánchez, A.; Artola, A. Fungal biopesticide production: Process scale-up and sequential batch mode operation with Trichoderma harzianum using agro-industrial solid wastes of different biodegradability. Chem. Eng. J. 2021, 425, 131620. [Google Scholar] [CrossRef]
- Sala, A.; Echegaray, T.; Palomas, G.; Boggione, M.J.; Tubio, G.; Barrena, R.; Artola, A. Insights on fungal solid-state fermentation for waste valorization: Conidia and chitinase production in different reactor configurations. Sustain. Chem. Pharm. 2022, 26, 100624. [Google Scholar] [CrossRef]
- Biz, A.; Finkler, A.T.J.; Pitol, L.O.; Medina, B.S.; Krieger, N.; Mitchell, D.A. Production of pectinases by solid-state fermentation of a mixture of citrus waste and sugarcane bagasse in a pilot-scale packed-bed bioreactor. Biochem. Eng. J. 2016, 111, 54–62. [Google Scholar] [CrossRef]
- Pitol, L.O.; Finkler, A.T.J.; Dias, G.S.; Machado, A.S.; Zanin, G.M.; Mitchell, D.A.; Krieger, N. Optimization studies to develop a low-cost medium for production of the lipases of Rhizopus microsporus by solid-state fermentation and scale-up of the process to a pilot packed-bed bioreactor. Process Biochem. 2017, 62, 37–47. [Google Scholar] [CrossRef]
- Weber, F.J.; Tramper, J.; Rinzema, A. A simplified material and energy balance approach for process development and scale-up of Coniothyrium minitans conidia production by solid-state cultivation in a packed-bed reactor. Biotechnol. Bioeng. 1999, 65, 447–458. [Google Scholar] [CrossRef]
- Virtanen, V.; Nyyssölä, A.; Leisola, M.; Seiskari, P. An aseptically operatable static solid state bioreactor consisting of two units. Biochem. Eng. J. 2008, 39, 594–597. [Google Scholar] [CrossRef]
- Mazutti, M.A.; Zabot, G.; Boni, G.; Skovronski, A.; Oliveira, D.; Luccio, M.; Rodrigues, M.I.; Treichel, H.; Maugeri, F. Kinetics of inulinase production by solid-state fermentation in a packed-bed bioreactor. Food Chem. 2010, 120, 163–173. [Google Scholar] [CrossRef]
- Mazutti, M.A.; Zabot, G.; Boni, G.; Skovronski, A.; Oliveira, D.; Luccio, M.; Rodrigues, M.I.; Maugeri, F.; Treichel, H. Mathematical modeling of Kluyveromyces marxianus growth in solid-state fermentation using a packed-bed bioreactor. Ind. Microbiol. Biotechnol. 2010, 37, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Srivastava, N.; Gupta, B.S.; Kuhad, R.C.; Gomes, J. Lovastatin production by Aspergillus terreus using lignocellulose biomass in large scale packed bed reactor. Food Bioprod. Process. 2014, 92, 416–424. [Google Scholar] [CrossRef]
- Silveira, C.L.; Mazutti, M.A.; Salau, N.P.G. Modeling the microbial growth and temperature profile in a fixed-bed bioreactor. Bioprocess Biosyst. Eng. 2014, 37, 1945–1954. [Google Scholar] [CrossRef] [PubMed]
- Manan, M.A.; Webb, C. Control strategies with variable air arrangements, forcefully aerated in single circular tray solid state bioreactors with modified Gompertz model and analysis of a distributed parameter gas balance. Biotechnol. Biotechnol. Equip. 2018, 32, 1455–1467. [Google Scholar] [CrossRef]
- Barrera, M.C.; Gómez, M.I.; Serrato Bermúdez, J.C. Towards the production of fungal biocontrol candidates using inert supports: A case of study of Trichoderma asperellum in a pilot fixed bed fermenter. Biocontrol Sci. Technol. 2019, 29, 162–184. [Google Scholar] [CrossRef]
- Zhang, X.; Jiang, W. Development and temperature gradient online monitoring of a vehicular rotary solid-state bioreactor: A novel device for large-scale preparation of Aspergillus niger spore inoculum. J. Chem. Technol. Biotechnol. 2019, 94, 3883–3894. [Google Scholar] [CrossRef]
- Nascimento, F.V.; Castro, A.M.; Secchi, A.R.; Coelho, M.A.Z. Insights into media supplementation in solid-state fermentation of soybean hulls by Yarrowia lipolytica: Impact on lipase production in tray and insulated packed-bed bioreactors. Biochem. Eng. J. 2021, 166, 107866. [Google Scholar] [CrossRef]
- Álvarez Pallín, M.; González-Rodríguez, S.; Eibes, G.; López-Abelairas, M.; Moreira, M.T.; Lema, J.M.; Lú-Chau, T.A. Towards industrial application of fungal pretreatment in 2G biorefinery: Scale-up of solid-state fermentation of wheat straw. Biomass Conv. Bioref. 2022, in press. [Google Scholar] [CrossRef]
- Bhattacharya, R.; Arora, S.; Ghosh, S. Utilization of waste pine needles for the production of cellulolytic enzymes in a solid state fermentation bioreactor and high calorific value fuel pellets from fermented residue: Towards a biorefinery approach. Renew. Energy 2022, 195, 1064–1076. [Google Scholar] [CrossRef]
- Finkler, A.T.J.; Biz, A.; Pitol, L.O.; Medina, B.S.; Luithardt, H.; Luz, L.F.L., Jr.; Krieger, N.; Mitchell, D.A. Intermittent agitation contributes to uniformity across the bed during pectinase production by Aspergillus niger grown in solid-state fermentation in a pilot-scale packed-bed bioreactor. Biochem. Eng. J. 2017, 121, 1–12. [Google Scholar] [CrossRef]
- Arora, S.; Dubey, M.; Singh, P.; Rani, R.; Ghosh, S. Effect of mixing events on the production of a thermo-tolerant and acid-stable phytase in a novel solid-state fermentation bioreactor. Process Biochem. 2017, 61, 12–23. [Google Scholar] [CrossRef]
- Arora, S.; Singh, P.; Rani, R.; Ghosh, S. Oxygen uptake rate as a tool for on-line estimation of cell biomass and bed temperature in a novel solid-state fermentation bioreactor. Bioprocess Biosyst. Eng. 2018, 41, 917–929. [Google Scholar] [CrossRef]
- Zhang, C.; Khan, R.A.A.; Wei, H.Y.; Wang, R.; Hou, J.M.; Liu, T. Rapid and mass production of biopesticide Trichoderma Brev T069 from cassava peels using newly established solid-state fermentation bioreactor system. J. Environ. Manage. 2022, 313, 114981. [Google Scholar] [CrossRef] [PubMed]
- Schutyser, M.A.I.; Pagter, P.; Weber, F.J.; Briels, W.J.; Boom, R.M.; Rinzema, A. Substrate aggregation due to aerial hyphae during discontinuously mixed solid-state fermentation with fermentation with Aspergillus oryzae: Experiments and modeling. Biotechnol. Bioeng. 2003, 83, 503–513. [Google Scholar] [CrossRef]
- Lu, M.Y.; Maddox, I.S.; Brooks, J.D. Application of a multi-layer packed-bed reactor to citric acid production in solid-state fermentation using Aspergillus niger. Process Biochem. 1998, 33, S0032–S9592. [Google Scholar] [CrossRef]
- Lüth, P.; Eiben, U. Solid-State Fermenter and Method for Solid-State Fermentation. World Patent No. WO 99/57239, 27 April 1999. [Google Scholar]
- Vargas, W.L.; Murcia, J.C.; Palacio, L.E.; Dominguez, D.M. Fractional diffusion model for force distribution in static granular media. Phys. Rev. E 2003, 68, 021302. [Google Scholar] [CrossRef] [PubMed]
- Chysirichote, T. Cellulase production by Aspergillus niger ATCC 16888 on copra waste from coconut milk process in layered packed-bed bioreactor. Chem. Biochem. Eng. Q. 2018, 32, 267–274. [Google Scholar] [CrossRef]
- Casciatori, F.P.; Casciatori, P.A.; Thoméo, J.C. Cellulase production in packed bed bioreactor by solid-state fermentation. In Proceedings of the 21st European Biomass Conference and Exhibition, Copenhagen, Denmark, 3–7 June 2013; pp. 1539–1546. Available online: http://www.etaflorence.it/proceedings/ (accessed on 27 June 2020).
- Manan, M.A.; Webb, C. Newly designed multi-stacked circular tray solid-state bioreactor: Analysis of a distributed parameter gas balance during solid-state fermentation with influence of variable initial moisture content arrangements. Bioresour. Bioprocess. 2020, 7, 16. [Google Scholar] [CrossRef]
- Oliveira, S.P.; Rodrigues, N.A.; Casciatori-Frassatto, P.A.; Casciatori, F.P. Solid-liquid extraction of cellulases from fungal solid-state cultivation in a packed bed bioreactor. Korean. J. Chem. Eng. 2020, 37, 1530–1540. [Google Scholar] [CrossRef]
- Rodrigues, N.A.; Katayama, E.T.; Casciatori, F.P. Alternative strategies to perform solid-state cultivation in a multilayer packed-bed bioreactor: Continuous and cyclic operations. Chem. Eng. J. 2022, 448, 137726. [Google Scholar] [CrossRef]
- Mitchell, D.A.; Cunha, L.E.N.; Machado, A.V.L. A model-based investigation of the potential advantages of multi-layer packed beds in solid-state fermentation. Biochem. Eng. J. 2010, 48, 195–203. [Google Scholar] [CrossRef]
- Maïga, Y.; Carboué, Q.; Hamrouni, R.; Tranier, M.S.; Menadi, Y.B.; Roussos, S. Development and evaluation of a disposable solid-state culture packed-bed bioreactor for the production of conidia from Trichoderma asperellum grown under water stress. Waste Biomass Valorization 2022, 12, 3223–3231. [Google Scholar] [CrossRef]
- Chen, H.Z.; He, Q.; Ding, W.Y. Modeling of ethanol separation from continuous solid-state fermentation coupled with online separation by CO2 gas stripping and heat pump technology. J. Chem. Technol. Biotechnol. 2014, 90, 1897–1905. [Google Scholar] [CrossRef]
- Shahryari, Z.; Fazaelipoor, M.H.; Shaabani, M.S.; Ghasemi, Y. Production of fungal phytase in an innovative trickle bed bioreactor. Waste Biomass Valorization 2020, 11, 3273–3280. [Google Scholar] [CrossRef]
- Brijwani, K.; Vadlani, P.V.; Hohn, K.L.; Maier, D.E. Experimental and theoretical analysis of a novel deep-bed solid-state bioreactor for cellulolytic enzymes production. Biochem. Eng. J. 2011, 58–59, 110–123. [Google Scholar] [CrossRef]
- Perez, C.L.; Casciatori, F.P.; Thoméo, J.C. Improving enzyme production by solid-state cultivation in packed-bed bioreactors by changing bed porosity and airflow distribution. Bioprocess Biosyst. Eng. 2021, 44, 537–548. [Google Scholar] [CrossRef]
- Durand, A.; Chereau, D. A new pilot reactor for solid-state fermentation: Application to the protein enrichment of sugar beet pulp. Biotechnol. Bioeng. 1988, 31, 476–486. [Google Scholar] [CrossRef]
- Sangsurasak, P.; Mitchell, D.A. Validation of a model describing two-dimensional heat transfer during solid-state fermentation in packed bed bioreactors. Biotechnol. Bioeng. 1998, 60, 739–749. [Google Scholar] [CrossRef]
- Ashley, V.M.; Mitchell, D.A.; Howes, T. Evaluating strategies for overcoming overheating problems during solid-state fermentation in packed bed bioreactors. Biochem. Eng. J. 1999, 3, 141–150. [Google Scholar] [CrossRef]
- Mitchell, D.A.; von Meien, O.F. Mathematical modeling as a tool to investigate the design and operation of the Zymotis packed-bed bioreactor for solid-state fermentation. Biotechnol. Bioeng. 2000, 68, 127–135. [Google Scholar] [CrossRef]
- von Meien, O.F.; Mitchell, D.A. A two-phase model for water and heat transfer within an intermittently-mixed solid-state fermentation bioreactor with forced aeration. Biotechnol. Bioeng. 2002, 79, 416–428. [Google Scholar] [CrossRef] [PubMed]
- Bück, A.; Casciatori, F.P.; Thoméo, J.C.; Tsotsas, E. Model-based control of enzyme yield in solid-state fermentation. Procedia Eng. 2015, 102, 362–371. [Google Scholar] [CrossRef]
- von Meien, O.F.; Luz, L.F.L., Jr.; Mitchell, D.A.; Pérez-Correa, J.R.; Agosin, E.; Fernández-Fernández, M.; Arcas, J.A. Control strategies for intermittently mixed, forcefully aerated solid-state fermentation bioreactors based on the analysis of a distributed parameter model. Chem. Eng. Sci. 2004, 59, 4493–4504. [Google Scholar] [CrossRef]
- Mitchell, D.A.; von Meien, O.F.; Luz, L.F.L., Jr.; Krieger, N. Evaluation of productivity of Zymotis solid-state bioreactor based on total reactor volume. Food Technol. Biotechnol. 2002, 40, 135–144. Available online: https://www.ftb.com.hr/images/pdfarticles/2002/April-June/40-135.pdf (accessed on 29 April 2008).
- Dorai, F.; Teixeira, C.M.; Rolland, M.; Climent, E.; Marcoux, M.; Wachs, A. Fully resolved simulations of the flow through a packed bed of cylinders: Effect of size distribution. Chem. Eng. Sci. 2015, 129, 180–192. [Google Scholar] [CrossRef]
- Ngu, T.N.W.; Chu, C.M.; Janaun, J.A. Simulation of air velocity in a vertical perforated air distributor. IOP Conf. Ser. Earth Environ. Sci. 2016, 36, 012047. [Google Scholar] [CrossRef]
- Malekjani, N.; Jafari, S.M. Simulation of food drying processes by Computational Fluid Dynamics (CFD); recent advances and approaches. Trends Food Sci. Technol. 2018, 78, 206–223. [Google Scholar] [CrossRef]
- Casciatori, F.P.; Laurentino, C.L.; Taboga, S.R.; Casciatori, P.A.; Thoméo, J.C. Structural properties of beds packed with agro-industrial solid by-products applicable for solid-state fermentation: Experimental data and effects on process performance. Chem. Eng. J. 2014, 255, 214–224. [Google Scholar] [CrossRef]
- Fanaei, M.A.; Vaziri, B.M. Modeling of temperature gradients in packed-bed solid-state bioreactors. Chem. Eng. Process. Process Intensif. 2009, 48, 446–451. [Google Scholar] [CrossRef]
- Cunha, D.C.; Souza, J.A.; Rocha, L.A.O.; Costa, J.A.V. Hexahedral modular bioreactor for solid state bioprocesses. World, J. Microbiol. Biotechnol. 2009, 25, 2173–2178. [Google Scholar] [CrossRef]
- Bathe, G.A.; Patil, V.S.; Deshpande, T.D.; Gujrathi, A.M. Temperature studies in the growth of Aspergillus oryzae on jowar straw in packed-bed solid state fermenter (PBSSF)–A modeling approach. Res. Rev. J. Eng. Technol. 2013, 2, 43–49. Available online: https://www.rroij.com/open-access/temperature-studies-in-the-growth-of-aspergillus-oryzae-on-jowar-straw-in-packedbed-solid-state-fermenter-pbssf-a-modeling-approach-43-49.pdf (accessed on 20 September 2020).
- Silveira, C.L.; Mazutti, M.A.; Salau, N.P.G. Solid-state fermentation model for a packed-bed bioreactor, Blucher Chem. Eng. Proc. 2015, 1, 12998–13004. [Google Scholar] [CrossRef]
- Zolfaghari-Esmaeelabadi, M.; Hejazi, P. Dynamic mathematical modeling of heat and mass transfer incorporating with the local nutrient and biomass limitation of growth in a packed-bed solid-state bioreactor. Prep. Biochem. Biotechnol. 2019, 49, 230–243. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekar, V.; Ganapathy, S.; Karthikeyan, S.; Nambi, E.; Pandiselvam, R. Numerical modeling and simulation of temperature profiles in finger millet bed during solid state fermentation. J. Food Process Eng. 2020, 43, e13282. [Google Scholar] [CrossRef]
- Casciatori, F.P.; Laurentino, C.L.; Costa, K.K.L.; Casciatori, P.A.; Thoméo, J.C. Hygroscopic properties of orange pulp and peel. J. Food Process Eng. 2013, 36, 803–810. [Google Scholar] [CrossRef]
- Casciatori, F.P.; Laurentino, C.L.; Zanelato, A.I.; Thoméo, J.C. Hygroscopic properties of solid agro-industrial by-products used in solid-state fermentation. Ind. Crops Prod. 2015, 64, 114–123. [Google Scholar] [CrossRef]
- Marques, B.C.; Barga, M.C.; Balmant, W.; Luz, L.F.L., Jr.; Krieger, N.; Mitchell, D.A. A model of the effect of the microbial biomass on the isotherm of the fermenting solids in solid-state fermentation. Food Technol. Biotechnol. 2006, 44, 457–463. [Google Scholar]
- Casciatori, F.P.; Laurentino, C.L.; Lopes, K.C.M.; Souza, A.G.; Thoméo, J.C. Stagnant effective thermal conductivity of agro-industrial residues for solid-state fermentation. Int. J. Food Prop. 2013, 16, 1578–1593. [Google Scholar] [CrossRef]
- Canedo, M.S.; Figueiredo, M.F.S.; Thomik, M.; Vorhauer-Huget, N.; Tsotsas, E.; Thoméo, J.C. Porosity and pore size distribution of beds composed by sugarcane bagasse and wheat bran for solid-state cultivation. Powder Technol. 2021, 386, 166–175. [Google Scholar] [CrossRef]
- Singh, A.; Rodríguez Jasso, R.M.; Gonzalez-Gloria, K.D.; Rosales, M.; Cerda, R.B.; Aguilar, C.N.; Singhania, R.R.; Ruiz, H.A. The enzyme biorefinery platform for advanced biofuels production. Bioresour. Technol. Rep. 2019, 7, 100257. [Google Scholar] [CrossRef]

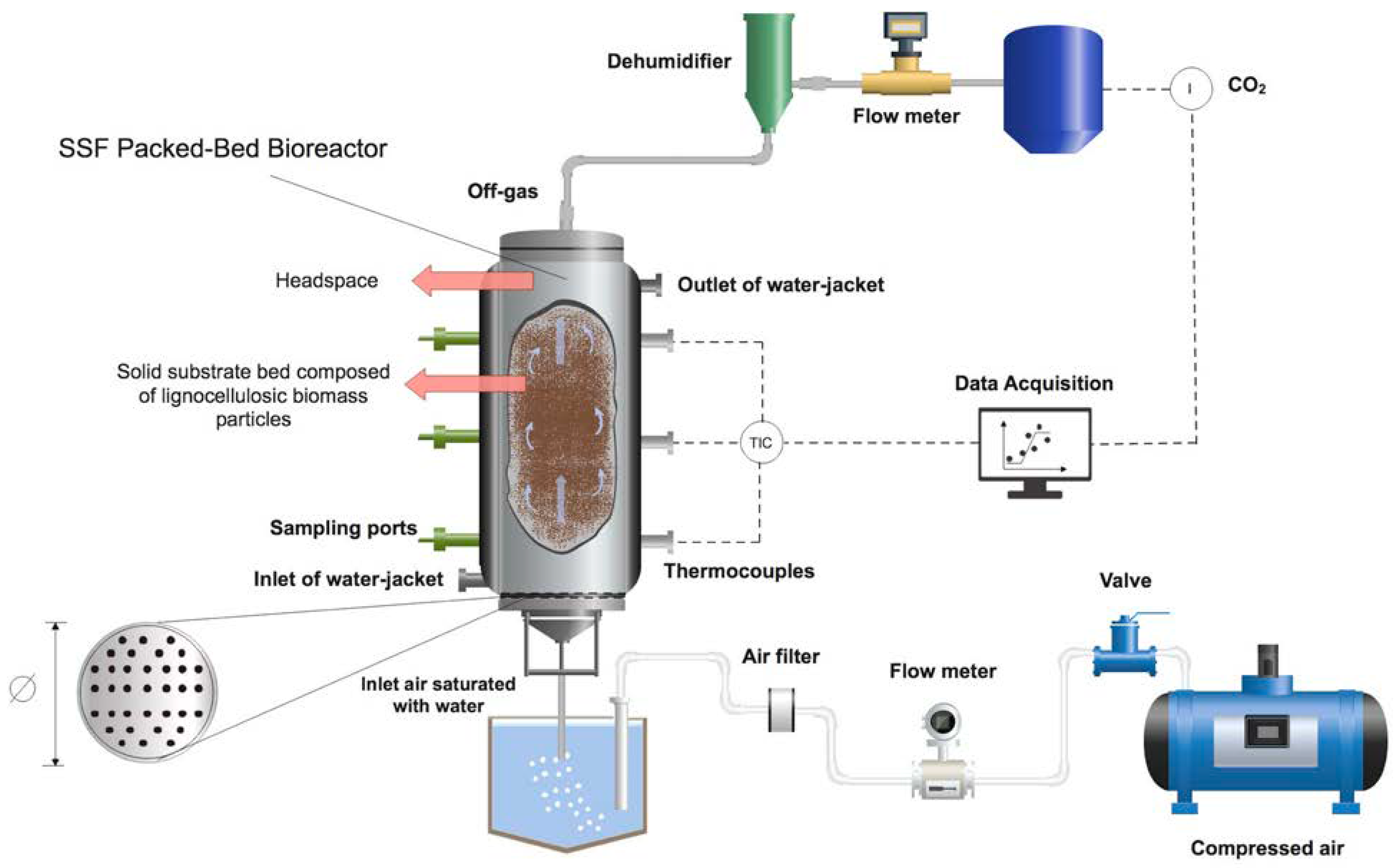
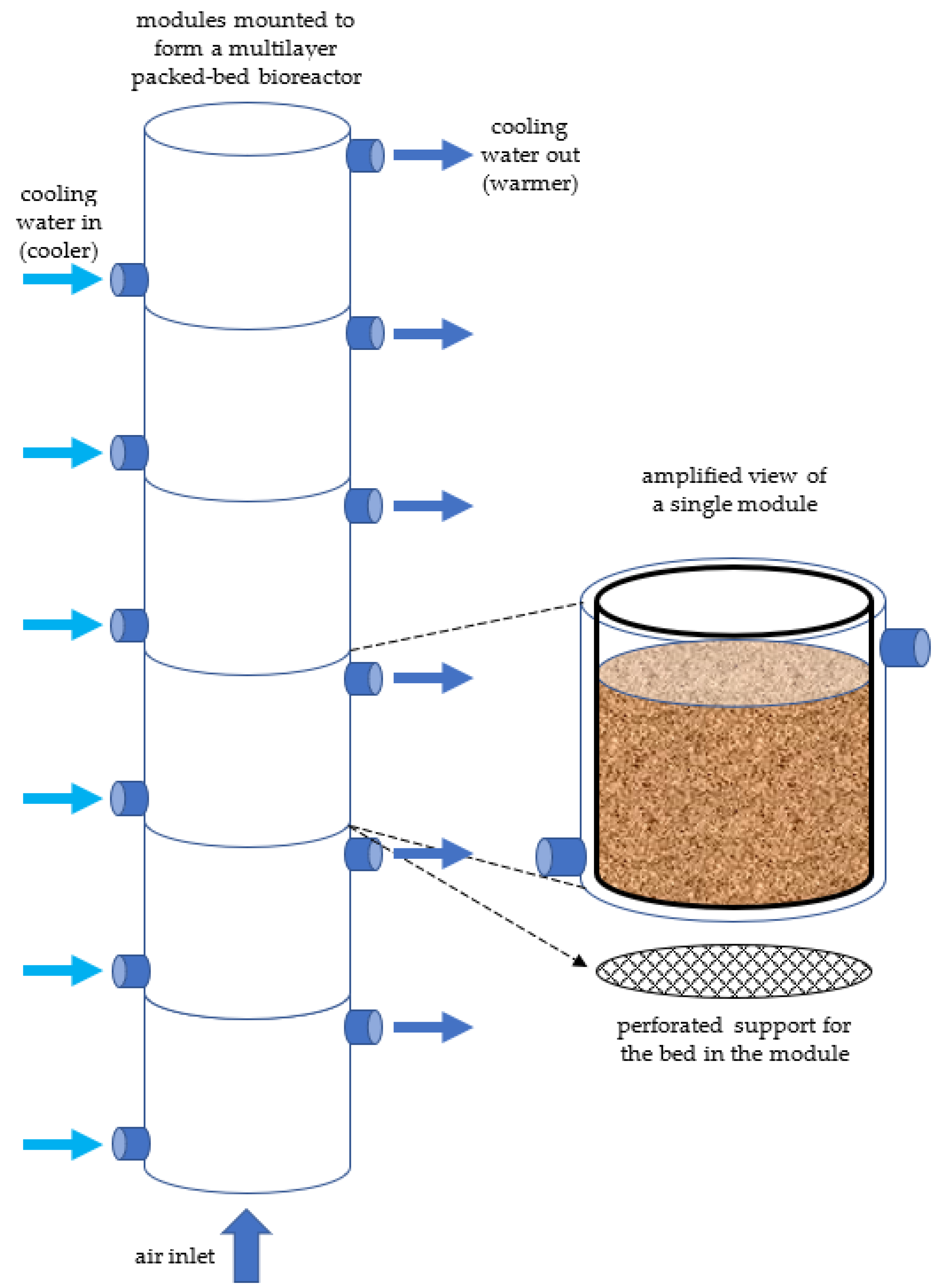

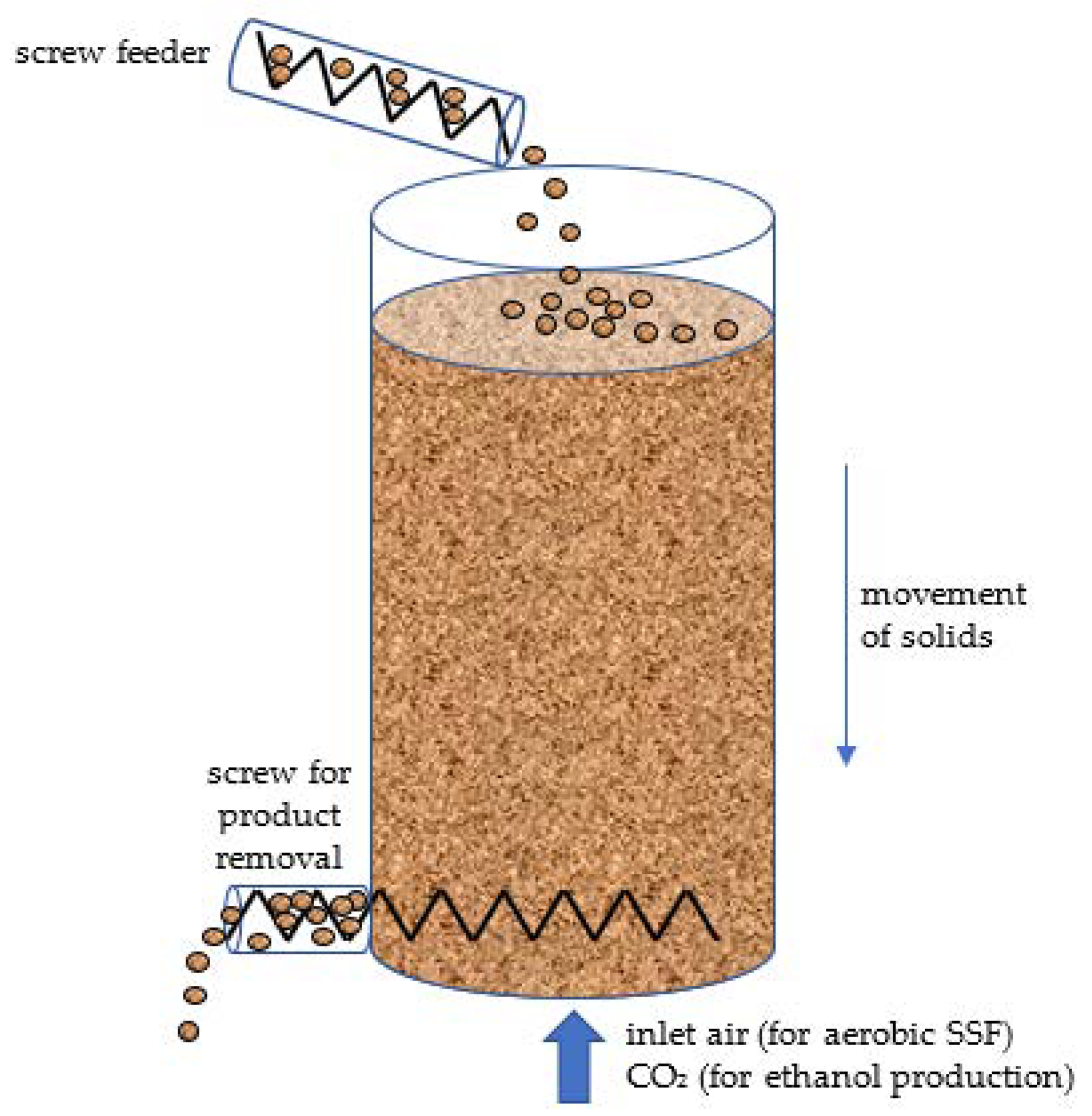



| Organism [Ref.] | Substrate a | Bioreactor Design and Operation b | Comments |
|---|---|---|---|
| Myceliophtora sp. Zanelato et al. [3] | Sugarcane bagasse (70%) and wheat bran (30%); 45 g (presumably wet) per module IMC = 75% (presumably wet basis) | Column: 5 water-jacketed modules, each h = 10 cm and ∅ = 7.62 cm. Total hbed = 80 cm Water jacket at (i) 45 °C or (ii) 50 °C (same temperature as the inlet air) Air at (i) 45 °C or (ii) 50 °C. VZ = 0.49 cm s−1 | Temperature control not good, considering the small column diameter. For the fermentation performed with the water jacket and inlet air at 45 °C, Tbed peaked at 51 °C |
| None Casciatori and Thoméo [4] | (1) Sugarcane bagasse (2) Sugarcane bagasse (20%), orange pulp and peel (40%) and wheat bran (40%) IMC = 70% | Column h = 21 cm, ∅ = 4.67 cm hbed up to 18 cm Water jacket at 65 °C Air at 40 °C VZ from 6.5 to 19.5 cm s−1 | Focus was on wall–bed heat transfer, which is not important in wide packed beds. Wall effects occurred due to the small bed diameter. VZ values are of the order necessary for good control of Tbed |
| Pseudomonas aeruginosa Ranjbar and Hejazi [5] | Mixture (0.34 kg) of dried extracted oil-corn germ meal (70%) and corn bran (30%) IMC = 70% | Column h = 60 cm, ∅ = 6 cm Water jacket at 30 °C Humidified air at 30 °C VZ = 1.67 cm s−1 | Spatiotemporal temperature profiles suggest uniform air flow, with T5cm < T20cm < T45cm < T60cm throughout the fermentation. However, temperature control poor, with Tbed reaching 43 °C |
| Myceliophtora thermophila Perez et al. [6] | Sugarcane bagasse (70%) and wheat bran (30%) (1) Bench scale = 45 g per module (2) Pilot scale = 620 g per module IMC = 75% Bed porosity = 0.75 | (1) Bench-scale column: 8 water-jacketed modules of h = 10 cm and ∅ = 7.62 cm. Total hbed = 80 cm. VZ up to 0.426 cm s−1 (2) Pilot-scale column: 4 water-jacketed modules of h = 20 cm and ∅ = 20 cm. Total hbed = 80 cm. VZ = 0.213 cm s−1 Water jacket at 45 °C Saturated air at 45 °C | Spatiotemporal temperature profiles suggest non-uniform airflow occurred in pilot-scale column. VZ < 1 cm s−1 |
| Metarhizium anisopliae Cunha et al. [7] | Rice (90%) and bagasse (10%) (1) Narrow bioreactor: 400 g of solids (360 g rice and 40 g bagasse) (2) Wide bioreactor: 4500 g of solids (4050 g rice and 450 g bagasse) IMC = 48% Bed porosity = 0.63 | (1) Narrow column: 3 water-jacketed modules, each h = 10 cm and ∅ = 10 cm. Total hbed = 33 cm. VZ = 0.21 cm s−1 (2) Wide column: 3 water-jacketed modules, each h = 20 cm and ∅ = 20 cm. Perforated metal plates between modules. Total hbed = 69 cm. VZ = 0.64 cm s−1 Water jackets at 28 °C | Spatiotemporal temperature profiles suggest non-uniform airflow occurred in both bioreactors. VZ < 1 cm s−1 |
| Aspergillus niger Chysirichote [8] | Banana peel, 3.5 kg (unclear if wet or dry mass) | Column h = 30 cm and ∅ = 30 cm. hbed ~15 cm. Air jacket fed with air at 30 °C Inlet air at 30 °C VZ = 0.5 cm s−1 | Without air jacket, Tbed reached 42 °C. With air jacket, Tbed peaked at ~38 °C. In both cases, peak Tbed > Tair outlet, suggesting non-uniform airflow occurred. |
| Myceliophthora thermophila Casciatori-Frassatto et al. [9] | Sugarcane bagasse (70%) and wheat bran (30); 45 g per module IMC = 75% | Column: 8 water-jacketed modules, each h = 10 cm and ∅ = 7.62 cm. Total hbed = 80 cm Water jacket at 45 °C Moist air at 45 °C VZ = 1.46 cm s−1 | Large variations in production of cellulases with bed height Temperature controlled well, remaining below 47 °C Spatiotemporal temperature profiles suggest non-uniform airflow: T20cm > T50cm > T80cm, (opposite of expected gradient for bottom-to-top airflow) |
| Organism [Ref.] | Substrate a | Bioreactor Design and Operation b | Comments |
|---|---|---|---|
| Aspergillus niger Karimi et al. [17] | Milled wheat bran (250 g) IMC = 55% | Column h = 20 cm, ∅ = 2.5 cm. Placed in a 30 °C water bath Air saturated at 30 °C. VZ = 1.70 cm s−1 | Focus on porosity changes during the fermentation. Spatiotemporal temperature profiles indicate uniform airflow through bed. Peak Tbed = 33 °C |
| Bacillus subtilis Piedrahíta-Aguirre et al. [18] | Mixture (100 g, presumably dry mass) of defatted soybean meal (64%), wheat bran (13%) and rice (23%) IMC = 70% | Column h = 30 cm, ∅ = 5 cm, hbed = 15 cm. Moist air saturated at 30 °C. VZ = 0.34 and 0.68 cm s−1 | Maximum Tbed reached 33–33.5 °C (difficult to interpret this result because Tair fluctuated by 3–4 °C). Spatiotemporal temperature profiles suggest non-uniform airflow |
| Aspergillus awamori Castro et al. [19] | Babassu cake (400 g) IMC = 62% | Column h = 30 cm, ∅ = 10 cm. hbed = 23.9 cm No water jacket. Room temperature = 22 °C Humidified air at 22.9 °C. VZ up to 2.2 cm s−1 | Temperature control very poor, with peak Tbed ~45 °C At the time of peak heat production at 48 h, Tbed increased monotonically with bed height, but at 72 h, Tbed decreased monotonically with bed height. |
| Aspergillus awamori Melikoglu et al. [20] | Pieces (20 mm) of waste bread: (1) 80 g; (2) 170 g IMC = 64% | Columns water-jacketed with silicon tubing (1) h = 15 cm, ∅ = 8 cm, hbed = 10 cm. VZ from 0.07 to 0.40 cm s−1 (2) h = 30 cm, ∅ = 8 cm, hbed = 20 cm. VZ from 0.33 to 0.99 cm s−1 Air 40% RH. Temperature presumably ~30 °C | Temperature control not good, considering the small bioreactor size (1) Tbed peaked at 36–37 °C (2) Tbed peaked at 36–38 °C VZ < 1 cm s−1 |
| Aspergillus niger Pitol et al. [10] | (1) 20 kg wheat bran (2) 18 kg wheat bran + 2 kg sugarcane bagasse (3) 27 kg wheat bran + 3 kg sugarcane bagasse IMC = 62% | Rectangular fermentation box, base 70 cm × 60 cm, h = 50 cm (1) hbed = 23 cm (2) hbed = 27 cm (3) hbed = 40 cm Saturated air at 30 °C VZ = 9.9 cm s−1 | Bed shrank away from walls, allowing preferential airflow around the bed. Tbed reached values as high as 47 °C Poor uniformity of pectinase production |
| Organism [Ref.] | Substrate a | Bioreactor Design and Operation b | Comments |
|---|---|---|---|
| Metarhizium anisopliae van Breukelen et al. [26] | Hemp fiber (2.5 kg) impregnated with nutrient solution containing glucose (5 kg), yeast extract (0.625 kg) and bacteriological peptone (0.625 kg) Initial moisture content not reported, but 7 L water added in the impregnation step, and hemp has high water-holding capacity | Air-jacketed column, h = 60 cm, ∅ = 20 cm. hbed = 60 cm Top to bottom airflow with saturated air. Inlet air temperature unclear. VZ varied manually based on off-gas temperatures. VZ ranged from 2.7 to 8.0 cm s−1 | Authors say that “Perfect temperature control was achieved” |
| Trichoderma harzianum Sala et al. [27,28] | Rice husk (presumably wet masses): (i) 300 g; (ii) 3 kg IMC: (i) 64%; (ii) 59% Initial porosity ~0.85 | (1) 1.5 L column: h = 21 cm, ∅ = 10.5 cm. hbed = 15.5 cm. VZ ≤ 0.02 cm s−1 c (2) 22 L column: h = 48 cm, ∅ = 24.5 cm. hbed = 43 cm. VZ ≤ 0.045 cm s−1 c Inlet air temperature and RH unclear | Tbed varied between 20 and 30 °C at both scales, but difficult to interpret temporal Tbed profiles, since Tair is unclear and may have varied. Spatial temperature gradients in the 22 L bioreactor ≤ 3 °C. |
| Trichoderma harzianum Sala et al. [27,28] | (i) Mixture (300 g, presumably wet mass) of beer draff (70%) and wood chips (30%) (ii) Mixture (4 kg, presumably wet mass) of beer draff (40%) and wood chips (60%) IMC: (i) 64%; (ii) 55% Initial porosity (i) ~0.70; (ii) ~0.80 | (1) 1.5 L column: h = 21 cm, ∅ = 10.5 cm. hbed = 15.5 cm. VZ ≤ 0.055 cm s−1 c (2) 22 L column: h = 48 cm, ∅ = 24.5 cm. hbed = 43 cm. VZ ≤ 0.125 cm s−1 c Inlet air temperature and RH unclear | Difficult to interpret temporal Tbed profiles, since Tair is unclear and may have varied. Spatial (radial) temperature gradients in the 22 L bioreactor >10 °C |
| Aspergillus oryzae Biz et al. [29] | 7.26 kg of sugarcane bagasse and 7.74 kg of citrus pulp IMC = 78% | Rectangular box, base 70 cm × 60 cm, h = 50 cm. hbed = 40 cm Saturated air at 30–32 °C VZ = 9.9 cm s−1 | Good temperature control due to high porosity and high VZ |
| Rhizopus microsporus Pitol et al. [30] | 7.5 kg of wheat bran and 7.5 kg of sugarcane bagasse | Rectangular box, base 70 cm × 60 cm, h = 50 cm. hbed = 40 cm Saturated air at (1) 34 °C and (2) 40 °C. VZ = 6 cm s−1 | Good temperature control. Axial temperature gradient negligible |
| Organism [Ref.] | Substrate a | Bioreactor Design and Operation b | Comments |
|---|---|---|---|
| (1) Phlebiopsis gigantea (basidiomycete) (2) Streptomyces sp. Virtanen et al. [32] | 40 kg (unclear whether wet or dry basis) (1) Water (58.3%), condensed starch mash (16.2%), silica (24.3%), dolomite lime (1.2%) (2) Water (66%), silica (29.1%), corn steep solids (1.4%), lactose (1.4%), dolomite lime (1.4%) | Column h = 48 cm, ∅ = 50 cm. hbed unclear Top-to-bottom airflow. Tair and humidity unclear (no attempt made to saturate the air). VZ = 0.03 cm s−1 | Temperature control not good, considering the slow-growing organisms used. Tbed increased from 23 to 30 °C for Phlebiopsis gigantea and from 23 to 27.5 °C for Streptomyces sp. (in both cases, the ambient temperature was 22 °C when the peak was reached) |
| Kluyveromyces marxianus Mazutti et al. [33,34] | Sugarcane bagasse (2 kg) supplemented with cane molasses, corn steep liquor and soybean bran IMC = 65% | Column h = 50 cm, ∅ = 34 cm, hbed = 40 cm Inlet air temperatures of 27, 30 and 33 °C and VZ = 0.61, 0.73 and 0.92 cm s−1 Air 95–100% RH | Temperature control poor, with Tbed peaking at values from 47 to 50 °C in different experiments VZ < 1 cm s−1 |
| Aspergillus terreus Kumar et al. [35] | Wheat straw and wheat bran. Mixtures ranged from “36% wheat straw + 64% wheat bran” to “64% wheat straw + 36% wheat bran”. Total of 50 kg IMC = 65% | Column 1200 L. ∅ = 94 cm. hbed = 122.5 cm. Provision for intermittent agitation (but agitation not described) Vz up to 0.59 cm s−1 Inlet air at 65% RH, with Tair controlled with PI controllers | Poor temperature control, with peak Tbed of 41.7, compared to optimum temperature for growth of 30 °C |
| Kluyveromyces marxianus Silveira et al. [36] | Sugarcane bagasse (3 kg) supplemented with pretreated cane molasses (10%), corn steep liquor (30%) and soybean bran (20%) IMC = 65% | Column h = 50 cm, ∅ = 34 cm. hbed = 50 cm Air with 95–100% RH. VZ = 0.61 cm s−1 | Temperature control poor. Tbed = 27.5 °C at the bottom compared to 49.6 °C at the top |
| Aspergillus awamori and Aspergillus oryzae Manan and Webb [37] | Wheat bran. Average particle size ~0.5 mm IMC = 65% | Column reactor h = 6 cm and ∅ = 10 cm. hbed = 2 cm Moist air at 30 °C VZ = 0.21, 0.42, 0.85 and 1.27 cm s−1 | Considering the small hbed of 2 cm, temperature control poor at VZ < 1 cm s−1. With Aspergillus awamori, Tbed reached ~35 °C. With Aspergillus awamori, Tbed reached ~34 °C |
| Trichoderma asperellum Barrera et al. [38] | Polyurethane foam cubes with 2 cm sides or 80-mesh rice husk, also a mixture of the two. Additionally, mixture of white rice (81.2%) and wheat bran (18.8% w/w) | PROPHYTA® L05 fixed-bed fermenter (16 L): 4 bed layers with cooling coils between layers. hbed values of 5, 7 and 8 cm tested. Square base 25 cm × 25 cm Incubation temperatures (temperature of water supplied to the cooling coils) of 23, 25 and 28 °C Air at 15, 50 and 95% RH VZ = 0.19, 0.34 and 0.50 cm s−1 | Temperature gradients reported, but not useful because the air gaps between layers are included in the calculation, but the sizes of the air gaps between layers are not specified |
| Aspergillus niger Zhang and Jiang [39] | Woody part of corn cobs (6–8 mm) (1) 4.5 kg woody part of corn cobs + 4.5 kg of water (2) 3.2 kg woody part of corn cobs + 2.56 kg of water | Vehicular rotary solid-state bioreactor (VRSBR). Column h = 40 cm, ∅ = 35 cm Water jacketed. Twater possibly 30 or 35 °C VZ depends on necessity for bed cooling. Varied from 0.07 to 0.90 cm s−1 During static operation, the 40 cm column is vertical. For intermittent mixing, bioreactor rotated 90° (such that the 40 cm column is horizontal); rotated like a rotating-bed bioreactor (20 rotations for each mixing event) (1) hbed = 22 cm. Mixing events at 10, 12, 19, 24, 30, 48, 72, 98, 145 and 192 h (2) hbed = 15 cm. Mixing events at 18, 24, 38, 48, 72, 96, 120, 144 and 168 h | Interesting design for intermittent mixing. Spatiotemporal temperature profiles indicate uniform airflow through the bed (1) Temperature control not good, with gradients as large as 20 °C at time of peak heat generation (26 °C at bottom of bed and 46 °C at top of bed) (2) Temperature control better, but still with gradients as large as 9 °C at time of peak heat generation (earlier, 26 °C at bottom of bed and 35 °C at top of bed; later, 28 °C at bottom of bed and 37 °C at top of bed) |
| Yarrowia lipolytica Nascimento et al. [40] | Soybean hulls (400 g) supplemented with a nutrient solution containing either glucose or soybean oil IMC = 55% (presumably on a wet basis) | Column h = 36 cm, ∅ = 10 cm, hbed = 28 cm Side walls insulated by a vacuum-sealed jacket to mimic the situation at a large scale in a wide packed bed. Moist air at ~26.5–28 °C (not specified clearly) VZ = 1.06 cm s−1 | Temperature control not good, with temperatures as high as 35 °C being reached Radial temperature gradients of up to 2 °C detected in the upper sections of the bioreactor, even though the side walls were insulated (which should eliminate such gradients). No visual evidence of the formation of preferential flow paths |
| Irpex lacteus (basidiomycete) Álvarez Pallín et al. [41] | Chopped wheat straw (5.35 kg, presumably wet basis) IMC = 82% (presumably wet basis) | Column h = 28 cm, ∅ = 14 cm Tair switched between lower and higher values (not stated) to maintain the bed temperature at 30 °C. Inlet air humidity modified manually by controlling the airflow through the humidifier. Vz = 0.35 cm s−1 | Although Irpex lacteus grows slowly, Tbed peaked at 36 °C. Moreover, Tbed was consistently higher than the outlet air temperature, suggesting that air was flowing around the bed rather than through it |
| Aspergillus niger Bhattacharya et al. [42] | Substrate loadings of (i) 100 g, (ii) 150 g, (iii) 200 g, (iv) 300 g, (v) 400 g IMC = 66% (presumably wet basis) | Column ∅ = 15 cm. hbed = (i) 0.5 cm, (ii) 1 cm, (iii) 2 cm, (iv) 3 cm, (v) 4 cm Air at 30 °C VZ = 0.07 cm s−1 | Poor temperature control, with Tbed reaching 35 °C No visual evidence of bed compaction nor formation of preferential flow paths |
| Organism [Ref.] | Substrate a | Bioreactor Design and Operation b | Comments |
|---|---|---|---|
| Aspergillus niger Finkler et al. [43] | 27 kg of wheat bran and 3 kg of sugarcane bagasse IMC = 62% | Rectangular box, base 70 cm × 60 cm, h = 50 cm. hbed = 40 cm Air saturated at 30 °C VZ = 9.9 cm s−1 Intermittently mixed by rotating whole bioreactor | In absence of mixing, Tbed had reached 43 °C, and there was evidence of non-uniform airflow [10]. With intermittent mixing, maximum Tbed < 40 °C, airflow more uniform |
| Rhizopus oryzae Arora et al. [44] | 200 g of a 1:1 mixture of wheat bran and linseed oil cake IMC = 34.5% (corresponds to 55% dry basis) | Column ∅ = 15 cm. hbed = 3.5 cm Agitated intermittently Compared (1) unmixed (2) mixing every 6 h (3) mixing every 10 min Air at 30 °C and 80% RH VZ = 0.38 cm s−1 | Poor temperature control, despite small scale. The unmixed bed compacted, causing non-uniform airflow, with Tbed reaching ~41 °C. With mixing, Tbed reached from 38 to 40 °C |
| Rhizopus oryzae Arora et al. [45] | 1:1 mixture of wheat bran and linseed oil cake (presumably 200 g) | Column ∅ = 15 cm. hbed = 3.5 cm Mixing events every 1 h Air at 30 °C, in different experiments, 60, 70 and 80% RH. In different experiments VZ = 0.28, 0.38 and 0.47 cm s−1 | Poor temperature control, despite small scale. In different experiments, Tbed peaked at values ranging from 34 to 38.5 °C |
| “Trichoderma Brev T069” [46] | Cassava peel. Values used were (i) 250 kg, (ii) 500 kg, (iii) 750 kg (presumably wet masses) IMC = 56% (presumably wet basis) | Rectangular perforated stainless-steel plates 900 cm × 200 cm. hbed values of (i) 7 cm, (ii) 14 cm, (iii) 21 cm. Agitator moves from one end of the bed to the other, with motors mounted on a carriage. Design of agitator blades not specified. Agitator used intermittently. Air temperature and RH unclear. Airflow adjusted based on temperatures measured in bed, reaching 50 m3 min−1, which corresponds to VZ = 4.6 cm s−1 | Temperature control poor, considering the small bed heights. For the 7 cm bed, Tbed increased from 28 to 35 °C. For the 14 cm bed, Tbed increased from 28 to 39 °C. The fermentation failed with the 21 cm bed height (ammonia odor was produced) |
| Organism [Ref.] | Substrate a | Bioreactor Design and Operation b | Comments |
|---|---|---|---|
| Aspergillus niger Chysirichote [51] | Copra waste, 2.0 kg IMC = 60% | Column. h = 80 cm and ∅ = 45 cm. Total hbed = 36 cm. In different experiments:
| VZ of 0.12 cm s−1 is low for a fast-growing organism like A. niger. Peak Tbed values (~50 °C) were higher than the outlet air temperature, suggesting non-uniform airflow occurred |
| Myceliophthora sp. Casciatori et al. [52] | Sugarcane bagasse and wheat bran. Proportions unclear IMC = 75% | Column: 8 water-jacketed modules, each h = 10 cm and ∅ = 7.62 cm. Total hbed = 80 cm Water jacket presumably at 45 °C Air at 45 °C and 95% RH VZ values of 0.73 and 1.46 cm s−1 | With VZ = 0.73 cm s−1, Tbed peaked ~52 °C in the bottom section of the bed, at which time Tbed was lower in the middle and higher sections, suggesting non-uniform airflow. With VZ = 1.46 cm s−1, Tbed peaked at ~50 °C at all three bed heights, suggesting non-uniform airflow. Significant drying occurred, with final bed moisture contents <15% at all bed heights below 60 cm. |
| (1) Aspergillus awamori (2) Aspergillus oryzae Manan and Webb [53] | Wheat bran (12 g per module) IMC ranging from 50 to 75% (in different modules) | Column with six modules with perforated bases, each h = 5 cm and ∅ = 10 cm, tightly stacked one above the other (total height of 33.5 cm). hbed in each module = 1.5 cm Moist air at 30 °C VZ = 0.42 cm s−1. | Temperature profiles for individual modules not reported, only averages over the 6 modules. Bed temperatures over 40 °C occurred, despite the small amount of substrate in the bioreactor |
| Myceliophthora thermophila Oliveira et al. [54] | Sugarcane bagasse (70%) and wheat bran (30%) IMC = 75% | Both cases: Column with 4 water-jacketed modules, each h = 10 cm and ∅ = 13 cm. Perforated metal at base of each module Water jacket at 45 °C Air saturated at 45 °C VZ = 0.73 cm s−1 (1) Batch operation: Same 4 modules throughout, in the same positions (2) “Descending” semicontinuous operation (Figure 4):
| Batch operation: Spatiotemporal temperature profiles at time of peak heat production suggest non-uniform airflow occurred. Maximum Tbed was 47.9 °C for batch operation and 47.7 °C for “descending” semicontinuous operation. |
| Myceliophthora thermophila Rodrigues et al. [55] | Sugarcane bagasse (70%) and wheat bran (30%) IMC = 75% | Column with 8 water-jacketed modules, each h = 10 cm and ∅ = 13 cm. Perforated metal at base of each module Water jacket at 45 °C Air saturated at 45 °C VZ = 0.73 cm s−1 (1) Batch operation: Same 8 modules throughout, in the same positions (2) Cyclic operation (Figure 4). Same 8 modules throughout. Every 24 h, 2 modules moved from the top to the bottom, with the other 6 modules moved upwards (3) “Ascending” semicontinuous operation (Figure 4):
| Cyclic operation: bottom-to-top axial temperature gradient quickly reestablished after movement of modules. Peak Tbed of 48.2 °C “Ascending” semi-continuous operation: Peak Tbed of 48.3 °C |
| Organism [Ref.] | Substrate a | Bioreactor Design and Operation b | Comments |
|---|---|---|---|
| Trichoderma asperellum Maïga et al. [57] | Grapevine shoots (33.3%), sugarcane bagasse (10%), wheat bran (33.3%), olive pomace (13.3%) and potato flakes (10%) Total mass = 6 kg IMC = 66% | Disposable plastic bag reactor. Rectangular base 52 cm × 40 cm, working height up to 19 cm. hbed = 15 cm Air at 25 °C. VZ values from 0.08 to 0.36 cm s−1 | Not intended for scale-up to larger scales. The intention is for the bioreactor to be portable and disposable. VZ < 1 cm s−1 Peak Tbed = 48 °C |
| None (but would presumably be Saccharomyces cerevisiae) Chen et al. [58] | Chopped sweet sorghum stalks Feed moisture content = 80% Feed and discharge at 2.5 kg wet substrate h−1 | Continuous packed-bed bioreactor. Column with hbed = 120 cm, ∅ = 40 cm. Working volume 150 L Inoculated new substrate added at top. Spent solids removed from bottom. Residence time of solids = 24 h CO2 circulated through bed to strip ethanol; ethanol condensed externally and then CO2 reheated and returned to bed | Plug-flow continuous system Focus on ethanol stripping in the absence of growth. However, with aeration with air, could be adapted for aerobic SSF |
| Aspergillus ficuum Shahryari et al. [59] | Wheat straw (particle size 0.70–0.14 mm) | Column h = 30 cm and ∅ = 10 cm. hbed = 19.5 cm (1) Trickle bed. Intermittently agitated with helical screw. After day 3, 195 mL of liquid (usually water) trickled into the bed daily over 1-min period (2) Static bed, no helical screw. No trickling of liquid Both bioreactors held in a 30 °C chamber Aeration for 2 h, twice a day. Air temperature presumably 30 °C. VZ = 0.21 cm s−1 (during aeration period) | VZ < 1 cm s−1 and aeration infrequent (1) Good temperature control, peak Tbed ~31 °C (2) Reasonable temperature control, peak Tbed ~34.5 °C Aeration intermittent, so, for most of the time, did not operate as a true packed bed. However, could be adapted for continuous aeration |
| mixed culture of Trichoderma reesei and Aspergillus oryzae Brijwani et al. [60] | Soybean hulls (1) 2.51 kg (2) 3.51 kg IMC = 70% | Cube 30 cm × 30 cm × 30 cm, with wire-mesh walls. (1) hbed = 15 cm; (2) hbed = 25 cm Central perforated tube with perforated horizontal branches at 3 heights Cube incubated in chamber with air at 30 °C and 95% RH Air saturated at 25 °C. Aeration of 50 L min−1 equivalent to VZ = 0.93 cm s−1 if aeration were end-to-end. | Temperature control poor, with Tbed reaching 44 °C Aeration rate low (equivalent to VZ < 1 cm s−1) |
| Myceliophthora thermophila Perez et al. [61] | Sugarcane bagasse (70%) and wheat bran (30%) (1) 45 g of substrate per module. (2) 620 g of substrate per module IMC = 75% Porosity = 0.75 | Column with water-jacketed modules, each module with a perforated grid at bottom (1) 8 modules, each h = 10 cm and ∅ = 7.62 cm. Total hbed = 80 cm. VZ = 0.43 cm s−1 for “Normal” aeration, also operated with “Split” aeration (2) 4 water-jacketed modules, each h = 20 cm and ∅ = 20 cm. Total hbed = 80 cm. Operated with split and radial aeration. Information insufficient to calculate VZ Water jacket at 45 °C Saturated air at 45 °C Aeration strategies: (i) Normal: All air introduced at the bottom of the column. Performed only in the narrow column (ii) Split: Half the air enters bottom of column and half through a perforated ring near the wall at half bed height. Performed in bioreactors (1) and (2) (iii) Radial: Air introduced through perforated tube at central vertical axis. Performed only in bioreactor (2) | (1) Good temperature control (Tbed < 48 °C) with both Normal and Split aeration (2) Temperature control not good. Tbed reached ~55 °C for both Split and Radial aeration In both cases, spatiotemporal temperature profiles indicate non-uniform airflow |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mitchell, D.A.; Ruiz, H.A.; Krieger, N. A Critical Evaluation of Recent Studies on Packed-Bed Bioreactors for Solid-State Fermentation. Processes 2023, 11, 872. https://doi.org/10.3390/pr11030872
Mitchell DA, Ruiz HA, Krieger N. A Critical Evaluation of Recent Studies on Packed-Bed Bioreactors for Solid-State Fermentation. Processes. 2023; 11(3):872. https://doi.org/10.3390/pr11030872
Chicago/Turabian StyleMitchell, David Alexander, Héctor A. Ruiz, and Nadia Krieger. 2023. "A Critical Evaluation of Recent Studies on Packed-Bed Bioreactors for Solid-State Fermentation" Processes 11, no. 3: 872. https://doi.org/10.3390/pr11030872
APA StyleMitchell, D. A., Ruiz, H. A., & Krieger, N. (2023). A Critical Evaluation of Recent Studies on Packed-Bed Bioreactors for Solid-State Fermentation. Processes, 11(3), 872. https://doi.org/10.3390/pr11030872







