Anaerobic Membrane Bioreactor (AnMBR) for the Removal of Dyes from Water and Wastewater: Progress, Challenges, and Future Perspectives
Abstract
1. Introduction
2. Types of Dyes
| Dye | Structure | Chemical Formula | MW * (g/mol) | Log KOW | CAS Number | Reference |
|---|---|---|---|---|---|---|
| Azo | ||||||
| Aniline Yellow |  | C12H11N3 | 197.24 | 3.41 | 60-09-3 | [52] |
| Reactive Orange 16 |  | C20H17N3Na2O11S3 | 617.5 | NR * | 20262-58-2 | [53] |
| Orange II |  | C16H11N2NaO4S | 350.3 | NR | 633-96-5 | [54] |
| Acid Orange 12 | 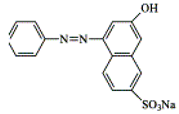 | C16H11N2NaO4S | 350.3 | NR | 1934-20-9 | [55] |
| Congo Red | 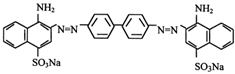 | C32H22N6Na2O6S2 | 696.7 | NR | 573-58-0 | [56] |
| Oil Red | 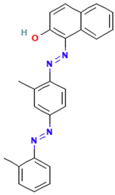 | C24H20N4O | 380.4 | NR | 85-83-6 | [57] |
| Direct Blue-1 | 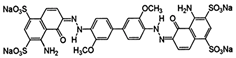 | C34H24N6Na4O16S4 | 992.8 | NR | 2610-05-1 | [58] |
| Reactive Black 5 | 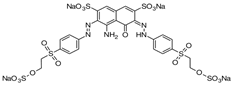 | C26H21N5Na4O19S6 | 991.8 | NR | 17095-24-8 | [59] |
| Sudan Black-B | 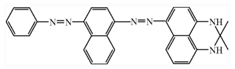 | C29H24N6 | 456.5 | NR | 4197-25-5 | [60] |
| Direct Blue-71 |  | C40H23N7Na4O13S4 | 1029.9 | NR | 4399-55-7 | [61] |
| Direct Black-22 |  | C44H32N13Na3O11S3 | 1084 | NR | 6473-13-8 | [62] |
| Phthalocyanines | ||||||
| Reactive Blue 21 |  | C40H25CuN9O14S5 | 1079.6 | NR | 12236-86-1 | [63] |
| Reactive Blue 7 | 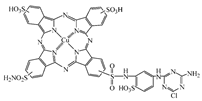 | C41H24CuN15O13S5Cl | 1194.1 | NR | - | [64] |
| Xanthene | ||||||
| Rhodamine B | 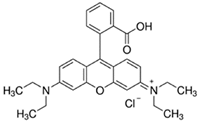 | C28H31ClN2O3 | 479.0 | NR | 81-88-9 | [65] |
| Phloxine B |  | C20H2Br4Cl4Na2O5 | 829.6 | NR | 18472-87-2 | [66] |
| Erythrosine B | 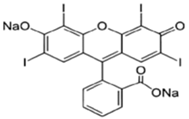 | C20H6I4Na2O5 | 879.9 | NR | 16423-68-0 | [67] |
| Nitro | ||||||
| Picric Acid | 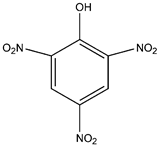 | C6H3N3O7 | 229.10 | 1.44 | 88-89-1 | [68] |
| Naphthol Yellow S | 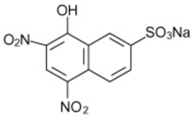 | C10H4N2Na2O8S | 358.19 | NR | 846-70-8 | [69] |
| Acid Orange 3 |  | C18H13N4NaO7S | 452.4 | -0.69 | 6373-74-6 | [70] |
| C.I. Disperse Yellow 42 |  | C18H15N3O4S | 369.4 | NR | 5124-25-4 | [71] |
| C.I. Disperse Yellow 70 | 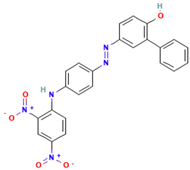 | C24H17N5O5 | 455.4 | NR | 12223-91-5 | [72] |
| Quinoline | ||||||
| Quinoline Yellow | 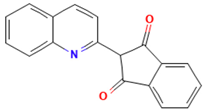 | C18H11NO2 | 273.3 | 4.10 | 8003-22-3 | [73] |
| Indigo | ||||||
| Indigo Carmine |  | C16H8N2Na2O8S2 | 466.4 | NR | 860-22-0 | [74] |
| Acridine | ||||||
| Acridine Orange | 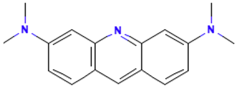 | C17H20ClN3 | 301.8 | NR | 65-61-2 | [75] |
| Acridine Yellow G |  | C15H16ClN3 | 273.76 | NR | 135-49-9 | [76] |
| Azine | ||||||
| Methylene Blue | 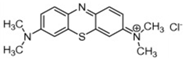 | C16H18N3ClS | 319.8 | 0.75 | 61-73-4 | [77] |
| Thionin | 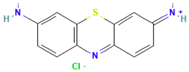 | C12H10ClN3S | 263.75 | NR | 581-64-6 | [78] |
| Safranin O | 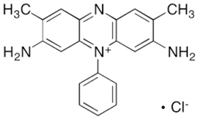 | C20H19ClN4 | 350.85 | NR | 477-73-6 | [79] |
| Anthraquinone | ||||||
| Acid Blue 25 |  | C20H13N2NaO5S | 416.4 | NR | 6408-78-2 | [80] |
| Disperse Red 11 | 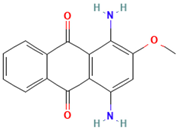 | C15H12N2O3 | 268.27 | NR | 2872-48-2 | [81] |
| Reactive Brilliant Blue | 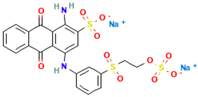 | C22H16N2Na2O11S3 | 626.5 | NR | 2580-78-1 | [82,83] |
| Triarylmethane | ||||||
| Basic Fuchsin | 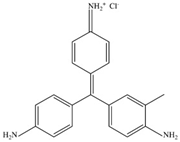 | C20H20ClN3 | 337.8 | NR | 632-99-5 | [84] |
| Malachite Green | 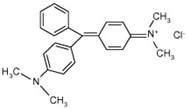 | C23H25N2Cl | 364.92 | 0.62 | 569-64-2 | [85] |
| Methyl Violet | 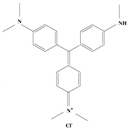 | C25H30ClN3 | 408.0 | 0.51 | 548-62-9 | [86,87] |
3. Toxicity of Dyes on Aquatic Environments
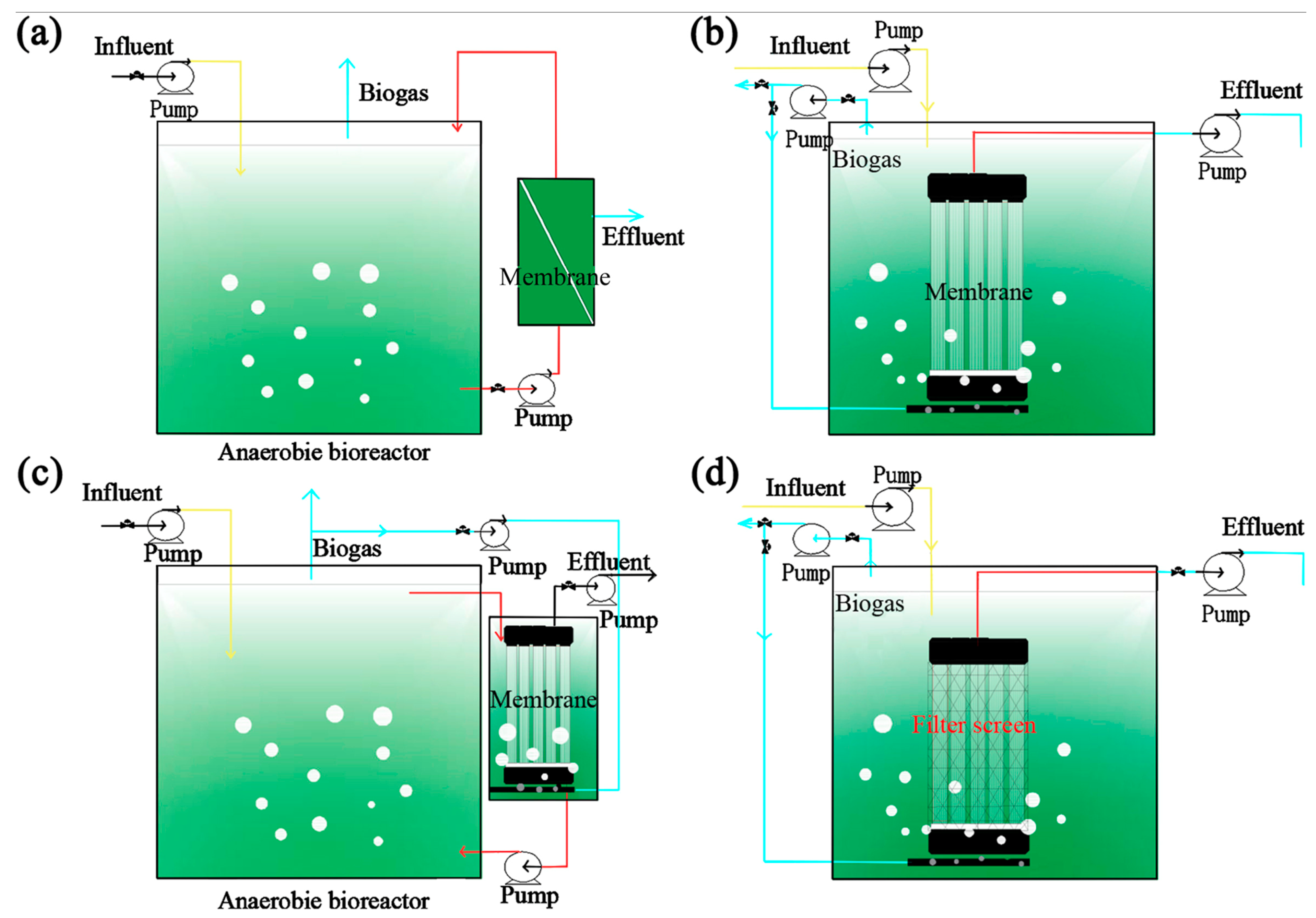
4. Membrane Bioreactor (MBR) for Removal of Dyes

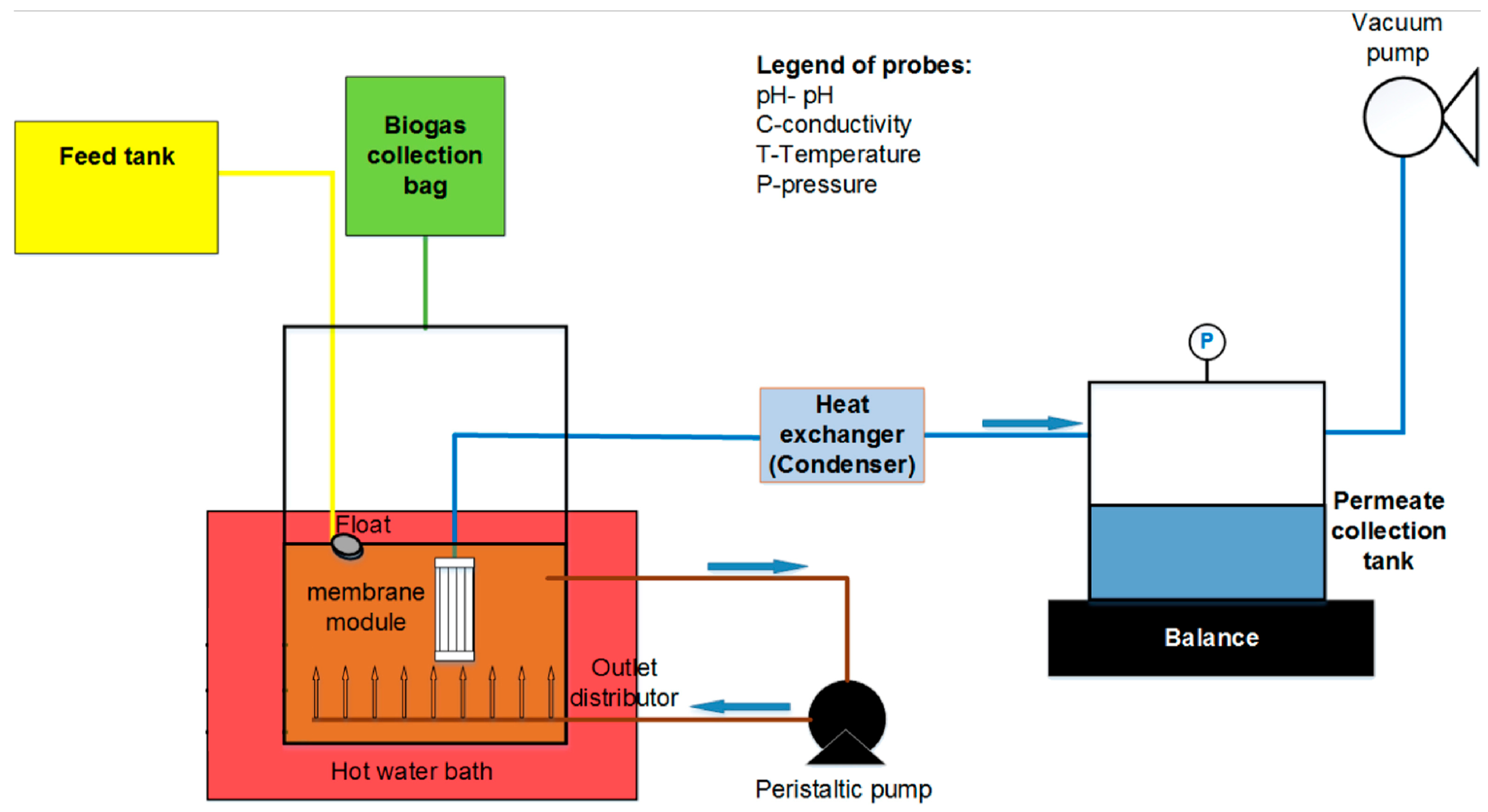
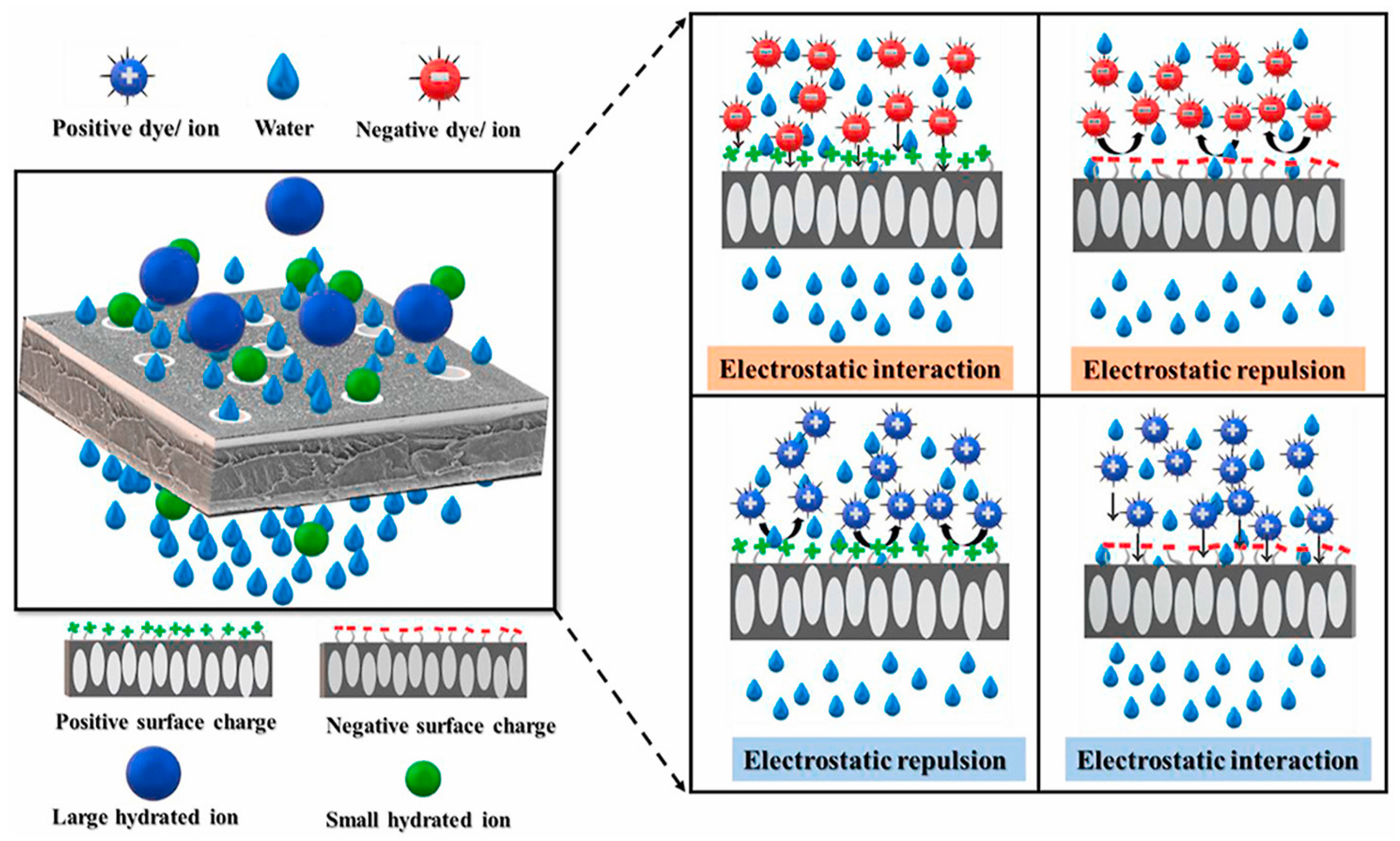
4.1. Removal Mechanisms and Vital Factors
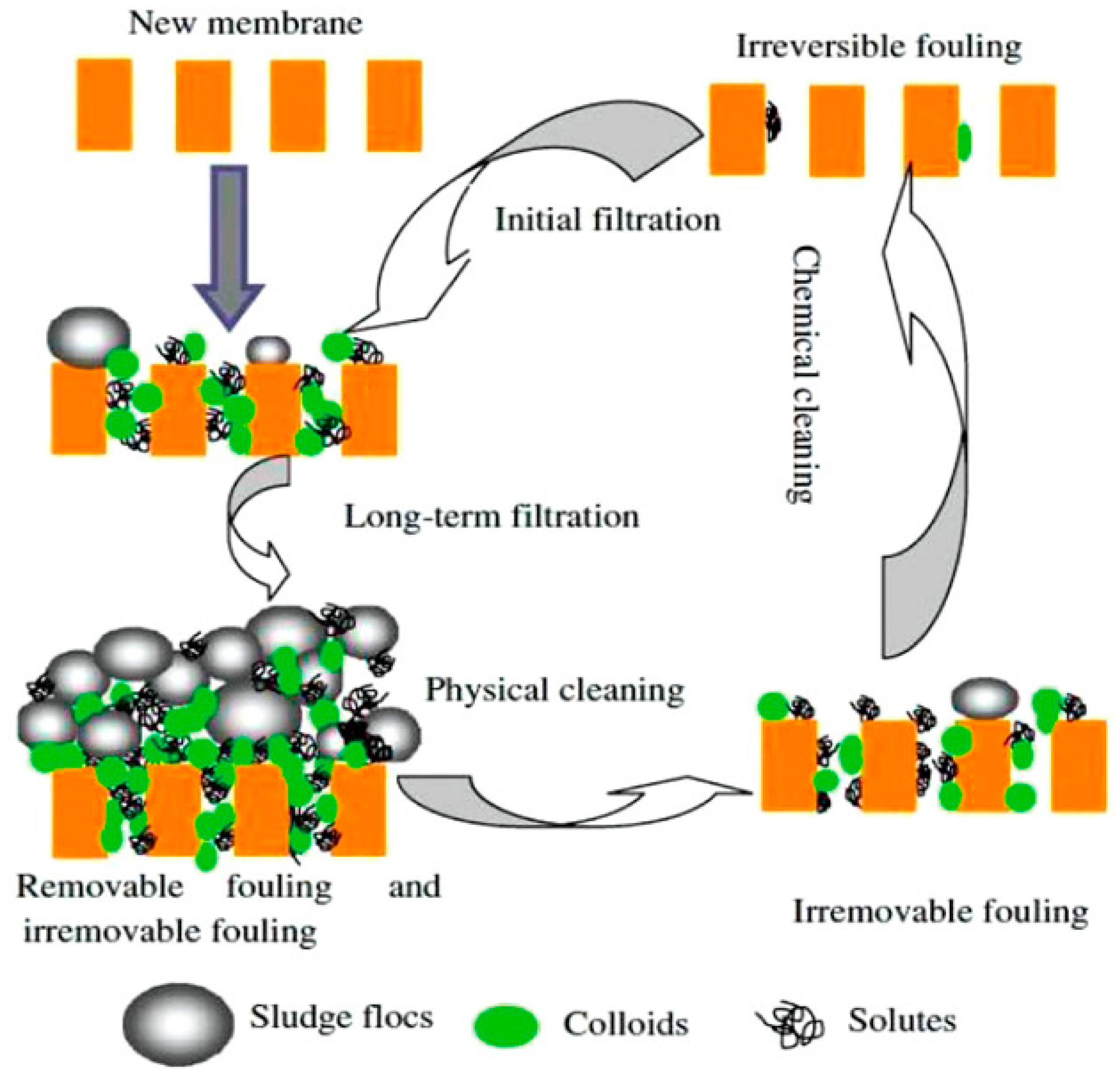
4.2. Fouling and Cleaning
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, H.; Li, H.; Gao, D.; Yu, H. Source identification of surface water pollution using multivariate statistics combined with physicochemical and socioeconomic parameters. Sci. Total Environ. 2022, 806, 151274. [Google Scholar] [CrossRef] [PubMed]
- Arif, M. Extraction of iron (III) ions by core-shell microgel for in situ formation of iron nanoparticles to reduce harmful pollutants from water. J. Environ. Chem. Eng. 2023, 11, 109270. [Google Scholar] [CrossRef]
- Wang, M.; Janssen, A.B.G.; Bazin, J.; Strokal, M.; Ma, L.; Kroeze, C. Accounting for interactions between Sustainable Development Goals is essential for water pollution control in China. Nat. Commun. 2022, 13, 730. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Thakur, C.; Chaudhari, P.K. Anaerobic biological treatment of dye bearing water in anaerobic sequencing batch reactor: Performance and kinetics studies. J. Indian Chem. Soc. 2022, 99, 100673. [Google Scholar] [CrossRef]
- Naseem, K.; Farooqi, Z.H.; Begum, R.; Irfan, A. Removal of Congo red dye from aqueous medium by its catalytic reduction using sodium borohydride in the presence of various inorganic nano-catalysts: A review. J. Clean. Prod. 2018, 187, 296–307. [Google Scholar] [CrossRef]
- Blanchard, R.; Mekonnen, T.H. Synchronous pyrolysis and activation of poly (ethylene terephthalate) for the generation of activated carbon for dye contaminated wastewater treatment. J. Environ. Chem. Eng. 2022, 10, 108810. [Google Scholar] [CrossRef]
- Mahmood, K.; Amara, U.; Siddique, S.; Usman, M.; Peng, Q.; Khalid, M.; Hussain, A.; Ajmal, M.; Ahmad, A.; Sumrra, S.H.; et al. Green synthesis of Ag@CdO nanocomposite and their application towards brilliant green dye degradation from wastewater. J. Nanostruct. Chem. 2022, 12, 329–341. [Google Scholar] [CrossRef]
- Ajmal, M.; Siddiq, M.; Aktas, N.; Sahiner, N. Magnetic Co–Fe bimetallic nanoparticle containing modifiable microgels for the removal of heavy metal ions, organic dyes and herbicides from aqueous media. RSC Adv. 2015, 5, 43873–43884. [Google Scholar] [CrossRef]
- Benny, C.K.; Chakraborty, S. Dyeing wastewater treatment in horizontal-vertical constructed wetland using organic waste media. J. Environ. Manag. 2023, 331, 117213. [Google Scholar] [CrossRef]
- Arif, M. Catalytic degradation of azo dyes by bimetallic nanoparticles loaded in smart polymer microgels. RSC Adv. 2023, 13, 3008–3019. [Google Scholar] [CrossRef]
- Selim, M.T.; Salem, S.S.; Mohamed, A.A.; El-Gamal, M.S.; Awad, M.F.; Fouda, A. Biological Treatment of Real Textile Effluent Using Aspergillus flavus and Fusarium oxysporium and Their Consortium along with the Evaluation of Their Phytotoxicity. J. Fungi 2021, 7, 193. [Google Scholar] [CrossRef]
- Tan, X.; Acquah, I.; Liu, H.; Li, W.; Tan, S. A critical review on saline wastewater treatment by membrane bioreactor (MBR) from a microbial perspective. Chemosphere 2019, 220, 1150–1162. [Google Scholar] [CrossRef]
- Castrogiovanni, F.; Borea, L.; Corpuz, M.V.A.; Buonerba, A.; Vigliotta, G.; Ballesteros, F.J.; Hasan, S.W.; Belgiorno, V.; Naddeo, V. Innovative encapsulated self-forming dynamic bio-membrane bioreactor (ESFDMBR) for efficient wastewater treatment and fouling control. Sci. Total Environ. 2022, 805, 150296. [Google Scholar] [CrossRef]
- Zhang, J.; Xiao, K.; Liu, Z.; Gao, T.; Liang, S.; Huang, X. Large-Scale Membrane Bioreactors for Industrial Wastewater Treatment in China: Technical and Economic Features, Driving Forces, and Perspectives. Engineering 2021, 7, 868–880. [Google Scholar] [CrossRef]
- Deng, L.; Guo, W.; Ngo, H.H.; Zhang, X.; Chen, C.; Chen, Z.; Cheng, D.; Ni, S.-Q.; Wang, Q. Recent advances in attached growth membrane bioreactor systems for wastewater treatment. Sci. Total Environ. 2022, 808, 152123. [Google Scholar] [CrossRef]
- Rondon, H.; El-Cheikh, W.; Boluarte, I.A.R.; Chang, C.-Y.; Bagshaw, S.; Farago, L.; Jegatheesan, V.; Shu, L. Application of enhanced membrane bioreactor (eMBR) to treat dye wastewater. Bioresour. Technol. 2015, 183, 78–85. [Google Scholar] [CrossRef]
- Ravadelli, M.; da Costa, R.E.; Lobo-Recio, M.A.; Akaboci, T.R.V.; Bassin, J.P.; Lapolli, F.R.; Belli, T.J. Anoxic/oxic membrane bioreactor assisted by electrocoagulation for the treatment of azo-dye containing wastewater. J. Environ. Chem. Eng. 2021, 9, 105286. [Google Scholar] [CrossRef]
- El-Kammah, M.; Elkhatib, E.; Gouveia, S.; Cameselle, C.; Aboukila, E. Cost-effective ecofriendly nanoparticles for rapid and efficient indigo carmine dye removal from wastewater: Adsorption equilibrium, kinetics and mechanism. Environ. Technol. Innov. 2022, 28, 102595. [Google Scholar] [CrossRef]
- Nada, A.A.; Bekheet, M.F.; Roualdes, S.; Gurlo, A.; Ayral, A. Functionalization of MCM-41 with titanium oxynitride deposited via PECVD for enhanced removal of methylene blue. J. Mol. Liq. 2019, 274, 505–515. [Google Scholar] [CrossRef]
- Artifon, W.; Cesca, K.; de Andrade, C.J.; Ulson de Souza, A.A.; de Oliveira, D. Dyestuffs from textile industry wastewaters: Trends and gaps in the use of bioflocculants. Process Biochem. 2021, 111, 181–190. [Google Scholar] [CrossRef]
- Liu, Q. Pollution and Treatment of Dye Waste-Water. IOP Conf. Ser. Earth Environ. Sci. 2020, 514, 052001. [Google Scholar] [CrossRef]
- Saba, B.; Kjellerup, B.V.; Christy, A.D. Eco-friendly bio-electro-degradation of textile dyes wastewater. Bioresour. Technol. Rep. 2021, 15, 100734. [Google Scholar] [CrossRef]
- Shabir, G.; Arif, M.; Saeed, A.; Hussain, G. Synthesis and Optical Study of Sensitive and Selective Calix[4] Based Cu2+ Ion Detection Probes. Russ. J. Gen. Chem. 2019, 89, 813–818. [Google Scholar] [CrossRef]
- Liu, Y.; Li, C.; Bao, J.; Wang, X.; Yu, W.; Shao, L. Degradation of Azo Dyes with Different Functional Groups in Simulated Wastewater by Electrocoagulation. Water 2022, 14, 123. [Google Scholar] [CrossRef]
- Salem, S.; Fouda, A.; Mohamed, A.; El-Gamal, M.; Talat, M. Biological decolorization of azo dyes from textile wastewater effluent by Aspergillus niger. Egypt. J. Chem. 2019, 62, 1799–1813. [Google Scholar] [CrossRef]
- Benkhaya, S.; El Harfi, S.; El Harfi, A. Classifications, properties and applications of textile dyes: A review. Appl. J. Environ. Eng. Sci. 2017, 3, 311–320. [Google Scholar]
- Tajiki, A.; Abdouss, M. Synthesis and Characterization of Graphene Oxide Nano-sheets for Effective Removal of Copper Phthalocyanine from Aqueous Media. Iran J. Chem. Chem. Eng. 2017, 36, 1–9. [Google Scholar]
- Lipatova, I.M.; Makarova, L.I.; Yusova, A.A. Adsorption removal of anionic dyes from aqueous solutions by chitosan nanoparticles deposited on the fibrous carrier. Chemosphere 2018, 212, 1155–1162. [Google Scholar] [CrossRef]
- El Aggadi, S.; El Abbassi, Z.; El Hourch, A. Color removal from dye-containing aqueous solutions by electrooxidation. Desalin. Water Treat. 2021, 215, 232–236. [Google Scholar] [CrossRef]
- Silva, M.C.; Corrêa, A.D.; Amorim, M.T.S.P.; Parpot, P.; Torres, J.A.; Chagas, P.M.B. Decolorization of the phthalocyanine dye reactive blue 21 by turnip peroxidase and assessment of its oxidation products. J. Mol. Catal. B Enzym. 2012, 77, 9–14. [Google Scholar] [CrossRef]
- Beyhill, M.I.; Matthews, R.D.; Pavlostathis, S.G. Decolorization of a reactive copper-phthalocyanine dye under methanogenic conditions. Water Sci. Technol. 2001, 43, 333–340. [Google Scholar] [CrossRef]
- Apostol, L.C.; Pereira, L.; Pereira, R.; Gavrilescu, M.; Alves, M.M. Biological decolorization of xanthene dyes by anaerobic granular biomass. Biodegradation 2012, 23, 725–737. [Google Scholar] [CrossRef]
- Kiernan, J. Classification and naming of dyes, stains and fluorochromes. Biotech. Histochem. 2001, 76, 261–278. [Google Scholar] [CrossRef]
- Niu, P.; Liang, X.; Lu, X.; Wang, S.; Li, Y.; Wang, L.; Guo, Y. Preparation of magnetic carbonized polyaniline nanotube and its adsorption behaviors of xanthene colorants in beverage and fish samples. J. Chromatogr. A 2019, 1605, 460369. [Google Scholar] [CrossRef]
- Ikeno, T.; Nagano, T.; Hanaoka, K. Silicon-substituted Xanthene Dyes and Their Unique Photophysical Properties for Fluorescent Probes. Chem. Asian J. 2017, 12, 1435–1446. [Google Scholar] [CrossRef]
- Kent, J.A. Kent and Riegel’s Handbook of Industrial Chemistry and Biotechnology; Kent, J.A., Ed.; Springer: Boston, MA, USA, 2007; ISBN 978-0-387-27842-1. [Google Scholar]
- dos Santos, G.C.; Oliveira, E.F.; Lavarda, F.C.; da Silva-Filho, L.C. Designing new quinoline-based organic photosensitizers for dye-sensitized solar cells (DSSC): A theoretical investigation. J. Mol. Model. 2019, 25, 75. [Google Scholar] [CrossRef]
- Gupta, V.K.; Jain, R.; Agarwal, S.; Nayak, A.; Shrivastava, M. Photodegradation of hazardous dye quinoline yellow catalyzed by TiO2. J. Colloid Interface Sci. 2012, 366, 135–140. [Google Scholar] [CrossRef]
- Wahyuningsih, S.; Ramelan, A.H.; Wardani, D.K.; Aini, F.N.; Sari, P.L.; Tamtama, B.P.N.; Kristiawan, Y.R. Indigo Dye Derived from Indigofera Tinctoria as Natural Food Colorant. IOP Conf. Ser. Mater. Sci. Eng. 2017, 193, 012048. [Google Scholar] [CrossRef]
- Balan, D.S.L.; Monteiro, R.T.R. Decolorization of textile indigo dye by ligninolytic fungi. J. Biotechnol. 2001, 89, 141–145. [Google Scholar] [CrossRef]
- Shaikh, M.; Swamy, Y.M.; Pal, H. Supramolecular host–guest interaction of acridine dye with cyclodextrin macrocycles: Photophysical, pKa shift and quenching study. J. Photochem. Photobiol. A Chem. 2013, 258, 41–50. [Google Scholar] [CrossRef]
- Liu, S.P.; Chen, S.; Liu, Z.F.; Hu, X.L.; Li, T.S. Resonance Rayleigh scattering spectra of interaction of sodium carboxymethylcellulose with cationic acridine dyes and their analytical applications. Anal. Chim. Acta 2005, 535, 169–175. [Google Scholar] [CrossRef]
- Yang, J.; Li, C.; Yang, Y.; Gu, J.; Bao, Q.; Lu, J.; Su, L.; Yang, L. Efficiency and mechanism of acridine orange removal by citric-acid-crosslinked β-cyclodextrin. Mater. Lett. 2022, 312, 131688. [Google Scholar] [CrossRef]
- Devadiga, D.; Selvakumar, M.; Devadiga, D.; Paramasivam, S.; Ahipa, T.N.; Shetty, P.; Kumar, S.S. Organic sensitizer with azine π-conjugated architecture as co-sensitizer and polymer-based electrolyte for efficient dye-sensitized solar cell. Surf. Interfaces 2022, 33, 102236. [Google Scholar] [CrossRef]
- Acar, E.T. An experimental and theoretical investigation of cationic azine dye adsorption on natural sepiolite in single and multi-component systems. Chem. Eng. Res. Des. 2022, 187, 507–515. [Google Scholar] [CrossRef]
- Fanchiang, J.-M.; Tseng, D.-H. Degradation of anthraquinone dye C.I. Reactive Blue 19 in aqueous solution by ozonation. Chemosphere 2009, 77, 214–221. [Google Scholar] [CrossRef]
- Deng, D.; Guo, J.; Zeng, G.; Sun, G. Decolorization of anthraquinone, triphenylmethane and azo dyes by a new isolated Bacillus cereus strain DC11. Int. Biodeterior. Biodegrad. 2008, 62, 263–269. [Google Scholar] [CrossRef]
- Li, X.; Tang, S.; Yuan, D.; Tang, J.; Zhang, C.; Li, N.; Rao, Y. Improved degradation of anthraquinone dye by electrochemical activation of PDS. Ecotoxicol. Environ. Saf. 2019, 177, 77–85. [Google Scholar] [CrossRef]
- Ogugbue, C.J.; Sawidis, T. Bioremediation and Detoxification of Synthetic Wastewater Containing Triarylmethane Dyes by Aeromonas hydrophila Isolated from Industrial Effluent. Biotechnol. Res. Int. 2011, 2011, 967925. [Google Scholar] [CrossRef]
- Jeyaram, S. Intermolecular charge transfer in donor–acceptor substituted triarylmethane dye for NLO and optical limiting applications. J. Mater. Sci. Mater. Electron. 2021, 32, 9368–9376. [Google Scholar] [CrossRef]
- Sakti, S.C.W.; Laily, R.N.; Aliyah, S.; Indrasari, N.; Fahmi, M.Z.; Lee, H.V.; Akemoto, Y.; Tanaka, S. Re-collectable and recyclable epichlorohydrin-crosslinked humic acid with spinel cobalt ferrite core for simple magnetic removal of cationic triarylmethane dyes in polluted water. J. Environ. Chem. Eng. 2020, 8, 104004. [Google Scholar] [CrossRef]
- Pagalan Jr, E.; Sebron, M.; Gomez, S.; Salva, S.J.; Ampusta, R.; Macarayo, A.J.; Joyno, C.; Ido, A.; Arazo, R. Activated carbon from spent coffee grounds as an adsorbent for treatment of water contaminated by aniline yellow dye. Ind. Crops Prod. 2020, 145, 111953. [Google Scholar] [CrossRef]
- Won, S.W.; Choi, S.B.; Yun, Y.-S. Performance and mechanism in binding of Reactive Orange 16 to various types of sludge. Biochem. Eng. J. 2006, 28, 208–214. [Google Scholar] [CrossRef]
- Daneshvar, N.; Ashassi-Sorkhabi, H.; Tizpar, A. Decolorization of orange II by electrocoagulation method. Sep. Purif. Technol. 2003, 31, 153–162. [Google Scholar] [CrossRef]
- Wong, Y.C.; Szeto, Y.S.; Cheung, W.H.; McKay, G. Adsorption of acid dyes on chitosan—Equilibrium isotherm analyses. Process Biochem. 2004, 39, 695–704. [Google Scholar] [CrossRef]
- Fu, Y.; Viraraghavan, T. Removal of Congo Red from an aqueous solution by fungus Aspergillus niger. Adv. Environ. Res. 2002, 7, 239–247. [Google Scholar] [CrossRef]
- PubChem Sudan IV. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/62330 (accessed on 9 March 2023).
- Singh, G.; Kumar, V.; Dwivedi, S.K. Comparative Investigation of Congo Red and Direct Blue-1 Adsorption on Mycosynthesized Iron Nanoparticle. J. Clust. Sci. 2022, 33, 1889–1905. [Google Scholar] [CrossRef]
- Beluci, N.d.C.L.; Mateus, G.A.P.; Miyashiro, C.S.; Homem, N.C.; Gomes, R.G.; Fagundes-Klen, M.R.; Bergamasco, R.; Vieira, A.M.S. Hybrid treatment of coagulation/flocculation process followed by ultrafiltration in TIO2-modified membranes to improve the removal of reactive black 5 dye. Sci. Total Environ. 2019, 664, 222–229. [Google Scholar] [CrossRef]
- Zhao, X.; Zhou, S.; Chen, M.; Wu, L. Effective encapsulation of Sudan black B with polystyrene using miniemulsion polymerization. Colloid Polym. Sci. 2009, 287, 969–977. [Google Scholar] [CrossRef]
- Bulut, Y.; Gözübenli, N.; Aydın, H. Equilibrium and kinetics studies for adsorption of direct blue 71 from aqueous solution by wheat shells. J. Hazard. Mater. 2007, 144, 300–306. [Google Scholar] [CrossRef]
- Alexandre, J.I.d.S.; Santos Neto, S.M.d.; Coutinho, A.P.; Melo, T.D.A.T.d.; Gonçalves, E.A.P.; Gondim, M.V.S.; Antonino, A.C.D.; Rabelo, A.E.C.d.G.d.C.; Oliveira, A.L.d. Sorption of the Direct Black 22 dye in alluvial soil. Ambient. Agua-An Interdiscip. J. Appl. Sci. 2020, 15, 1. [Google Scholar] [CrossRef]
- PubChem Reactive Blue 21. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Reactive-Blue-21 (accessed on 9 March 2023).
- Lee, Y.H.; Matthews, R.D.; Pavlostathis, S.G. Biological Decolorization of Reactive Anthraquinone and Phthalocyanine Dyes Under Various Oxidation-Reduction Conditions. Water Environ. Res. 2006, 78, 156–169. [Google Scholar] [CrossRef] [PubMed]
- Imam, S.S.; Babamale, H.F. A Short Review on the Removal of Rhodamine B Dye Using Agricultural Waste-Based Adsorbents. Asian J. Chem. Sci. 2020, 7, 25–37. [Google Scholar] [CrossRef]
- Walthall, W.; Stark, J. The acute and chronic toxicity of two xanthene dyes, fluorescein sodium salt and phloxine B, to Daphnia pulex. Environ. Pollut. 1999, 104, 207–215. [Google Scholar] [CrossRef]
- Rashtbari, Y.; Afshin, S.; Hamzezadeh, A.; Gholizadeh, A.; Ansari, F.J.; Poureshgh, Y.; Fazlzadeh, M. Green synthesis of zinc oxide nanoparticles loaded on activated carbon prepared from walnut peel extract for the removal of Eosin Y and Erythrosine B dyes from aqueous solution: Experimental approaches, kinetics models, and thermodynamic studies. Environ. Sci. Pollut. Res. 2022, 29, 5194–5206. [Google Scholar] [CrossRef]
- Bhatt, D.R.; Maheria, K.C.; Parikh, J.K. Highly efficient micellar extraction of toxic picric acid into novel ionic liquid: Effect of parameters, solubilization isotherm, evaluation of thermodynamics and design parameters. J. Hazard. Mater. 2015, 300, 338–346. [Google Scholar] [CrossRef]
- Jain, R.; Gupta, V.K.; Sikarwar, S. Adsorption and desorption studies on hazardous dye Naphthol Yellow S. J. Hazard. Mater. 2010, 182, 749–756. [Google Scholar] [CrossRef]
- Li, J.T.; Bai, B.; Song, Y.L. Degradation of Acid orange 3 in aqueous solution by combination of Fly ash/H2O2 and ultrasound irradiation. Indian J. Chem. Technol. 2010, 17, 198–203. [Google Scholar]
- Hashemian, S.; Sadeghi, B.; Mozafari, F.; Salehfar, H.; Salari, K. Adsorption of Disperse of Yellow 42 onto Bentonite and Organo-Modified Bentonite by Tetra Butyl Ammonium Iodide (B-TBAI). Pol. J. Environ. Stud. 2013, 22, 1363–1370. [Google Scholar]
- PubChem C.I. Disperse Yellow 70. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/135666422 (accessed on 9 March 2023).
- PubChem Quinoline Yellow. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/6731 (accessed on 9 March 2023).
- Ramesh, T.N.; Kirana, D.V.; Ashwini, A.; Manasa, T.R. Calcium hydroxide as low cost adsorbent for the effective removal of indigo carmine dye in water. J. Saudi Chem. Soc. 2017, 21, 165–171. [Google Scholar] [CrossRef]
- PubChem 3,6-Bis(dimethylamino)acridine Hydrochloride. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/517204 (accessed on 9 March 2023).
- Solanki, V.S.; Ameta, K.L.; Pare, B.; Jonnalagadda, S.B.; Gupta, P. Investigation of photocatalytic mineralisation of Acridine Yellow G dye by BaCrO4 in the presence of eco-friendly LEDs irradiation. J. Indian Chem. Soc. 2022, 99, 100340. [Google Scholar] [CrossRef]
- Ahmad, A.; Rafatullah, M.; Sulaiman, O.; Ibrahim, M.H.; Hashim, R. Scavenging behaviour of meranti sawdust in the removal of methylene blue from aqueous solution. J. Hazard. Mater. 2009, 170, 357–365. [Google Scholar] [CrossRef]
- PubChem Thionine. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/65043 (accessed on 9 March 2023).
- Ghosh, I.; Kar, S.; Chatterjee, T.; Bar, N.; Das, S.K. Adsorptive removal of Safranin-O dye from aqueous medium using coconut coir and its acid-treated forms: Adsorption study, scale-up design, MPR and GA-ANN modeling. Sustain. Chem. Pharm. 2021, 19, 100374. [Google Scholar] [CrossRef]
- Daneshvar, E.; Sohrabi, M.S.; Kousha, M.; Bhatnagar, A.; Aliakbarian, B.; Converti, A.; Norrström, A.-C. Shrimp shell as an efficient bioadsorbent for Acid Blue 25 dye removal from aqueous solution. J. Taiwan Inst. Chem. Eng. 2014, 45, 2926–2934. [Google Scholar] [CrossRef]
- PubChem Disperse Red 11. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/17885 (accessed on 9 March 2023).
- PubChem Reactive Blue 19. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/17409 (accessed on 9 March 2023).
- Janaki, V.; Vijayaraghavan, K.; Ramasamy, A.K.; Lee, K.-J.; Oh, B.-T.; Kamala-Kannan, S. Competitive adsorption of Reactive Orange 16 and Reactive Brilliant Blue R on polyaniline/bacterial extracellular polysaccharides composite—A novel eco-friendly polymer. J. Hazard. Mater. 2012, 241–242, 110–117. [Google Scholar] [CrossRef]
- Huang, L.; Kong, J.; Wang, W.; Zhang, C.; Niu, S.; Gao, B. Study on Fe(III) and Mn(II) modified activated carbons derived from Zizania latifolia to removal basic fuchsin. Desalination 2012, 286, 268–276. [Google Scholar] [CrossRef]
- Sartape, A.S.; Mandhare, A.M.; Jadhav, V.V.; Raut, P.D.; Anuse, M.A.; Kolekar, S.S. Removal of malachite green dye from aqueous solution with adsorption technique using Limonia acidissima (wood apple) shell as low cost adsorbent. Arab. J. Chem. 2017, 10, S3229–S3238. [Google Scholar] [CrossRef]
- PubChem Gentian Violet. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/11057 (accessed on 9 March 2023).
- Shamsipur, M.; Rajabi, H.R. Study of photocatalytic activity of ZnS quantum dots as efficient nanoparticles for removal of methyl violet: Effect of ferric ion doping. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 122, 260–267. [Google Scholar] [CrossRef]
- Gita, S.; Hussan, A.; Choudhury, T.G. Impact of Textile Dyes Waste on Aquatic Environments and its Treatment. Environ. Ecol. 2017, 35, 2349–2353. [Google Scholar]
- Hernández-Zamora, M.; Martínez-Jerónimo, F.; Cristiani-Urbina, E.; Cañizares-Villanueva, R.O. Congo red dye affects survival and reproduction in the cladoceran Ceriodaphnia dubia. Effects of direct and dietary exposure. Ecotoxicology 2016, 25, 1832–1840. [Google Scholar] [CrossRef]
- Krishna Moorthy, A.; Govindarajan Rathi, B.; Shukla, S.P.; Kumar, K.; Shree Bharti, V. Acute toxicity of textile dye Methylene blue on growth and metabolism of selected freshwater microalgae. Environ. Toxicol. Pharmacol. 2021, 82, 103552. [Google Scholar] [CrossRef]
- Gita, S.; Shukla, S.P.; Deshmukhe, G.; Choudhury, T.G.; Saharan, N.; Singh, A.K. Toxicity Evaluation of Six Textile Dyes on Growth, Metabolism and Elemental Composition (C, H, N, S) of Microalgae Spirulina platensis: The Environmental Consequences. Bull. Environ. Contam. Toxicol. 2021, 106, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Marimuthu, S.; Antonisamy, A.J.; Malayandi, S.; Rajendran, K.; Tsai, P.-C.; Pugazhendhi, A.; Ponnusamy, V.K. Silver nanoparticles in dye effluent treatment: A review on synthesis, treatment methods, mechanisms, photocatalytic degradation, toxic effects and mitigation of toxicity. J. Photochem. Photobiol. B Biol. 2020, 205, 111823. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Kopp-Schneider, A. Summarizing EC50 estimates from multiple dose-response experiments: A comparison of a meta-analysis strategy to a mixed-effects model approach. Biom. J. 2014, 56, 493–512. [Google Scholar] [CrossRef] [PubMed]
- Skjolding, L.M.; Jørgensen, L.G.; Dyhr, K.S.; Köppl, C.J.; McKnight, U.S.; Bauer-Gottwein, P.; Mayer, P.; Bjerg, P.L.; Baun, A. Assessing the aquatic toxicity and environmental safety of tracer compounds Rhodamine B and Rhodamine WT. Water Res. 2021, 197, 117109. [Google Scholar] [CrossRef]
- Novotný, Č.; Dias, N.; Kapanen, A.; Malachová, K.; Vándrovcová, M.; Itävaara, M.; Lima, N. Comparative use of bacterial, algal and protozoan tests to study toxicity of azo- and anthraquinone dyes. Chemosphere 2006, 63, 1436–1442. [Google Scholar] [CrossRef]
- Gita, S.; Shukla, S.P.; Prakash, C.; Saharan, N.; Deshmukhe, G. Evaluation of Toxicity of a Textile Dye (Optilan Red) towards a Green Microalga Chlorella vulgaris. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 3346–3355. [Google Scholar] [CrossRef]
- Vinitnantharat, S.; Chartthe, W.; Pinisakul, A. Toxicity of reactive red 141 and basic red 14 to algae and waterfleas. Water Sci. Technol. 2008, 58, 1193–1198. [Google Scholar] [CrossRef]
- Srivastava, S.; Sinha, R.; Roy, D. Toxicological effects of malachite green. Aquat. Toxicol. 2004, 66, 319–329. [Google Scholar] [CrossRef]
- Köktürk, M. In vivo toxicity assessment of Remazol Gelb–GR (RG-GR) textile dye in zebrafish embryos/larvae (Danio rerio): Teratogenic effects, biochemical changes, immunohistochemical changes. Sci. Total Environ. 2022, 852, 158473. [Google Scholar] [CrossRef]
- Hussain, B.; Sajad, M.; Usman, H.; Al-Ghanim, K.A.; Riaz, M.N.; Berenjian, A.; Mahboob, S.; Show, P.L. Assessment of hepatotoxicity and nephrotoxicity in Cirrhinus mrigala induced by trypan blue—An azo dye. Environ. Res. 2022, 215, 114120. [Google Scholar] [CrossRef]
- Parrott, J.L.; Bartlett, A.J.; Balakrishnan, V.K. Chronic toxicity of azo and anthracenedione dyes to embryo-larval fathead minnow. Environ. Pollut. 2016, 210, 40–47. [Google Scholar] [CrossRef]
- Elmoutez, S.; Abushaban, A.; Necibi, M.C.; Sillanpää, M.; Liu, J.; Dhiba, D.; Chehbouni, A.; Taky, M. Design and operational aspects of anaerobic membrane bioreactor for efficient wastewater treatment and biogas production. Environ. Chall. 2023, 10, 100671. [Google Scholar] [CrossRef]
- Wijaya, J.; Oh, S. Machine learning reveals the complex ecological interplay of microbiome in a full-scale membrane bioreactor wastewater treatment plant. Environ. Res. 2023, 222, 115366. [Google Scholar] [CrossRef]
- Lin, H.; Peng, W.; Zhang, M.; Chen, J.; Hong, H.; Zhang, Y. A review on anaerobic membrane bioreactors: Applications, membrane fouling and future perspectives. Desalination 2013, 314, 169–188. [Google Scholar] [CrossRef]
- George, J.; Kumar, V.V. Designing a novel poly (methyl vinyl ether maleic anhydride) based polymeric membrane with enhanced antifouling performance for removal of pentachlorophenol from aqueous solution. Environ. Res. 2023, 223, 115404. [Google Scholar] [CrossRef]
- Baig, U.; Waheed, A. An efficient and simple strategy for fabricating a polypyrrole decorated ceramic-polymeric porous membrane for purification of a variety of oily wastewater streams. Environ. Res. 2023, 219, 114959. [Google Scholar] [CrossRef]
- Huang, J.; Chen, H.; Yang, J.; Zhou, T.; Zhang, H. Effects of particle size on microstructure and mechanical strength of a fly ash based ceramic membrane. Ceram. Int. 2023, in press. [Google Scholar] [CrossRef]
- Zhao, M.; Liu, Y.; Zhang, J.; Jiang, H.; Chen, R. Janus ceramic membranes with asymmetric wettability for high-efficient microbubble aeration. J. Memb. Sci. 2023, 671, 121418. [Google Scholar] [CrossRef]
- Aalami-Aleagha, M.E.; Madaeni, S.S.; Daraei, P. A New Application of Thermal Spray in Preparation of Metallic Membrane for Concentration of Glucose Solution. J. Therm. Spray Technol. 2009, 18, 519–524. [Google Scholar] [CrossRef]
- Yurtsever, A.; Sahinkaya, E.; Aktaş, Ö.; Uçar, D.; Çınar, Ö.; Wang, Z. Performances of anaerobic and aerobic membrane bioreactors for the treatment of synthetic textile wastewater. Bioresour. Technol. 2015, 192, 564–573. [Google Scholar] [CrossRef]
- Batstone, D.J.; Virdis, B. The role of anaerobic digestion in the emerging energy economy. Curr. Opin. Biotechnol. 2014, 27, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Kakade, A.; Yu, Z.; Khan, A.; Liu, P.; Li, X. Anaerobic membrane bioreactors for treatment of emerging contaminants: A review. J. Environ. Manag. 2020, 270, 110913. [Google Scholar] [CrossRef] [PubMed]
- Maaz, M.; Yasin, M.; Aslam, M.; Kumar, G.; Atabani, A.E.; Idrees, M.; Anjum, F.; Jamil, F.; Ahmad, R.; Khan, A.L.; et al. Anaerobic membrane bioreactors for wastewater treatment: Novel configurations, fouling control and energy considerations. Bioresour. Technol. 2019, 283, 358–372. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Jiang, J.; Li, J.; He, C.; Luo, Y.; Wei, L. Anaerobic membrane bioreactors for wastewater treatment: Mechanisms, fouling control, novel configurations, and future perspectives. Environ. Eng. Res. 2022, 28, 210575. [Google Scholar] [CrossRef]
- Fairley-Wax, T.; Raskin, L.; Skerlos, S.J. Recirculating Anaerobic Dynamic Membrane Bioreactor Treatment of Municipal Wastewater. ACS ES&T Eng. 2022, 2, 842–852. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, H.; Jie, M.; Zhang, K.; Qian, Y.; Ma, J. Performance and microbial communities of different biofilm membrane bioreactors with pre-anoxic tanks treating mariculture wastewater. Bioresour. Technol. 2020, 295, 122302. [Google Scholar] [CrossRef]
- Raj Deena, S.; Kumar, G.; Vickram, A.S.; Rani Singhania, R.; Dong, C.; Rohini, K.; Anbarasu, K.; Thanigaivel, S.; Ponnusamy, V.K. Efficiency of various biofilm carriers and microbial interactions with substrate in moving bed-biofilm reactor for environmental wastewater treatment. Bioresour. Technol. 2022, 359, 127421. [Google Scholar] [CrossRef]
- Zhao, K.; Su, F.; Gu, K.; Qi, J.; Liu, R.; Hu, C. Antifouling potential and microbial characterization of an electrochemical anaerobic membrane bioreactor utilizing membrane cathode and iron anode. Bioresour. Technol. 2021, 334, 125230. [Google Scholar] [CrossRef]
- Yao, M.; Woo, Y.C.; Ren, J.; Tijing, L.D.; Choi, J.-S.; Kim, S.-H.; Shon, H.K. Volatile fatty acids and biogas recovery using thermophilic anaerobic membrane distillation bioreactor for wastewater reclamation. J. Environ. Manag. 2019, 231, 833–842. [Google Scholar] [CrossRef]
- Moideen, S.N.F.; Krishnan, S.; Li, Y.-Y.; Hassim, M.H.; Kamyab, H.; Nasrullah, M.; Din, M.F.M.; Halim, K.A.; Chaiprapat, S. Performance evaluation and energy potential analysis of anaerobic membrane bioreactor (AnMBR) in the treatment of simulated milk wastewater. Chemosphere 2023, 317, 137923. [Google Scholar] [CrossRef]
- Chen, H.; Yuan, J.; Yang, E.; Yang, T.; Shi, L.; Liu, Z.; Yu, H.; Cao, J.; Kong, Z.; Zhou, Q.; et al. Swine wastewater treatment using combined up-flow anaerobic sludge blanket and anaerobic membrane bioreactor: Performance and microbial community diversity. Bioresour. Technol. 2023, 373, 128606. [Google Scholar] [CrossRef]
- Ramadan, L.; Deeb, R.; Sawaya, C.; El Khoury, C.; Wazne, M.; Harb, M. Anaerobic membrane bioreactor-based treatment of poultry slaughterhouse wastewater: Microbial community adaptation and antibiotic resistance gene profiles. Biochem. Eng. J. 2023, 192, 108847. [Google Scholar] [CrossRef]
- Li, N.; He, L.; Lu, Y.-Z.; Zeng, R.J.; Sheng, G.-P. Robust performance of a novel anaerobic biofilm membrane bioreactor with mesh filter and carbon fiber (ABMBR) for low to high strength wastewater treatment. Chem. Eng. J. 2017, 313, 56–64. [Google Scholar] [CrossRef]
- Keerthi; Suganthi, V.; Mahalakshmi, M.; Balasubramanian, N. Development of hybrid membrane bioreactor for tannery effluent treatment. Desalination 2013, 309, 231–236. [Google Scholar] [CrossRef]
- Yurtsever, A.; Basaran, E.; Ucar, D.; Sahinkaya, E. Self-forming dynamic membrane bioreactor for textile industry wastewater treatment. Sci. Total Environ. 2021, 751, 141572. [Google Scholar] [CrossRef]
- Sari Erkan, H.; Çağlak, A.; Soysaloglu, A.; Takatas, B.; Onkal Engin, G. Performance evaluation of conventional membrane bioreactor and moving bed membrane bioreactor for synthetic textile wastewater treatment. J. Water Process Eng. 2020, 38, 101631. [Google Scholar] [CrossRef]
- Yurtsever, A.; Basaran, E.; Ucar, D. Process optimization and filtration performance of an anaerobic dynamic membrane bioreactor treating textile wastewaters. J. Environ. Manag. 2020, 273, 111114. [Google Scholar] [CrossRef]
- Katuri, K.P.; Werner, C.M.; Jimenez-Sandoval, R.J.; Chen, W.; Jeon, S.; Logan, B.E.; Lai, Z.; Amy, G.L.; Saikaly, P.E. A Novel Anaerobic Electrochemical Membrane Bioreactor (AnEMBR) with Conductive Hollow-fiber Membrane for Treatment of Low-Organic Strength Solutions. Environ. Sci. Technol. 2014, 48, 12833–12841. [Google Scholar] [CrossRef]
- Azimi, B.; Abdollahzadeh-Sharghi, E.; Bonakdarpour, B. Anaerobic-aerobic processes for the treatment of textile dyeing wastewater containing three commercial reactive azo dyes: Effect of number of stages and bioreactor type. Chin. J. Chem. Eng. 2021, 39, 228–239. [Google Scholar] [CrossRef]
- Manzoor, K.; Khan, S.J.; Yasmeen, M.; Jamal, Y.; Arshad, M. Assessment of anaerobic membrane distillation bioreactor hybrid system at mesophilic and thermophilic temperatures treating textile wastewater. J. Water Process Eng. 2022, 46, 102603. [Google Scholar] [CrossRef]
- Manzoor, K.; Khan, S.J.; Khan, A.; Abbasi, H.; Zaman, W.Q. Woven-fiber microfiltration coupled with anaerobic forward osmosis membrane bioreactor treating textile wastewater: Use of fertilizer draw solutes for direct fertigation. Biochem. Eng. J. 2022, 181, 108385. [Google Scholar] [CrossRef]
- Bai, Y.-N.; Wang, X.-N.; Zhang, F.; Wu, J.; Zhang, W.; Lu, Y.-Z.; Fu, L.; Lau, T.-C.; Zeng, R.J. High-rate anaerobic decolorization of methyl orange from synthetic azo dye wastewater in a methane-based hollow fiber membrane bioreactor. J. Hazard. Mater. 2020, 388, 121753. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xiong, J.; Tian, C.; Gao, B.; Wang, L.; Jia, X. The degradation of methyl orange and membrane fouling behavior in anaerobic baffled membrane bioreactor. Chem. Eng. J. 2018, 338, 719–725. [Google Scholar] [CrossRef]
- Castro, F.D.; Bassin, J.P.; Alves, T.L.M.; Sant’Anna, G.L.; Dezotti, M. Reactive Orange 16 dye degradation in anaerobic and aerobic MBBR coupled with ozonation: Addressing pathways and performance. Int. J. Environ. Sci. Technol. 2021, 18, 1991–2010. [Google Scholar] [CrossRef]
- Choerudin, C.; Arrahmah, F.I.; Daniel, J.K.; Watari, T.; Yamaguchi, T.; Setiadi, T. Evaluation of combined anaerobic membrane bioreactor and downflow hanging sponge reactor for treatment of synthetic textile wastewater. J. Environ. Chem. Eng. 2021, 9, 105276. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, F.; Wang, D.; Jin, Y. Impact of reactor configuration on treatment performance and microbial diversity in treating high-strength dyeing wastewater: Anaerobic flat-sheet ceramic membrane bioreactor versus upflow anaerobic sludge blanket reactor. Bioresour. Technol. 2018, 269, 269–275. [Google Scholar] [CrossRef]
- Sahinkaya, E.; Yurtsever, A.; Çınar, Ö. Treatment of textile industry wastewater using dynamic membrane bioreactor: Impact of intermittent aeration on process performance. Sep. Purif. Technol. 2017, 174, 445–454. [Google Scholar] [CrossRef]
- Baêta, B.E.L.; Lima, D.R.S.; Silva, S.Q.; Aquino, S.F. Influence of the applied organic load (OLR) on textile wastewater treatment using submerged anaerobic membrane bioreactors (SAMBR) in the presence of redox mediator and powdered activated carbon (PAC). Braz. J. Chem. Eng. 2016, 33, 817–825. [Google Scholar] [CrossRef]
- Jin, Y.; Wang, D.; Zhang, W. Newly Designed Hydrolysis Acidification Flat-Sheet Ceramic Membrane Bioreactor for Treating High-Strength Dyeing Wastewater. Int. J. Environ. Res. Public Health 2019, 16, 777. [Google Scholar] [CrossRef]
- Jegatheesan, V.; Pramanik, B.K.; Chen, J.; Navaratna, D.; Chang, C.-Y.; Shu, L. Treatment of textile wastewater with membrane bioreactor: A critical review. Bioresour. Technol. 2016, 204, 202–212. [Google Scholar] [CrossRef]
- Mutamim, N.S.A.; Noor, Z.Z.; Hassan, M.A.A.; Olsson, G. Application of membrane bioreactor technology in treating high strength industrial wastewater: A performance review. Desalination 2012, 305, 1–11. [Google Scholar] [CrossRef]
- Januário, E.F.D.; Vidovix, T.B.; Beluci, N.d.C.L.; Paixão, R.M.; Silva, L.H.B.R.d.; Homem, N.C.; Bergamasco, R.; Vieira, A.M.S. Advanced graphene oxide-based membranes as a potential alternative for dyes removal: A review. Sci. Total Environ. 2021, 789, 147957. [Google Scholar] [CrossRef]
- Joshi, R.K.; Carbone, P.; Wang, F.C.; Kravets, V.G.; Su, Y.; Grigorieva, I.V.; Wu, H.A.; Geim, A.K.; Nair, R.R. Precise and Ultrafast Molecular Sieving Through Graphene Oxide Membranes. Science 2014, 343, 752–754. [Google Scholar] [CrossRef]
- Diogo Januário, E.F.; de Camargo Lima Beluci, N.; Vidovix, T.B.; Vieira, M.F.; Bergamasco, R.; Salcedo Vieira, A.M. Functionalization of membrane surface by layer-by-layer self-assembly method for dyes removal. Process Saf. Environ. Prot. 2020, 134, 140–148. [Google Scholar] [CrossRef]
- Rashidi, H.R.; Sulaiman, N.M.N.; Hashim, N.A.; Hassan, C.R.C.; Ramli, M.R. Synthetic reactive dye wastewater treatment by using nano-membrane filtration. Desalin. Water Treat. 2015, 55, 86–95. [Google Scholar] [CrossRef]
- Ding, J.; Pu, L.; Zou, D.; Cao, M.; Shan, C.; Zhang, Q.; Gao, G.; Pan, B. Removal of model dyes on charged UF membranes: Experiment and simulation. Chemosphere 2020, 240, 124940. [Google Scholar] [CrossRef]
- Zhong, P.S.; Widjojo, N.; Chung, T.-S.; Weber, M.; Maletzko, C. Positively charged nanofiltration (NF) membranes via UV grafting on sulfonated polyphenylenesulfone (sPPSU) for effective removal of textile dyes from wastewater. J. Memb. Sci. 2012, 417–418, 52–60. [Google Scholar] [CrossRef]
- Gnanasekaran, G.; Sudhakaran, M.S.P.; Kulmatova, D.; Han, J.; Arthanareeswaran, G.; Jwa, E.; Mok, Y.S. Efficient removal of anionic, cationic textile dyes and salt mixture using a novel CS/MIL-100 (Fe) based nanofiltration membrane. Chemosphere 2021, 284, 131244. [Google Scholar] [CrossRef]
- Bromley-Challenor, K. Decolorization of an azo dye by unacclimated activated sludge under anaerobic conditions. Water Res. 2000, 34, 4410–4418. [Google Scholar] [CrossRef]
- Türgay, O.; Ersöz, G.; Atalay, S.; Forss, J.; Welander, U. The treatment of azo dyes found in textile industry wastewater by anaerobic biological method and chemical oxidation. Sep. Purif. Technol. 2011, 79, 26–33. [Google Scholar] [CrossRef]
- Khalili, Z.; Bonakdarpour, B. Statistical Optimization of Anaerobic Biological Processes for Dye Treatment. CLEAN-Soil Air Water 2010, 38, 942–950. [Google Scholar] [CrossRef]
- Fang, F.; Cao, J.S.; Chen, L.; Chen, L.; Feng, Q.; Xu, H. Enhanced performance of dyeing wastewater reclamation by PAC addition in a membrane bioreactor. J. Food Agric. Environ. 2012, 10, 1138–1141. [Google Scholar]
- Zhou, Z.; Tao, Y.; Zhang, S.; Xiao, Y.; Meng, F.; Stuckey, D.C. Size-dependent microbial diversity of sub-visible particles in a submerged anaerobic membrane bioreactor (SAnMBR): Implications for membrane fouling. Water Res. 2019, 159, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Niu, C.; Pan, Y.; Lu, X.; Wang, S.; Zhang, Z.; Zheng, C.; Tan, Y.; Zhen, G.; Zhao, Y.; Li, Y.-Y. Mesophilic anaerobic digestion of thermally hydrolyzed sludge in anaerobic membrane bioreactor: Long-term performance, microbial community dynamics and membrane fouling mitigation. J. Memb. Sci. 2020, 612, 118264. [Google Scholar] [CrossRef]
- Liao, Y.; Wang, Y.; Ouyang, L.; Dong, Y.; Zhou, J.; Hu, Q.; Qiu, B. Conductive Polyaniline Enhanced Decolorization of Azo Dyes in Anaerobic Wastewater Treatment. ES Food Agrofor. 2021, 6, 35–42. [Google Scholar] [CrossRef]
- Chaudhari, A.; Paul, D.; Thamke, V.; Bagade, A.; Bapat, V.A.; Kodam, K.M. Concurrent removal of reactive blue HERD dye and Cr(VI) by aerobic bacterial granules. J. Clean. Prod. 2022, 367, 133075. [Google Scholar] [CrossRef]
- Yang, B.; Xu, H.; Yang, S.; Bi, S.; Li, F.; Shen, C.; Ma, C.; Tian, Q.; Liu, J.; Song, X.; et al. Treatment of industrial dyeing wastewater with a pilot-scale strengthened circulation anaerobic reactor. Bioresour. Technol. 2018, 264, 154–162. [Google Scholar] [CrossRef]
- Chen, H.; Liu, Y.; Xu, X.; Sun, M.; Jiang, M.; Xue, G.; Li, X.; Liu, Z. How does iron facilitate the aerated biofilter for tertiary simultaneous nutrient and refractory organics removal from real dyeing wastewater? Water Res. 2019, 148, 344–358. [Google Scholar] [CrossRef]
- Qiu, B.; Xu, X.; Dang, Y.; Wang, Q.; Sun, D.; Wei, S.; Guo, Z. Biotransformative removal of cationic Red X-GRL by anaerobic activated sludge. RSC Adv. 2015, 5, 25699–25707. [Google Scholar] [CrossRef]
- Zhang, L. Molecular diversity of bacterial community of dye wastewater in an anaerobic sequencing batch reactor. Afr. J. Microbiol. Res. 2012, 6, 6444–6453. [Google Scholar] [CrossRef]
- Dai, Q.; Zhang, S.; Liu, H.; Huang, J.; Li, L. Sulfide-mediated azo dye degradation and microbial community analysis in a single-chamber air cathode microbial fuel cell. Bioelectrochemistry 2020, 131, 107349. [Google Scholar] [CrossRef]
- Kong, Z.; Li, L.; Xue, Y.; Yang, M.; Li, Y.-Y. Challenges and prospects for the anaerobic treatment of chemical-industrial organic wastewater: A review. J. Clean. Prod. 2019, 231, 913–927. [Google Scholar] [CrossRef]
- Hamedi, H.; Ehteshami, M.; Mirbagheri, S.A.; Rasouli, S.A.; Zendehboudi, S. Current Status and Future Prospects of Membrane Bioreactors (MBRs) and Fouling Phenomena: A Systematic Review. Can. J. Chem. Eng. 2019, 97, 32–58. [Google Scholar] [CrossRef]
- Huang, Z.; Ong, S.L.; Ng, H.Y. Submerged anaerobic membrane bioreactor for low-strength wastewater treatment: Effect of HRT and SRT on treatment performance and membrane fouling. Water Res. 2011, 45, 705–713. [Google Scholar] [CrossRef]
- Nabi, M.; Liang, H.; Zhou, Q.; Cao, J.; Gao, D. In-situ membrane fouling control and performance improvement by adding materials in anaerobic membrane bioreactor: A review. Sci. Total Environ. 2023, 865, 161262. [Google Scholar] [CrossRef]
- Gul, A.; Hruza, J.; Dvorak, L.; Yalcinkaya, F. Chemical Cleaning Process of Polymeric Nanofibrous Membranes. Polymers 2022, 14, 1102. [Google Scholar] [CrossRef]
- Shahid, M.K.; Kashif, A.; Rout, P.R.; Aslam, M.; Fuwad, A.; Choi, Y.; Banu, J.R.; Park, J.H.; Kumar, G. A brief review of anaerobic membrane bioreactors emphasizing recent advancements, fouling issues and future perspectives. J. Environ. Manag. 2020, 270, 110909. [Google Scholar] [CrossRef]

| Dye | Dye Category | Species | EC50 or LC50 (mg/L) | Remark | Reference |
|---|---|---|---|---|---|
| Algae | |||||
| Rhodamine B | Xanthene | Raphidocelis subcapitata | 14 | EC50 reported for 72 h exposure to dye. | [94] |
| Reactive Orange 16 Congo Red | Azo | S. capricornutum | 7.8 4.8 | EC50 reported for 96 h exposure to dye. | [95] |
| Optilan red | Azo | Chlorella vulgaris | 23.1 | EC50 reported for 96 h exposure to dye. | [96] |
| Methylene blue | Azine | Chlorella vulgaris S. platensis | 61.8 to 5.4 5.8 to 1.0 | EC50 reported for 24 to 96 h exposure to dye. | [90] |
| Reactive red 114 Basic red 14 | Azo Xanthene | Chlorella vulgaris | 95.5 10.8 | EC50 reported for 96 h exposure to dye. | [97] |
| Optilan red | Azo | Spirulina platensis | 7.6 | EC50 reported for 96 h exposure to dye. | [91] |
| Fish | |||||
| Rhodamine B | Xanthene | Danio rerio | 18 | LC50 reported for 72 h exposure to dye. | [94] |
| Malachite green | Triarylmethane | Lepormis macrochirus Ictarulus punctatus | 2.1 0.2 to 1.3 | LC50 reported for 6 h exposure to dye. | [98] |
| Remazol gelb-GR | Azo | Zebrafish embryos | 151.9 | LC50 reported for 96 h exposure to dye. | [99] |
| Trypan blue | Azo | C. mrigala | 40 | LC50 reported for 96 h exposure to dye. | [100] |
| Sudan Red G Disperse Yellow 7 | Azo | Fathead minnow (P. promelas) | 0.02 0.01 | - | [101] |
| Dye | Water Bodies | Removal (%) | Treatment | Cleaning | Operation Condition | Reference |
|---|---|---|---|---|---|---|
| Azo (Black 5, Black WNN, and Red 3BS | Synthetic wastewater | 79.9 | * HAnMBR (SBR-AnMBR) | * NR | HRT = 48 h; concentration of color = 80 mg/L; Temperature = 30 °C | [129] |
| Methylene blue, Cibacron blue, and Cibacron yellow | Synthetic wastewater | 99.0 | Modified AnMBR (anaerobic membrane distillation bioreactor) | Physical (Backwash) | Polymeric membrane (polytetrafluoroethylene-PTFE and polypropylene-PP); color concentration = 1000 Pt.Co; Temperature > 35 °C; HRT = 12 h | [130] |
| Methylene blue, Cibacron blue, and Cibacron yellow | Synthetic wastewater | 92.3 | Modified AnMBR (Anaerobic Forward Osmosis Membrane Bioreactor (An-FOMBR)) | Chemical (NaOCl) | Polymeric membrane (polyamide-PA and polysulfone-PSF); HRT = 12–24 h; Temperature = 36 °C; SRT = 60 days; color concentration of color = 1000 Pt. Co. | [131] |
| Methyl orange | Synthetic wastewater | 100 | Modified AnMBR (methane-based hollow fiber membrane bioreactor) | NR | HRT = 1.5–2 days; Polymeric membrane (polyvinylidene fluoride-PVDF); concentration of MO = 400 mg/L | [132] |
| Methyl orange | Synthetic wastewater | 100 | Modified AnMBR (anaerobic baffled membrane bioreactor) | Physical and chemical (HCl) | HRT = 15–17 h; Polymeric membrane; concentration of MO = 50 mg/L | [133] |
| Reactive orange 16 | Synthetic wastewater | 97 | HAnMBR (integrated with ozonation) | NR | HRT = 12 h; 30 min ozonation; temperature = 22–25 °C | [134] |
| Reactive orange 16 | Synthetic wastewater | 61 | AnMBR | NR | HRT = 12 h; Dye concentration = 5 mg/L; temperature = 22–25 °C | [134] |
| Reactive Black 5 | Synthetic wastewater | 95 | HAnMBR (integrated with downflow hanging sponge) | Physical and chemical (NaOCl) | HRT = 12–24 h; temperature = 30 °C; polymeric membrane; dye concentration = 50 mg/L | [135] |
| Mostly azo dyes | Real dying wastewater | >90 | Modified AnMBR (Anaerobic flat-sheet ceramic membrane bioreactor) | Physical and chemical (NaOCl) | HRT = 24 h; Ceramic membrane; temperature 34 °C | [136] |
| Remazol Brillant Violet 5R | Synthetic wastewater | 97 | DMBR | Physical (Vacuum line) | HRT = 11.2 h–14.5 h; polymeric membrane (PVC); dye concentration = 50 mg/L; temperature 34 °C; SRT = 60 days | [137] |
| Dyes | Textile wastewater | Up to 87 | S-AnMBR | NR | HRT = 12 h–24 h; polymeric membrane; dye concentration = 50 mg/L; temperature 35 °C | [138] |
| Navy blue | Real dying wastewater | 99 | Modified AnMBR (Hydrolysis acidification flat-sheet ceramic membrane bioreactor) | Chemical (pumping 1000 mg/L NaOCl into the inner space of the membranes) | HRT = 12 h; Ceramic membrane; temperature 34 °C | [139] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mojiri, A.; Zhou, J.L.; KarimiDermani, B.; Razmi, E.; Kasmuri, N. Anaerobic Membrane Bioreactor (AnMBR) for the Removal of Dyes from Water and Wastewater: Progress, Challenges, and Future Perspectives. Processes 2023, 11, 855. https://doi.org/10.3390/pr11030855
Mojiri A, Zhou JL, KarimiDermani B, Razmi E, Kasmuri N. Anaerobic Membrane Bioreactor (AnMBR) for the Removal of Dyes from Water and Wastewater: Progress, Challenges, and Future Perspectives. Processes. 2023; 11(3):855. https://doi.org/10.3390/pr11030855
Chicago/Turabian StyleMojiri, Amin, John L. Zhou, Bahareh KarimiDermani, Elham Razmi, and Norhafezah Kasmuri. 2023. "Anaerobic Membrane Bioreactor (AnMBR) for the Removal of Dyes from Water and Wastewater: Progress, Challenges, and Future Perspectives" Processes 11, no. 3: 855. https://doi.org/10.3390/pr11030855
APA StyleMojiri, A., Zhou, J. L., KarimiDermani, B., Razmi, E., & Kasmuri, N. (2023). Anaerobic Membrane Bioreactor (AnMBR) for the Removal of Dyes from Water and Wastewater: Progress, Challenges, and Future Perspectives. Processes, 11(3), 855. https://doi.org/10.3390/pr11030855








