Abstract
Although numerous investigations have revealed the gas physisorption characteristics of porous media, the essence of physisorption behavior of gas within nanoscale space is still indistinct. We speculated that the physisorption behavior of a complex molecular system (e.g., CH4 and CO2) exhibits a quantum effect due to the confinement effect of nanopores. Gas molecules occur in varied orbitals following certain probabilities and, therefore, have separate energy levels inside a nanoscale space. Energy level transition of molecules from excited state to ground state triggers gas physisorption, while non-uniform spatial distribution of energy-quantized molecules within nanopores dominates the gas physisorption behavior. The spatial distribution of gas molecules can be adjusted by temperature, pressure and potential energy field. Based on the quantum effect, we developed a physisorption equation from the perspective of quantum mechanics to re-understand the basic principles of gas physisorption within nanopores.
1. Introduction
Climate change induced by increasing emission of greenhouse gases of methane (CH4) and carbon dioxide (CO2) threatens the living environment of human beings [1,2,3,4]. To reduce carbon emissions, carbon capture and storage (CCS) has long been a concern of global scholars and government organizations [5,6,7,8]. Physical adsorption is one of the significant properties utilized to accomplish CCS by nanoporous media. There are two types of storage media that have received widespread attention, and they are: (a) naturally formed organic-rich rocks (coal, shale), which are rich in nanoscale pores and are an important geological place for CH4 and CO2 storage [9,10,11,12,13]; (b) artificially synthesized nanoporous materials such as metal-organic frameworks (MOFs), which have been widely used for the separation and storage of CH4 and CO2 due to their super adsorption capacity and highly adsorptive selectivity [14,15,16,17,18].
During the past century, the nature of the gas physisorption has been investigated in light of various possible theories, such as monolayer adsorption [19], multi-molecular layer adsorption [20], potential theory [21,22], and volume filling of micropores [23]. The most famous adsorption theory proposed by Langmuir regards gas physisorption behavior as the dynamic equilibrium of evaporation-condensation of gas on plane surfaces [19]. Although it has been successfully applied to characterize the physisorption behavior of CO2 and CH4, essential issues involving how gas physisorption is triggered within nanoscale pores and what dominates the gas physisorption behavior are still thought provoking.
In this presentation, we proposed a new theory from the perspective of quantum mechanics improving our understanding of the basic principles of the physisorption of gases within confined nanoscale space and provided a new idea to predict the physisorption behavior of CH4 and CO2 within nanoporous media. Especially in the fields of energy and the environment, including geo-resources (coalbed methane and shale gas) assessment and extraction, gas storage and transport, gas separation and purification, and CCS technique, the quantum physisorption theory proposed here would be demonstrated as a powerful tool.
2. Theory and Modelling
2.1. Quantum Effect of Gas Physisorption
A conceptually physical model of quantum physisorption for CH4 and CO2 gases was established. Gas molecules within the confined nanoscale space make up the Boltzmann system, which is characterized by the same molecular properties, negligible intermolecular interaction, and discrete energy levels with an unlimited number of molecules at each energy level. The molecular energy of the Boltzmann system is quantized, that is, the energy levels are separated, which is manifested as a quantum effect. This quantum effect embodies two aspects: (a) The potential energy of interaction between pore surface and molecule shows a discontinuously quantized distribution, and the spatial potential energy field consists of n + 1 energy levels in different orbitals with a number set of M= (Figure 1A). The potential energy in different orbitals is an integer multiple of the smallest potential energy unit E0, that is, Epi = (i−n)E0, and the lowest potential energy −nE0 occurs near the pore surface (Figure 1B,C). (b) The kinetic energy of molecules is simplified to two states according to phase difference, in which the non-adsorbed molecules have the same kinetic energy, that is ; while the adsorbed molecule locates in the orbital with the lowest potential energy, and its kinetic energy is . Therefore, gas molecules in different orbitals have separate energy levels, i.e., . The width of the excited molecular orbital should be an integer multiple of the de Broglie wavelength, which satisfies the condition of maintaining standing wave. Molecules can exist stably in the orbital when this condition is met (Figure 1D). Based on this understanding, the maximum quantum number n can be discriminated by a function of considering the gas type, molecular kinetic diameter, and temperature of molecular system, expressed as (see Appendix A):
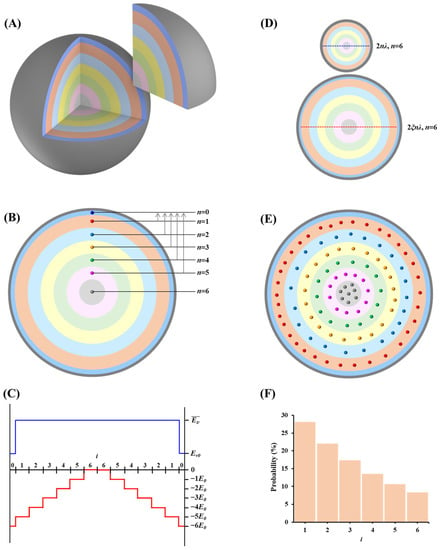
Figure 1.
Conceptually physical model of quantum physisorption for gases of CO2 and CH4 (take n = 6 for example). (A) Spatial distribution of quantized energy field within nanopore; (B) An ideal pattern of energy level transition of molecules, showing a profile in (A); (C) Energy distribution within a nanopore showing a straight line in (B), where the blue line indicates kinetic energy, and the red line indicates potential energy. (D) Taking n = 6 as a case, individual orbital width equals to the de Broglie wavelength of the molecule and total width (2nλ, length of the blue dotted line) equals to the molecular kinetic diameter; in larger pores, individual orbital width equals to the integer multiples of the de Broglie wavelength of the molecule and total width is 2ξnλ (length of the blue dotted line). (E) The figure shows an ideal case of the distribution of 100 molecules following the probability distribution (T = 298 K, E0 = 10−21 J) presented in (F).
It can be further expressed as and for CH4 and CO2, respectively. Because the value of n should be a positive integer, the smaller integer is assigned to the value of n when the calculated n value is between two integers. Theoretically, the maximum quantum number of CH4 and CO2 gradually increases with the increasing temperature. Under the same temperature conditions, the maximum quantum number of CO2 is greater than that of CH4.
After molecules are injected into the nanoscale space, the specific location of molecules at the initial moment before the molecules are adsorbed cannot be predicted, but in the quantized energy field, gas molecules will be distributed in orbitals (1 ≤ i ≤ n) by a certain probability distribution (Figure 1E,F), and the distribution conforms to the classic Boltzmann distribution law [24], that is:
Molecules in the same orbital have a uniform quantum state, and have diverse quantum states in different orbitals. Ideally, from the surface (the lowest potential energy, orbital i = 0) to the inside of a pore, the molecular energy level gradually increases. Excited gas molecules in orbitals i = 1~n − 1 are unstable and will spontaneously return to the ground state (orbital i = 0) near the pore surface. When the molecule returns to the ground state orbital, it appears to be adsorbed on the pore surface. Therefore, the physisorption behavior of gas molecules is exactly the result of molecular energy level transition, and the amount of adsorption is the number of molecular transitions.
Energy level transition of gas molecules occurs only when the kinetic energy and potential energy decrease simultaneously and follows the principles: (a) Only transitions from i = 1~n − 1 orbitals (excited state) to i = 0 orbital (ground state) occur, showing a transition selectivity; (b) It is not that all the molecules in an excited state will undergo energy level transition, but that they have a certain probability of transition, that is, transition is probabilistic; (c) The probability of energy level transition is equal to each other for all molecules due to the exactly identical molecular properties; (d) Molecular transition speed is equal to the average velocity of molecular motion; (e) During the molecular transition, energy releases including both the kinetic energy and potential energy and is numerically equal to the difference in energy level, i.e., .
The orbital of i = n belongs to the zero potential energy region, and molecules in this orbital are always in a free state because they get rid of the constraints of gas-solid interaction. In addition, desorption is the reverse process of the adsorption and can be regarded as the process of molecules returning to the excited state from the ground state when receiving external energy.
2.2. Equation of Quantum Physisorption
Gas physisorption isotherm is parameterized according to the above physical model. Based on the quantum statistical physics, a quantum equation for the CH4 and CO2 physisorption within nanoporous media was established (see Appendix B). The total amount (na) of gas adsorption is the sum of molecular transition from energy levels of i = 1~n−1 and can be expressed as:
where nai is the adsorption amount of gas molecules at i-th energy level and is equal to the number of transitions of gas molecules at the i-th energy level, which can be determined by the quantum physisorption equation:
where,
In Formula (4), ki is the adsorption coefficient of gas molecules at the i-th energy level, which reflects the adsorption saturation speed of gas during pressurization process, generally . A smaller ki value indicates a faster physisorption saturation. Gases of different energy levels start to undergo transition at varied initial pressures, which are equal to . In other words, the transition occurs when the molecules reach a certain concentration. During the process of gas pressurization, when the pressure p reaches the initial pressure, the molecules of the i-th energy level begin to undergo energy level transitions, which conform to the quantum physisorption equation.
Additionally, the results obtained by widely used volumetric and gravimetric methods are the Gibbs excess adsorption (ne), which is not the actual amount of adsorbed gas (na). When the gas-adsorbed volumes cannot be ignored especially at high pressure, they can be converted into each other through the density ratio of free gas to adsorbed gas [25], which is mathematically expressed as:
3. Application Method of Quantum Physisorption Theory
The quantum physisorption theory is applicable to the adsorption processes of CH4 and CO2 in various nanoporous media, such as metal organic frameworks (MOFs), porous organic frameworks (POFs), molecular sieve, activated carbon, coal rock, shale rock, and clay minerals etc. For a set of isotherm adsorption data of CH4 or CO2 measured by widely used volumetric and gravimetric methods, the adsorption isotherm can be parameterized by using the equation of quantum physisorption.
Firstly, the maximum quantum number can be determined by Equation (1) for CH4 and CO2, respectively. Because the value of n should be a positive integer, the smaller integer is assigned to the value of n when the calculated n value is between two integers. Details are presented in Appendix A.
Secondly, to determine the free phase density and compressibility factor of gases at experimental temperature and pressure conditions, the data can be directly obtained from the Reference Fluid Thermodynamic and Transport Properties Database (REFPROP), which was developed by the National Institute of Standards and Technology (NIST). REFPROP is a computer program, distributed through the Standard Reference Data Program of NIST, that provides thermophysical properties of pure fluids and mixtures over a wide range of fluid conditions including liquid, gas, and supercritical phases.
Thirdly, it is necessary to analyze the isotherm adsorption data (absolute adsorption amount vs. gas pressure) by combining Equations (3)–(5). If the adsorption data is the Gibbs excess adsorption, Equation (6) also needs to be employed. The next thing that needs to be done is to obtain the undetermined parameters in these adsorption equations, including E0, nL, ki, and ρa (for excess adsorption). In this study, we developed a computer program based on particle swarm algorithm, which can get the above parameters by fitting the experimental data (m points) with these adsorption equations and minimizing the relative error (Equation (7)). After determining the parameters, the amounts of adsorbed molecules at different energy levels can also be calculated by using Equation (4), so as to predict the physisorption behavior.
In this study, relative error δ was used instead of residual (as shown in literature [26]) taking into account the large difference in adsorption amounts at low and high gas pressures.
4. Results and Discussion
4.1. Calculated Results
This study is not focused on analyzing the differences of gas physisorption in various porous media but on verifying the feasibility of the theory and exploring the gas physisorption mechanism. Therefore, two MOFs (Cu3(BTC)2, MOF-2) and two shale rocks were presented here as a case study. Related parameters obtained by using the average of repetitive analyses were listed in Table 1. MOFs have homogeneous pore structure [15], while shale is a chemically heterogeneous porous rock [12]. The quantum physisorption equation can well predict the adsorption behavior of CH4 and CO2 in the above nanoporous media, and the adsorption of gases with different energy levels is markedly distinct (Figure 2a–d). We can see that the contribution to total adsorption amount decreases as molecular energy level increases, particularly at the stage of rapid increase in adsorption amount (Figure 2h). The gap between the contributions of different energy levels is dominated by probability distribution of molecules in different orbitals. As gas adsorption trends towards saturation, the gap gradually decreases until it reaches stability. As shown in Figure 3, it can be seen that with the increase in the quantum number, maximum the adsorption amount of molecules (nmi) decreases in an exponential form while the adsorption coefficient of molecules (ki) gradually increases. This indicates that a larger adsorption amount and a fast adsorption saturation speed will occur in molecules with relatively lower energy level. This also implies that gas molecules near the pore surface are more likely to adsorb.

Table 1.
Analyzed results of the studied cases.
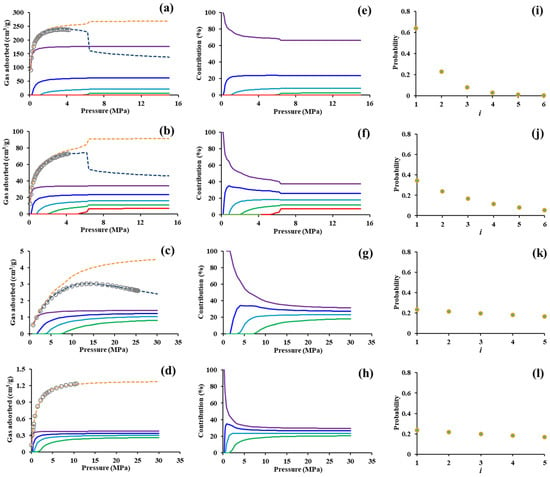
Figure 2.
Application cases of quantum physisorption theory. (a,e,i). Cu3(BTC)2 adsorbs CO2 at 298 K [15]; (b,f,j). MOF-2 adsorbs CO2 at 298 K [15]; (c,g,k). Sleen shale adsorbs CH4 at 338.15 K [27]; (d,h,i). Grange Hill shale adsorbs CH4 at 333.15 K [12]. (e,f,g,h) present the contribution of molecules with different energy level to total adsorption amount. (i,j,k,l) show the probability distribution of molecules at different energy levels. The gray circle indicates experimental data; the dark blue dotted line indicates simulated Gibbs excess adsorption; the orange dotted line indicates simulated absolute adsorption; purple, blue, cyan, green and red solid lines indicate molecules with i = 1, i = 2, i = 3, i = 4, and i = 5, respectively.
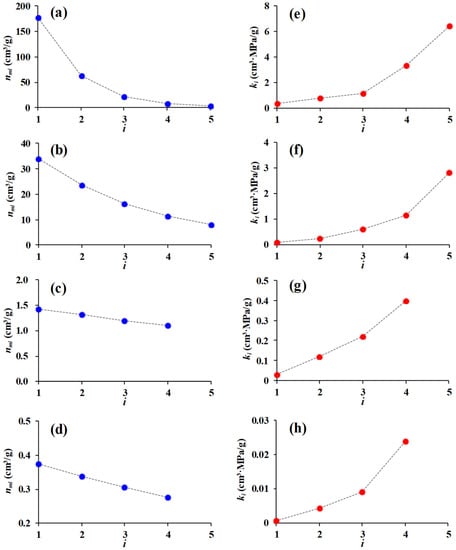
Figure 3.
Relationships of quantum number (i) with maximum adsorption amount (nmi) and adsorption coefficient (ki). (a,e). Cu3(BTC)2; (b,f). MOF-2; (c,g). Sleen shale; (d,h). Grange Hill shale.
4.2. Mechanism of Quantum Physisorption
The physisorption behavior of complex molecular systems of CH4 and CO2 confined within nanopores is essentially resulted from energy level transition of gas molecules from excited state to ground state. The ground state orbital is located near the internal pore surface, which resembles the elementary space described by Langmuir [19]. The maximum adsorption capacity theoretically equals to the total space occupied by the ground state orbital, which positively associates with internal surface area of pores. Vast specific surface area for MOFs may be the main reason that the MOFs have larger adsorption capacity compared to shale rock. The actual adsorption amount of gas closely relates to the gas pressure, gas temperature, and potential energy field of the gas-solid system. Under certain temperature and pressure conditions, excited gas molecules are initially unevenly distributed in the orbitals (i = 1~n) following the Boltzmann distribution law. The molecular amount of energy level transition is a function of the total number of molecules with the same transition probability. Therefore, the amount of gas physisorption gradually increases due to the increase in the total amount of molecules in all the orbitals during pressurization process. Change in pressure, however, does not change the probability of molecular transition. In addition, there is an initial pressure at which adsorption occurs; that is, the law of quantum physisorption is followed when a certain amount of molecules is reached.
Potential energy of gas-solid interaction decrease or temperature increase will promote a more even molecular distribution within nanoscale space (Figure 2i–l), which enhances the difficulty of gas physisorption. As an extreme case, the gas molecules evenly distribute inside nanopores and will be non-adsorptive for a gas-solid system without interaction or under an extremely high temperature condition. With an enhanced potential energy field, the percentage of molecules with a low quantum number (especially i = 1 and 2) will increase (Figure 4), which causes an increasing adsorption amount. Temperature also adjusts the probability distribution of molecules in orbitals together with a potential energy field. As shown in Figure 5, the increase of temperature will cause a dramatic decrease in the percentage of molecules with a low quantum number. Therefore, the temperature increase makes it more difficult for gas physisorption. Previously, the impacts of specific surface area of porous medium and temperature on the adsorbed amount have been widely observed. These phenomena can be reasonably explained by the quantum physisorption theory.
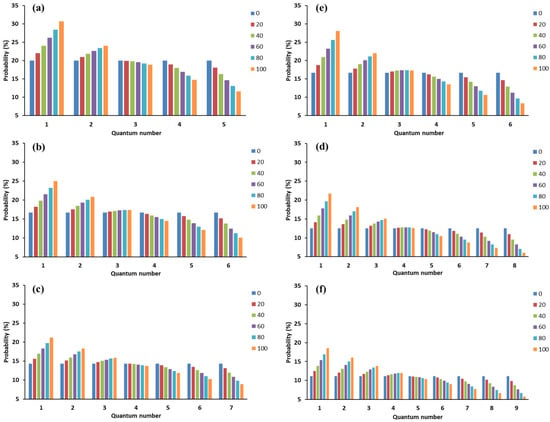
Figure 4.
Impact of potential energy field (E0 = 0~10−21 J in this case) on molecular distribution within nanoscale space. (a–c) indicate the temperature conditions of 298.15 K, 398.15 K, and 498.15 K for CH4, respectively; (d–f) indicate the temperature conditions of 298.15 K, 398.15 K, and 498.15 K for CO2, respectively.
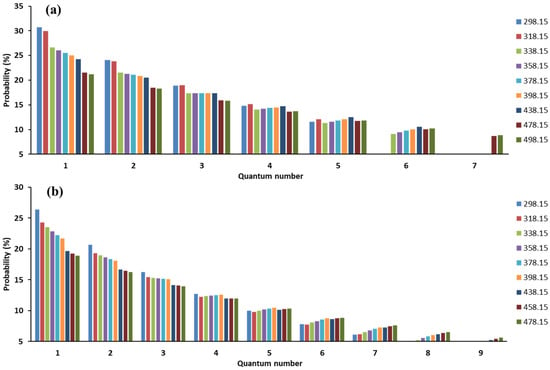
Figure 5.
Impact of temperature (T = 298.15~498.15 J in this case) on molecular distribution within nanoscale space. (a) indicates the CH4 with a potential energy field of E0 = 10−21 J; (b) indicates the CO2 with a potential energy field of E0 = 10−21 J.
5. Conclusions
In summary, this study attempts to explore the essence of the physisorption behavior of gas (CH4 and CO2) within nanoscale space. We proposed a new mechanism to re-understand the basic principles of gas physisorption within nanoscale space and found that the physisorption behavior of the complex molecular system of CH4 and CO2 within nanoporous media exhibits a quantum effect; that is energy level transition induces gas physisorption. Based on this phenomenon, we established a quantum physisorption equation, which matches well with experimental data for various nanoporous media. This discovery differs from any traditional theory and is expected to open a new field involving in the quantum physisorption of gases. Confidently, the quantum physisorption theory proposed will be of significance to the researchers of multidisciplinary sciences (energy, environment, physics, and materials).
Funding
This research was funded by the National Natural Science Foundation (Grant No. 41972123).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data supporting reported results can be found in the main text.
Acknowledgments
The author would like to thank Li Wenbiao, He Taohua and Li Junkun for their assistance during the preparation of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Nomenclature
| ne | excess adsorption amount, mol or mol/g |
| na | actual (absolute) adsorption amount, mol or mol/g |
| ρg | density of free gas, cm3/g |
| ρa | density of adsorbed gas, cm3/g |
| T | gas temperature, K |
| p | gas pressure, MPa |
| z | gas compressibility factor, dimensionless |
| M | molar mass, kg/mol |
| n | maximum quantum number, integer |
| i | quantum number, integer |
| E0 | smallest potential energy unit, J |
| Epi | potential energy of molecules with different energy level, J |
| mean kinetic energy for free gas molecules, J | |
| Evi | kinetic energy of molecules with i-th energy level, J |
| Ev0 | kinetic energy of adsorbed gas molecules, J |
| nai | actual (absolute) adsorption amount of molecules with i-th energy level, mol, or mol/g |
| nmi | maximum adsorption amount of molecules with i-th energy level, mol, or mol/g |
| ki | adsorption coefficient of molecules with i-th energy level, mol·MPa or mol·Mpa/g |
| nL | maximum adsorption amount, mol, or mol/g |
| KB | Boltzmann’s constant, J/K |
| NA | Avogadro’s constant, mol−1 |
| h | Planck’s constant, J·s |
| R | universal gas constant, 8.314 J/mol/K |
| d | kinetic diameter of molecule, m |
| ξ | positive integer ratio of orbital width to molecular de Broglie wavelength, integer |
| fi | probability, fraction |
| δ | relative error, dimensionless |
| calculated i-th value by the equation of quantum physisorption, mol, or mol/g | |
| measured i-th value obtained from adsorption experiment, mol, or mol/g |
Appendix A. Determination of Maximum Quantum Number (n)
The orbital width occupied by excited molecules should be an integer multiple of the De Broglie wavelength based on the theory of matter wave, which is the condition of maintaining standing wave. Therefore, molecules can exist stably in the orbital when this condition is met.
A total of 2n orbitals are formed inside the nanopores for accommodating excited molecules and satisfy the following conditions:
of which,
where, n is the maximum quantum number, integer; h is the Planck’s constant, J·s; m0 is the rest mass of molecule, kg; v is the molecular velocity, m/s; is the mean velocity of gas molecules, m/s; λ is the de Broglie wavelength, m; L is the total width of 2n orbitals in pores, m; R is the universal gas constant, 8.314 J/mol/K; M is the molar mass, kg/mol.
We can obtain:
In a complex network of nanopores, molecules can enter all the interconnected pores with a size of larger than the kinetic diameter of the molecule (CH4, 0.38 nm; CO2, 0.33 nm). When the width L is equal to the molecular kinetic diameter, it is the smallest width at which the molecule can exist stably. In this case, the largest quantum number n can appear when ξ =1. In larger pores, the individual orbital width is equal to ξλ. Therefore, gas molecules can exist stably in all the interconnected pores that molecules can enter, and the following relationships are established to discriminate the largest quantum number.
Thus, the maximum quantum number n can be discriminated by a function of considering the gas type, molecular kinetic diameter, and temperature of molecular system, expressed as:
For methane (CH4):
For carbon dioxide (CO2):
Because the value of n should be a positive integer, the smaller integer is assigned to the value of n when the calculated n value is between two integers. For example, the calculated n value is equal to 4.78 for methane at 298 K. The maximum quantum number n should be assigned the value 4 to ensure that the kinetic diameter of the molecule is smaller than the pore size.
Appendix B. Establish of the Equation of Quantum Physisorption
Appendix B.1. Energy Level Transition
The number of molecular transitions is exactly the amount of gas adsorption. Only transitions from i = 1~n−1 orbitals (excited state) to i = 0 orbital (ground state) occur, showing a transition selectivity. With respect to a nanoporous medium with a unit mass (e.g., one gram), the frequency of the energy level transition of gas molecules for the i-th energy level under certain temperature and pressure conditions can be expressed as:
where F is the frequency of energy level transition of gas molecules, that is the number of molecules undergoing energy level transition per second, s−1; Se is the effective surface area providing for the ground state position, cm2.
where Sm is the cross-sectional area of adsorbed molecule, cm2; NA is the Avogadro’s constant, 6.022 × 1023 mol−1; θim is the maximum coverage rate of adsorbed molecules from the i-th energy level orbital on the ground state orbital, fraction; θi is the actual coverage rate of adsorbed molecules from the i-th energy level orbital on the ground state orbital, fraction; nm is the maximum adsorbed amount from all the energy level molecules, mol.
In Equation (B1), dN/dt is the number of molecular transition to unit area (1 cm2) ground state orbital during unit time (1 s); based on the assumption that molecular transition velocity is equal to the average velocity of molecular motion, it can be described as:
where V is the pore volume, cm3; N is the total number of molecules in the pore, dimensionless; is the mean velocity of gas molecules, cm/s. In Equation (A10), N/V indicates a mean gas concentration, that is the molecular number per unit volume.
According to Maxwell’s velocity distribution theory, mean velocity of gas molecules can be written as:
R is the universal gas constant, 8.314 J/mol/K; M is the molar mass, kg/mol; T is the temperature, K.
Further, we can obtain:
According to the equation of gas state:
and the relationship:
where p is the apparent mean gas pressure in pore, MPa; V is the pore volume, cm3; z is the gas compressibility factor, dimensionless; n is the amount of substance of gas, mol; R is the universal gas constant, 8.314 J/mol/K; T is the temperature, K; N is the total number of gas molecule, number.
We can obtain:
where
Appendix B.2. Energy Release
During the process of molecular energy level transition (i.e., gas physisorption), the release of molecular energy is accompanied. During the energy level transition, the energy released by the molecule includes both the loss of kinetic energy and potential energy.
The kinetic energy loss () of molecules is:
where, is the mean kinetic energy for free gas molecules (see Equation (A11)), J; Ev0 is the kinetic energy of adsorbed gas molecules, J; Evi is the kinetic energy of molecules with i-th energy level, J; i is the quantum number, integer (1, 2, …, n−1).
The potential energy loss () of molecules is:
where, Epi is the potential energy of molecules with i-th energy level and is mathematically described as Epi = (i−n)E0, J; E0 is the smallest potential energy unit, J; i is the quantum number, integer (1, 2, …, n−1).
Total energy loss of molecules during energy level transition can be obtained as:
The carrier of this energy released may be an infrared light radiation, which has a frequency as:
where h is the Planck’s constant, J·s; νi is the frequency of infrared light radiation released by the i-th energy level molecules, s−1.
Simultaneously, we can obtain the energy released by one mole molecules as:
where Qi is the energy released by one mole molecules at the i-th energy level, J/mol.
From the perspective of adsorption energy, the frequency of energy level transition of a gas molecule can also be written as:
where Ai is the frequency factor for the molecules at the i-th energy level, which is a constant independent of temperature and concentration of gas, s−1.
Combining Equations (A15) and (A21), we can obtain:
Further, it can be obtained as:
It can be further simplified as:
where nai is the actual (absolute) adsorption amount of molecules with the i-th energy level, mol; nmi is the maximum adsorption amount of molecules with i-th energy level, mol.
For a classic Boltzmann system composed by gas molecules, molecules have identical properties under a certain temperature during physisorption. Some parameters (M, T, Ai, Qi) can be considered as constant values. Thus, we put these parameters into one item and define it as the adsorption coefficient (ki). It can be expressed as:
where ki is the adsorption coefficient of molecules with the i-th energy level, mol·MPa, generally . A smaller ki value indicates a faster molecular adsorption saturation during pressurization process.
Appendix B.3. Equation of Quantum Physisorption
Combining Equations (A24) and (A25), the equation of quantum physisorption describing the physisorption isotherm of gas molecule with a certain energy level can be obtained as:
Gas molecules of different energy levels begin to adsorb at varied gas pressures. Before adsorption begins, the adsorption amount is of zero. The initial pressures for physisorption of molecules with a different energy level can be determined by the Equation (A26), and can be written as:
When the pressure p reaches the initial pressure pi, the molecules of the i-th energy level begin to adsorb, and the adsorption capacity increases as the pressure increases according to the equation of quantum physisorption.
The total amount (na) of gas physisorption is the sum of molecular transition from energy levels of i = 1 ~ n−1 onto i = 0, and can be expressed as:
Additionally, the results obtained by widely used volumetric and gravimetric methods are the Gibbs excess adsorption (ne), which is not the actual amount of adsorbed gas (na). When the gas-adsorbed volumes cannot be ignored especially at high pressure, they can be converted into each other through the density ratio of free gas to adsorbed gas, which is mathematically expressed as:
where ρg is the density of free gas, cm3/g; ρa is the density of adsorbed gas, cm3/g.
Appendix B.4. Determination of Adsorption Capacity
According to the equation of quantum physisorption, the adsorption amount of each energy level gradually increases as the gas pressure increases. When the pressure tends toward infinity, adsorption of molecules of different energy levels towards saturation. Under an infinite pressure, the number of molecules at each energy level (i = 1 ~ n) conforms to the Boltzmann distribution before adsorption occurs, which can be written as:
where, fi is the probability of the occurrence of molecules at the i-th energy level, fraction; E0 is the smallest potential energy unit, J; i is the quantum number, integer; kB is the Boltzmann’s constant, J/K; T is the temperature, K.
It is not that all the molecules in an excited state (i = 1~n − 1) will undergo energy level transition, but that they have a certain probability of transition, that is, transition is probabilistic. Moreover, the probability of energy level transition is equal for all molecules due to the identical molecular properties. Since molecules in the i = 1~n − 1 orbitals have the same probability of adsorption, the adsorption amounts of molecules at different energy levels is proportional to the number of molecules at each energy level. According to this principle, the proportional relationship between nmi and the maximum adsorption amount nL can be determined as:
where, nmi is the maximum adsorption amount of molecules at the i-th energy level, mol; nL is the maximum adsorption amount (including all the energy levels), mol.
References
- Wigley, T.; Raper, S. Thermal expansion of sea water associated with global warming. Nature 1987, 330, 127–131. [Google Scholar] [CrossRef]
- Lashof, D.; Ahuja, D. Relative contributions of greenhouse gas emissions to global warming. Nature 1990, 344, 529–531. [Google Scholar] [CrossRef]
- Wuebbles, D.J.; Hayhoe, K. Atmospheric methane and global change. Earth-Sci. Rev. 2002, 57, 177–210. [Google Scholar] [CrossRef]
- Cowan, T.; Undorf, S.; Hegerl, G.C.; Harrington, L.J.; Otto, F.E. Present-day greenhouse gases could cause more frequent and longer Dust Bowl heatwaves. Nat. Clim. Chang. 2020, 10, 505–510. [Google Scholar] [CrossRef]
- Haugan, P.; Drange, H. Sequestration of CO2 in the deep ocean by shallow injection. Nature 1992, 357, 318–320. [Google Scholar] [CrossRef]
- Lackner, K.S. A Guide to CO2 Sequestration. Science 2003, 300, 1677. [Google Scholar] [CrossRef]
- Gasda, S.E.; Nordbotten, J.M.; Celia, M.A. Vertical equilibrium with sub-scale analytical methods for geological CO2 sequestration. Comput. Geosci. 2009, 13, 469–481. [Google Scholar] [CrossRef]
- Cooper, A. Cooperative carbon capture. Nature 2015, 519, 294–295. [Google Scholar] [CrossRef]
- Viete, D.R.; Ranjith, P.G. The effect of CO2 on the geomechanical and permeability behavior of brown coal: Implications for coal seam CO2 sequestration. Int. J. Coal Geol. 2006, 66, 204–216. [Google Scholar] [CrossRef]
- Lastoskie, C. Caging carbon dioxide. Science 2010, 330, 595. [Google Scholar] [CrossRef]
- Weniger, P.; Kalkreuth, W.; Busch, A.; Krooss, B.M. High-pressure methane and carbon dioxide sorption on coal and shale samples from the Paraná Basin, Brazil. Int. J. Coal Geol. 2010, 84, 190–205. [Google Scholar] [CrossRef]
- Whitelaw, P.; Uguna, C.N.; Stevens, L.A.; Meredith, W.; Snape, C.E.; Vane, C.H.; Moss-Hayes, V.; Carr, A.D. Shale gas reserve evaluation by laboratory pyrolysis and gas holding capacity consistent with field data. Nat. Commun. 2019, 10, 3659. [Google Scholar] [CrossRef]
- Lyu, Q.; Tan, J.; Li, L.; Ju, Y.; Busch, A.; Wood, D.A.; Ranjith, P.G.; Middleton, R.; Shu, B.; Hu, C.; et al. The role of supercritical carbon dioxide for recovery of shale gas and sequestration in gas shale reservoirs. Energy Environ. Sci. 2021, 14, 4203–4227. [Google Scholar] [CrossRef]
- Eddaoudi, M.; Kim, J.; Rosi, N.; Vodak, D.; Wachter, J.; O’Keeffe, M.; Yaghi, O.M. Systematic design of pore size and functionality in isoreticular MOFs and their application in methane storage. Science 2002, 295, 469–472. [Google Scholar] [CrossRef]
- Millward, A.R.; Yaghi, O.M. Metal-organic frameworks with exceptionally high capacity for storage of carbon dioxide at room temperature. J. Am. Chem. Soc. 2005, 127, 17998–17999. [Google Scholar] [CrossRef]
- Furukawa, H.; Ko, N.; Go, Y.B.; Aratani, N.; Choi, S.B.; Choi, E.; Yazaydin, A.Ö.; Snurr, R.Q.; O’Keeffe, M.; Kim, J.; et al. Ultrahigh porosity in metal-organic frameworks. Science 2010, 329, 424–428. [Google Scholar] [CrossRef]
- Boyd, P.G.; Chidambaram, A.; García-Díez, E.; Ireland, C.P.; Daff, T.D.; Bounds, R.; Gładysiak, A.; Schouwink, P.; Moosavi, S.M.; Maroto-Valer, M.M.; et al. Data-driven design of metal-organic frameworks for wet flue gas CO2 capture. Nature 2019, 576, 253–256. [Google Scholar] [CrossRef]
- Chen, Z.; Li, P.; Anderson, R.; Wang, X.; Zhang, X.; Robison, L.; Redfern, L.R.; Moribe, S.; Islamoglu, T.; Gómez-Gualdrón, D.A.; et al. Balancing volumetric and gravimetric uptake in highly porous materials for clean energy. Science 2020, 368, 297–303. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Polanyi, M. Section III.—Theories of the adsorption of gases. A general survey and some additional remarks. Introductory paper to section III. Trans. Fraraday Soc. 1932, 28, 316–333. [Google Scholar] [CrossRef]
- Polanyi, M. The Potential Theory of Adsorption. Science 1963, 141, 1010–1013. [Google Scholar] [CrossRef]
- Dubinin, M.M.; Astakhov, V.A. Development of the concepts of volume filling of micropores in the adsorption of gases and vapors by microporous adsorbents. Communication 2. General bases of the theory of adsorption of gases and vapors on zeolites. Bull. Acad. Sci. USSR Div. Chem. Sci. 1971, 20, 8–12. [Google Scholar] [CrossRef]
- Cerreia-Vioglio, S.; Maccheroni, F.; Marinacci, M.; Rustichini, A. Axiomatic tests for the Boltzmann distribution. J. Stat. Mech. Theory Exp. 2021, 2021, 013406. [Google Scholar] [CrossRef]
- Sircar, S. Gibbsian surface excess for gas adsorptions-revisited. Ind. Eng. Chem. Res. 1999, 38, 3670–3682. [Google Scholar] [CrossRef]
- Do, D.D.; Do, H.D. A desorption of supercritical fluids in non-porous and porous carbons: Analysis of adsorbed phase volume and density. Carbon 2003, 41, 1777–1791. [Google Scholar] [CrossRef]
- Gasparik, M.; Ghanizadeh, A.; Bertier, P.; Gensterblum, Y.; Bouw, S.; Krooss, B.M. High-pressure methane sorption isotherms of black shales from the Netherlands. Energy Fuel 2012, 26, 4995–5004. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).