Abstract
A sustainable separation concept for large-scale recycling of NdFeB magnets under atmospheric pressure was developed by utilizing a combination of two separation concepts known from the literature: (I) selective pre-separation by in situ chlorination and evaporation of ground oxidized NdFeB material and (II) subsequent distillation for high-purity recovery of all recyclable chlorinated material components, especially its Rare Earth Elements (REEs). Theoretically, simplified estimations of the time conversion curves at 1173 K, 1273 K, and 2000 K of a single particle resulted in the idea of realizing chlorination in some kind of combustion chamber, fluidized bed, or continuous combustion chamber. After chlorination, all non-volatile components, such as REE chlorides, are condensed out of the vapor phase in a single-stage phase separator. For subsequent fine separation by distillation (1292–1982 K for Rare Earth Chlorides and 418–867 K at 2500 kPa for boron and zirconium chloride recovery), simplified simulations were performed in a total-reflux column under ideal phase equilibrium conditions to show the estimated minimum separation effort. Using two composition examples from the literature, high-purity separation of the major Rare Earth Chlorides within a twelve-stage distillation column as a residual heavy boiling product has been demonstrated to be potentially technically feasible.
1. Introduction
Neodymium and dysprosium are important components of NdFeB magnets, which are widely used in vehicle and wind turbine technology. As sufficiently described in [1] and [2], the shares for neodymium, praseodymium, and dysprosium already increased enormously from 2015 to 2020. According to the predictions presented in [1,2,3], the total demand on NdFeB magnets will increase from 54.7 kt in 2020 to 207.6 kt in 2030, with the share of electrical devices remaining almost constant at about 10 kt [2]. Thus, the enormous total increase results from electric vehicles and wind turbines contributing equally. Considering the increasing demand, especially for NdFeB magnets for electric vehicles, and the possible resource shortage of these Rare Earth Elements (REEs), combined with the costly raw material extraction and the environmental problems arising in the mining regions, a sustainable recycling into valuable materials will be of increasing importance in the future. In addition, these raw materials are also essential as valuable materials in every cell phone, computer technology, and almost every electronic device. The intense use of such materials illustrates the great importance of future recycling instead of consuming natural resources.
Recycling methods for Light Rare Earth Elements (LREEs) from NdFeB magnets applicable today are explained in detail in [3,4]. In summary, there is, on the one hand, the possibility of direct mechanical recycling or the application of numerous wet metallurgical or hydrometallurgical separation techniques such as [5,6] low temperature extraction or ion exchange processes with many necessary agents and solvents. On the other hand, dry high-temperature-based pyrometallurgical separation methods can be used for the avoidance of extraction agents or solvents and the reduction in other possibly required chemical agents. Alternatively, in wet-based separation processes, the choice of ionic liquids according to [3] as solvents or the use of a phosphorus-based trichloride ionic liquid in combination with the leaching and stripping separation steps to recover the metallic valuable materials is applicable as a new separation process according to [7]. For this purpose, ball mills can be used to grind the material of NdFeB magnets as fine as possible, according to [7]. Other combination processes for separation by leaching, as well as dissolution of metals and subsequent precipitation of the sought components, are also listed in [8], whose separation processes were developed in the 1990s. The selective separation of REEs from NdFeB magnetic material by utilizing the volatilizing chlorination of non-REE material with simultaneous selective evaporation has already been experimentally investigated in [9]. This process, which combines thermal and physical separation, provides good separation of up to 95 mole% of the components, as shown by previous studies on chlorination and volatilization in a two-stage combustion chamber in [10] and further developments as in [11,12,13], or [14] (using NH4Cl). In contrast, purities of 99% are required for the recycling and reuse of neodymium for the production of NdFeB magnets [15]. However, in general, marketable and recycled neodymium would be available on the market with purities ranging from 95 mass% to 99.999% [15,16], thus REEs should be recovered within these purity requirements.
The main objective of this work is to achieve high-purity recovery of mixtures containing reusable REEs from NdFeB magnets by using distillation as an option for fine separation. In this separation process route, the raw materials from the magnets could also be recycled on an industrial scale without using solvents and auxiliary materials. Based on the process already developed in [9] for the separation of rare earths by chlorination and selective evaporation of non-rare earth components, the novelty of this work is to perform chlorination in a more industrial way by chlorination of fly ash in a kind of combustion chamber and subsequent distillation as a separation process with a higher separation accuracy. Even the chlorine component can be recovered after combustion with atmospheric oxygen. Chlorination is used to lower the boiling points for the application of distillation-based separation processes of the contaminated non-REEs substance components from the raw material to be recycled. The original idea for the distillation of metal chlorides in this work is based on the Kroll process, which is used to extract titanium from titanium ores by chlorination and subsequent distillation (see: report of investigations on the pilot plant in [17]). The distillative separation process was developed by William Kroll of Luxembourg in the 1930s–1940s as an alternative to the environmentally harmful sulfate process, which is based on the thermal decomposition of titanium ores with sulfuric acid at equally high temperatures [17,18]. The developed, more economical and far more environmentally friendly separation process based on distillation, therefore, still represents one of the most important processes for raw material extraction today. Similar to the Kroll process, chlorination and distillation were also successfully used for recycling metal alloys such as niobium–tantalum alloys, as described in [19]. In this paper, an effective separation concept extended to distillation is demonstrated and ideally simulated, which provides high purity separation results without the need for solvents, extractants, and auxiliary materials in compact separation apparatus.
2. Proposed Separation Concept and Calculation Procedure
A separation concept based on chlorination, idealized flash evaporation, condensation, and distillation in distillation columns was simulated using self-developed simplified models implemented in Octave (analogous to MATLAB, a freely available software tool for solving mathematical problems) under ideal phase equilibrium conditions. The conversion–time curves in the chlorination have been calculated using the shrinking core models of spherical solid particles with different porosities [20]. According to [20], the total chlorination time could be determined from the film and pore diffusion of the chlorinating agent into the material and the reaction time in a modified shrinking core model from [20,21] as a function of the conversion.
The diffusion coefficients in the gas phase were estimated by the methods described in [20] or given as experimental transport property data, e.g., from [22,23], for the diffusion simplified assumed in air. The kinetic data for chlorination were given in [24] for oxidation, in [25] for chlorination of Nd2O3 by elemental chlorine, and in [26,27,28] for the chlorination of Fe2O3. The diffusion of the chlorides out of the material was neglected. Carbon tetrachloride or a mixture of carbon and elemental chlorine could be used as the chlorinating agent for the chlorination of the NdFeB oxide material, which was injected into the combustion chamber.
The chlorination step was followed by a selective preseparation unit following the work of [9,10], but using a pressure relaxation-cooling step, instead, for the preseparation of more volatile chlorides, such as boron chloride, zirconium tetrachloride from the REE chlorides, and other dichloride components. This separation step was calculated as an isothermal flash evaporation using the equations from [29] (p. 13–25f).
Subsequently, the two separated fractions were distilled in separate distillation columns at different pressures (see [29] p. 13–35f “approximate multicomponent distillation methods”). In the distillation of the light boilers, such as boron trichloride and zirconium tetrachloride, higher pressures up to 2500 kPa were necessary so that all chlorides involved could have a liquid phase as a pure substance component. In this fraction, REEs are no longer present, but zirconium and other raw materials contained therein were recovered.
The remaining heavy boiling molten salt fraction contains the REEs to be recycled as chlorides, which were to be obtained as the remaining heavy boiling distillation products. In this process, the distillation was carried out under high temperatures but at normal pressures of 101.325 kPa. For the simulative estimation, a total reflux equilibrium stage column was modeled in Octave, where the entire distillation product was fed back to the column (see: [29] p. 13–39f “rigorous methods for multicomponent distillation-type separations” for modeling) is simulated. In addition, only ideal enthalpy changes were used for the expression of the energy balance for each separation stage, which were expressed in simplified form with the integral over the heat capacities. The vapor–liquid equilibrium was estimated ideally according to Raoult’s and Dalton’s law (see [29] p. 13–10f), in which the activity coefficients always had the value γ_i = 1, because no measured VLE data were available for the chlorinated NdFeB-magnet example. For low pressures, the generalized vapor–liquid equilibrium condition was (with x, y liquid, vapor mole fraction, p total pressure, pure component vapor pressure in VLE-operation, activity coefficient each of substance component i):
The set of equations of each separation stage into a total-reflux column consists only of the ideal phase equilibrium relation equation from Raoult and Dalton’s law and the energy balance each separation stage, whereby only the mixture is cooled down as a saturated liquid at the condenser. Due to the total-reflux condition, the evaporated fraction and total mass should be the same as from the condensation stage. Furthermore, because of the total-reflux condition, heating and cooling power was equal (without considering heat losses to the environment). The cooling capacity in the condenser is calculated by means of the energy balance. Thus, the calculation of the mass in the Octave model was performed with the help of the energy balance in each column stage, while the calculation of the mole fraction was performed independently with equilibrium relation equations and a sum-rate condition for the sum of all mole fraction values. For this, required vapor pressure data, heat capacities, and other necessary substance properties for simulation were available in [30,31], as well as from numerous other literature references.
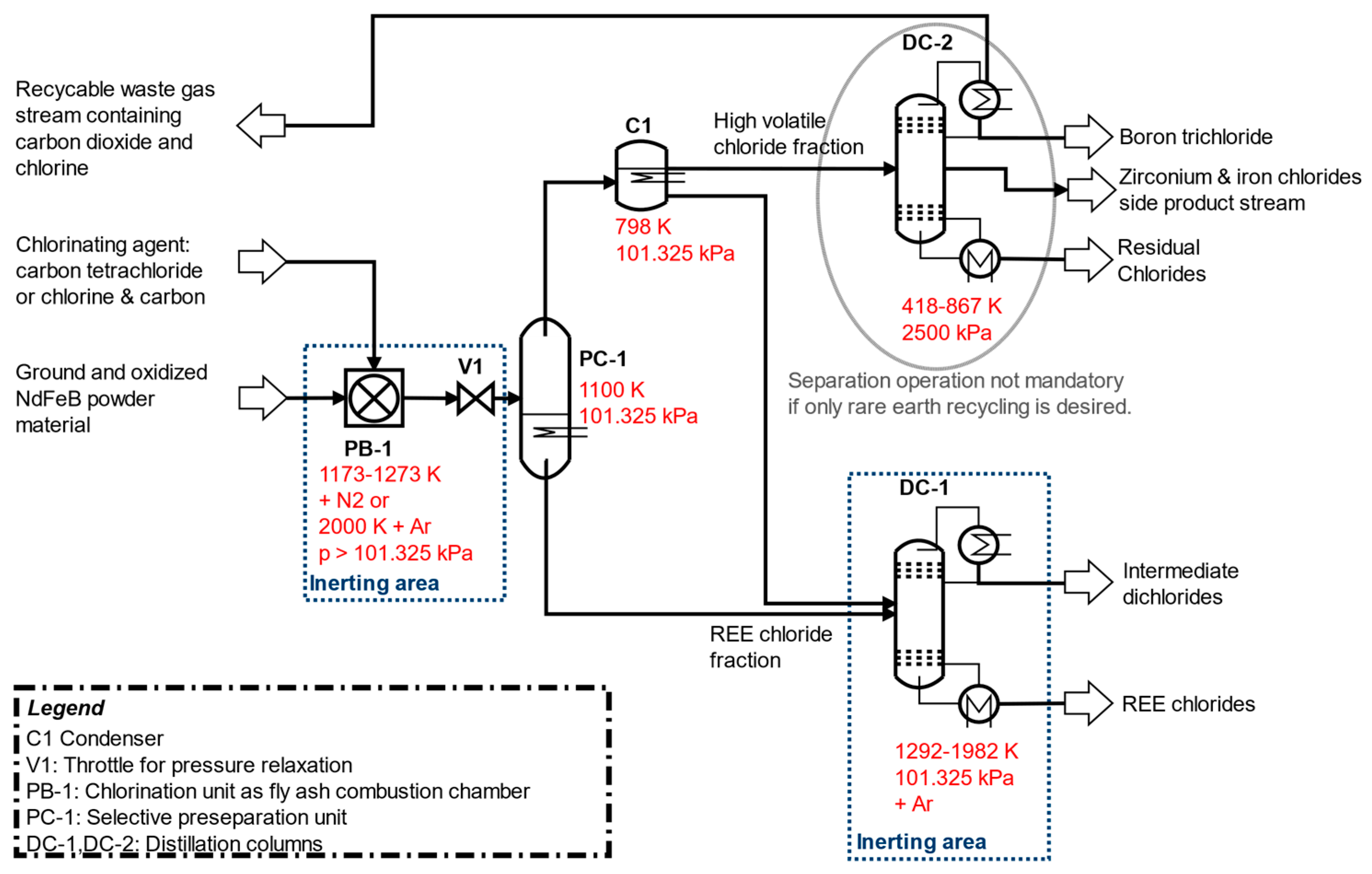
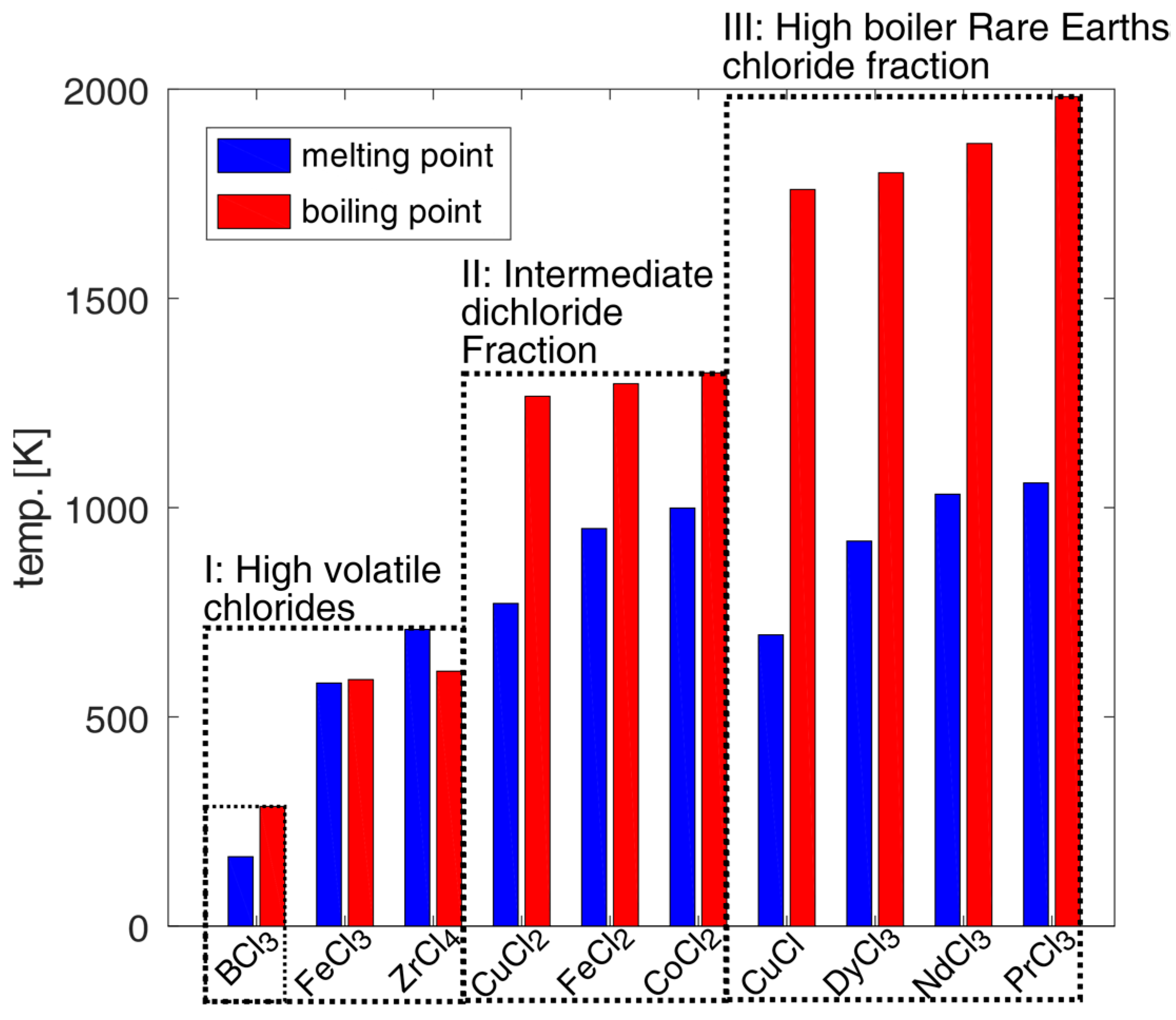
The developed separation process based on distillation designed under the simplified assumption of idealized liquid–vapor equilibrium conditions is shown in Figure 1. The separation concept could be better understood by considering the melting and boiling points in Figure 2 of the pure chlorides involved. Chlorination and selective vapor separation according to [9,10] has achieved a rough separation of other chlorides from a fraction of heavy boiling REE chlorides. This process could be refined by distillation of the fractions in Figure 2.
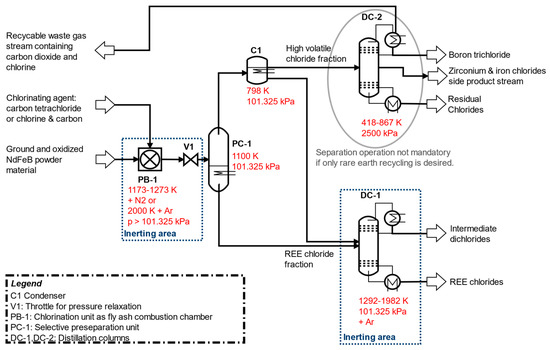
Figure 1.
Recommended separation process for distillation-based recovery of REEs and other raw materials (including boron and zirconium recovery in DC-2) from ground NdFeB magnet material. (1 atm = 101.325 kPa).
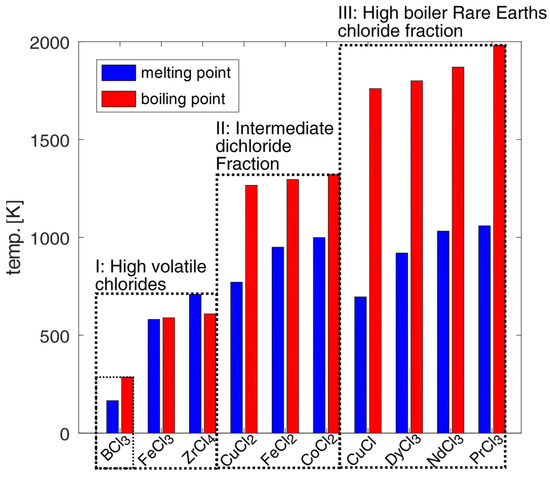
Figure 2.
Melting and boiling points of involved chloride components from fully chlorinated NdFeB magnet material based on the composition examples of [9,10,24].
In PB-1, the chlorination of ground and fully oxidized NdFeB magnetic material takes place in the combustion chamber among three possible technical chlorination designs. The first possibility is the experimental process idea of [9,10] due to chlorination and selective separation of volatiles at 1173 K to 1273 K in the N2-Cl2 vapor carrier gas from roasted coarsely crushed NdFeB material as described in [10], but with the selective removal of only one single volatile fraction of boron, zirconium, and iron chlorides with minor impurities of other chlorides into the vapor fraction. This is consistent with the combined consideration of PB-1 and PC-1 in Figure 1. During this procedure, the REE residues remain with possible impurities of dichlorides, such as cobalt dichloride.
Another dry technical chlorination option alternative could be the realization of a combustion chamber for chlorination, analogous to the technical design of a synthesis gas production according to the Winkler process from pulverized coal (compare: [32]). In this technical modification, there are two detailed potential separation options for the chlorination of NdFeB magnet material: Chlorination at temperatures around 1173–1273 K, but longer reaction times up to two hours or chlorination at very high temperatures of 2000 K under significantly lower reaction times in the range of minutes with almost complete conversion as estimated within the shrinking core model. Cooling and deposition in PC-1 of the still-contaminated REEs occurs at 1100 K. At this temperature, the chlorides of the REEs are still present in the vapor stream at a maximum of only few ppm according to the following calculations.
In all variants, other chlorides are present in the separated vapor from PC-1, which must be almost completely separated by condensation in C1 and further purification in the DC-1 distillation column. In DC-1, the fine separation of the REEs as trichlorides takes place to the high purity heavy boiler product. In addition, dichlorides of iron, cobalt, and copper can be obtained as a high-boiling distillation product according to following simulation results.
The purified vapors from C1 no longer contained rare earth components. However, by means of distillation in DC-2, a recovery of high-purity boron (as boron trichloride) and a zirconium recovery (as zirconium tetrachloride) with larger impurities of other dichloride components could be achieved, but only at process pressures of 2500 kPa. With the aid of a partial condenser, the waste gas (chlorine and carbon dioxide) could be obtained.
3. Results and Discussion on the Potential Technical Feasibility
For the process simulation, a plant with a realistic 25 kt/a was assumed. High-temperature operations require the inertization of some process steps in Figure 1. Here, nitrogen can be used without a reaction up to 1100 K, but at higher temperatures argon is a better inerting agent. Inerting serves to strictly exclude reactive oxygen and moisture from the molten salt inside the separation units.
3.1. Chlorination
The reduction of the required chlorination time plays a significant role not only in the separation process but also in an entire large-scale plant to recycle the largest possible batches of NdFeB magnets. Therefore, estimates of chlorination times and high product yields were crucial where only conversion rates were calculated. For simplified calculations using the shrinking-core model, the components of neodymium and iron from [9] were used for ordinary NdFeB-Dy magnets by neglecting all other components that were below 1.05 mass%.
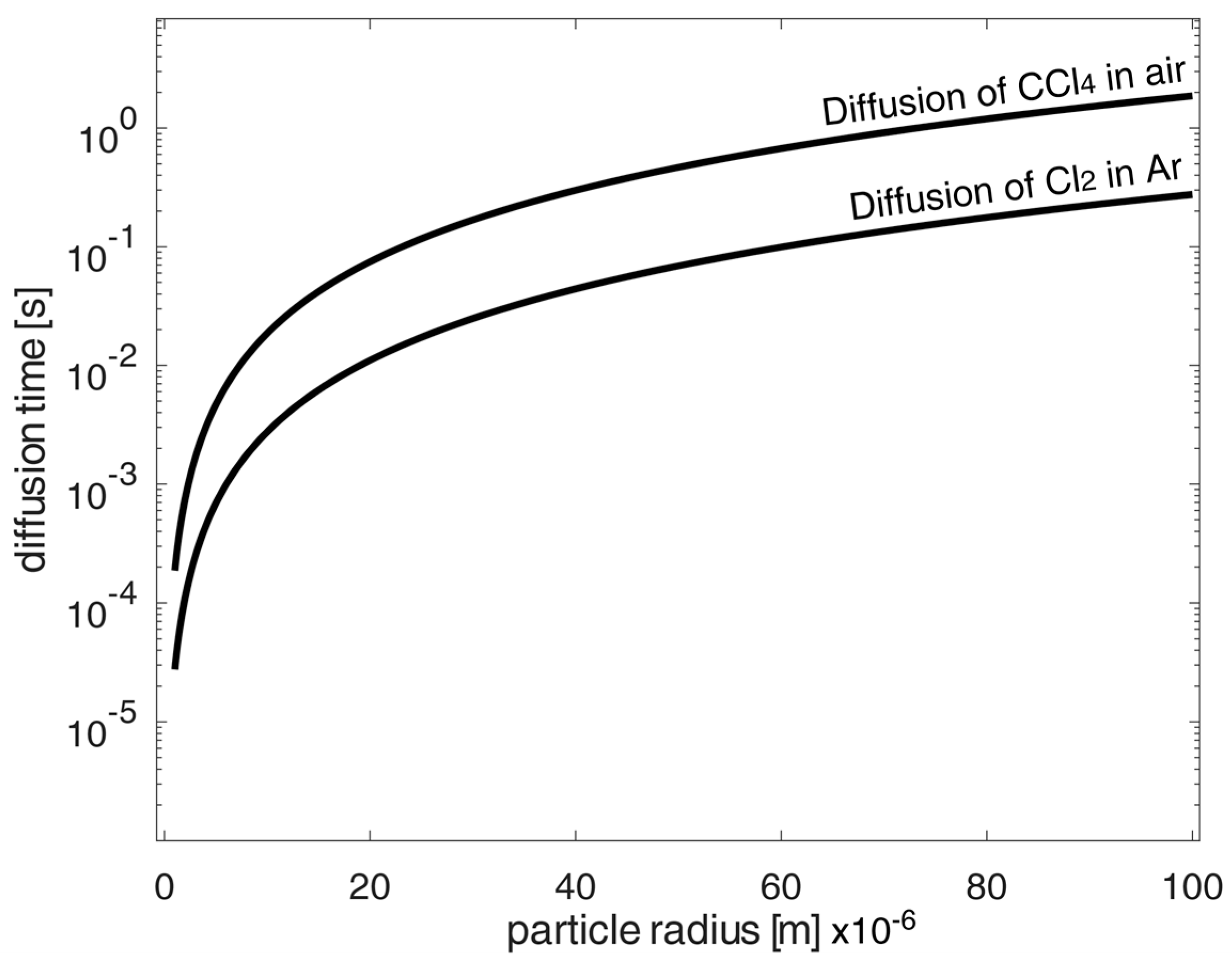
According to the calculation of the required total chlorination time, it was shown for the diffusion time excerpt of the in-air carbon tetrachloride, as well as for elementary chlorine in argon, that the diffusion time alone is negligibly small in contrast to the reaction time. Here, the diffusion coefficient of carbon tetrachloride in air was given in [22], whereas diffusion coefficients of chlorine or carbon tetrachloride in argon were estimated only by Fuller’s equation from [33] (pp. 11.10–11.11). Figure 3 shows the calculated diffusion times of the chlorinating agents carbon tetrachloride in air and chlorine in elemental argon. It can be seen that these diffusion times were small compared to the calculated reaction times as determined for low porosity values according to Figure 4 and Figure 5. When comparing diffusion and reaction, the ratio is 720–43,200 as the ratio of reaction time to diffusion time. Thus, the diffusion time is negligibly small in the calculations.
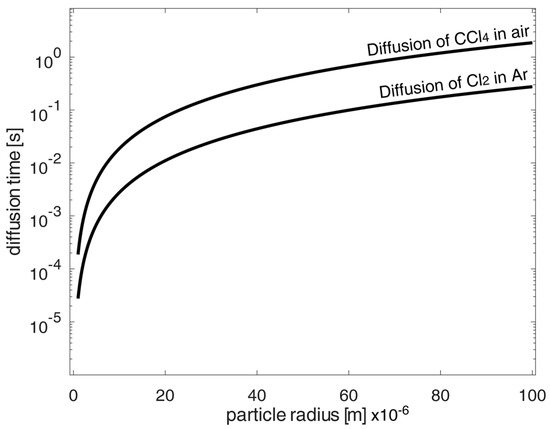
Figure 3.
Exemplary calculated diffusion time form the shrinking core model of spherical oxidized NdFeB magnetic material particle at high porosities p to 95 mole% under chlorination conversion rate of 100%.
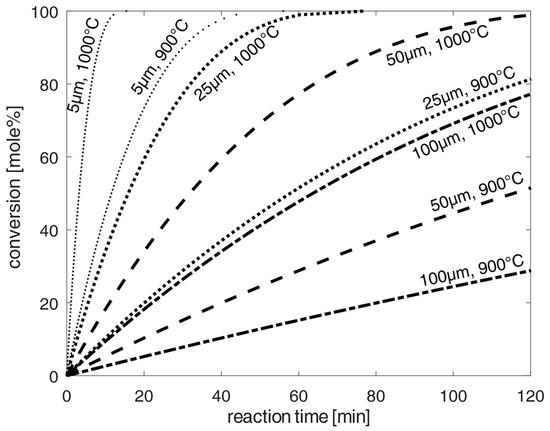
Figure 4.
Estimation of the progress of chlorination of spherical oxidized NdFeB-magnet material particles at 1173–1273 K according to the shrinking-core model [20] and the kinetic data from [25,26,27,28,34], and diffusion according to [22] for diffusion in air. Uncertainties of calculated values are mainly caused by uncertainties of kinetic constants and activation energies given in the literature. The variation in these parameters results in the average uncertainty of 25%, slightly depending on the reaction temperature.
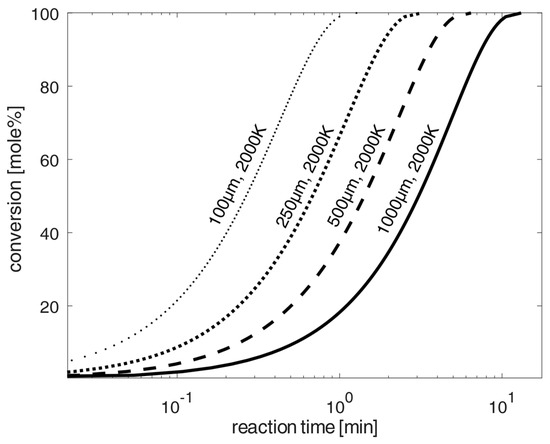
Figure 5.
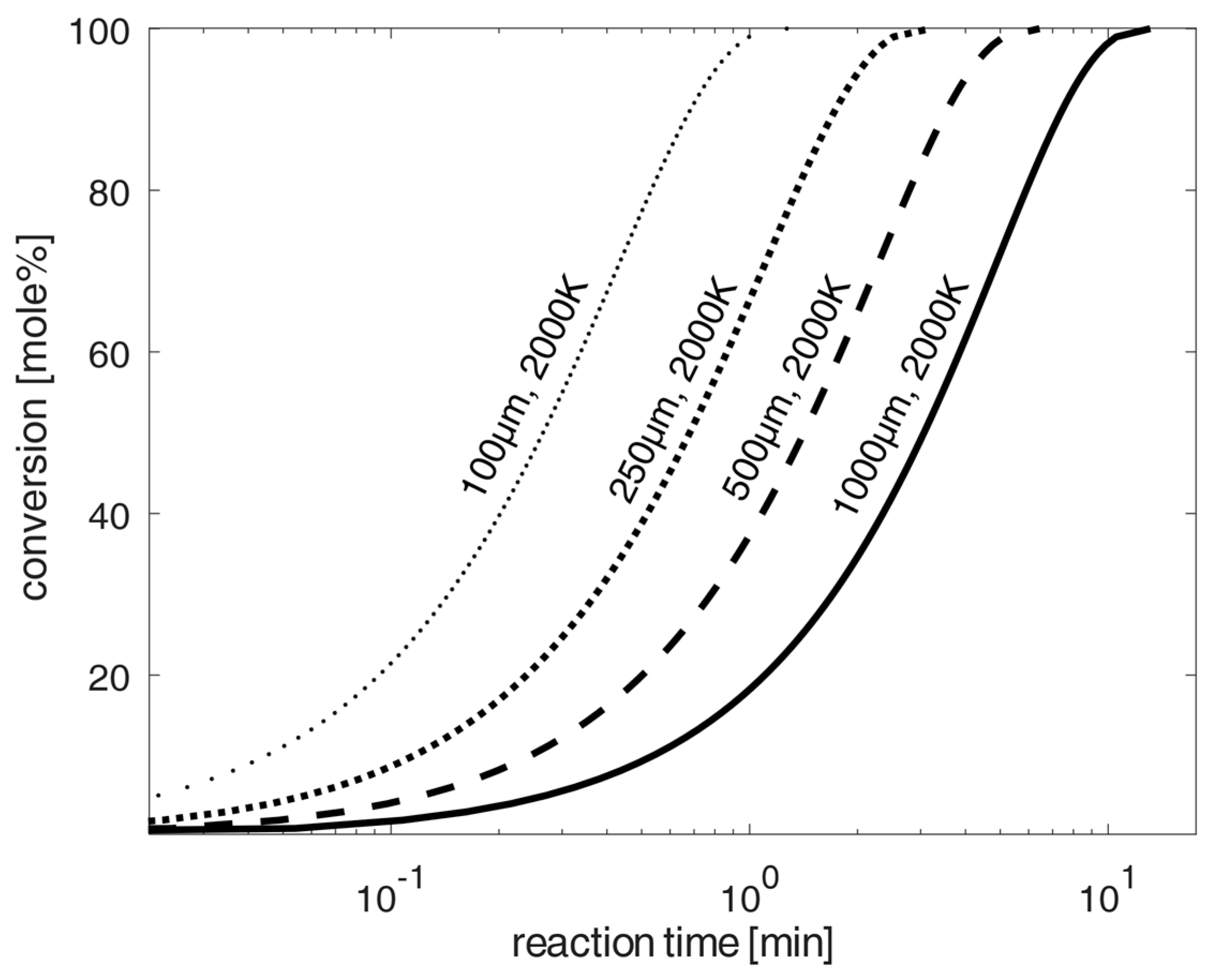
Schemes follow the same formatting. Estimation of the progress of chlorination of spherical oxidized NdFeB-magnet material particles at 2000 K according to the shrinking-core model [20] and the kinetic data from [4,24,26,27,28], and diffusion according to [22] for diffusion in air. Uncertainties of calculated values are mainly caused by uncertainties of kinetic constants and activation energies given in the literature. The variation in these parameters results in the average uncertainty of 15%.
The reaction time was determined from the following highly simplified reaction network for the diffusion of carbon tetrachloride and the available kinetic data from [25,26,27,28,31]:
CCl4 → C + 2Cl2,
Fe2O3 + 3Cl2 → 2FeCl3 + 3/2O2,
Nd2O3 + 3Cl2 → 2NdCl3 + 3/2O2,
C + O2 → CO2
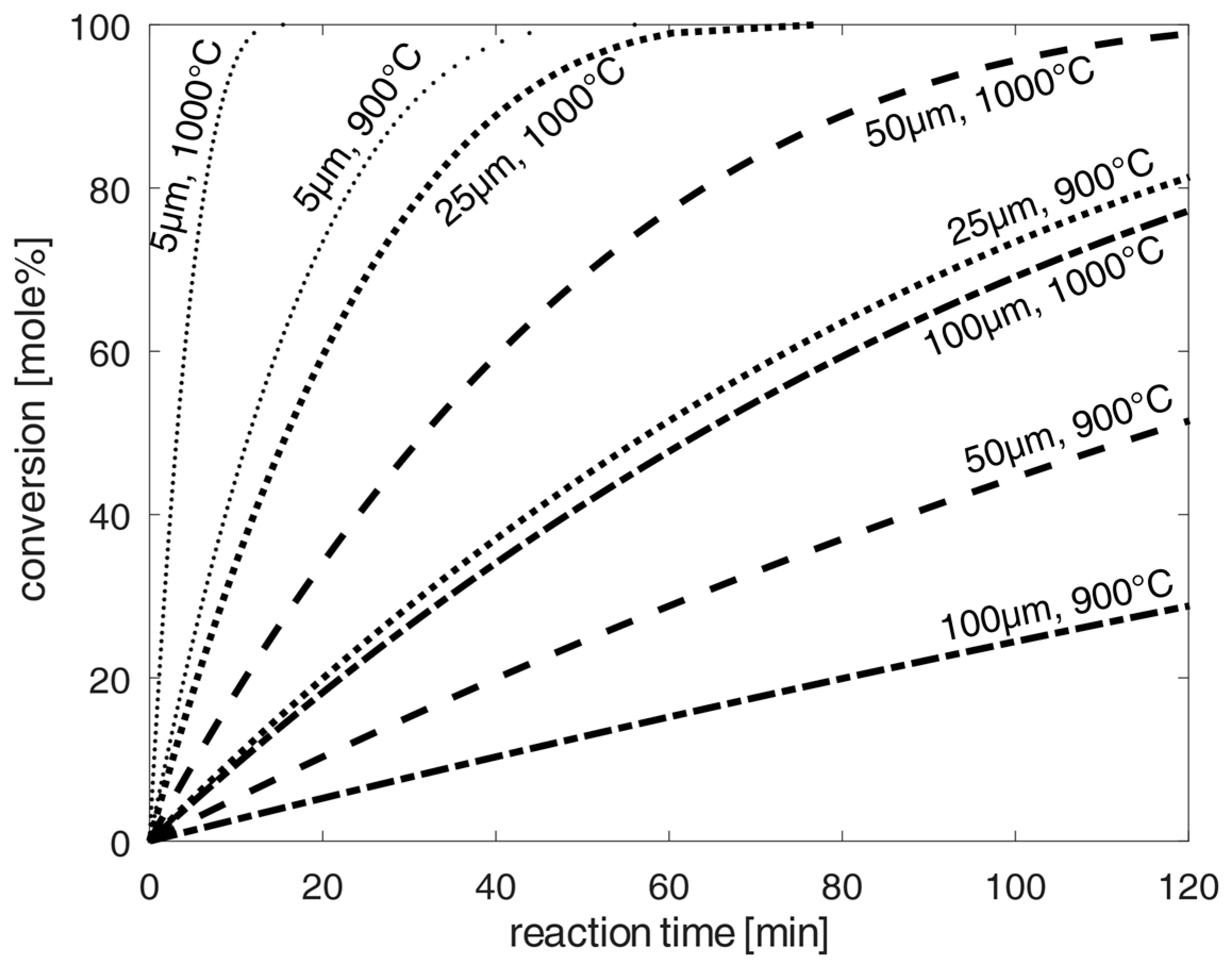
Due to the significantly higher calculated reaction times compared to the diffusion times, diffusion played a negligible role for the calculation of the total time for chlorination. Estimation of the chlorination of spherical oxidized NdFeB-magnet dust particles at 1173–1273 K according to the shrinking-core model [20] is presented in Figure 4. Analogous to [9], the calculated chlorination time at 1173–1273 K ranged from 120–160 min to reach the highest conversion rates of up to 100 mole%. Finer grinding increased the surface-to-volume ratio. However, for half-sized 50 µm dust particles, the total chlorination time at 1173 K was still 120 min. To achieve very low chlorination times at high conversions, very fine grinding would be required. Thus, temperatures of 1273 K and 5 µm grind size would be required to move into the range of a few minutes. However, such grinding size distributions with small particles in the range of 5–25 µm would be very difficult to manufacture, for example, in ball mills, as used in [7] for particle sizes of about 125 µm. Therefore, minimal particle sizes of 25–100 µm in terms of grinding effort and a high conversion rate of up to 100% has been optimized under these chlorination conditions at 1173–1273 K. Such chlorination could, for example, be technically implemented on an industrial scale by means of parallel-installable closed combustion chambers with the circulation of the dust particles by chlorine or initial carbon tetrachloride gas after subsequent thermal decomposition at higher temperatures up to 1223 K, according to the stability limit of carbon tetrachloride at 1268 K (see: [31]).
In order to significantly reduce the required chlorination time, a high temperature chlorination variant with chlorine at 2000 K is being investigated using much larger particles for chlorination. For this purpose, Figure 5 shows the application of high temperatures at 2000 K, as can usually be achieved in industrial burners. This shows, according to the calculations of the shrinking core model, that the total chlorination times are significantly reduced to maximums of 10 min (for 1 mm particles), achieving high conversion-rates of up to 100% for the chlorination of the oxidized NdFeB material. For particle sizes in direction to 100 µm, the required total chlorination time is only about one minute. The total time will be reduced below 100 µm in a logarithmic scale in the same expected reduced particle size sizes from the 1 mm to 100 µm reduction. A technically possible implementation of chlorination could be chlorination in a continuously operated combustion chamber with the recirculation of unchlorinated material and particle sizes below 100 µm, analogous to the Winkler process, for synthesis gas production according to [32], or a type of continuously operated fly ash combustion chamber under very small particle sizes. On the other hand, a fluidized bed process with the injection of chlorine under prior mixing with carbon could also be technically applicable for greater particle sizes up to 1 mm.
For the process variant in [9], a technical implementation according to [10] is also conceivable by the parallelization of each chlorination reactor, on the basis of the results for the required chlorination time, for extrapolation on a larger industrial scale.
3.2. Preseparation of Volatile Components at 1 atm (101.325 kPa)
In order to separate the volatile chlorides (boron trichloride, iron(III) chloride, zirconium tetrachloride), the selective separation through subsequent condensation of remaining non-volatile chlorides is necessary. Table 1 gives the composition of chlorinated NdFeB magnet components with respect to their original contributions according to [9] and [24].

Table 1.
Different feed compositions in mole% of complete chlorinated NdFeB magnets.
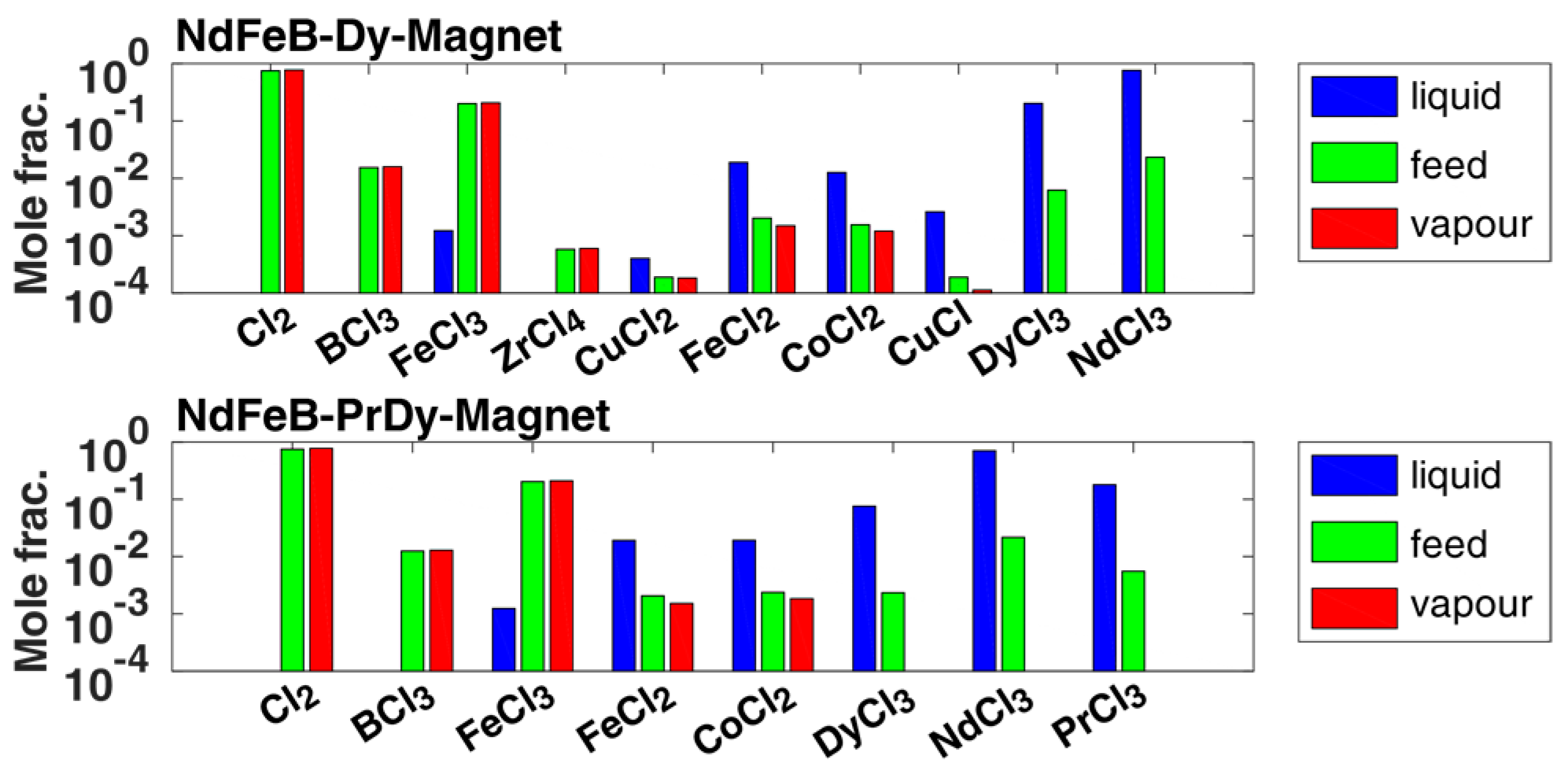
The first separation operation of volatile chlorides in PC-1 was simulated according to the flow diagram in Figure 6, simplified as isothermal flash evaporation calculated with the composition examples from Table 1. The results are summarized in Figure 6 (as the crucial separation takes place in different interesting orders of magnitude in composition values and moves down to very small separation accuracies, a logarithmic y-axis representation is shown in all distillation-based simulation results) for both separation examples, where the following excess amount of chlorine was also assumed in the feed composition.
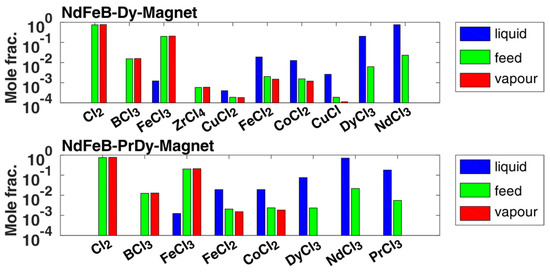
Figure 6.
First pre-separation by cooling and pressure release, simplified and calculated as isothermal flash evaporation, as explained in [28]: Upper part: Composition from [9] for the generalized NdFeB-Dy magnet example (first feed example given in [9]) within a maximum deviation of a component of 0.8% lower limit and 1.3% upper limit. The components neodymium and dysprosium trichloride show the highest deviation values. Lower part: Composition from [24] of the first given NdFeB-DyPr magnet example within a maximum deviation of a component of 0.9% lower limit and 1.2% upper limit. The component neodymium trichloride shows the highest deviation values.
The results show a high-pure separation of boron and zirconium from the feed into the vapor phase or vapor phase transport with excess chlorine with a separation accuracy of 99.99 mole% in both separation examples. Only a small amount of iron(III) chloride, up to 0.1 mole%, condenses out in the liquid fraction. Other non-volatile chlorides such as cobalt or iron(II) chloride in the range of 0.1 mole% were also obtained in the vapor phase. Those and other impurities were completely removed from the vapor phase by a second condensation step in C1, according to Figure 1, at temperatures of 798 K with a separation accuracy of 99.99 mole%, to ensure only the volatile chlorides were obtained. The rare earth trichlorides were so low in volatility that their composition values in the liquid fraction were even below 1 × 10−4 mole%, i.e., at most only available in the ppm range with respect to the mass fractions (in PC-1). Thus, the separation of the volatile chlorides could be performed successfully assuming the ideal phase equilibrium according to Equation (1), with .
3.3. Distillation for REE Purification at 1 atm (101.325 kPa)
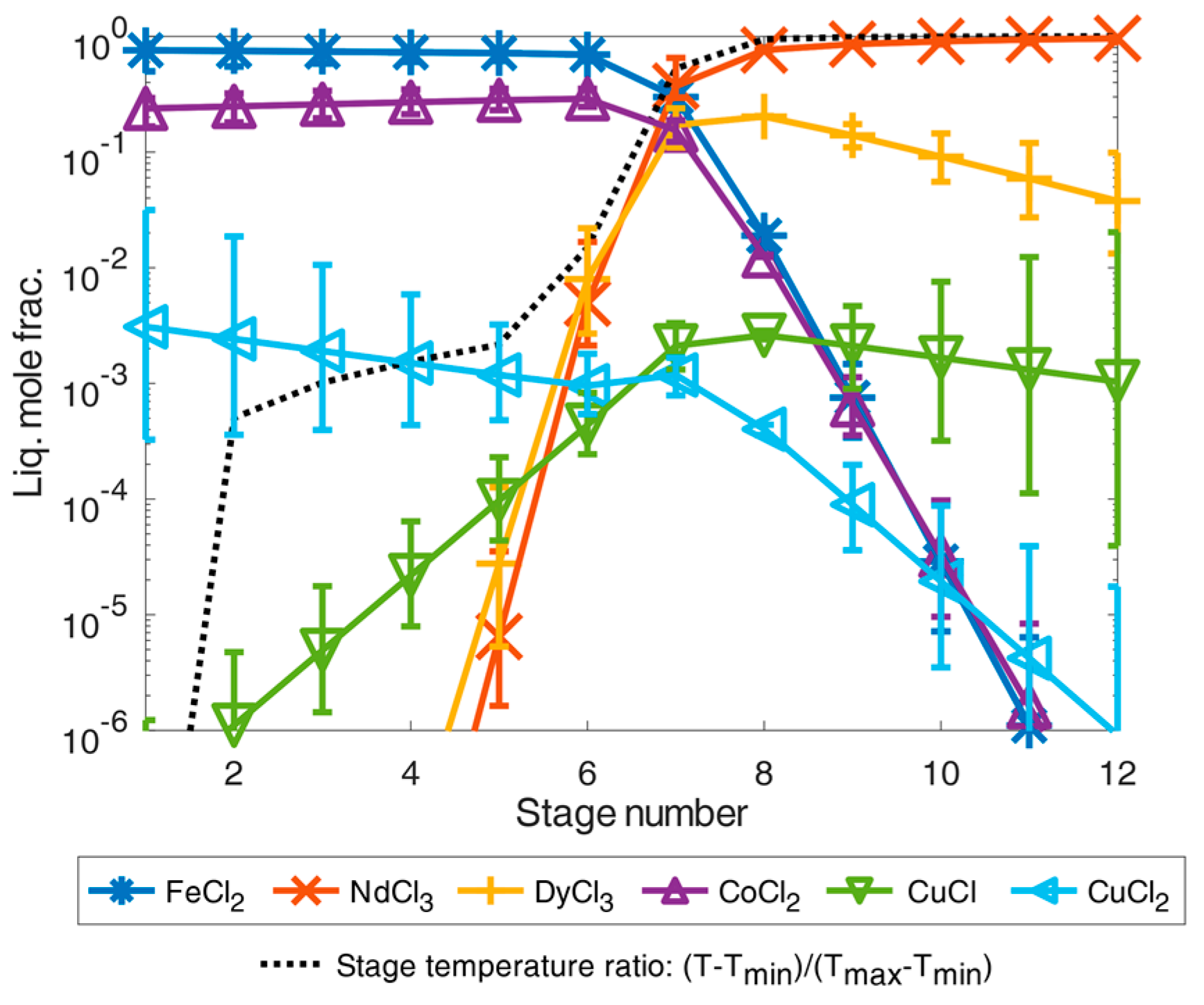
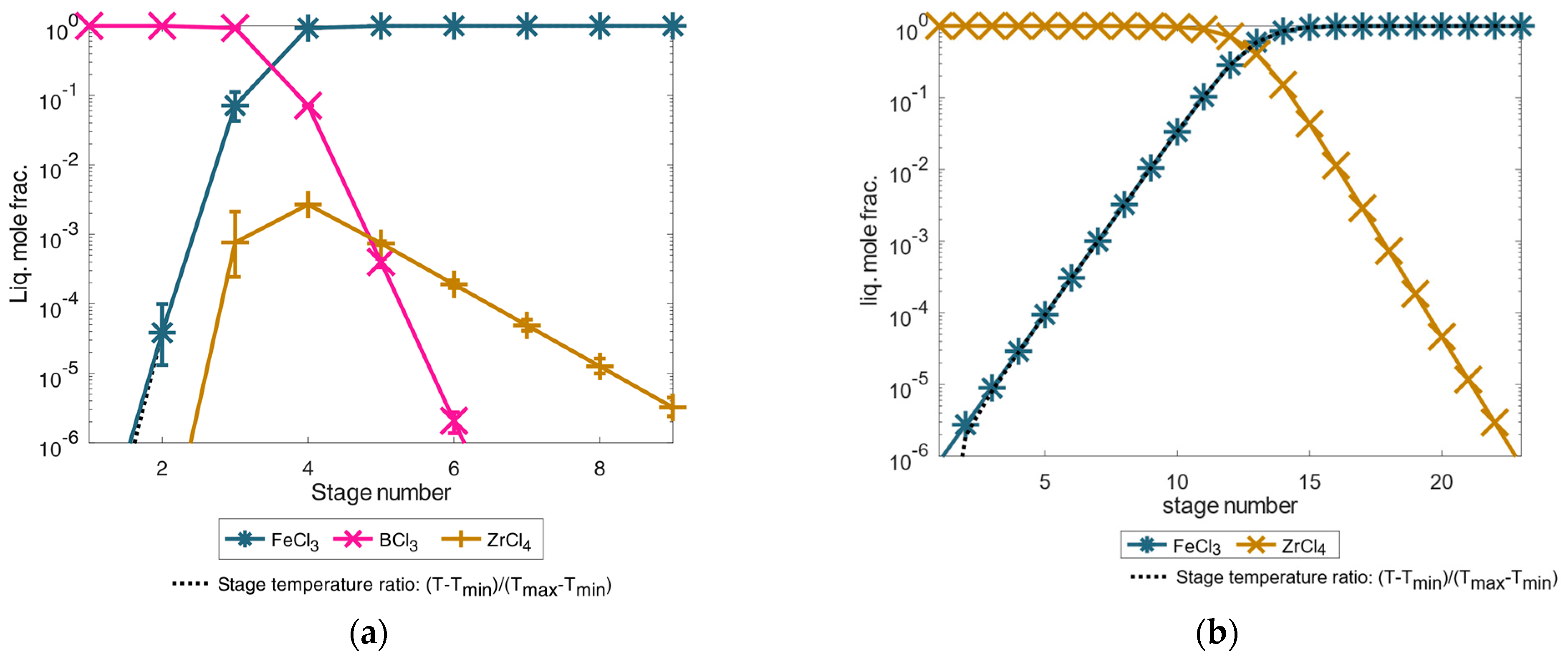
From the separation results of the volatile chlorides as shown in Figure 6, the REEs remained in the liquid as low volatile trichlorides together with other dichloride components presented in Figure 2. Before the simulation, a twelve-stage separation column was given, with a predominant feed composition at the eighth stage as a further simplifying assumption. On the feed example according to [9], the simulation results of the total-reflux column are shown in Figure 7. In both separation examples, the dichlorides of iron(II) chloride, cobalt dichloride, and the possible small contribution of copper(II) chloride accumulated as high boiler components. They were highly purified from the other chlorides and REE chlorides in the first separation stage(s) with very high separation accuracies of 99.9999 mole% (contamination far below 1 × 10−4 mole%). From the sixth separation stage onwards, the REE chlorides were present in a concentration just above 1 × 10−2 mole%, and the fraction value increased with the increasing separation stage number. Thus, even under equilibrium conditions, the boiling temperature of the mixture must increase significantly from the sixth separation stage as shown by the defined temperature fraction curve between minimum and maximum temperature in the column with and . From the eighth separation stage on, the neodymium trichloride boiling fraction then dominated in the total-reflux column model, corresponding to the stage temperature ratio close to the boiling temperature 1870 K of the neodymium trichloride (see Figure 7). Analogous to the increase in the separation curves of the rare earth trichlorides, the separation curves of the iron(II) and cobalt dichloride components as light boiling components decreased analogously. It can still be observed that copper(I) chloride tends to concentrate in the heavy boiling range in the column in contrast to copper(II) chloride, if these chlorides are simplified and assumed to be thermally stable. Regarding the achievable separation accuracy for the neodymium trichloride as a heavy boiling distillation product, it can be concluded that only a mixture with dysprosium was achievable by distillation. Impurities of copper via assumed copper(I) chloride was only approx. 0.1 mole%, meaning that a mixture of neodymium trichloride and dysprosium trichloride of 99.959 mass% could be achieved by distillation separation which exceeds the necessary industrial purity of 99 mass% [15,16].
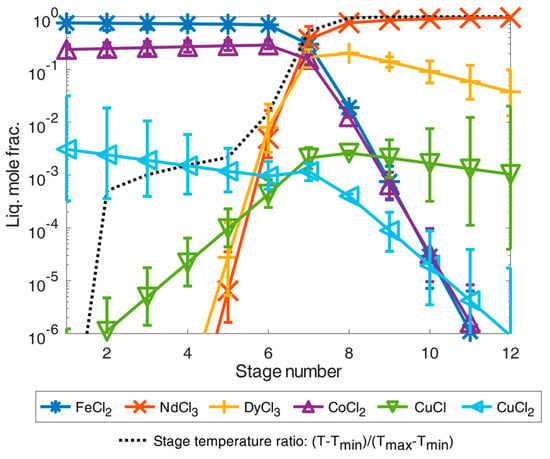
Figure 7.
Illustration of separation of high-purity REEs by distillation simulated in an exemplary total-reflux column under simplified idealized phase equilibrium conditions according to Equation (1) for the feed composition case from [9]. The error bars show the results for a deviation of the calculated temperature of ±5%.
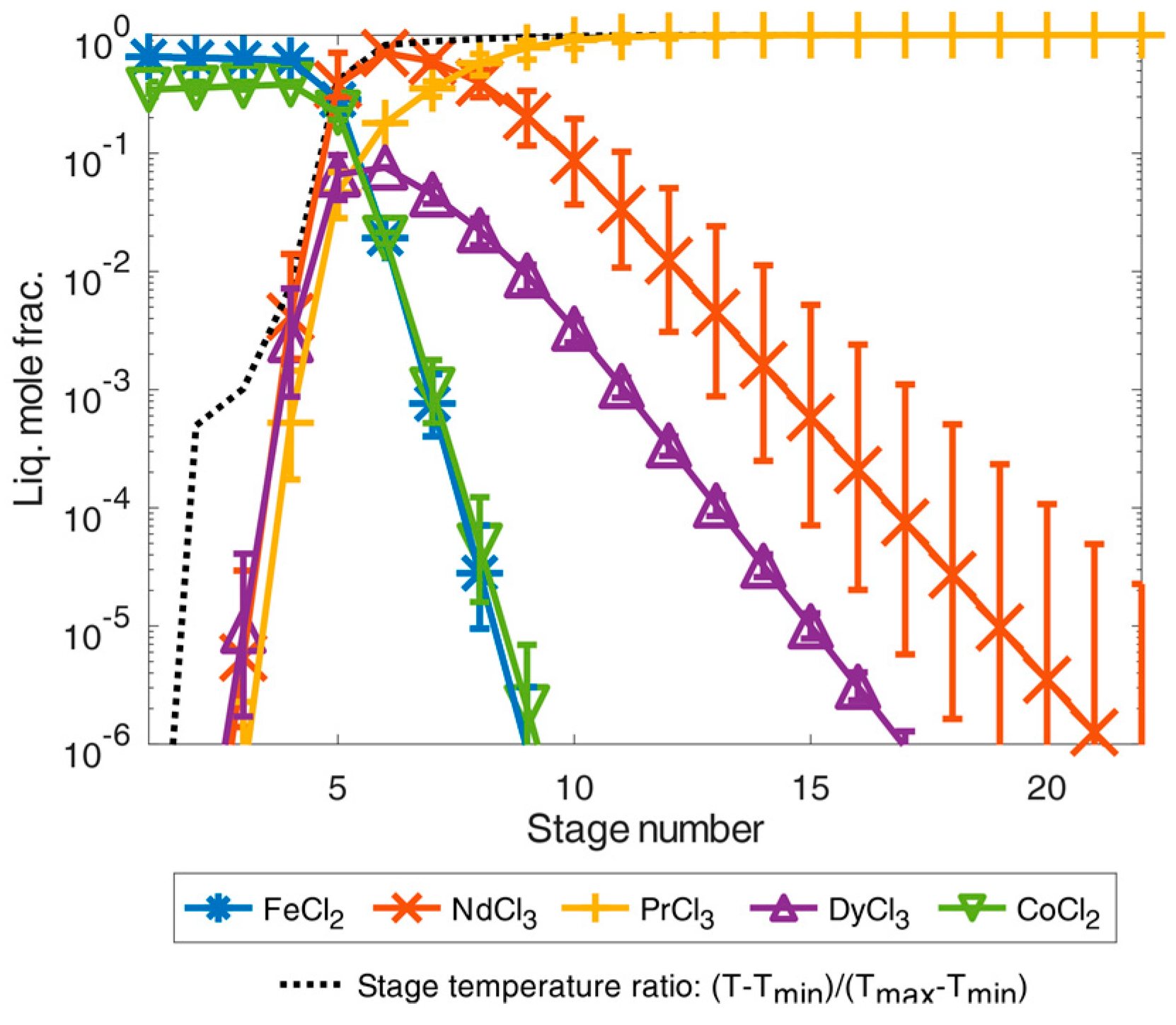
If the feed example according to [24] is used for the simulation instead of [9], considerably more separation stages are required for additional praseodymium trichloride purification as shown in the simulation results in Figure 8.
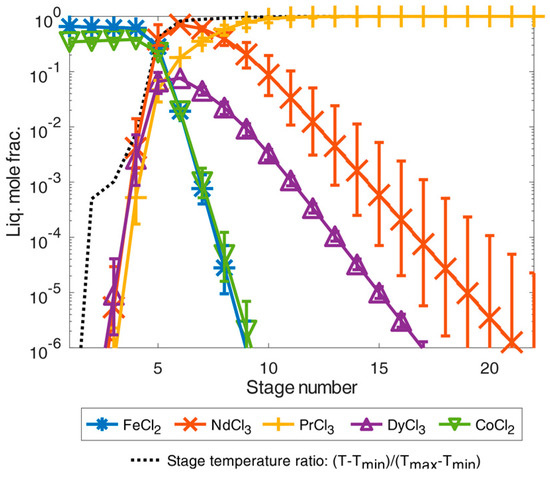
Figure 8.
Illustration of separation of high-purity REEs by distillation simulated in an exemplary total-reflux column under simplified idealized phase equilibrium conditions according to Equation (1) for the feed composition case from [24]. The error bars show the results for a deviation of the calculated temperature of ±5%.
A mixture of iron(II) and cobalt dichloride accumulated in the first separation stage of the simulated feed example from [24]. From the fourth stage onward, the proportion of REEs consisting of neodymium, dysprosium, and praseodymium trichloride increased significantly as the number of separation stages increased and as the temperature also rose. From the tenth separation stage onwards, a mixture of all trichlorides could already be obtained by distillation with a high purity of 99.9999 mole% for REE trichlorides. By further distillation with a temperature close to the boiling temperature of praseodymium trichloride, the proportion of neodymium trichloride and dysprosium trichloride could then be decreased from the separation stages 10–22 to obtain high-pure praseodymium trichloride in the last separation stages. Thus, high-pure praseodymium trichloride could be achieved in the 22nd separation stage with purities far below 99.9999 mole%.
For the technical implementation, besides the high temperatures required in the column, the large temperature differences between the column separation stages might be a technical problem. Therefore, it is recommended to realize the distillation in a fractionated distillation column operating in a discontinuous manner, in which the separation fractions are carried out at intervals, starting with the most volatile chloride fraction. After separation of the fractions, the REE chlorides remain in the distillation column as a high-boiling residual fraction.
3.4. Zirconium and Boron Purification Using High-Pressure Distillation (High-Volatile Separation at 2500 kPa)
Distillative separation of the volatile components in the DC-2 separation unit at higher pressures of 2500 kPa essentially deals with the substance separation problem of mixtures of boron trichloride, iron(III) chloride, zirconium tetrachloride, and low impurity contents of copper dichloride. All other residual less volatile chlorides have been previously separated in the C1 condensation unit.
Figure 9 shows the Octave simulation results of the total-reflux column at 2500 kPa. Here, the first figure (separation of boron trichloride and iron(III) chloride) shows the high purity boron trichloride-iron(III) chloride separation to achieve the separation accuracy of 99.9999 mole% in a ten-stage separation column. In contrast, the second figure below (separation based on purification of zirconium tetrachloride) shows the separation result of high pure zirconium tetrachloride-based separation with a separation accuracy of 99.9999 mole%, neglecting the boron trichloride component. The simulation results show the potential high-purity separability of boron trichloride and the more difficult separation of zirconium tetrachloride as a boiling intermediate in a distillation column at 2500 kPa. In addition, the separation result for a boron trichloride- and zirconium tetrachloride-based distillation shown here does not influence the good separation result for the distillative recycling of REEs in the separation column DC-1.
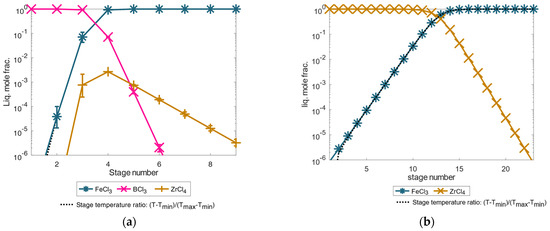
Figure 9.
Illustration of high-purity boron trichloride (a) and zirconium tetrachloride (b) separation excerpt example by high-pressure distillation at 2500 kPa, simulated in an exemplary total-reflux column under simplified idealized phase equilibrium conditions according to Equation (1). The error bars show the results for a deviation of the calculated temperature of ±5%.
4. Conclusions
The present study demonstrated the possibility of high purity separation of rare earth mixtures from various NdFeB-DyPr magnet recyclates by chlorination and subsequent distillation. The simulation results of the developed distillation process have shown that the required 99 mol% purity for NdFeB magnets can be easily achieved and thus could be applied as a new recycling method on an industrial scale. Chlorination can be carried out in a combustion chamber at temperatures of 1173–1273 K as proposed in [7,9] or at much higher temperatures up to 2000 K under significantly lower reaction times of a few minutes. The simulation results have particularly shown that all rare earths, such as neodymium, dysprosium, and praseodymium can be separated with separation accuracies of 99.9999 mol% and are thus suitable for the reuse of NdFeB magnets depending on the required minimum purity of 99 mol% (see: [15]). Only for the zirconium and copper recovery can a higher separation effort with higher impurifications be expected. Based on the increasing demand of NdFeB magnets up to 2030 [1,2,3], the possibility of recycling worldwide waste can be realized completely by distillation-based separation processes. With a few separation plants, 25,000 t of recyclables needed in 2030 could thus be recovered with a total rate of 3125 kg/h, which seems to be very well manageable for separation technology in reasonable quantities.
Analogous to the Kroll process for distillation-separated titanium recovery by chlorination and distillation, space-saving compact distillation apparatuses with low number of separation stages can also be developed for the NdFeB magnet recycling on the basis of the results presented here for an industrial large-scale separation process. However, further experimental studies on vapor–liquid equilibria are still needed to better model the distillation process.
Author Contributions
Conceptualization, Data curation, Formal analysis, lnvestigation, Methodology, Software, Visualization, Writing—original stuff, D.B.; Visualization, Writing—review & editing, K.C.; Conceptualization, Validation, Writing—reviewer, S.G.; Supervision, A.H.; Supervision, G.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pavel, C.; Thiel, C.; Degreif, S.; Blagoeva, D.; Buchert, M.; Schüler, D.; Tzimas, E. Role of substitution in mitigating the supply pressure of rare earths in electric road transport applications. Sustain. Mater. Technol. 2017, 12, 62–72. [Google Scholar] [CrossRef]
- Rademaker, J.H.; Kleijn, R.; Yang, Y. Recycling as a Strategy against Rare Earth Element Criticality: A Systemic Evaluation of the Potential Yield of NdFeB Magnet Recycling. Environ. Sci. Technol. 2013, 47, 10129–10136. [Google Scholar] [CrossRef]
- Yang, Y.; Walton, A.; Sheridan, R.; Güth, K.; Gauß, R.; Gutfleisch, O.; Buchert, M.; Steenari, B.M.; Gerven, T.V.; Jones, P.T.; et al. REE Recovery from End-of-Life NdFeB Permanent Magnet Scrap: A Critical Review. J. Sustain. Metall. 2016, 3, 122–149. [Google Scholar] [CrossRef]
- Royen, H.; Fortkamp, U. Rare Earth Elements—Purification, Separation and Recycling; IVL Swedish Environmental Research Institute: Stockholm, Sweden, 2016. [Google Scholar]
- Allam, E.M.; Lashen, T.A.; Abou El-Enein, S.A.; Hassanin, M.A.; Sakr, A.K.; Cheira, M.F.; Almuqrin, A.; Hanfi, M.Y.; Sayyed, M.I. Rare Earth Group Separation after Extraction Using Sodium Diethyldithiocarbamate/Polyvinyl Chloride from Lamprophyre Dykes Leachate. Materials 2022, 15, 1211. [Google Scholar] [CrossRef] [PubMed]
- Weshahy, A.R.; Gouda, A.A.; Atia, B.M.; Sakr, A.K.; Al-Otaibi, J.S.; Almuqrin, A.; Hanfi, M.Y.; Sayyed, M.I.; Sheikh, R.E.; Radwan, H.A.; et al. Efficient Recovery of Rare Earth Elements and Zinc from Spent Ni–Metal Hydride Batteries: Statistical Studies. Nanomaterials 2022, 12, 2305. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, Z.; Orefice, M.; Binnemans, K. Metal Recovery from Spent Samarium-Cobalt Magnets Using a Trichloride Ionic Liquid. ACS Sustain. Chem. Eng. 2018, 7, 2578–2584. [Google Scholar] [CrossRef] [PubMed]
- Lyman, J.; Palmer, G. Recycling of Rare Earths and Iron from NdFeB Magnet Scrap High Temperature Materials and Processes. Walter De Gruyter GmbH 1993, 11, 175–188. [Google Scholar]
- Mochizuki, Y.; Tsubouchi, N.; Sugawara, K. Selective Recovery of Rare Earth Elements from Dy containing NdFeB Magnets by Chlorination. ACS Sustain. Chem. Eng. 2013, 1, 655–662. [Google Scholar] [CrossRef]
- Murase, K.; Ozaki, T.; Machida, K.; Adachi, G. Extraction and mutual separation of rare earths from concentrates and crude oxides using chemical vapor transport. J. Alloys Compd. 1996, 233, 96–106. [Google Scholar] [CrossRef]
- Carter, A. Chlorination and Vapor Phase Extraction of Rare Earth Element Concentrate from the Bear Lodge Property Wyoming. Ph.D. Thesis, Metallurgical and Mineral Process Engineering, Montana Tech of The University of Montana, Butte, MT, USA, 2013. [Google Scholar]
- Okabe, P.; Newton, M.; Rappleye, D.; Simpson, M.F. Gas-solid reaction pathway for chlorination of rare earth and actinide metals using hydrogen and chlorine gas. J. Nucl. Mater. 2020, 534, 152–156. [Google Scholar] [CrossRef]
- Gaede, D. Chlorination and Selective Vaporization of Rare Earth Elements. Master Thesis, Montana Tech of the University of Montana, Butte, MT, USA, 2016. [Google Scholar]
- Itoh, M.; Miura, K.; Machida, K. Novel rare earth recovery process on Nd–Fe–B magnet scrap by selective chlorination using NH4Cl. J. Alloys Compd. 2009, 477, 484–487. [Google Scholar] [CrossRef]
- Öhl, J. Beitrag zur Entwicklung Eines Energieeffizienten Elektrolyseverfahrens für Neodym in Geschmolzenen Chloriden; Frauenhofer Verlag: Stuttgart, Germany, 2021. [Google Scholar]
- Adler, B.; Müller, R. Seltene Erden: Seltene Erdmetalle: Gewinnung, Verwendung und Recycling, Berichte aus der Biomechatronik 10; Universitätsverlag Ilmenau: Ilmenau, Germany, 2014. [Google Scholar]
- Stoddard, C.K.; Pietz, E. Pilot-Plant Distillation and Purification of Titanium Tetrachloride (Report of Investigations); Bureau of Mines: Pittsburgh, PA, USA, 1947. [Google Scholar]
- Kapp, R. Titanium Tetrachloride. In Encyclopedia of Toxicology; Elsevier: Amsterdam, The Netherlands, 2014; pp. 586–589. [Google Scholar]
- Eckert, J. Niobium and Niobium Compounds. Int. J. Refract. Met. Hard Mater. 1993, 12, 335–340. [Google Scholar] [CrossRef]
- Levenspiel, O. Chemical Reaction Engineering, 3rd ed.; Wiley-VCH: Weinheim, Germany, 1999; ISBN 0-471-25424-X. [Google Scholar]
- Cussler, E. Diffusion: Mass Transfer in Fluid Systems, 3rd ed.; Cambridge University Press: Cambridge, UK, 1997; ISBN 978-0-521-87121-1. [Google Scholar]
- Yaws, C. Transport Properties of Chemicals and Hydrocarbons: Viscosity, Thermal Conductivity, and Diffusivity for More Than 7800 Hydrocarbons and Chemicals, Including C1 to C100 Organics and Ac to Zr Inorganics; William Andrew: New York, NY, USA, 2009; ISBN 0815520395. [Google Scholar]
- Yaws, C. Chemical Properties Handbook: Physical, Thermodynamics, Engironmental Transport, Safety & Health Related Properties for Organic & Inorganic Chemical; McGraw-Hill Education: New York, NY, USA, 1998. [Google Scholar]
- Asabe, K.; Saguchi, A.; Takahashi, W.; Suzuki, R.O.; Ono, K. Recycling of Rare Earth Magnet Scraps: Part I Carbon Removal by High Temperature Oxidation. Mater. Transit. 2001, 42, 2487–2491. [Google Scholar] [CrossRef]
- Bosco, M.V.; Fouga, G.G.; Bohé, A.E. Kinetic study of neodymium oxide chlorination with gaseous chlorine. Thermochim. Acta 2012, 540, 98–106. [Google Scholar] [CrossRef]
- Kanari, N.; Mishra, D.; Mochón, J.; Verdeja, L.F.; Diot, F.; Allain, E. Some kinetics aspects of chlorine-solids reactions. Rev. Metal. 2010, 46, 22–36. [Google Scholar] [CrossRef]
- Kanari, N.; Mishra, D.; Filippov, L.; Diot, F.; Mochón, J.; Allain, E. Kinetics of hematite chlorination with Cl2 and Cl2 + O2: Part I. Chlorination with Cl2. Thermochim. Acta 2010, 497, 52–59. [Google Scholar] [CrossRef]
- Kanari, N.; Mishra, D.; Filippov, L.; Diot, F.; Mochón, J.; Allain, E. Kinetics of hematite chlorination with Cl2 and Cl2 + O2. Part II. Chlorination with Cl2 + O2. Thermochim. Acta 2010, 506, 34–40. [Google Scholar] [CrossRef]
- Perry, R.; Green, D. Perry’s Chemical Engineers Handbook 7th, Section 13: Distillation; McGraw-Hill: New York, NY, USA, 1997. [Google Scholar]
- Knacke, O.; Kubaschewski, O. Thermochemical Properties of Inorganic Substances; Hesselmann, E., Ed.; Springer: Berlin/Heidelberg, Germany, 1991. [Google Scholar]
- Barin, I. Thermochemical Data of Pure Substances, 3rd ed.; (2 Volume Set); Wiley-VCH: Weinheim, Germany, 1997. [Google Scholar]
- Baern, M.; Behr, A.; Gmehling, J.; Hofmann, H.; Onken, U. Technische Chemie; Wiley-VCH: Weinheim, Germany, 2013. [Google Scholar]
- Poling, B.; Prausnitz, J.M. The Properties of Gases and Liquids, 5th ed.; McGraw-Hill: New York, NY, USA, 2001; ISBN 0-07-011682-2. [Google Scholar]
- Gaviria, J.; Bohé, A. The Kinetics of the Chlorination of Yttrium Oxide. Metall. Mater. Trans. 2009, 40, 45–53. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).