Fatty Acid Alkyl Ester Production by One-Step Supercritical Transesterification of Beef Tallow by Using Ethanol, Iso-Butanol, and 1-Butanol
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Transesterification
2.3. Quantification
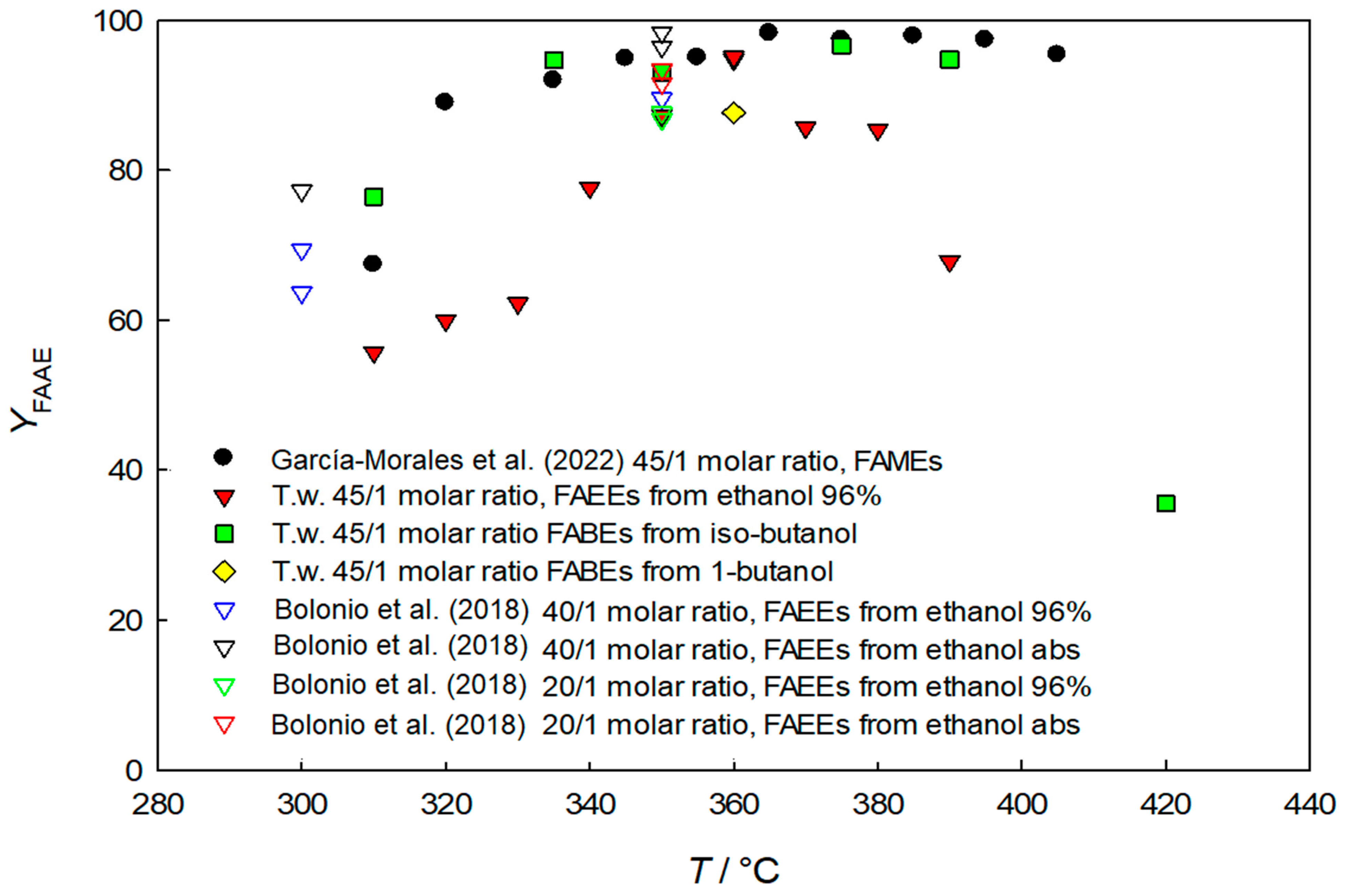
3. Results and Discussion
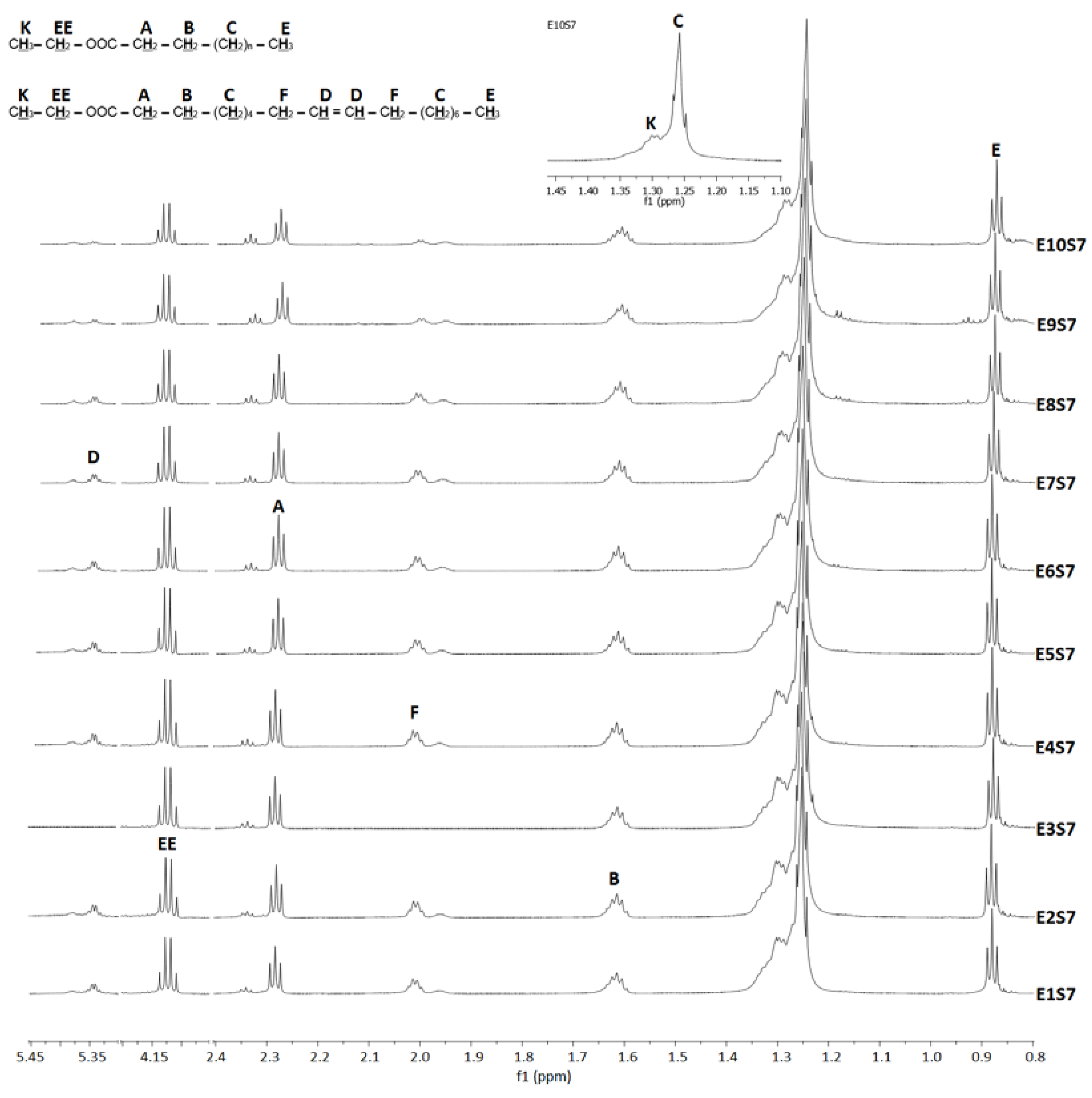
3.1. Fatty Acid Ethyl Esters (FAEEs)
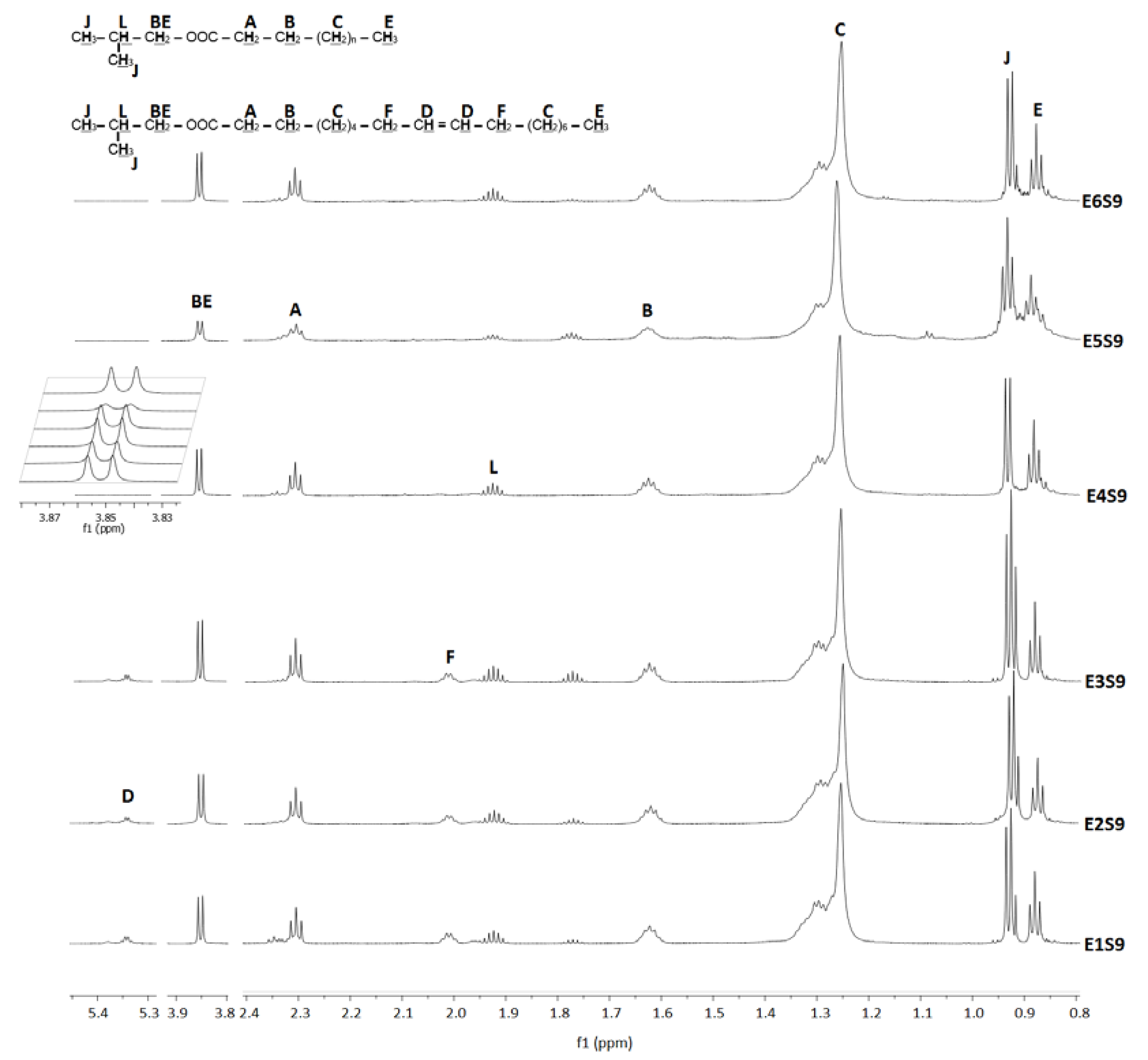
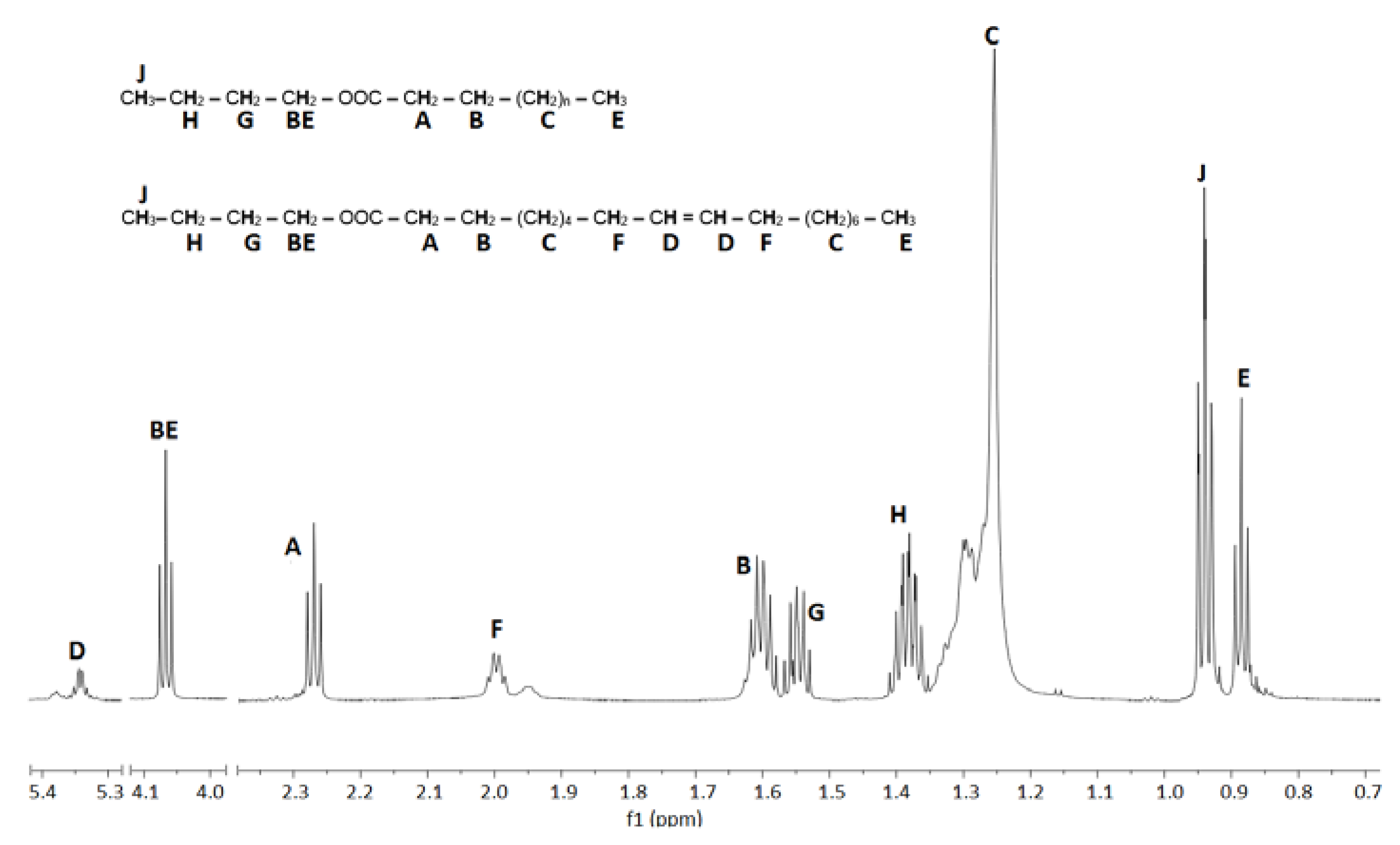
3.2. Fatty Acid Butyl Esters (FABEs)
3.3. Fatty Acid Alkyl Ester Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- U.S. Energy Information Administration. Annual Energy Outlook 2022. Washington, DC, USA . March 2022. Available online: https://www.eia.gov/outlooks/aeo/pdf/AEO2022_ChartLibrary_full.pdf (accessed on 1 June 2022).
- Gharehghani, A.; Fakhari, A.H. Biodiesel as a clean fuel for mobility. In Clean Fuels for Mobility. Energy, Environment, and Sustainability; Di Blasio, G., Agarwal, A.K., Belgiorno, G., Shukla, P.C., Eds.; Springer: Singapore, 2022; pp. 141–168. [Google Scholar] [CrossRef]
- Gotovuša, M.; Pucko, I.; Racar, M.; Faraguna, M. Biodiesel produced from propanol and longer chain alcohols—Synthesis and properties. Energies 2022, 15, 4996. [Google Scholar] [CrossRef]
- Kurczyński, D.; Wcisło, G.; Leśniak, A.; Kozak, M.; Łagowski, P. Production and testing of butyl and methyl esters as new generation biodiesels from fatty wastes of the leather industry. Energies 2022, 15, 8744. [Google Scholar] [CrossRef]
- Canabarro, N.I.; Silva-Ortiz, P.; Nogueira, L.A.H.; Cantarella, H.; Maciel-Filho, R.; Souza, G.M. Sustainability assessment of ethanol and biodiesel production in Argentina, Brazil, Colombia, and Guatemala. Renew. Sustain. Energy Rev. 2023, 171, 113019. [Google Scholar] [CrossRef]
- Dutta, N.; Usman, M.; Ashraf, M.A.; Luo, G.; El-Din, M.G.; Zhang, S. Methods to convert lignocellulosic waste into biohydrogen, biogas, bioethanol, biodiesel and value-added chemicals: A review. Environ. Chem. Lett. 2022. [Google Scholar] [CrossRef]
- Culaba, A.B.; Mayol, A.P.; San Juan, J.L.G.; Ubando, A.T.; Bandala, A.A.; Concepcion, R.S., II; Alipio, M.; Chen, W.H.; Show, P.L.; Chang, J.S. Design of biorefineries towards carbon neutrality: A critical review. Biores. Technol. 2023, 369, 128256. [Google Scholar] [CrossRef] [PubMed]
- Dahiya, A. Bioenergy. Biomass to Biofuels and Waste to Energy, 2nd ed.; Academic Press: London, UK, 2020. [Google Scholar] [CrossRef]
- Carlucci, C. An Overview on the production of biodiesel enabled by continuous flow methodologies. Catalysts 2022, 12, 717. [Google Scholar] [CrossRef]
- Vernier, L.J.; Barrachini-Nunes, A.L.; Albarello, M.; de Castilhos, F. Continuous production of fatty acid methyl esters from soybean oil deodorized distillate and methyl acetate at supercritical conditions. J. Supercrit. Fluids 2022, 186, 105603. [Google Scholar] [CrossRef]
- da Silva, P.R.; Alhadeff, E.M. Biodiesel from beef tallow: A technological patent mapping. Braz. J. Dev. 2022, 8, 38061–38075. [Google Scholar] [CrossRef]
- dos Reis Carraro, A.; da Silva César, A.; Conejero, M.A.; Ribeiro, E.C.B.; Mozer, T.S. The potential use of beef tallow for biodiesel production in Brazil. Revista Valore 2021, 6, e-6006. [Google Scholar] [CrossRef]
- U.S. Energy Information Administration. Monthly Biofuels Capacity and Feedstocks Update. Washington, DC. May 2022. Available online: https://www.eia.gov/biofuels/update/table2.pdf (accessed on 1 June 2022).
- Vignesh, P.; Kumar, A.R.P.; Ganesh, N.S.; Jayaseelan, V.; Sudhakar, K. Biodiesel and green diesel generation: An overview. Oil Gas Sci. Technol. Rev. IFP Energies Nouvelles 2021, 76, 6. [Google Scholar] [CrossRef]
- Toldrá-Reig, F.; Mora, L.; Toldrá, F. Developments in the use of lipase transesterification for biodiesel production from animal fat waste. Appl. Sci. 2020, 10, 5085. [Google Scholar] [CrossRef]
- Pinotti, L.M.; Salomão, G.S.B.; Benevides, L.C.; Antunes, P.W.P.; Cassini, S.T.A.; de Oliveira, J.P. Lipase-catalyzed biodiesel production from grease trap. Arab. J. Sci. Eng. 2022, 47, 6125–6133. [Google Scholar] [CrossRef]
- Toldrá-Reig, F.; Mora, L.; Toldrá, F. Trends in biodiesel production from animal fat waste. Appl. Sci. 2020, 10, 3644. [Google Scholar] [CrossRef]
- Samanta, S.; Sahoo, R.R. Waste cooking (palm) oil as an economical source of biodiesel production for alternative green fuel and efficient lubricant. BioEnergy Res. 2021, 14, 163–174. [Google Scholar] [CrossRef]
- Farrokheh, A.; Tahvildari, K.; Nozari, M. Comparison of biodiesel production using the oil of chlorella vulgaris micro-algae by electrolysis and reflux methods using CaO/KOH-Fe3O4 and KF/KOH-Fe3O4 as magnetic nano catalysts. Waste Biomass Valorization 2021, 12, 3315–3329. [Google Scholar] [CrossRef]
- Jambulingam, R.; Srinivasan, G.R.; Palani, S.; Munir, M.; Saeed, M.; Mohanam, A. Process optimization of biodiesel production from waste beef tallow using ethanol as co-solvent. SN Appl. Sci. 2020, 2, 1454. [Google Scholar] [CrossRef]
- Rosson, E.; Sgarbossa, P.; Pedrielli, F.; Mozzon, M.; Bertani, R. Bioliquids from raw waste animal fats: An alternative renewable energy source. Biomass Convers. Biorefin. 2021, 11, 1475–1490. [Google Scholar] [CrossRef]
- Erchamo, Y.S.; Mamo, T.T.; Workneh, G.A.; Mekonnen, Y.S. Improved biodiesel production from waste cooking oil with mixed methanol–ethanol using enhanced eggshell-derived CaO nano-catalyst. Sci. Rep. 2021, 11, 6708. [Google Scholar] [CrossRef]
- Demir, V.; Akgün, M. New catalysts for biodiesel production under supercritical conditions of alcohols: A comprehensive review. ChemistrySelect 2022, 7, e202104459. [Google Scholar] [CrossRef]
- Ghosh, N.; Halder, G. Current progress and perspective of heterogeneous nanocatalytic transesterification towards biodiesel production from edible and inedible feedstock: A review. Energy Convers. Manag. 2022, 270, 116292. [Google Scholar] [CrossRef]
- Mahlia, T.M.I.; Syazmi, Z.A.H.S.; Mofijur, M.; Abas, A.E.P.; Bilad, M.R.; Ong, H.C.; Silitonga, A.S. Patent landscape review on biodiesel production: Technology updates. Renew. Sustain. Energy Rev. 2020, 118, 109526. [Google Scholar] [CrossRef]
- Rathnam, V.M.; Madras, G. Conversion of Shizochitrium limacinum microalgae to biodiesel by non-catalytic transesterification using various supercritical fluids. Bioresour. Technol. 2019, 288, 121538. [Google Scholar] [CrossRef]
- Vega-Guerrero, D.B.; Gómez-Castro, F.I.; López-Molina, A. Production of biodiesel with supercritical ethanol: Compromise between safety and costs. Chem. Eng. Res. Des. 2022, 184, 79–89. [Google Scholar] [CrossRef]
- Sakdasri, W.; Sawangkeaw, R.; Ngamprasertsith, S. An entirely renewable biofuel production from used palm oil with supercritical ethanol at low molar ratio. Braz. J. Chem. Eng. 2017, 34, 1023–1034. [Google Scholar] [CrossRef]
- Yuliana, M.; Santoso, S.P.; Soetaredjo, F.E.; Ismadji, S.; Ayucitra, A.; Angkawijaya, A.E.; Ju, Y.H.; Nguyen, P.L.T. A one-pot synthesis of biodiesel from leather tanning waste using supercritical ethanol: Process optimization. Biomass Bioenergy 2020, 142, 105761. [Google Scholar] [CrossRef]
- Hegel, P.E.; Martín, L.A.; Popovich, C.A.; Damiani, C.; Leonardi, P.I. Biodiesel production from Halamphora coffeeaeformis microalga oil by supercritical ethanol transesterification. Chem. Eng. Process. Process Intensif. 2019, 145, 107670. [Google Scholar] [CrossRef]
- Poudel, J.; Karki, S.; Sanjel, N.; Shah, M.; Oh, S.C. Comparison of biodiesel obtained from virgin cooking oil and waste cooking oil using supercritical and catalytic transesterification. Energies 2017, 10, 546. [Google Scholar] [CrossRef]
- Sanjel, N.; Gu, J.H.; Oh, S.C. Transesterification kinetics of waste vegetable oil in supercritical alcohols. Energies 2014, 7, 2095–2106. [Google Scholar] [CrossRef]
- Akkarawatkhoosith, N.; Kaewchada, A.; Jaree, A. Production of biodiesel from palm oil under supercritical ethanol in the presence of ethyl acetate. Energy Fuels 2019, 33, 5322–5331. [Google Scholar] [CrossRef]
- Bolonio, D.; Marco Neu, P.; Schober, S.; García-Martínez, M.J.; Mittelbach, M.; Canoira, L. Fatty acid ethyl esters from animal fat using supercritical ethanol process. Energy Fuels 2018, 32, 490–496. [Google Scholar] [CrossRef]
- Farobie, O.; Leow, Z.Y.M.; Samanmulya, T.; Matsumura, Y. In-depth study of continuous production of biodiesel using supercritical 1-butanol. Energy Convers. Manag. 2017, 132, 410–417. [Google Scholar] [CrossRef]
- dos Santos, K.C.; Hamerski, F.; Voll, F.A.P.; Corazza, M.L. Experimental and kinetic modeling of acid oil (trans)esterification in supercritical ethanol. Fuel 2018, 224, 489–498. [Google Scholar] [CrossRef]
- Sun, Y.; Reddy, H.K.; Muppaneni, T.; Ponnusamy, S.; Patil, P.D.; Li, C.; Jiang, L.; Deng, S. A comparative study of direct transesterification of camelina oil under supercritical methanol, ethanol and 1-butanol conditions. Fuel 2014, 135, 530–536. [Google Scholar] [CrossRef]
- Geuens, J.; Kremsner, J.M.; Nebel, B.A.; Schober, S.; Dommisse, R.A.; Mittelbach, M.; Tavernier, S.; Kappe, C.O.; Maes, B.U.W. Microwave-assisted catalyst-free transesterification of triglycerides with 1-butanol under supercritical conditions. Energy Fuels 2008, 22, 643–645. [Google Scholar] [CrossRef]
- Singh, C.S.; Kumar, N.; Gautam, R. Supercritical transesterification route for biodiesel production: Effect of parameters on yield and future perspectives. Environ. Prog. Sustain. Energy 2021, 40, e13685. [Google Scholar] [CrossRef]
- Tobar, M.; Núñez, G.A. Supercritical transesterification of microalgae triglycerides for biodiesel production: Effect of alcohol type and co-solvent. J. Supercrit. Fluids 2018, 137, 50–56. [Google Scholar] [CrossRef]
- Hoang, D.; Bensaid, S.; Saracco, G.; Pirone, R.; Fino, D. Investigation on the conversion of rapeseed oil via supercritical ethanol condition in the presence of a heterogeneous catalyst. Green Process. Synth. 2017, 6, 91–101. [Google Scholar] [CrossRef]
- Trentini, C.P.; Postaue, N.; Cardozo-Filho, L.; Reisa, R.R.; Sampaio, S.C.; da Silva, C. Production of esters from grease trap waste lipids under supercritical conditions: Effect of water addition on ethanol. J. Supercrit. Fluids 2019, 147, 9–16. [Google Scholar] [CrossRef]
- Poudel, J.; Shah, M.; Karki, S.; Oh, S.C. Qualitative analysis of transesterification of waste pig fat in supercritical alcohols. Energies 2017, 10, 265. [Google Scholar] [CrossRef]
- Trentini, C.P.; Fonseca, J.M.; Cardozo-Filho, L.; Reis, R.R.; Sampaio, S.C.; da Silva, C. Assessment of continuous catalyst-free production of ethyl esters from grease trap waste. J. Supercrit. Fluids 2018, 136, 157–163. [Google Scholar] [CrossRef]
- Shah, M.; Poudel, J.; Kwak, H.; Oh, S.C. Kinetic analysis of transesterification of waste pig fat in supercritical alcohols. Process Saf. Environ. Prot. 2015, 98, 239–244. [Google Scholar] [CrossRef]
- Nematian, T.; Fatehi, M.; Hosseinpour, M.; Barati, M. One-pot conversion of sesame cake to low N-content biodiesel via nano-catalytic supercritical methanol. Renew. Energy 2021, 170, 964–973. [Google Scholar] [CrossRef]
- Sherpa, K.C.; Kundu, D.; Banerjee, S.; Ghangrekar, M.M.; Banerjee, R. An integrated biorefinery approach for bioethanol production from sugarcane tops. J. Clean. Prod. 2022, 352, 131451. [Google Scholar] [CrossRef]
- Su, G.; Chan, C.; He, J. Enhanced biobutanol production from starch waste via orange peel doping. Renew. Energy 2022, 193, 576–583. [Google Scholar] [CrossRef]
- Maity, S.; Mallick, N. Trends and advances in sustainable bioethanol production by marine microalgae: A critical review. J. Clean. Prod. 2022, 345, 131153. [Google Scholar] [CrossRef]
- Devi, A.; Bajar, S.; Kour, H.; Kothari, R.; Pant, D.; Singh, A. Lignocellulosic biomass valorization for bioethanol production: A circular bioeconomy approach. BioEnergy Res. 2022, 15, 1820–1841. [Google Scholar] [CrossRef]
- Iyyappan, J.; Bharathiraja, B.; Varjani, S.; Praveenkumar, R.; Muthu Kumar, S. Anaerobic biobutanol production from black strap molasses using Clostridium acetobutylicum MTCC11274: Media engineering and kinetic analysis. Bioresour. Technol. 2022, 346, 126405. [Google Scholar] [CrossRef]
- Das, M.; Maiti, S.K. Current knowledge on cyanobacterial biobutanol production: Advances, challenges, and prospects. Rev. Environ. Sci. Biotechnol. 2022, 21, 483–516. [Google Scholar] [CrossRef]
- Tse, T.J.; Wiens, D.J.; Reaney, M.J.T. Production of bioethanol—A review of factors affecting ethanol yield. Fermentation 2021, 7, 268. [Google Scholar] [CrossRef]
- Andreo-Martínez, P.; Ortiz-Martínez, V.M.; Salar-García, M.J.; Veiga-del-Baño, J.M.; Chica, A.; Quesada-Medina, J. Waste animal fats as feedstock for biodiesel production using non-catalytic supercritical alcohol transesterification: A perspective by the PRISMA methodology. Energy Sustain. Dev. 2022, 69, 150–163. [Google Scholar] [CrossRef]
- Marulanda-Buitrago, P.A.; Marulanda-Cardona, V.F. Supercritical transesterification of beef tallow for biodiesel production in a batch reactor. Cienc. Tecnol. Futuro 2014, 5, 55–73. [Google Scholar] [CrossRef]
- García-Morales, R.; Zúñiga-Moreno, A.; Verónico-Sánchez, F.J.; Domenzain-González, J.; Pérez-López, H.I.; Bouchot, C.; Elizalde-Solis, O. Fatty acid methyl esters from waste beef tallow using supercritical methanol transesterification. Fuel 2022, 313, 122706. [Google Scholar] [CrossRef]
- Reid, R.C.; Prausnitz, J.M.; Poling, B.E. The Properties of Gases and Liquids, 4th ed.; McGraw-Hill: New York, NY, USA, 1987. [Google Scholar]
- Postaue, N.; Borba, C.E.; da Silva, C. Transesterification under high pressure as a sequential step from pressurized liquid extraction: Effect of operational parameters and characterization. J. Supercrit. Fluids 2023, 193, 105814. [Google Scholar] [CrossRef]
- Corazza, M.L.; Fouad, W.A.; Chapman, W.G. Application of molecular modeling to the vapor–liquid equilibrium of alkyl esters (biodiesel) and alcohols systems. Fuel 2015, 161, 34–42. [Google Scholar] [CrossRef]
- Ghesti, G.F.; de Macedo, J.L.; Resck, J.A.; Dias, I.S.; Dias, S.C.L. FT-Raman spectroscopy quantification of biodiesel in a progressive soybean oil transesterification reaction and its correlation with 1H NMR spectroscopy methods. Energy Fuels 2007, 21, 2475–2480. [Google Scholar] [CrossRef]
- Osmieri, L.; Alipour Moghadam Esfahani, R.; Recasens, F. Continuous biodiesel production in supercritical two-step process: Phase equilibrium and process design. J. Supercrit. Fluids 2017, 124, 57–71. [Google Scholar] [CrossRef]
- Roze, F.; Pignat, P.; Ferreira, O.; Pinho, S.P.; Jaubert, J.N.; Coniglio, L. Phase equilibria of mixtures involving fatty acid ethyl esters and fat alcohols between 4 and 27 kPa for bioproduct production. Fuel 2021, 306, 121304. [Google Scholar] [CrossRef]
- Farobie, O.; Matsumura, Y. A comparative study of biodiesel production using methanol, ethanol, and tert-butyl methyl ether (MTBE) under supercritical conditions. Bioresour. Technol. 2015, 191, 306–311. [Google Scholar] [CrossRef]
- Teixeira, L.S.G.; Assis, J.C.R.; Mendonça, D.R.; Santos, I.T.V.; Guimarães, P.R.B.; Pontes, L.A.M.; Teixeira, J.S.R. Comparison between conventional and ultrasonic preparation of beef tallow biodiesel. Fuel Process. Technol. 2009, 90, 1164–1166. [Google Scholar] [CrossRef]
- Ehiri, R.C.; Ikelle, I.I.; Ozoaku, O.F. Acid-catalyzed transesterification reaction of beef tallow for biodiesel production by factor variation. Am. J. Eng. Res. 2014, 3, 174–177. [Google Scholar]
- Da Rós, P.C.M.; Silva, G.A.M.; Mendes, A.A.; Santos, J.C.; de Castro, H.F. Evaluation of the catalytic properties of Burkholderia cepacia lipase immobilized on non-commercial matrices to be used in biodiesel synthesis from different feedstocks. Bioresour. Technol. 2010, 101, 5508–5516. [Google Scholar] [CrossRef]
- Knothe, G. A comprehensive evaluation of the cetane numbers of fatty acid methyl esters. Fuel 2014, 119, 6–13. [Google Scholar] [CrossRef]
- Ramírez-Verduzco, L.F.; Rodríguez-Rodríguez, J.E.; Jaramillo-Jacob, A.R. Predicting cetane number, kinematic viscosity, density and higher heating value of biodiesel from its fatty acid methyl ester composition. Fuel 2012, 91, 102–111. [Google Scholar] [CrossRef]
- Yanowitz, J.; Ratcliff, M.A.; McCormick, R.L.; Taylor, J.D.; Murphy, M.J. Compendium of Experimental Cetane Numbers. In National Renewable Energy Lab Technical Report NREL/TP-5400-67585; National Renewable Energy Laboratory: Golden, CO, USA, February 2017. [Google Scholar] [CrossRef]
- Official Mexican Standard. NMX-F-101-SCFI-2012. Foods—Vegetable or Animal Fats and Oils—Determination of Free Fatty Acids—Test Method. Official Gazette of the Federation. Mexico. 2012. Available online: http://www.economia-nmx.gob.mx/normas/nmx/2010/nmx-f-101-scfi-2012.pdf (accessed on 13 January 2023).
- Kusdiana, D.; Saka, S. Effects of water on biodiesel fuel production by supercritical methanol treatment. Bioresour. Technol. 2004, 91, 289–295. [Google Scholar] [CrossRef]
- Xu, J.; Jiang, Z.; Li, L.; Fang, T. A review of multi-phase equilibrium studies on biodiesel production with supercritical methanol. RSC Adv. 2014, 4, 23447–43455. [Google Scholar] [CrossRef]
- Seames, W.; Luo, Y.; Ahmed, I.; Aulich, T.; Kubátová, A.; Štávová, J.; Kozliak, E. The thermal cracking of canola and soybean methyl esters: Improvement of cold flow properties. Biomass Bioenergy 2010, 34, 939–946. [Google Scholar] [CrossRef]
| Property | Value |
|---|---|
| ρ at 308.15 K/ | 0.890 |
| 737.7 ± 2.0 a | |
| 742.5 ± 1.8 b | |
| HHV/ | 39,143.7 |
| water content/ppm | 200 |
| SI | 189.1 |
| 3.55 ± 0.04 | |
| 3.94 ± 0.03 | |
| 3.91 ± 0.04 |
| Reaction | T/°C | P/bar | IFAEEs Area | IFAEEs Interval | ITAG Area | ITAG Interval | CP/°C | CN | CG | FFASA /% | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ethanol | |||||||||||
| E1S7 | 310 | 152.0 | 95,455.9 | 4.18–4.09 | 19,980.7 | 4.35–4.17 | 55.74 | 20 | 79.7 | 3.6 | 8.1 |
| E2S7 | 320 | 171.6 | 101,315.3 | 4.17–4.10 | 18,443.4 | 4.35–4.17 | 59.96 | 18 | 79.2 | 4.0 | 7.2 |
| E3S7 | 330 | 217.3 | 94,003.9 | 4.17–4.10 | 15,789.8 | 4.34–4.17 | 62.28 | 18 | 86.8 | 4.1 | 7.6 |
| E4S7 | 340 | 254.9 | 86,045.8 | 4.17–4.09 | 7509.2 | 4.26–4.17 | 77.71 | 19 | 79.7 | 3.5 | 7.6 |
| E5S7 | 350 | 280.4 | 77,754.0 | 4.17–3.97 | 3585.4 | 4.22–4.17 | 87.33 | 17 | 79.5 | 3.8 | 8.3 |
| E6S7 | 360 | 344.6 | 141,698.7 | 4.17–4.09 | 2565.2 | 4.34–4.17 | 94.76 | 16 | 80.1 | 4.1 | 9.2 |
| E7S7 | 360 | 344.2 | 83,930.6 | 4.17–4.07 | 1410.7 | 4.36–4.17 | 95.12 | 16 | 80.8 | 4.1 | 9.2 |
| E8S7 | 370 | 397.1 | 81,931.6 | 4.17–4.09 | 4318.1 | 4.34–4.17 | 85.70 | 14 | 80.8 | 5.4 | 10.9 |
| E9S7 | 380 | 397.9 | 50,956.1 | 4.17–4.10 | 2745.7 | 4.35–4.17 | 85.41 | 14 | 81.1 | 5.7 | 15.0 |
| E10S7 | 390 | 397.1 | 70,722.1 | 4.18–4.10 | 9636.4 | 4.36–4.17 | 67.88 | 14 | 81.2 | 7.4 | 18.7 |
| Iso-butanol | |||||||||||
| E1S9 | 310 | 63.7 | 70,200.5 | 3.87–3.77 | 25,722.0 | 4.35–3.89 | 76.47 | 18 | 4.6 | 6.0 | |
| E2S9 | 335 | 147.1 | 73,763.8 | 3.90–3.79 | 5817.2 | 4.34–3.90 | 94.73 | 17 | 2.7 | 5.3 | |
| E3S9 | 350 | 205.9 | 68,245.6 | 3.90–3.79 | 7571.5 | 4.33–3.88 | 93.06 | 14 | 2.4 | 4.7 | |
| E4S9 | 375 | 269.6 | 65,417 | 3.89–3.77 | 1266.7 | 4.34–3.88 | 96.65 | 10 | 4.8 | 6.2 | |
| E6S9 | 390 | 279.4 | 71,736.0 | 3.89–3.80 | 1946.8 | 4.35–3.88 | 94.80 | 9 | 5.7 | 8.2 | |
| E5S9 | 420 | 343.2 | 34,260.4 | 3.90–3.78 | 12,063.0 | 4.37–3.89 | 35.55 | 11 | 7.3 | 8.2 | |
| 1-butanol | |||||||||||
| E1S10 | 360 | 210.7 | 237,318.6 | 3.01–4.10 | 20,289.96 | 4.31–4.11 | 87.70 | 8 | 5.0 | 6.4 | |
| Experiment | Chemical | Formula | MW | wt% | mol% | MWFAEEs |
|---|---|---|---|---|---|---|
| E1S7 | Ethyl palmitate | C18H36O2 | 284.4772 | 26.492 | 28.306 | 303.960 |
| Ethyl stearate | C20H40O2 | 312.5304 | 41.598 | 40.458 | ||
| Ethyl oleate | C20H38O2 | 310.5145 | 31.910 | 31.236 | ||
| E2S7 | Ethyl palmitate | C18H36O2 | 284.4772 | 25.856 | 27.641 | 304.108 |
| Ethyl stearate | C20H40O2 | 312.5304 | 40.281 | 39.196 | ||
| Ethyl oleate | C20H38O2 | 310.5145 | 33.862 | 33.164 | ||
| E3S7 | Ethyl palmitate | C18H36O2 | 284.4772 | 20.239 | 21.800 | 306.415 |
| Ethyl stearate | C20H40O2 | 312.5304 | 79.761 | 78.200 | ||
| E4S7 | Ethyl palmitate | C18H36O2 | 284.4772 | 26.650 | 28.472 | 303.921 |
| Ethyl stearate | C20H40O2 | 312.5304 | 41.835 | 40.683 | ||
| Ethyl oleate | C20H38O2 | 310.5145 | 31.515 | 30.846 | ||
| E5S7 | Ethyl palmitate | C18H36O2 | 284.4772 | 26.338 | 28.145 | 303.990 |
| Ethyl stearate | C20H40O2 | 312.5304 | 41.008 | 39.887 | ||
| Ethyl oleate | C20H38O2 | 310.5145 | 32.654 | 31.968 | ||
| E6S7 | Ethyl palmitate | C18H36O2 | 284.4772 | 25.985 | 27.782 | 304.143 |
| Ethyl stearate | C20H40O2 | 312.5304 | 43.961 | 42.782 | ||
| Ethyl oleate | C20H38O2 | 310.5145 | 30.054 | 29.437 | ||
| E7S7 | Ethyl palmitate | C18H36O2 | 284.4772 | 25.927 | 27.727 | 304.226 |
| Ethyl stearate | C20H40O2 | 312.5304 | 47.416 | 46.156 | ||
| Ethyl oleate | C20H38O2 | 310.5145 | 26.658 | 26.118 | ||
| E8S7 | Ethyl palmitate | C18H36O2 | 284.4772 | 29.913 | 31.868 | 303.069 |
| Ethyl stearate | C20H40O2 | 312.5304 | 43.568 | 42.249 | ||
| Ethyl oleate | C20H38O2 | 310.5145 | 26.519 | 25.883 | ||
| E9S7 | Ethyl palmitate | C18H36O2 | 284.4772 | 30.763 | 32.749 | 302.844 |
| Ethyl stearate | C20H40O2 | 312.5304 | 43.861 | 42.502 | ||
| Ethyl oleate | C20H38O2 | 310.5145 | 25.376 | 24.749 | ||
| E10S7 | Ethyl palmitate | C18H36O2 | 284.4772 | 27.426 | 29.291 | 303.817 |
| Ethyl stearate | C20H40O2 | 312.5304 | 47.416 | 46.094 | ||
| Ethyl oleate | C20H38O2 | 310.5145 | 25.158 | 24.615 |
| Experiment: | E1S9 | E2S9 | E3S9 | E4S9 | E6S9 | E5S9 | ||
|---|---|---|---|---|---|---|---|---|
| Chemical | Formula | MW | Area % | |||||
| isobutyl butyrate | C8H16O2 | 144.2114 | 1.293 | 1.698 | ||||
| 4-methyl-2ethyl-pentanol | C8H18O | 130.1800 | 3.237 | |||||
| isobutyl caproate | C10H20O2 | 172.2646 | 1.469 | 4.537 | 4.132 | |||
| butyl heptanoate | C11H22O2 | 186.2912 | 1.567 | |||||
| isobutyl enanthate | C11H22O2 | 186.2912 | 1.133 | 1.765 | ||||
| isobutyl caprylate | C12H24O2 | 200.3178 | 0.067 | 0.119 | 0.735 | 1.472 | 1.523 | |
| 3-methyl-undecane | C12H26 | 170.3300 | 2.958 | |||||
| isobutyl pelargonate | C13H26O2 | 214.3443 | 0.687 | 1.226 | 1.424 | |||
| tridecane | C13H28 | 184.3700 | 1.867 | |||||
| isobutyl caprate | C14H28O2 | 228.3709 | 0.095 | 0.168 | 0.972 | 1.418 | 1.914 | |
| tetradecane | C14H30 | 198.3900 | 1.225 | |||||
| isobutyl undecylenate | C15H30O2 | 242.4030 | 1.798 | 1.555 | ||||
| n-butyl laurate | C16H32O2 | 256.4241 | 1.314 | |||||
| isobutyl laurate | C16H32O2 | 256.4241 | 0.158 | 0.247 | 1.953 | |||
| hexadecane | C16H34 | 226.4100 | 4.342 | |||||
| isobutyl tridecanoate | C17H34O2 | 270.4570 | 1.559 | 4.458 | 4.528 | |||
| isobutyl myristate | C18H36O2 | 284.4772 | 6.065 | 5.477 | 5.019 | 5.016 | 4.010 | 4.211 |
| isobutyl pentadecanoate | C19H38O2 | 298.5038 | 1.291 | 3.106 | 2.918 | 1.131 | 5.148 | 4.165 |
| nonadecane | C19H40 | 268.5100 | 4.981 | |||||
| 2-methyl-octadecane | C19H40 | 268.5100 | 0.874 | |||||
| isobutyl palmitate | C20H40O2 | 312.5304 | 27.119 | 27.507 | 27.652 | 25.951 | 28.426 | 18.358 |
| isobutyl margarate | C21H42O2 | 326.5570 | 4.738 | 6.105 | 5.938 | 5.182 | ||
| 2-methyl-eicosane | C21H44 | 296.6000 | 0.797 | |||||
| isobutyl oleate | C22H42O2 | 338.5677 | 24.679 | 22.784 | 23.499 | |||
| isobutyl stearate | C22H44O2 | 340.5836 | 33.536 | 31.609 | 32.185 | 47.921 | 41.353 | 32.125 |
| Experiment | Chemical | Formula | MW | Area % |
|---|---|---|---|---|
| E1S10 | Butyl myristate | C18H36O2 | 284.4772 | 1.904 |
| Butyl hexadecanoate | C20H40O2 | 312.5304 | 25.549 | |
| 2-Methyl propyl octadecanoate | C22H44O2 | 340.592 | 50.282 | |
| Pentyl octadecenoate | C23H44O2 | 352.5940 | 22.264 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Morales, R.; Verónico-Sánchez, F.J.; Zúñiga-Moreno, A.; González-Vargas, O.A.; Ramírez-Jiménez, E.; Elizalde-Solis, O. Fatty Acid Alkyl Ester Production by One-Step Supercritical Transesterification of Beef Tallow by Using Ethanol, Iso-Butanol, and 1-Butanol. Processes 2023, 11, 742. https://doi.org/10.3390/pr11030742
García-Morales R, Verónico-Sánchez FJ, Zúñiga-Moreno A, González-Vargas OA, Ramírez-Jiménez E, Elizalde-Solis O. Fatty Acid Alkyl Ester Production by One-Step Supercritical Transesterification of Beef Tallow by Using Ethanol, Iso-Butanol, and 1-Butanol. Processes. 2023; 11(3):742. https://doi.org/10.3390/pr11030742
Chicago/Turabian StyleGarcía-Morales, Ricardo, Francisco J. Verónico-Sánchez, Abel Zúñiga-Moreno, Oscar A. González-Vargas, Edgar Ramírez-Jiménez, and Octavio Elizalde-Solis. 2023. "Fatty Acid Alkyl Ester Production by One-Step Supercritical Transesterification of Beef Tallow by Using Ethanol, Iso-Butanol, and 1-Butanol" Processes 11, no. 3: 742. https://doi.org/10.3390/pr11030742
APA StyleGarcía-Morales, R., Verónico-Sánchez, F. J., Zúñiga-Moreno, A., González-Vargas, O. A., Ramírez-Jiménez, E., & Elizalde-Solis, O. (2023). Fatty Acid Alkyl Ester Production by One-Step Supercritical Transesterification of Beef Tallow by Using Ethanol, Iso-Butanol, and 1-Butanol. Processes, 11(3), 742. https://doi.org/10.3390/pr11030742









