Research Progress of Co-Catalysts in Photocatalytic CO2 Reduction: A Review of Developments, Opportunities, and Directions
Abstract
1. Introduction
2. Application of Co-Catalysts in CO2 Photocatalytic Reduction
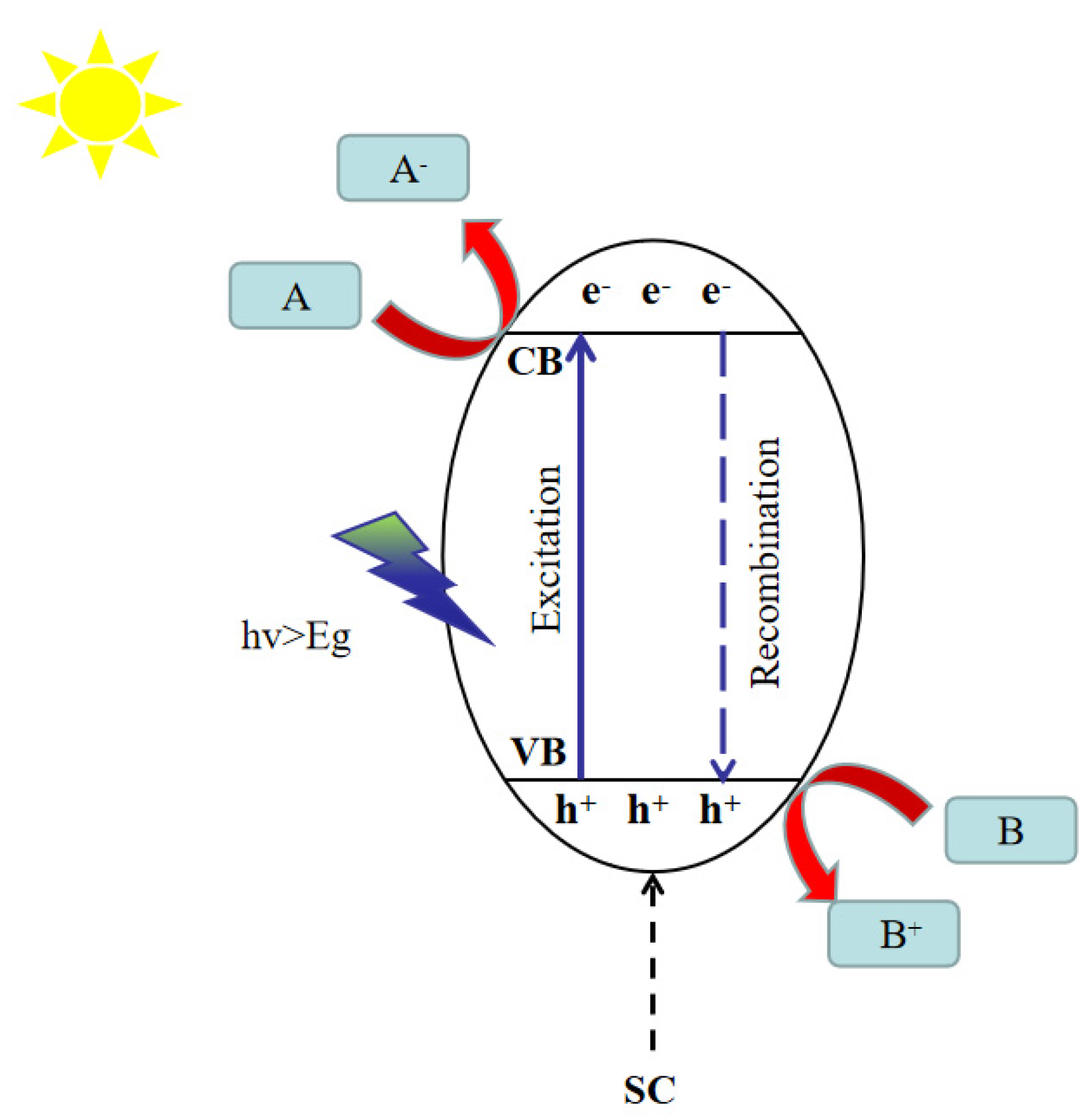
2.1. Catalytic Reduction of CO2 Reaction Mechanism
Mechanistic Role of Co-Catalysts in CO2 Photocatalytic Reduction
3. Noble Metal-Based Co-Catalysts
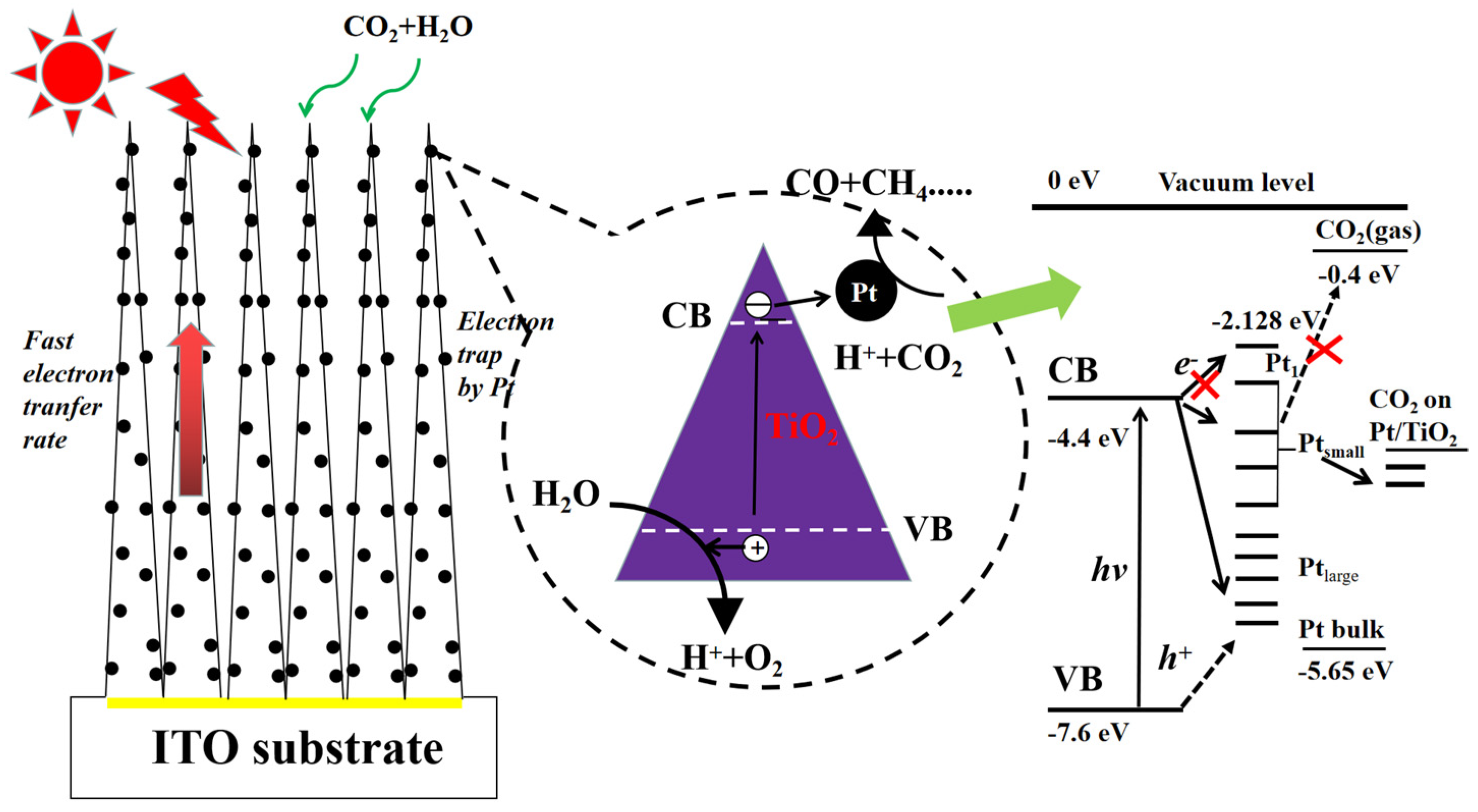
3.1. Pt-Based Co-Catalysts
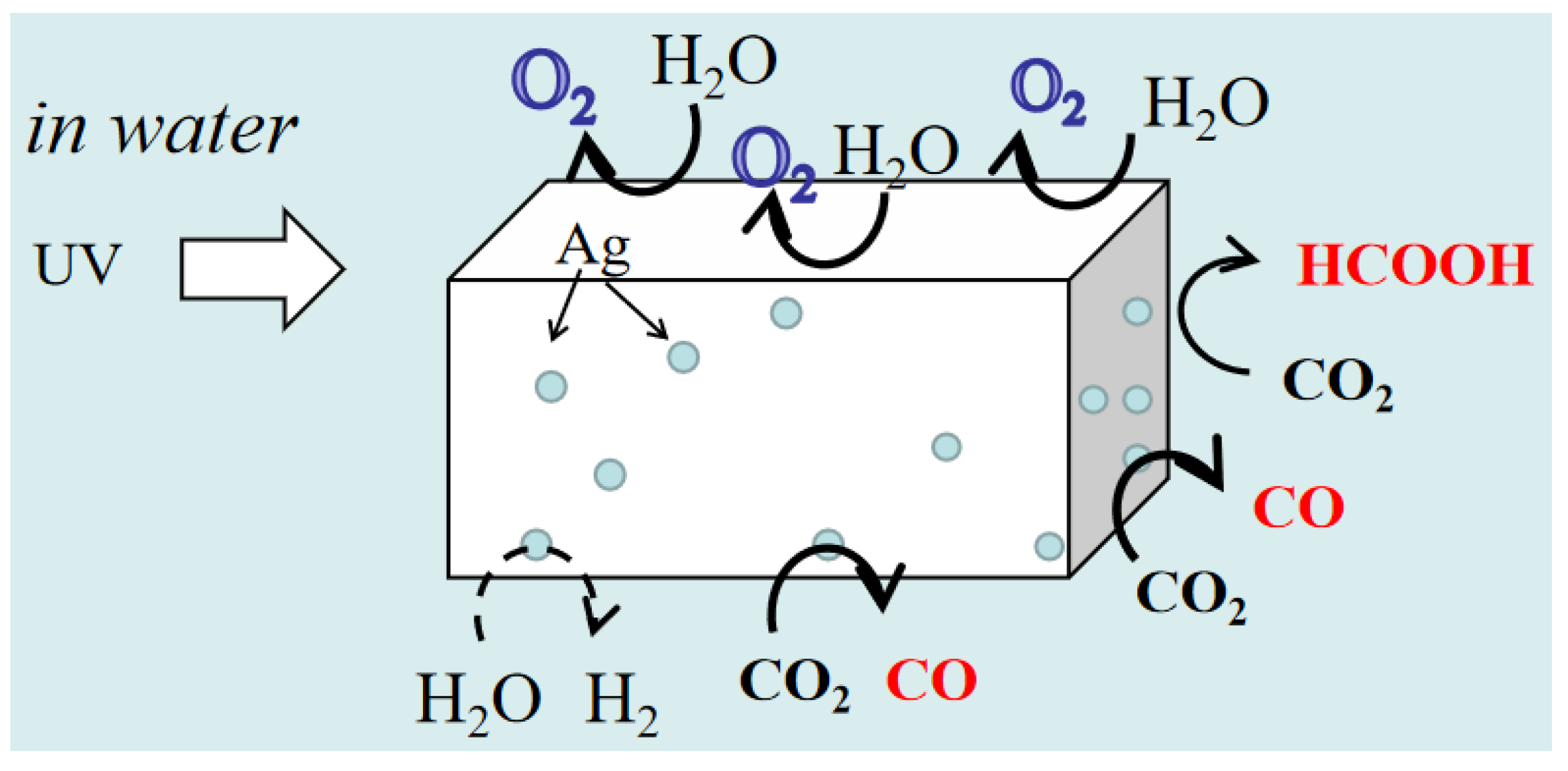
3.2. Ag-Based Co-Catalysts
3.3. Pd-Based Co-Catalysts
3.4. Ru-Based Co-Catalysts
3.5. Alloy Co-Catalysts
4. Precious Metal-Free Co-Catalysts
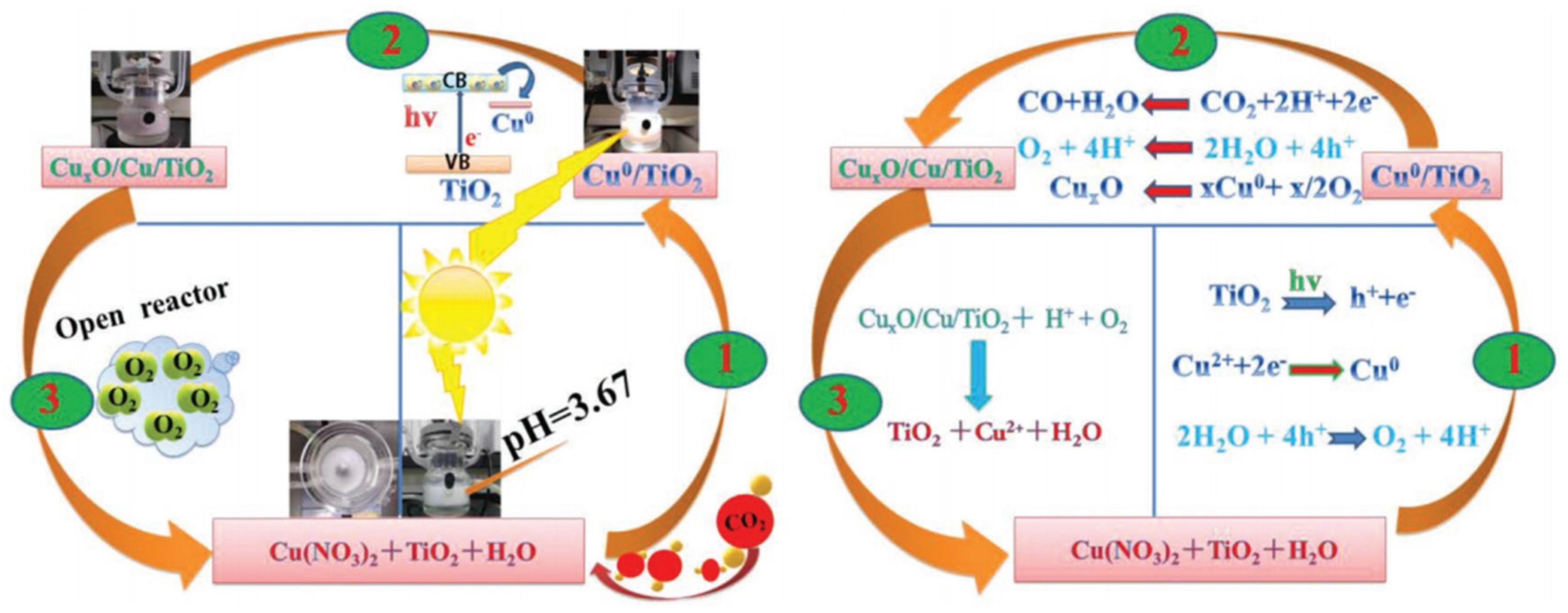
4.1. Cu-Based Co-Catalysts
4.2. Ni-Based Co-Catalysts
4.3. Graphene Co-Catalysts
4.3.1. Composite Method
4.3.2. Wrapping Method
4.3.3. Participation of Graphene Itself in Photocatalytic Reactions
4.4. Single-Atom Co-Catalysts
5. Challenges and Perspectives
6. Conclusions
- The activity of CO2 reduction could be improved by controlling the preparation of the catalyst. Currently, monometallic and bimetallic co-catalysts are the most studied in the literature, but there are few studies on three or more polymetallic co-catalysts. In future research, we should focus on developing multi-metal and multi-functional co-catalysts. Finally, it must be noted that co-catalysts that require environmental friendliness, energy efficiency, and other advantages are essential to achieve the scale of industrial application of photocatalysts.
- We aimed to explore high selectivity, high activity, and low-cost co-catalysts. According to previous studies, catalysts like Cu/Pt and Cu/Au are more effective for CO2 reduction. Given this, it could be tried to find new and more efficient photocatalysts, such as three metals or metal oxides of more than three metals, metal nitride, metal phosphide, bentonite, spinel, and chalcocite. Multi-component active ingredients with synergistic effects could enhance photocatalytic CO2 activity. In addition, the stability of semiconductor photocatalysts is also a significant challenge. Finding effective techniques to stop the chemical or photocorrosion of co-catalysts will be a crucial direction for future research.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Habisreutinger, S.N.; Schmidt-Mende, L.; Stolarczyk, J.K. Photocatalytic Reduction of CO2 on TiO2 and Other Semiconductors. Angew. Chem. Int. Ed. 2013, 52, 7372–7408. [Google Scholar] [CrossRef] [PubMed]
- Karamian, E.; Sharifnia, S. On the general mechanism of photocatalytic reduction of CO2. J. CO2 Util. 2016, 16, 194–203. [Google Scholar] [CrossRef]
- Mao, J.; Li, K.; Peng, T. Recent advances in the photocatalytic CO2 reduction over semiconductors. Catal. Sci. Technol. 2013, 3, 2481–2498. [Google Scholar] [CrossRef]
- Cook, J.; Oreskes, N.; Doran, P.T.; Anderegg, W.R.L.; Verheggen, B.; Maibach, E.W.; Carlton, J.S.; Lewandowsky, S.; Skuce, A.G.; Green, S.A.; et al. Consensus on consensus: A synthesis of consensus estimates on human-caused global warming. Environ. Res. Lett. 2016, 11, 048002. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, C.; Cui, P. Ultrahigh Photocatalytic CO2 Reduction Efficiency and Selectivity Manipulation by Single-Tungsten-Atom Oxide at the Atomic Step of TiO2. Adv. Mater. 2022, 34, 2109074. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.M.; Kim, D.; Rungtaweevoranit, B.; Trickett, C.A.; Barmanbek, J.T.; Alshammari, A.S.; Yang, P.; Yaghi, O.M. Plasmon-enhanced photocatalytic CO2 conversion within metal-organic frameworks under visible light. J. Am. Chem. Soc. 2017, 139, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Gondal, M.A.; Dastageer, M.A.; Oloore, L.E.; Baig, U. Laser induced selective photo-catalytic reduction of CO2 into methanol using In2O3-WO3 nano-composite. J. Photochem. Photobiol. A 2017, 343, 40–50. [Google Scholar] [CrossRef]
- Liu, J.; Liu, M.; Yang, X.L.; Chen, H.; Liu, S.Z.F.; Yan, J.Q. Photo-Redeposition Synthesis of Bimetal Pt−Cu Co-catalysts for TiO2 Photocatalytic Solar-Fuel Production. ACS Sustain. Chem. Eng. 2019, 6, 1900289. [Google Scholar] [CrossRef]
- Yu, J.; Low, J.; Xiao, W.; Zhou, P.; Jaroniec, M. Enhanced photocatalytic CO2 reduction activity of anatase TiO2 by coexposed {001} and {101} facets. J. Am. Chem. Soc. 2014, 136, 8839–8842. [Google Scholar] [CrossRef]
- Pu, Y.; Luo, Y.; Wei, X.; Sun, J.; Li, L.; Zou, W.; Dong, L. Synergistic effects of Cu2O-decorated CeO2 on photocatalytic CO2 reduction: Surface Lewis acid/base and oxygen defect. Appl. Catal. B 2019, 254, 580–586. [Google Scholar] [CrossRef]
- Nguyen, N.H.; Wu, H.Y.; Bai, H. Photocatalytic reduction of NO2 and CO2 using molybdenum-doped titania nanotubes. Chem. Eng. J. 2015, 269, 60–66. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, Z.; Cao, S.-W.; Xue, C. Au/Pt Nanoparticle-Decorated TiO2 Nanofibers with Plasmon-Enhanced Photocatalytic Activities for Solar-to-Fuel Conversion. J. Phys. Chem. C 2013, 117, 25939–25947. [Google Scholar] [CrossRef]
- Uner, D.; Oymak, M.M. On the mechanism of photocatalytic CO2 reduction with water in the gas phase. Catal. Today 2012, 181, 82–88. [Google Scholar] [CrossRef]
- Xiong, Z.; Wang, H.; Xu, N.; Li, H.; Fang, B.; Zhao, Y.; Zhang, J.; Zheng, C. Photocatalytic reduction of CO2 on Pt2+-Pt0/TiO2 nanoparticles under UV/Vis light irradiation: A combination of Pt2+ doping and Pt nanoparticles deposition. Int. J. Hydrogen Energy 2015, 40, 10049–10062. [Google Scholar] [CrossRef]
- Xie, S.; Wang, Y.; Zhang, Q.; Deng, W.; Wang, Y. MgO- and Pt-Promoted TiO2 as an Efficient Photocatalyst for the Preferential Reduction of Carbon Dioxide in the Presence of Water. ACS Catal. 2014, 4, 3644–3653. [Google Scholar] [CrossRef]
- Dukovic, G.; Merkle, M.G.; Nelson, J.H.; Hughes, S.M.; Alivisatos, A.P. Photodeposition of Pt on Colloidal CdS and CdSe/CdS Semiconductor Nanostructures. Adv. Mater. 2008, 20, 4306–4311. [Google Scholar] [CrossRef]
- Maimaitizi, H.; Abulizi, A.; Kadeer, K.; Talifu, D.; Tursun, Y. In situ synthesis of Pt and N co-doped hollow hierarchical BiOCl microsphere as an efficient photocatalyst for organic pollutant degradation and photocatalytic CO2 reduction. Appl. Surf. Sci. 2020, 502, 144083. [Google Scholar] [CrossRef]
- Fang, B.; Bonakdarpour, A.; Reilly, K.; Xing, Y.; Taghipour, F.; Wilkinson, D.P. Large-scale synthesis of TiO2 microspheres with hierarchical nanostructure for highly efficient photodriven reduction of CO2 to CH4. ACS Appl. Mater. Interfaces 2014, 6, 15488–15498. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wei, Y.; Wu, X.; Zheng, H.; Zhao, Z.; Liu, J.; Li, J. Graphene-wrapped Pt/TiO2 Photocatalysts with Enhanced Photogenerated Charges Separation and Reactant Adsorption for High Selective Photoreduction of CO2 to CH4. Appl. Catal. B 2018, 226, 360–372. [Google Scholar] [CrossRef]
- Feng, X.; Sloppy, J.D.; LaTempa, T.J.; Paulose, M.; Komarneni, S.; Bao, N.; Grimes, C.A. Synthesis and deposition of ultrafine Pt nanoparticles within high aspect ratio TiO2 nanotube arrays: Application to the photocatalytic reduction of carbon dioxide. J. Mater. Chem. 2011, 21, 13429–13433. [Google Scholar] [CrossRef]
- Li, K.; Peng, T.; Ying, Z.; Song, S.; Zhang, J. Ag-loading on brookite TiO2 quasi nanocubes with exposed {210} and {001} facets: Activity and selectivity of CO2 photoreduction to CO/CH4. Appl. Catal. B 2016, 180, 130–138. [Google Scholar] [CrossRef]
- Takayama, T.; Tanabe, K.; Saito, K.; Iwase, A.; Kudo, A. The KCaSrTa5O15 photocatalyst with tungsten bronze structure for water splitting and CO2 reduction. Phys. Chem. Chem. Phys. 2014, 16, 24417–24422. [Google Scholar] [CrossRef]
- Wang, Z.; Teramura, K.; Huang, Z.; Hosokawa, S.; Sakatad, Y.; Tanaka, T. Tuning the selectivity toward CO evolution in the photocatalytic conversion of CO2 by H2O through the modification of Ag-loaded Ga2O3 with a ZnGa2O4 layer. Catal. Sci. Technol. 2016, 6, 1025–1032. [Google Scholar] [CrossRef]
- Wang, Z.; Teramura, K.; Hosokawa, S.; Tanaka, T. Highly efficient photocatalytic conversion of CO2 into solid CO using H2O as a reductant over Ag-modified ZnGa2O4. J. Mater. Chem. A 2015, 3, 11313–11319. [Google Scholar] [CrossRef]
- Yamamoto, M.; Yoshida, T.; Yamamoto, N.; Nomoto, T.; Yamamoto, Y.; Yagi, S.; Yoshida, H. Photocatalytic reduction of CO2 with water promoted by Ag clusters in Ag/Ga2O3 photocatalysts. J. Mater. Chem. A 2015, 3, 16810–16816. [Google Scholar] [CrossRef]
- Ohno, T.; Higo, T.; Murakami, N.; Saito, H.; Zhang, Q.; Yang, Y.; Tsubota, T. Photocatalytic reduction of CO2 over exposed-crystal-face-controlled TiO2 nanorod having a brookite phase with co-catalyst loading. Appl. Catal. B 2014, 152–153, 309–316. [Google Scholar] [CrossRef]
- Zhao, C.; Krall, A.; Zhao, H.; Zhang, Q.; Li, Y. Ultrasonic spray pyrolysis synthesis of Ag/TiO2 nanocomposite photocatalysts for simultaneous H2 production and CO2 reduction. Int. J. Hydrogen Energy 2012, 37, 9967–9976. [Google Scholar] [CrossRef]
- Wang, Z.; Teramura, K.; Hosokawa, S.; Tanaka, T. Photocatalytic conversion of CO2 in water over Ag-modified La2Ti2O7. Appl. Catal. B 2015, 163, 241–247. [Google Scholar] [CrossRef]
- Sui, D.; Yin, X.; Dong, H.; Qin, S.; Chen, J.; Jiang, W. Photocatalytically Reducing CO2 to Methyl Formate in Methanol over Ag Loaded SrTiO3 Nanocrystal Catalysts. Catal. Lett. 2012, 142, 1202–1210. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Dong, P.; Huang, Z.; Nie, X.; Zhang, X. TiO2 Surfaces Self-Doped with Ag Nanoparticles Exhibit Efficient CO2 Photoreduction under Visible Light. RSC Adv. 2018, 8, 15991–15998. [Google Scholar] [CrossRef]
- Liu, L.; Pitts, D.T.; Zhao, H.; Zhao, C.; Li, Y. Silver-incorporated bicrystalline (anatase/brookite) TiO2 microspheres for CO2 photoreduction with water in the presence of methanol. Appl. Catal. A 2013, 467, 474–482. [Google Scholar] [CrossRef]
- Yui, T.; Kan, A.; Saitoh, C.; Koike, K.; Ibusuki, T.; Ishitani, O. Photochemical Reduction of CO2 Using TiO2: Effects of Organic Adsorbates on TiO2 and Deposition of Pd onto TiO2. ACS Appl. Mater. Interfaces 2011, 3, 2594–2600. [Google Scholar] [CrossRef]
- Kim, W.; Seok, T.; Choi, W. Nafion layer-enhanced photosynthetic conversion of CO2 into hydrocarbons on TiO2 nanoparticles. Energy Environ. Sci. 2012, 5, 6066–6070. [Google Scholar] [CrossRef]
- Koci, K.; Matejova, L.; Reli, M.; Capek, L.; Matejka, V.; Lacny, Z.; Kustrowski, P.; Obalbv, L. Sol-Gel Derived Pd Supported TiO2ZrO, and TiO, Photocatalysts; Their Examination in PhotocatalyticReduction of Carbon Dioxide. Catal. Today. 2014, 230, 20–26. [Google Scholar] [CrossRef]
- Hong, J.; Zhang, W.; Wang, Y.; Zhou, T.; Xu, R. Photocatalytic Reduction of Carbon Dioxide over Self-Assembled Carbon Nitride and Layered Double Hydroxide: The Role of Carbon Dioxide Enrichment. ChemCatChem 2014, 6, 2315–2321. [Google Scholar] [CrossRef]
- Kuai, L.; Chen, Z.; Liu, S.; Kan, E.; Yu, N.; Ren, Y.; Fang, C.; Li, X.; Li, Y.; Geng, B. Titania Supported Synergistic Palladium Single Atoms and Nanoparticles for Room Temperature Ketone and Aldehydes Hydrogenation. Nat. Commun. 2020, 11, 48. [Google Scholar] [CrossRef]
- Sasirekha, N.; Basha, S.J.S.; Shanthi, K. Photocatalytic performance of Ru doped anatase mounted on silica for reduction of carbon dioxide. Appl. Catal. B 2006, 62, 169–180. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, H.; Tang, S.; Liu, C.; Dong, F.; Yue, H.; Liang, B. Photocatalytic Oxidative Dehydrogenation of Ethane Using CO2 as a Soft Oxidant over Pd/TiO2 Catalysts to C2H4 and Syngas. ACS Catal. 2018, 8, 9280–9286. [Google Scholar] [CrossRef]
- Yan, S.C.; Ouyang, S.X.; Gao, J.; Yang, M.; Feng, J.Y.; Fan, X.X.; Wan, L.J.; Li, Z.S.; Ye, J.H.; Zhou, Y.; et al. A Room-Temperature Reactive-Template Route to Mesoporous ZnGa2O4 with Improved Photocatalytic Activity in Reduction of CO2. Angew. Chem. Int. Ed. 2010, 49, 6544–6548. [Google Scholar] [CrossRef]
- Yoshino, S.; Sato, K.; Yamaguchi, Y.; Iwase, A.; Kudo, A. Z-schematic CO2 reduction to CO through interparticle electron transfer between SrTiO3: Rh of a reducing photocatalyst and BiVO4 of a water oxidation photocatalyst under visible light. ACS Appl. Energy Mater. 2020, 3, 10001–10007. [Google Scholar] [CrossRef]
- Baran, T.; Wojtyła, S.; Dibenedetto, A.; Aresta, M.; Macyk, W. Zinc sulfide functionalized with ruthenium nanoparticles for photocatalytic reduction of CO2. Appl. Catal. B 2015, 178, 170–176. [Google Scholar] [CrossRef]
- Cao, L.; Sahu, S.; Anilkumar, P.; Bunker, C.E.; Xu, J.; Fernando, K.A.S.; Wang, P.; Guliants, E.A., II; Tackett, K.N.; Sun, Y.-P. Carbon Nanoparticles as Visible-Light Photocatalysts for Efficient CO2 Conversion and Beyond. J. Am. Chem.Soc. 2011, 133, 4754–4757. [Google Scholar] [CrossRef]
- Rossetti, I.; Villa, A.; Compagnoni, M.; Prati, L.; Ramis, G.; Pirola, C.; Bianchi, C.L.; Wang, W.; Wang, D. CO2 photoconversion to fuels under high pressure: Effect of TiO2 phase and of unconventional reaction conditions. Catal. Sci. Technol. 2015, 5, 4481–4487. [Google Scholar] [CrossRef]
- Wang, J.; Li, G.; Li, Z.; Tang, C.; Feng, Z.; An, H.; Liu, H.; Liu, T.; Li, C. A Highly Selective and Stable ZnO-ZrO2 Solid Solution Catalyst for CO2 Hydrogenation to Methanol. Sci. Adv. 2017, 3, e1701290. [Google Scholar] [CrossRef]
- Zhang, X.; Han, F.; Shi, B.; Farsinezhad, S.; Dechaine, G.P.; Shankar, K. Photocatalytic Conversion of Diluted CO2 into Light Hydrocarbons Using Periodically Modulated Multiwalled Nanotube Arrays. Angew. Chem. Int. Ed. 2012, 51, 12732–12735. [Google Scholar] [CrossRef]
- Baldoví, H.G.; Neatu, S.; Khan, A.; Asiri, A.M.; Kosa, S.A.; Garcia, H. Understanding the Origin of the Photocatalytic CO2 Reduction by Au- and Cu-Loaded TiO2: A Microsecond Transient Absorption Spectroscopy Study. J. Phys. Chem. C 2015, 119, 6819–6827. [Google Scholar] [CrossRef]
- Qin, L.; Si, G.; Li, X.; Kang, S.-Z. Synergetic effect of Cu−Pt bimetallic cocatalyst on SrTiO3 for efficient photocatalytic hydrogen production from water. RSC Adv. 2015, 5, 102593–102598. [Google Scholar] [CrossRef]
- Kang, Q.; Wang, T.; Li, P.; Liu, L.; Chang, K.; Li, M.; Ye, J. Photocatalytic Reduction of Carbon Dioxide by Hydrous Hydrazine over Au-Cu Alloy Nanoparticles Supported on SrTiO3/TiO2 Coaxial Nanotube Arrays. Angew. Chem. Int. Ed. 2015, 54, 841–845. [Google Scholar] [CrossRef]
- Fang, B.; Xing, Y.; Bonakdarpour, A.; Zhang, S.; Wilkinson, D.P. Hierarchical CuO-TiO2 Hollow Microspheres for Highly Efficient Photodriven Reduction of CO2 to CH4. ACS Sustain. Chem. Eng. 2015, 3, 2381–2388. [Google Scholar] [CrossRef]
- Ovcharov, M.L.; Mishura, A.M.; Shcherban, N.D.; Filonenko, S.M.; Granchak, V.M. Photocatalytic Reduction of CO2 usingNanostructured Cu2O with Foam-Like Structure. Sol. Energy 2016, 139, 452–457. [Google Scholar] [CrossRef]
- Zhang, Q.; Gao, T.; Andino, J.M.; Li, Y. Copper and iodine co-modified TiO2 nanoparticles for improved activity of CO2 photoreduction with water vapor. Appl. Catal. B 2012, 123–124, 257. [Google Scholar] [CrossRef]
- Li, Y.; Wang, W.-N.; Zhan, Z.; Woo, M.-H.; Wu, C.-Y.; Biswas, P. Photocatalytic reduction of CO2 with H2O on mesoporous silica supported Cu/TiO2 catalysts. Appl. Catal. B 2010, 100, 386. [Google Scholar] [CrossRef]
- Liu, L.; Gao, F.; Zhao, H.; Li, Y. Tailoring Cu valence and oxygen vacancy in Cu/TiO2 catalysts for enhanced CO2 photoreduction efficiency. Appl. Catal. B 2013, 134–135, 349–358. [Google Scholar] [CrossRef]
- Liu, D.; Fernández, Y.; Ola, O.; Mackintosh, S.; Maroto-Valer, M.; Parlett, C.M.A.; Lee, A.F.; Wu, J.C.S. On the impact of Cu dispersion on CO2 photoreduction over Cu/TiO2. Catal. Commun. 2012, 25, 78. [Google Scholar] [CrossRef]
- Yang, H.-C.; Lin, H.-Y.; Chien, Y.-S.; Wu, J.C.-S.; Wu, H.-H. Mesoporous TiO2/SBA-15, and Cu/TiO2/SBA-15 Composite Photocatalysts for Photoreduction of CO2 to Methanol. Catal. Lett. 2009, 131, 381. [Google Scholar] [CrossRef]
- Wang, W.-N.; Park, J.; Biswas, P. Rapid synthesis of nanostructured Cu-TiO2-SiO2 composites for CO2 photoreduction by evaporation driven self-assembly. Catal. Sci. Technol. 2011, 1, 593. [Google Scholar] [CrossRef]
- Liu, E.; Qi, L.; Bian, J.; Chen, Y.; Hu, X.; Fan, J.; Liu, H.; Zhu, C.; Wang, Q. A facile strategy to fabricate plasmonic Cu modified TiO2 nano-flower films for photocatalytic reduction of CO2 to methanol. Mater. Res. Bull. 2015, 68, 203. [Google Scholar] [CrossRef]
- Huang, L.; Duan, Z.; Song, Y.; Li, Q.; Chen, L. BiVO4 microplates with oxygen vacancies decorated with metallic Cu and Bi nanoparticles for CO2 photoreduction. ACS Appl. Nano Mater. 2021, 4, 3576–3585. [Google Scholar] [CrossRef]
- Bharad, P.A.; Nikam, A.V.; Thomas, F.; Gopinath, C.S. CuOx-TiO2 composites: Electronically integrated nanocomposites for solar hydrogen generation. Chemistryselect 2018, 3, 12022–12030. [Google Scholar] [CrossRef]
- Srinivas, B.; Shubhamangala, B.; Lalitha, K.; Reddy, P.A.K.; Kumari, V.D.; Subrahmanyam, M. Photocatalytic reduction of CO2 over Cu-TiO2/molecular sieve 5A composite. Photochem. Photobiol. 2011, 87, 995. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, G.; Naushad, M.; Ahamad, T.; Veses, R.C.; Stadler, F.J. Highly visible active Ag2CrO4/Ag/BiFeO3@RGO nano-junction for photoreduction of CO2 and photocatalytic removal of ciprofloxacin and bromate ions: The triggering effect of Ag and RGO. Chem. Eng. J. 2019, 370, 148–165. [Google Scholar] [CrossRef]
- Jeyalakshmi, V.; Mahalakshmy, R.; Krishnamurthy, K.R.; Viswanathan, B. Photocatalytic Reduction of Carbon Dioxide by Water: A Step towards Sustainable Fuels and Chemicals. Catal. Today 2016, 266, 160. [Google Scholar] [CrossRef]
- Wang, J.; Li, B.; Chen, J.; Li, N.; Zheng, J.; Zhao, J.; Zhu, Z. Enhanced photocatalytic H2 production activity of CdxZn1-xS nanocrystals by surface loading MS (M = Ni, Co, Cu) species. Appl. Surf. Sci. 2012, 259, 118–123. [Google Scholar] [CrossRef]
- Ruan, C.; Huang, Z.-Q.; Lin, J.; Li, L.; Liu, X.; Tian, M.; Huang, C.; Chang, C.-R.; Li, J.; Wang, X. Synergy of the catalytic activation on Ni and the CeO2−TiO2/Ce2Ti2O7 stoichiometric redox cycle for dramatically enhanced solar fuel production. Energy Environ. Sci. 2019, 12, 767–779. [Google Scholar] [CrossRef]
- Liou, P.Y.; Chen, S.C.; Wu, J.C.S.; Liu, D.; Mackintosh, S.; Maroto-Valerb, M.; Linforth, R. Photocatalytic CO2 reduction using an internally illuminated monolith photoreactor. Energy Environ. Sci. 2011, 4, 1487. [Google Scholar] [CrossRef]
- Miao, Y.F.; Guo, R.T.; Gu, J.W.; Liu, Y.Z.; Wu, G.L.; Duan, C.P.; Pan, W.G. Z-scheme Bi/Bi2O2CO3/Layered double-hydroxide nanosheet heterojunctions for photocatalytic CO2 reduction under visible light. ACS Appl. Nano Mater. 2021, 4, 4902–4911. [Google Scholar] [CrossRef]
- Yu, L.; Zhang, X.; Li, G.; Cao, Y.; Shao, Y.; Li, D. Highly efficient Bi2O2CO3/BiOCl photocatalyst based on heterojunction with enhanced dye-sensitization under visible light. Appl. Catal. B 2016, 187, 301–309. [Google Scholar] [CrossRef]
- Zhang, H.; Wei, J.; Dong, J.; Liu, G.; Shi, L.; An, P.; Zhao, G.; Kong, J.; Wang, X.; Meng, X.; et al. Efficient Visible-Light-Driven Carbon Dioxide Reduction by a Single-Atom Implanted Metal-Organic Framework. Angew. Chem. Int. Ed. 2016, 55, 14310. [Google Scholar] [CrossRef]
- Wei, L.; Yu, C.; Zhang, Q.; Liu, H.; Wang, Y. TiO2-Based Heterojunction Photocatalysts for Photocatalytic Reduction of CO2 into Solar Fuels. J. Mater. Chem. A 2018, 6, 22411–22436. [Google Scholar] [CrossRef]
- Nie, N.; Zhang, L.; Fu, J.; Cheng, B.; Yu, J. Self-assembled hierarchical direct Zscheme g-C3N4/ZnO microspheres with enhanced photocatalytic CO2 reduction performance. Appl. Surf. Sci. 2018, 441, 12–22. [Google Scholar] [CrossRef]
- Zhou, L.; Kamyab, H.; Surendar, A.; Maseleno, A.; Ibatova, A.Z.; Chelliapan, S.; Karachi, N.; Parsaee, Z. Novel Z-scheme composite Ag2CrO4/NG/polyimide as high performance nano catalyst for photoreduction of CO2: Design, fabrication, characterization and mechanism. J. Photochem. Photobiol. A 2019, 368, 30–40. [Google Scholar] [CrossRef]
- Xu, Q.L.; Xia, Z.H.; Zhang, J.M.; Wei, Z.Y.; Guo, Q.; Jin, H.L.; Tang, H.; Li, S.Z.; Pan, X.C.; Su, Z.; et al. Recent advances in solar-driven CO2 reduction over g-C3N4-based photocatalysts. Carbon Energy 2022. [Google Scholar] [CrossRef]
- Li, X.; Zhuang, Z.; Li, W.; Pan, H. Photocatalytic reduction of CO2 over noble metal-loaded and nitrogen-doped mesoporous TiO2. Appl. Catal. A 2012, 429–430, 31–38. [Google Scholar] [CrossRef]
- Kočí, K.; Matějů, K.; Obalová, L.; Krejčíková, S.; Lacný, Z.; Plachá, D.; Čapek, L.; Hospodková, A.; Šolcová, O. Effect of silver doping on the TiO2 for photocatalytic reduction of CO2. Appl. Catal. B-Environ. 2013, 96, 239–244. [Google Scholar] [CrossRef]
- Yuan, Y.-P.; Cao, S.-W.; Liao, Y.-S.; Yin, L.-S.; Xue, C. Red phosphor/g-C3N4 heterojunction with enhanced photocatalytic activities for solar fuels production. Appl. Catal. B 2013, 140–141, 164–168. [Google Scholar] [CrossRef]
- Gao, G.; Jiao, Y.; Waclawik, E.R.; Du, A. Single atom (Pd/Pt) supported on graphitic carbon nitride as efficient photocatalyst for visible-light reduction of carbon dioxide. J. Am. Chem. Soc. 2016, 138, 6292. [Google Scholar] [CrossRef]
- Li, X.; Li, W.; Zhuang, Z.; Zhong, Y.; Li, Q.; Wang, L. Photocatalytic Reduction of Carbon Dioxide to Methane over SiO2-Pillared HNb3O8. J. Phys. Chem. C 2012, 116, 16047. [Google Scholar] [CrossRef]
- Yu, X.; Wang, Y.; Kim, A.; Kim, Y.K. Observation of Temperature-Dependent Kinetics for Catalytic CO Oxidation over TiO2-Supported Pt Catalysts. Chem. Phys. Lett. 2017, 685, 282–287. [Google Scholar] [CrossRef]
- Asi, M.A.; Zhu, L.; He, C.; Sharma, V.K.; Shu, D.; Li, S.; Yang, J.; Xiong, Y. Visible-Light-Harvesting Reduction of CO2 to Chemical Fuels with Plasmonic Ag@AgBr/CNT Nanocomposites. Catal. Today 2013, 216, 268–275. [Google Scholar] [CrossRef]
- Iizuka, K.; Wato, T.; Miseki, Y.; Saito, K.; Kudo, A. Photocatalytic Reduction of Carbon Dioxide over Ag Cocatalyst-Loaded ALa4Ti4O15(A=Ca, Sr, and Ba) Using Water as a Reducing Reagent. J. Am. Chem. Soc. 2011, 133, 20863. [Google Scholar] [CrossRef]
- Bai, S.; Wang, X.; Hu, C.; Xie, M.; Jiang, J.; Xiong, Y. Two-dimensional g-C3N4: An ideal platform for examining facet selectivity of metal co-catalysts in photocatalysis. Chem. Commun. 2014, 50, 6094. [Google Scholar] [CrossRef] [PubMed]
- Tseng, I.-H.; Wu, J.C.-S. Photoreduction of CO2 using sol-gel derived titania and titania-supported copper catalysts. Appl. Catal. B 2002, 37, 37–48. [Google Scholar] [CrossRef]
- Wu, J.C.S.; Wu, T.H.; Chu, T.; Huang, H.; Tsai, D. Application of Optical-fiber Photoreactor for CO2 Photocatalytic Reduction. Top. Catal. 2008, 47, 131–136. [Google Scholar] [CrossRef]
- Tseng, I.-H.; Wu, J.C.-S. Effects of sol-gel procedures on the photocatalysis of Cu/TiO2 in CO2 photoreduction. J. Catal. 2004, 221, 432–440. [Google Scholar] [CrossRef]
- Xie, S.; Ma, W.; Wu, X.; Zhang, H.; Zhang, Q.; Wang, Y.; Wang, Y. Photocatalytic and Electrocatalytic Transformations of C1Molecules Involving C-C Coupling. Energy Environ. Sci. 2021, 14, 37–89. [Google Scholar] [CrossRef]
- Ajmal, S.; Yang, Y.; Tahir, M.; Li, K.A.; Bacha, A.-U.-R.; Nabi, I.; Liu, Y.; Wang, T.; Zhang, L. Boosting C2 Products in Electrochemical CO2 Reduction over Highly Dense Copper Nanoplates. Catal. Sci. Technol. 2020, 10, 4562–4570. [Google Scholar] [CrossRef]
- Slamet; Nasution, H.W.; Purnama, E.; Kosela, S.; Gunlazuardi, J. Photocatalytic reduction of CO2 on copper-doped Titania catalysts prepared by improved-impregnation method. Catal. Commun. 2005, 6, 313. [Google Scholar] [CrossRef]
- Zhao, H.; Duan, J.; Zhang, Z.; Wang, W. Bi-Ti-In trimetallic sites in the Indiumdoped Bi4Ti3O12-CuIn5S8 S-scheme heterojunction for controlling the selectivity of CO2 photoreduction. Fuel 2022, 325, 124993. [Google Scholar] [CrossRef]
- Adachi, K.; Ohta, K.; Mizuno, T. Photocatalytic reduction of carbon dioxide to hydrocarbon using copper-loaded titanium dioxide. Sol. Energy 1994, 53, 187. [Google Scholar] [CrossRef]
- Wang, Z.-Y.; Chou, H.-C.; Wu, J.C.; Tsai, D.P.; Mul, G. CO2 photoreduction using NiO/InTaO4 in optical-fiber reactor for renewable energy. Mul. Appl. Catal. A 2010, 380, 172. [Google Scholar] [CrossRef]
- Tsai, C.-W.; Chen, H.M.; Liu, R.-S.; Asakura, K.; Chan, T.-S. Ni@NiO Core-Shell Structure-Modified Nitrogen-Doped InTaO4 for Solar-Driven Highly Efficient CO2 Reduction to Methanol. J. Phys. Chem. C 2011, 115, 10180. [Google Scholar] [CrossRef]
- Li, N.; Jiang, R.; Li, Y.; Zhou, J.; Ma, Q.; Shen, S.; Liu, M. Plasma-Assisted Photocatalysis of CH4 and CO2 into Ethylene. ACS Sustain. Chem. Eng. 2019, 7, 11455–11463. [Google Scholar] [CrossRef]
- Sim, L.C.; Leong, K.H.; Saravanan, P.; Ibrahim, S. Rapid thermal reduced graphene oxide/Pt-TiO2 nanotube arrays for enhanced visible-light-driven photocatalytic reduction of CO2. Appl. Surf. Sci. 2015, 358, 122–129. [Google Scholar] [CrossRef]
- Shown, I.; Hsu, H.-C.; Chang, Y.-C.; Lin, C.-H.; Roy, P.K.; Ganguly, A.; Wang, C.-H.; Chang, J.-K.; Wu, C.-I.; Chen, L.-C.; et al. Highly Efficient Visible Light Photocatalytic Reduction of CO2 to Hydrocarbon Fuels by Cu-Nanoparticle Decorated Graphene Oxide. Nano Lett. 2014, 14, 6097. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Jin, J.; Cheng, B.; Jaroniec, M. A noble metal-free reduced graphene oxide-CdS nanorod composite for the enhanced visible-light photocatalytic reduction of CO2 to solar fuel. J. Mater. Chem. A 2014, 2, 3407. [Google Scholar] [CrossRef]
- Gui, M.M.; Chai, S.-P.; Xu, B.-Q.; Mohamed, A.R. Enhanced visible light responsive MWCNT/TiO2 core-shell nanocomposites as the potential photocatalyst for reduction of CO2 into methane. Sol. Energy Mater. Sol. Cells 2014, 122, 183. [Google Scholar] [CrossRef]
- Kuang, Y.; Shang, J.; Zhu, T. Photo-Activated Graphene Oxide to Enhance Photocatalytic Reduction of CO2. ACS Appl. Mater. Interfaces 2020, 12, 3580–3591. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Sun, W.; Miao, W.; Yuan, Z. Living Atomically Dispersed Cu Ultrathin TiO2 Nanosheet CO2 Reduction Photocatalyst. Adv. Sci. 2019, 6, 1900289. [Google Scholar] [CrossRef]
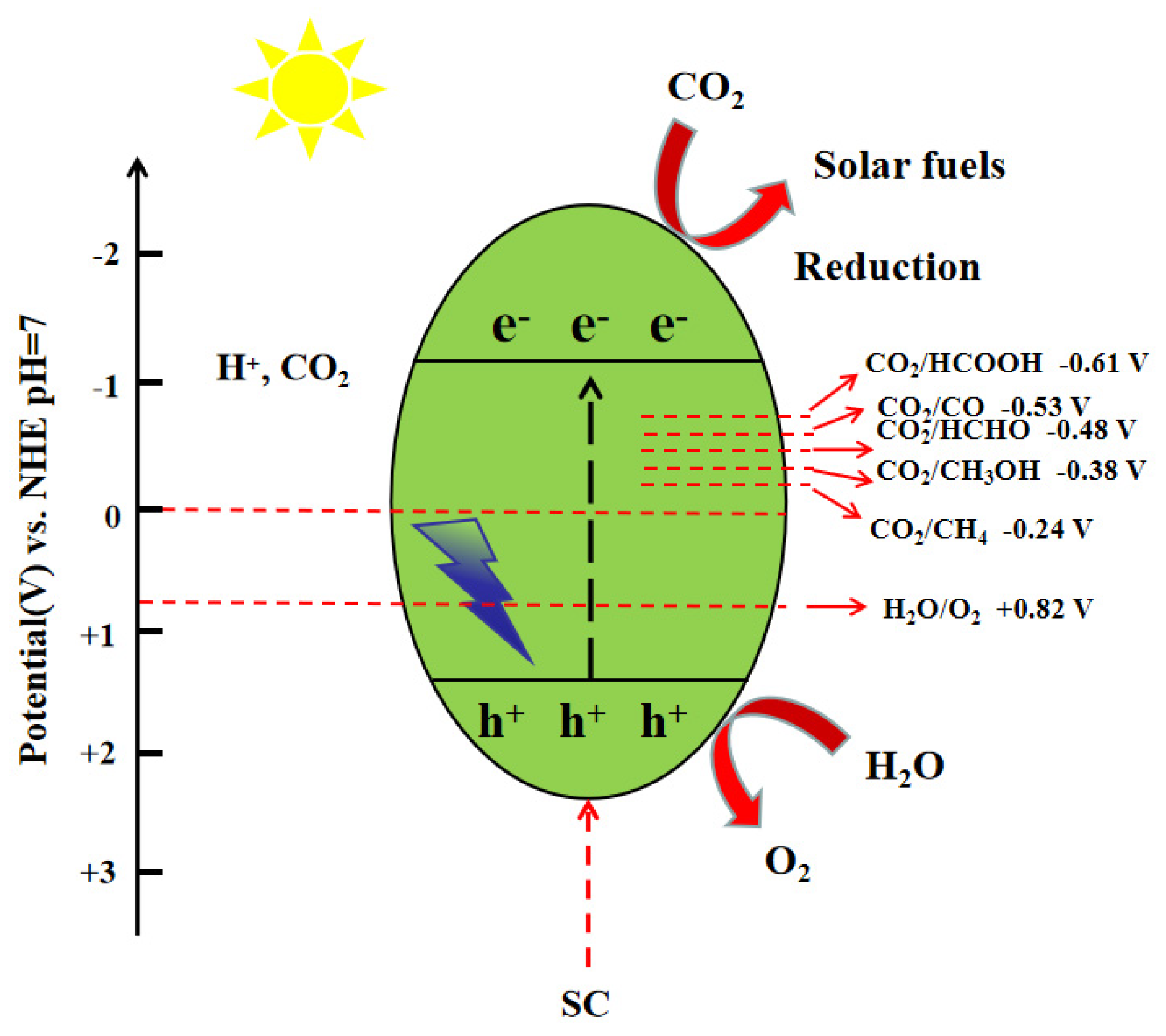

| Reaction | E0redox (vs. NHE)/V |
|---|---|
| CO2 + 2H+ + 2e−→HCOOH | −0.61 |
| CO2 + 2H+ + 2e−→CO + H2O | −0.53 |
| CO2 + 4H+ + 4e−→HCHO + H2O | −0.48 |
| CO2 + 4H+ + 4e−→C + 2H2O | −0.20 |
| CO2 + 6H+ + 6e−→CH3OH + H2O | −0.38 |
| CO2 + 8H+ + 8e−→CH4 + 2H2O | −0.24 |
| 2CO2 + 12H+ + 12e−→C2H4 + 4H2O | −0.34 |
| 2CO2 + 12H+ + 12e−→C2H5OH + 3H2O | −0.33 |
| 2CO2 + 14H+ + 14e−→HCOOH + C2H6 | −0.27 |
| 2H+ + 2e−→H2 | −0.42 |
| Co-Catalysts | Representational Preparation Method | Nitrogen Source | Major CO2 Reduction Products | Reaction Medium | References |
|---|---|---|---|---|---|
| Pt-based | Situ photodeposition, impregnation–calcination and microwave-assisted solvent-heat methods | N2 | CH4 CO | H2O | [15,19] |
| Ag-based | Sol-gel | N2 | CH4 | H2O | [79] |
| Alloy | Photodeposition | N2 | C2H4 C2H6 | H2O | [44,45] |
| Pd-based | Photodeposition | N2 | CH4 C2H6 | H2O | [33] |
| Cu-based | Thermal hydrolysis | N2 | CH3OH CO | H2O | [83,84] |
| Ni-based | Incipient wetness impregnation | N2 | CH3OH | H2O | [90,91] |
| Graphene | Hydrothermal method | N2 | CH4 | H2O | [92,93] |
| Names | Abbreviations |
|---|---|
| Density functional theory | DFT |
| Ultraviolet photoelectron spectroscopy | UPS |
| Nanotube arrays | NTAs |
| Nanotetrahydrogen | NT |
| Energy dispersive X-ray | EDX |
| Periodically modulated double-walled titanium dioxide nanotubes | PMTiNT |
| Reduced graphene oxide | RGO |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zuo, C.; Su, Q.; Yan, X. Research Progress of Co-Catalysts in Photocatalytic CO2 Reduction: A Review of Developments, Opportunities, and Directions. Processes 2023, 11, 867. https://doi.org/10.3390/pr11030867
Zuo C, Su Q, Yan X. Research Progress of Co-Catalysts in Photocatalytic CO2 Reduction: A Review of Developments, Opportunities, and Directions. Processes. 2023; 11(3):867. https://doi.org/10.3390/pr11030867
Chicago/Turabian StyleZuo, Cheng, Qian Su, and Xueyuan Yan. 2023. "Research Progress of Co-Catalysts in Photocatalytic CO2 Reduction: A Review of Developments, Opportunities, and Directions" Processes 11, no. 3: 867. https://doi.org/10.3390/pr11030867
APA StyleZuo, C., Su, Q., & Yan, X. (2023). Research Progress of Co-Catalysts in Photocatalytic CO2 Reduction: A Review of Developments, Opportunities, and Directions. Processes, 11(3), 867. https://doi.org/10.3390/pr11030867









