Investigation of Congo Red Toxicity towards Different Living Organisms: A Review
Abstract
1. Introduction
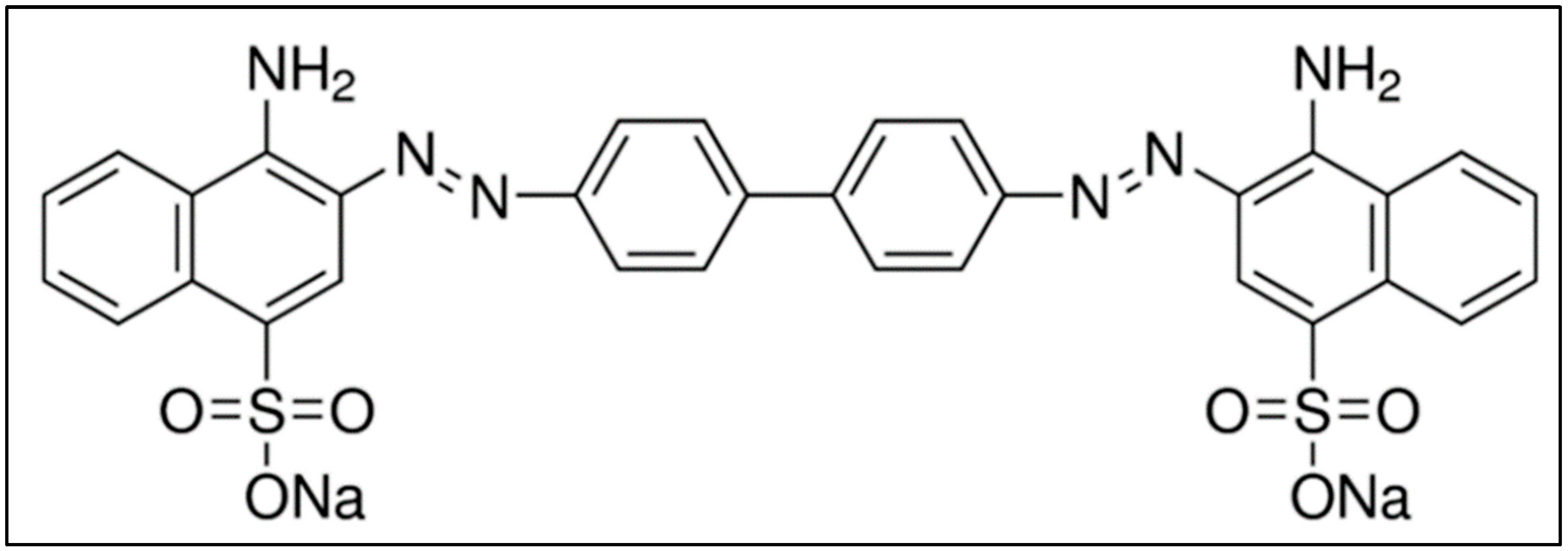
2. Congo Red
3. Toxicities Caused by CR
3.1. Toxic Effect of CR on Humans
3.2. The Toxic Effect of CR on Aquatic Species
3.2.1. Bacterial Test
3.2.2. Algal Test
3.2.3. Flash Bioluminescence Test
3.2.4. Ames Test
3.2.5. Protozoan Tests
3.2.6. Genetic Toxicity Test
3.3. The Toxic Effect of CR on Plants
3.4. Evaluation of Toxic, Mutagenic, Carcinogenic, and Teratogenic Properties
3.5. Groups of CR That Cause Toxicity
3.5.1. Aromatic Amines (AA)
3.5.2. Benzidine
3.6. Some Major Findings Related to CR
3.7. Mechanisms for Azo Dye Carcinogenicity
3.8. Lethal Dose Low
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mcyotto, F.; Wei, Q.; Macharia, D.K.; Huang, M.; Shen, C.; Chow, C.W.K. Effect of dye structure on color removal efficiency by coagulation. Chem. Eng. J. 2021, 405, 126674. [Google Scholar] [CrossRef]
- Lellis, B.; Fávaro-Polonio, C.Z.; Pamphile, J.A.; Polonio, J.C. Effects of textile dyes on health and the environment and bioremediation potential of living organisms. Biotechnol. Res. Innov. 2019, 3, 275–290. [Google Scholar] [CrossRef]
- Pargai, D.; Jahan, S.; Gahlot, M. Functional Properties of Natural Dyed Textiles. In Chemistry and Technology of Natural and Synthetic Dyes and Pigments; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Benkhaya, S.; M’rabet, S.; El Harfi, A. Classifications, properties, recent synthesis and applications of azo dyes. Heliyon 2020, 6, e03271. [Google Scholar] [CrossRef]
- Ardila-Leal, L.D.; Poutou-Piñales, R.A.; Pedroza-Rodríguez, A.M.; Quevedo-Hidalgo, B.E. A Brief History of Colour, the Environmental Impact of Synthetic Dyes and Removal by Using Laccases. Molecules 2021, 26, 3813. [Google Scholar] [CrossRef] [PubMed]
- Ismail, M.; Akhtar, K.; Khan, M.I.; Kamal, T.; Khan, M.A.; Asiri, A.M.; Seo, J.; Khan, S.B. Pollution, toxicity and carcinogenicity of organic dyes and their catalytic bio-remediation. Curr. Pharm. Des. 2019, 25, 3645–3663. [Google Scholar] [CrossRef]
- Levine, W.G. Metabolism of azo dyes: Implication for detoxication and activation. Drug Metab. Rev. 1991, 23, 253–309. [Google Scholar] [CrossRef]
- Gičević, A.; Hindija, L.; Karačić, A. Toxicity of Azo Dyes in Pharmaceutical Industry. In International Conference on Medical and Biological Engineering; Springer: Cham, Switzerland; Berlin/Heidelberg, Germany, 2019; pp. 581–587. [Google Scholar]
- Ventura-Camargo, B.D.C.; Marin-Morales, M.A. Azo Dyes: Characterization and Toxicity—A Review. Text. Light Ind. Sci. Technol. 2013, 2, 85–103. [Google Scholar]
- Yates, E.; Yates, A. Johann Peter Griess FRS (1829-88): Victorian brewer and synthetic dye chemist. Notes Rec. R. Soc. Lond. 2016, 70, 65–81. [Google Scholar] [CrossRef]
- Lekshmi, G.S.; Ramasamy, T.; Bazaka, O.; Levchenko, I.; Bazaka, K.; Govindan, R.; Mandhakini, M. Antioxidant, Anti-Bacterial, and Congo Red Dye Degradation Activity of AgxO-Decorated Mustard Oil-Derived rGO Nanocomposites. Molecules 2022, 27, 5950. [Google Scholar] [CrossRef]
- Tara, N.; Siddiqui, S.I.; Rathi, G.; Chaudhry, S.A.; Inamuddin; Asiri, A.M. Nano-engineered Adsorbent for the Removal of Dyes from Water: A Review. Curr. Anal. Chem. 2020, 16, 14–40. [Google Scholar] [CrossRef]
- Raval, N.P.; Shah, P.U.; Shah, N.K. Adsorptive amputation of hazardous azo dye Congo red from wastewater: A critical review. Environ. Sci. Pollut. Res. 2016, 23, 14810–14853. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 11313, Congo Red. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Congo-red (accessed on 5 February 2023).
- D’Souza, E.; Fulke, A.B.; Mulani, N.; Ram, A.; Asodekar, M.; Narkhede, N.; Gajbhiye, S.N. Decolorization of Congo red mediated by marine Alcaligenes species isolated from Indian West coast sediments. Environ. Earth Sci. 2017, 76, 721. [Google Scholar] [CrossRef]
- Clavijo, C.; Osma, J.F. Functionalized leather: A novel and effective hazardous solid waste adsorbent for the removal of the diazo dye congo red from aqueous solution. Water 2019, 11, 1906. [Google Scholar] [CrossRef]
- Dutta, S.; Gupta, B.; Srivastava, S.K.; Gupta, A.K. Recent advances on the removal of dyes from wastewater using various adsorbents: A critical review. Mater. Adv. 2021, 2, 4497–4531. [Google Scholar] [CrossRef]
- Mandal, S.; Calderon, J.; Marpu, S.B.; Omary, M.A.; Shi, S.Q. Mesoporous activated carbon as a green adsorbent for the removal of heavy metals and Congo red: Characterization, adsorption kinetics, and isotherm studies. J. Contam. Hydrol. 2021, 243, 103869. [Google Scholar] [CrossRef]
- Li, H.; Zhao, Y.; Yin, C.; Jiao, L.; Ding, L. WO3 nanocrystal prepared by self-assembly of phosphotungstic acid and dopamine for photocatalytic degradation of Congo red. Colloids Surf. A Physicochem. Eng. Asp. 2019, 572, 147–151. [Google Scholar] [CrossRef]
- Hernández-Zamora, M.; Martínez-Jerónimo, F.; Cristiani-Urbina, E.; Cañizares-Villanueva, R.O. Congo red dye affects survival and reproduction in the cladoceran Ceriodaphnia dubia. Effects of direct and dietary exposure. Ecotoxicology 2016, 25, 1832–1840. [Google Scholar] [CrossRef]
- Rani, K.C.; Naik, A.; Chaurasiya, R.S.; Raghavarao, K.S.M.S. Removal of toxic Congo red dye from water employing low-cost coconut residual fiber. Water Sci. Technol. 2017, 75, 2225–2236. [Google Scholar] [CrossRef]
- Oladoye, P.O.; Bamigboye, O.M.; Ogunbiyi, O.D.; Akano, M.T. Toxicity and decontamination strategies of Congo red dye. Groundw. Sustain. Dev. 2022, 19, 100844. [Google Scholar] [CrossRef]
- Litefti, K.; Freire, M.S.; Stitou, M.; González-Álvarez, J. Adsorption of an anionic dye (Congo red) from aqueous solutions by pine bark. Sci. Rep. 2019, 9, 16530. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V. Effective degradation of rhodamine B and Congo red dyes over biosynthesized silver nanoparticles-imbibed carboxymethyl cellulose hydrogel. Polym. Bull. 2020, 77, 3349–3365. [Google Scholar]
- Naseem, K.; Farooqi, Z.H.; Begum, R.; Irfan, A. Removal of Congo red dye from aqueous medium by its catalytic reduction using sodium borohydride in the presence of various inorganic nano-catalysts: A review. J. Clean. Prod. 2018, 187, 296–307. [Google Scholar] [CrossRef]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Chemical Agents and Related Occupations. Lyon (FR): International Agency for Research on Cancer; 2012. (IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, No. 100F. Dyes metabolized to benzidine. Available online: https://www.ncbi.nlm.nih.gov/books/NBK304402/ (accessed on 7 March 2023).
- Obi, F.O.; Maduka, H.C.C.; Zubairu, I. Assessment of congo red-induced liver damage by selected serum transaminase levels. J. Med. Sci. 2003, 3, 157–162. [Google Scholar] [CrossRef]
- Macht, D.I. Experimental studies on heparin and its influence on toxicity of digitaloids, congo red, cobra venom and other drugs. Ann. Intern. Med. 1943, 18, 772–791. [Google Scholar]
- Novotný, Č.; Dias, N.; Kapanen, A.; Malachová, K.; Vándrovcová, M.; Itävaara, M.; Lima, N. Comparative use of bacterial, algal and protozoan tests to study toxicity of azo-and anthraquinone dyes. Chemosphere 2006, 63, 1436–1442. [Google Scholar] [CrossRef]
- Hernández-Zamora, M.; Martínez-Jerónimo, F. Congo red dye diversely affects organisms of different trophic levels: A comparative study with microalgae, cladocerans, and zebrafish embryos. Environ. Sci. Pollut. Res. Int. 2019, 26, 11743–11755. [Google Scholar] [CrossRef]
- Smith, R.; John, G. Azo Dye Toxicity: A measure of toxic effect metabolized azo dyes have on the body. Biol. Chem. Eng. 2016, 2, 1–4. [Google Scholar]
- Busch, H.; Hagedoorn, P.L.; Hanefeld, U. Rhodococcus as A Versatile Biocatalyst in Organic Synthesis. Int. J. Mol. Sci. 2019, 20, 4787. [Google Scholar] [CrossRef]
- Imran, M.; Shaharoona, B.; Crowley, D.; Khalid, A.; Hussain, S.; Arshad, M. The stability of textile azo dyes in soil and their impact on microbial phospholipid fatty acid profiles. Ecotoxicol. Environ. Saf. 2015, 120, 163–168. [Google Scholar] [CrossRef]
- Sarkar, S.; Banerjee, A.; Halder, U.; Biswas, R.; Bandopadhyay, R. Degradation of synthetic azo dyes of textile industry: A sustainable approach using microbial enzymes. Water Conserv. Sci. Eng. 2017, 2, 121–131. [Google Scholar] [CrossRef]
- Lobiuc, A.; Olaru, S.; Hancu, E.I.; Costica, N.; Fortuna, M.E.; Zamfirache, M.M.; Constantinescu, G. Toxicity and removal of direct red 28 diazo dye in living polymeric systems. Rev. Chim. 2018, 69, 1628–1635. [Google Scholar] [CrossRef]
- Sharma, J.; Sharma, S.; Soni, V. Classification and impact of synthetic textile dyes on Aquatic Flora: A review. Reg. Stud. Mar. Sci. 2021, 45, 101802. [Google Scholar] [CrossRef]
- Zhou, X.; Xiang, X. Effect of different plants on azo-dye wastewater biodecolorization. Procedia Environ. Sci. 2013, 18, 540–546. [Google Scholar] [CrossRef]
- Rathi, G.; Siddiqui, S.I.; Pham, Q.; Nam, V.T. Nigella sativa seeds based antibacterial composites: A sustainable technology for water cleansing—A review. Sustain. Chem. Pharm. 2020, 18, 100332. [Google Scholar] [CrossRef]
- Siddiqui, S.I.; Naushad, M.; Chaudhry, S.A. Promising prospects of nanomaterials for arsenic water remediation: A comprehensive review. Process Saf. Environ. Protect. 2019, 126, 60–97. [Google Scholar] [CrossRef]
- Chequer, F.M.D.; Lizier, T.M.; de Felício, T.; Zanoni, M.V.B.; Debonsi, H.M.; Lopes, N.P.; Marcos, R.; de Oliveira, D.P. Analyses of the genotoxic and mutagenic potential of the products formed after the biotransformation of the azo dye Disperse Red 1. Toxicol. In Vitro 2011, 25, 2054–2063. [Google Scholar] [CrossRef]
- Chung, K.T. Azo dyes and human health: A review. J. Environ. Sci. Health C. Environ. Carcinog. Ecotoxicol. Rev. 2017, 35, 67. [Google Scholar] [CrossRef]
- Ngo, A.C.R.; Tischler, D. Microbial Degradation of Azo Dyes: Approaches and Prospects for a Hazard-Free Conversion by Microorganisms. Int. J. Environ. Res. Public Health 2022, 19, 4740. [Google Scholar] [CrossRef]
- Reza, M.S.A.; Hasan, M.M.; Kamruzzaman, M.; Hossain, M.I.; Zubair, M.A.; Bari, L.; Abedin, M.Z.; Reza, M.A.; Khalid-Bin-Ferdaus, K.M.; Haque, K.M.F. Study of a common azo food dye in mice model: Toxicity reports and its relation to carcinogenicity. Food Sci. Nutr. 2019, 7, 667–677. [Google Scholar] [CrossRef]
- Rinde, E.; Troll, W. Metabolic reduciton of benzidine azo dyes to benzidine in the Rhesus monkey. J. Natl. Cancer Inst. 1975, 55, 181–182. [Google Scholar] [CrossRef]
- Brown, M.A.; de Vito, S.C. Predicting Azo Dye Toxicity. Crit. Rev. Environ. Sci. Technol. 1993, 23, 249–324. [Google Scholar] [CrossRef]
- Feng, J.; Cerniglia, C.E.; Chen, H. Toxicological significance of azo dye metabolism by human intestinal microbiota. Front. Biosci. 2012, 4, 568–586. [Google Scholar] [CrossRef]
- Zollinger, H. Colour Chemistry Synthesis Properties and Application of Organic Dyes and Pigments; VCH: New York, NY, USA, 1991; pp. 92–102. [Google Scholar]
- Pielesz, A.; Baranowska, I.; Rybak, A.; Włochowicz, A. Detection and determination of aromatic amines as products of reductive splitting from selected azo dyes. Ecotoxicol. Environ. Saf. 2002, 53, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.T. The significance of azo-reduction in the mutagenesis and carcinogenesis of azo dyes. Mutat. Res. 1983, 114, 269–281. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.T.; Stevens, S.E., Jr.; Cerniglia, C.E. The reduction of azo dyes by the intestinal microflora. Crit. Rev. Microbiol. 1992, 18, 175–190. [Google Scholar] [CrossRef]
- Beaudoin, A.R. The teratogenicity of congo red in rats. Proceed. Soc. Exp. Biol. Med. 1964, 117, 176–179. [Google Scholar] [CrossRef]
- Preston, R.J.; Ross, J.A. 1.16—Mechanisms: DNA-Reactive Agents. In Comprehensive Toxicol; Charlene, A., Ed.; McQueen: London, UK, 2018; pp. 332–343. [Google Scholar]
- Attia, S.M. Deleterious effects of reactive metabolites. Oxid. Med. Cell. Longev. 2010, 3, 238–253. [Google Scholar] [CrossRef]
- Elson, L.A.; Goulden, F.; Warren, F.L. The metabolism of aromatic amines in relation to carcinogenesis. Br. J. Cancer 1958, 12, 108–115. [Google Scholar] [CrossRef]
- Aromatic Amines: An Assessment of the Biological and Environmental Effects, Chapter 2: Metabolism of Aromatic Amines; The National Academics of Sciences Engineering Medicine, The National Academic Press: Washington, DC, USA, 1981.
- Reid, T.M.; Morton, K.C.; Wang, C.Y.; King, C.M. Mutagenicity of azo dyes following metabolism by different reductive/oxidative systems. Environ. Mutagen. 1984, 6, 705–717. [Google Scholar] [CrossRef]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Some Aromatic Amines, Organic Dyes, and Related Exposures. Lyon (FR): International Agency for Research on Cancer, 2010. (IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, No. 99.) 4, Mechanistic and Other Relevant Data. Available online: https://www.ncbi.nlm.nih.gov/books/NBK385414/ (accessed on 7 March 2023).
- Kaushal, S.; Mathur, S.R.; Vijay, M.; Rustagi, A. Calcifying epithelial odontogenic tumor (Pindborg tumor) without calcification: A rare entity. J. Oral Maxillofac. Pathol. 2012, 16, 110–112. [Google Scholar]
- Haley, T.J. Benzidine revisited: A review of the literature and problems associated with the use of benzidine and its congeners. Clin. Toxicol. 1975, 8, 13–42. [Google Scholar] [CrossRef]
- McCann, J.; Spingarn, N.E.; Kobori, J.; Ames, B.N. Detection of carcinogens as mutagens: Bacterial tester strains with R factor plasmids. Proc. Natl. Acad. Sci. USA 1975, 72, 979–983. [Google Scholar] [CrossRef]
- Tanaka, K.I.; Mii, T.; Marui, S.; Matsubara, I.; Igaki, H. Mutagenicity of urinary metabolites of benzidine and benzidine-based azo dyes. Int. Arch. Occup. Environ. Health 1981, 49, 177–185. [Google Scholar] [CrossRef]
- Reid, T.M.; Morton, K.C.; Wang, C.Y.; King, C.M. Conversion of Congo red and 2-azoxyfluorene to mutagens following in vitro reduction by whole-cell rat cecal bacteria. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 1983, 117, 105–112. [Google Scholar] [CrossRef]
- Kennelly, J.C.; Shaw, A.; Martin, C.N. Reduction to benzidine is not necessary for the covalent binding of a benzidine azodye to rat liver DNA. Toxicology 1984, 32, 315–324. [Google Scholar] [CrossRef]
- Martin, C.N.; Kennelly, J.C. Rat liver microsomal azoreductase activity on four azo dyes derived from benzidine, 3,3′-dimethylbenzidine or 3,3′-dimethoxybenzidine. Carcinogenesis 1981, 2, 307–312. [Google Scholar] [CrossRef]
| Order | Toxic/Inhibitory Effect | Affected Targets | References |
|---|---|---|---|
| 1. | Carcinogenic | Humans and animals | [18] |
| 2. | Mutagenic | Humans and animals | [19] |
| 3. | Causing Infertility | Water flea (Ceriodaphnia dubia) | [20] |
| 4. | Increases COD | Water bodies and aquatic flora and fauna | [21] |
| 5. | It makes surface water unaesthetic | Water bodies | [22] |
| 6. | Allergic | Humans | [23] |
| 7. | Phytotoxicity | Plants | [24] |
| Order | Organism | Test Type | Route | Dose | Effect |
|---|---|---|---|---|---|
| 1 | Man | Ldlo | Oral | 143 mg/kg | Vascular: other changes |
| 2 | Man | Ldlo | Intravenous | 1429 ug/kg | Behavioral: convulsions or effect on seizure threshold; lungs, thorax, or respiration: dyspnea; blood: change in clotting factors |
| 3 | Rat | Ldlo | Inhalation | 50 gm/m3/1H | - |
| 4 | Rat | Ldlo | Intravenous | 160 mg/kg | Behavioral: somnolence (general depressed activity); lungs, thorax, or respiration: chronic pulmonary edema; blood: change in clotting factors |
| 5 | Mouse | Ldlo | Intravenous | 250 mg/kg | |
| 6 | Cat | Ldlo | Intravenous | 100 mg/kg | Behavioral: somnolence (general depressed activity); lungs, thorax, or respiration: chronic pulmonary edema; blood: change in clotting factors |
| 7 | Rabbit | Ldlo | Oral | 6 gm/kg | - |
| 8 | Rabbit | Ldlo | Skin | 4 gm/kg | - |
| 9 | Rabbit | Ldlo | Intravenous | 230 mg/kg | Behavioral: somnolence (general depressed activity); lungs, thorax, or respiration: chronic pulmonary edema; blood: change in clotting factors |
| 10 | Pigeon | Ldlo | Intravenous | 120 mg/kg | Behavioral: somnolence (general depressed activity); lungs, thorax, or respiration: chronic pulmonary edema; blood: change in clotting factors |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siddiqui, S.I.; Allehyani, E.S.; Al-Harbi, S.A.; Hasan, Z.; Abomuti, M.A.; Rajor, H.K.; Oh, S. Investigation of Congo Red Toxicity towards Different Living Organisms: A Review. Processes 2023, 11, 807. https://doi.org/10.3390/pr11030807
Siddiqui SI, Allehyani ES, Al-Harbi SA, Hasan Z, Abomuti MA, Rajor HK, Oh S. Investigation of Congo Red Toxicity towards Different Living Organisms: A Review. Processes. 2023; 11(3):807. https://doi.org/10.3390/pr11030807
Chicago/Turabian StyleSiddiqui, Sharf Ilahi, Esam S. Allehyani, Sami A. Al-Harbi, Ziaul Hasan, May Abdullah Abomuti, Hament Kumar Rajor, and Seungdae Oh. 2023. "Investigation of Congo Red Toxicity towards Different Living Organisms: A Review" Processes 11, no. 3: 807. https://doi.org/10.3390/pr11030807
APA StyleSiddiqui, S. I., Allehyani, E. S., Al-Harbi, S. A., Hasan, Z., Abomuti, M. A., Rajor, H. K., & Oh, S. (2023). Investigation of Congo Red Toxicity towards Different Living Organisms: A Review. Processes, 11(3), 807. https://doi.org/10.3390/pr11030807







