Intensification of endo-1,4-Xylanase Extraction by Coupling Microextractors and Aqueous Two-Phase System
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Chemicals
2.1.2. Raw Enzyme
2.2. Methods
2.2.1. Analytical Methods
2.2.2. Batch Extraction of Xylanase
Optimization of Extraction
2.2.3. Continuous Extraction in a Microextractor
2.2.4. Mathematical Modelling of the Xylanase Extraction in a Microextractor
- Xylanase concentration in raffinate phase (R):
- Xylanase concentration in extract phase (E):
3. Results and Discussion
3.1. Selection of the ATPS for Xylanase Extraction
3.2. Extraction Optimization
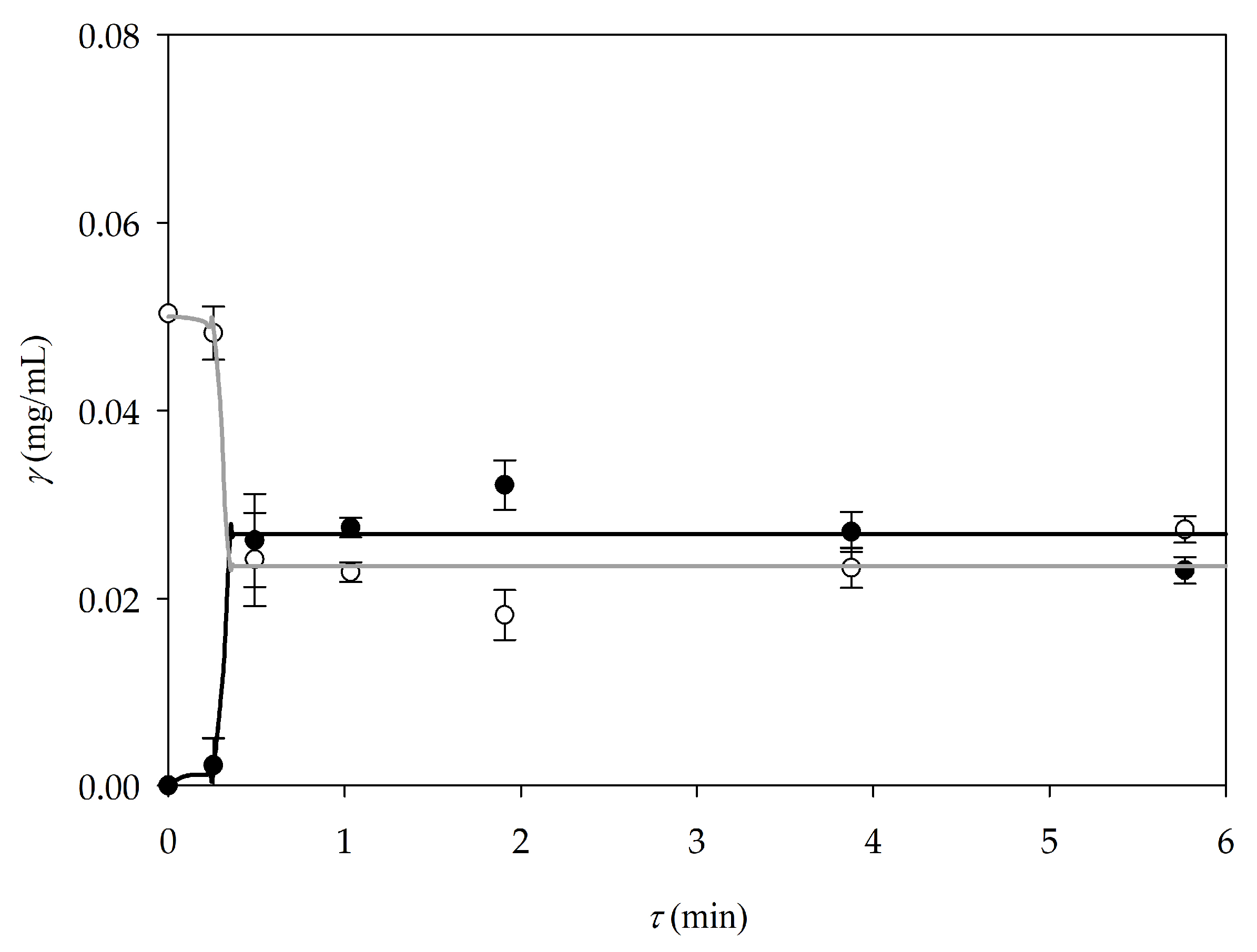
3.3. Extraction Intensification in a Microextractor
3.4. Extraction of Raw Xylanases Produced by Solid-State Fermentation of Thermomyces Lanuginosus
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Torres-Acosta, M.A.; Mayolo-Deloisa, K.; González-Valdez, J.; Rito-Palomares, M. Aqueous two-phase systems at large scale: Challenges and opportunities. Biotechnol. J. 2019, 14, 1800117. [Google Scholar] [CrossRef] [PubMed]
- Shahi, N.; Hasan, A.; Akhtar, S.; Siddiqui, M.H.; Sayeed, U. Xylanase: A promising enzyme. J. Chem. Pharm. Res. 2016, 8, 334–339. [Google Scholar]
- Gangwar, A.K.; Prakash, N.T.; Prakash, R. Applicability of microbial xylanases in paper pulp bleaching: A review. BioResources 2014, 9, 3733–3754. [Google Scholar] [CrossRef]
- Harris, A.D.; Ramalingam, C. Xylanases and its Application in Food Industry: A Review. J. Exp. Sci. 2010, 7, 1–11. [Google Scholar]
- Rahimpour, F.; Hatti-Kaul, R.; Mamo, G. Response surface methodology and artificial neural network modelling of an aqueous two-phase system for purification of a recombinant alkaline active xylanase. Process Biochem. 2016, 51, 452–462. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Kumar, B.; Verma, P. A detailed overview of xylanases: An emerging biomolecule for current and future prospective. Bioresour. Bioprocess. 2019, 6, 40. [Google Scholar] [CrossRef]
- Tonova, K.; Lazarova, Z. Reversed micelle solvents as tools of enzyme purification and enzyme-catalyzed conversion. Biotechnol. Adv. 2008, 26, 516–532. [Google Scholar] [CrossRef] [PubMed]
- Hatti-Kaul, R. Aqueous two-phase systems: A general overview. Mol. Biotechnol. 2002, 19, 269–278. [Google Scholar] [CrossRef]
- Yavari, M.; Pazuki, G.R.; Vossoughi, M.; Mirkhani, S.A.; Seifkordi, A.A. Partitioning of alkaline protease from Bacillus licheniformis (ATCC 21424) using PEG–K2HPO4 aqueous two-phase system. Fluid Phase Equilibria 2013, 337, 1–5. [Google Scholar] [CrossRef]
- Barbosa, J.M.P.; Souza, R.L.; Fricks, A.T.; Zanin, G.M.; Soares, C.M.F.; Lima, A.S. Purification of lipase produced by a new source of Bacillus in submerged fermentation using an aqueous two-phase system. J. Chromatogr. B 2011, 879, 3853–3858. [Google Scholar] [CrossRef]
- Sánchez-Trasviña, C.; Enriquez-Ochoa, D.; Arellano-Gurrola, C.; Tinoco-Valencia, R.; Rito-Palomares, M.; Serrano-Carreón, L.; Mayolo-Deloisa, K. Strategies based on aqueous two-phase systems for the separation of laccase from protease produced by Pleurotus ostreatus. Fluid Phase Equilibria 2019, 502, 112281. [Google Scholar] [CrossRef]
- Clavijo, V.; Torres-Acosta, M.A.; Vives-Flórez, M.J.; Rito-Palomares, M. Aqueous twophase systems for the recovery and purification of phage therapy products: Recovery of salmonella bacteriophage ϕSan23 as a case study. Sep. Purif. Technol. 2019, 211, 322–329. [Google Scholar] [CrossRef]
- González-Valdez, J.; Mayolo-Deloisa, K.; Rito-Palomares, M. Novel aspects and future trends in the use of aqueous two-phase systems as a bioengineering tool. J. Chem. Technol. Biotechnol. 2018, 93, 1836–1844. [Google Scholar] [CrossRef]
- Jiang, B.; Wang, L.; Wang, M.; Wu, S.; Wang, X.; Li, D.; Liu, C.; Feng, Z.; Chi, Y. Direct separation and purification of alphalactalbumin from cow milk whey by aqueous two-phase flotation of thermo-sensitive polymer/phosphate. J. Sci. Food Agric. 2021, 101, 4173–4182. [Google Scholar] [CrossRef]
- Jiang, B.; Wang, M.; Wang, X.; Wu, S.; Li, D.; Liu, C.; Feng, Z.; Li, J. Effective separation of prolyl endopeptidase from Aspergillus Niger by aqueous two phase system and its characterization and application. Int. J. Biol. Macromol. 2021, 169, 384–395. [Google Scholar] [CrossRef]
- Leong, H.Y.; Fu, X.Q.; Show, P.L.; Yao, S.J.; Lin, D.Q. Downstream processing of virus-like particles with aqueous two-phase systems: Applications and challenges. J. Sep. Sci. 2022, 45, 2064–2076. [Google Scholar] [CrossRef]
- Kruse, T.; Kampmann, M.; Rüddel, I.; Greller, G. An alternative downstream process based on aqueous two-phase extraction for the purification of monoclonal antibodies. Biochem. Eng. J. 2020, 161, 107703. [Google Scholar] [CrossRef]
- Jiang, B.; Wang, L.; Zhu, M.; Wu, S.; Wang, X.; Li, D.; Liu, C.; Feng, Z.; Tian, B. Separation, structural characteristics and biological activity of lactic acid bacteria exopolysaccharides separated by aqueous two-phase system. LWT 2021, 147, 111617. [Google Scholar] [CrossRef]
- Mokshina, N.Y.; Shkinev, V.M.; Shatalov, G.V.; Pakhomova, O.A.; Spivakov, B.Y. Extraction systems based on N-vinylformamide for the extraction and separation of cyclic amino acids. Dokl. Chem. 2020, 493, 113–116. [Google Scholar] [CrossRef]
- Grilo, A.L.; Aires-Barros, M.R.; Azevedo, A.M. Partitioning in aqueous two-phase systems: Fundamentals, applications and trends. Sep. Purif. Rev. 2016, 45, 68–80. [Google Scholar] [CrossRef]
- Pereira, F.B.; Freire, M.G.; Coutinho, J.A.P. Aqueous two-phase systems: Towards novel and more disruptive applications. Fluid Phase Equilibria 2020, 505, 112341. [Google Scholar] [CrossRef]
- Iqbal, M.; Tao, Y.; Xie, S.; Zhu, Y.; Chen, D.; Wang, X.; Huang, L.; Peng, D.; Sattar, A.; Shabbir, M.A.B.; et al. Aqueous two-phase system (ATPS): An overview and advances in its applications. Biol. Proced. Online 2016, 18, 18. [Google Scholar] [CrossRef]
- Magalhães, F.F.; Tavares, A.P.M.; Freire, M.G. Advances in aqueous biphasic systems for biotechnology applications. Curr. Opin. Green Sustain. Chem. 2021, 27, 100417. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, Z.; Wang, Q.; Wang, J.; Shang, L. Aqueous two-phase emulsions toward biologically relevant applications. Trends Chem. 2023, 5, 61–75. [Google Scholar] [CrossRef]
- Xu, Y.; He, G.; Li, J.-J. Effective extraction of elastase from Bacillus sp. fermentation broth using aqueous two-phase system. J. Zhejiang. Univ. Sci. B 2005, 6, 1087. [Google Scholar] [CrossRef]
- Mamo, G.; Hatti-Kaul, R.; Mattiasson, B. A thermostable alkaline active endo-β-1-4-xylanase from Bacillus halodurans S7: Purification and characterization. Enzyme Microb. Technol. 2006, 39, 1492–1498. [Google Scholar] [CrossRef]
- Taddia, A.; Rito-Palomares, M.; Mayolo-Deloisa, K.; Tubio, G. Purification of xylanase from Aspergillus niger NRRL3 extract by an integrated strategy based on aqueous two-phase systems followed by ion exchange chromatography. Sep. Purif. Technol. 2021, 255, 117699. [Google Scholar] [CrossRef]
- Moteshafi, H.; Jabbari, L.; Hashemi, M. Performance of Bacillus subtilis D3d xylanase separated through optimized aqueous two-phase system in bio-bleaching of sugar beet pulp. Process. Saf. Environ. Prot. 2022, 159, 749–756. [Google Scholar] [CrossRef]
- Kuan, D.H.; Wu, C.C.; Su, W.Y.; Huang, N.T. A microfluidic device for simultaneous extraction of plasma, red blood cells, and on-chip white blood cell trapping. Sci. Rep. 2018, 8, 15345. [Google Scholar] [CrossRef]
- Abdulbari, H.A.; Basheer, E.A.M. Microfluidics chip for directional solvent extraction desalination of seawater. Sci. Rep. 2019, 9, 12576. [Google Scholar] [CrossRef]
- Goja, A.M.; Yang, H.; Cui, M.; Li, C. Aqueous two-phase extraction advances for bioseparation. J. Bioprocess. Biotech. 2013, 4, 1000140. [Google Scholar] [CrossRef]
- Novak, U.; Pohar, A.; Plazl, I.; Žnidaršič-Plazl, P. Ionic liquid-based aqueous two-phase extraction within a microchannel system. Sep. Purif. Technol. 2012, 97, 172–178. [Google Scholar] [CrossRef]
- Šalić, A.; Tušek, A.; Fabek, D.; Rukavina, I.; Zelić, B. Aqueous two-phase extraction of polyphenols using a microchannel system—Process optimization and intensification. Food Technol. Biotechnol. 2011, 49, 495–501. [Google Scholar]
- Jurinjak Tušek, A.; Šalić, A.; Zelić, B. Mathematical modelling of polyphenol extraction by aqueous two-phase system in continuously operated macro- and micro-extractors. Sep. Sci. Technol. 2017, 52, 864–875. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosenbrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Alvarenga, B.G.; Virtuoso, L.S.; Lemes, N.H.T.; da Silva, L.A.; Mesquita, A.F.; Nascimento, K.S.; Hespanhol Da Silva, M.C.; Mendes Da Silva, L.H. Measurement and correlation of the phase equilibrium of aqueous two-phase systems composed of polyethylene(glycol) 1500 or 4000 + sodium sulfite + water at different temperatures. J. Chem. Eng. Data 2014, 59, 382–390. [Google Scholar] [CrossRef]
- Silvério, S.C.; Wegrzyn, A.; Lladosa, E.; Rodríguez, O.; MacEdo, E.A. Effect of aqueous two-phase system constituents in different poly(ethylene glycol)–Salt phase diagrams. J. Chem. Eng. Data 2012, 57, 1203–1208. [Google Scholar] [CrossRef]
- González-Amado, M.; Rodil, E.; Arce, A.; Soto, A.; Rodríguez, O. Polyethylene glycol (1500 or 600)—Potassium tartrate aqueous two-phase systems. Fluid Phase Equilibria 2018, 470, 120–125. [Google Scholar] [CrossRef]
- Garai, D.; Kumar, V. Aqueous two phase extraction of alkaline fungal xylanase in PEG/phosphate system: Optimization by Box–Behnken design approach. Biocatal. Agric. Biotechnol. 2013, 2, 125–131. [Google Scholar] [CrossRef]
- Young, M.E.; Carroad, P.A.; Bell, R.L. Estimation of diffusion coefficients of proteins. Biotechnol. Bioeng. 1980, 22, 947–955. [Google Scholar] [CrossRef]
- Abreu, D.C.; Figueiredo, K.C.D.S. Bromelain separation and purification process from pineapple extract. Braz. J. Chem. Eng. 2019, 36, 1029–1039. [Google Scholar] [CrossRef]
- Chaiwut, P.; Rawdkuen, S.; Benjakul, S. Extraction of protease from Calotropis procera latex by polyethylene glycol-salts biphasic system. Process Biochem. 2010, 45, 1148–1155. [Google Scholar] [CrossRef]
- Yuzugullu, Y.; Duman, Y.A. Aqueous two-phase (PEG4000/Na2SO4) extraction and characterization of an acid invertase from potato tuber (Solanum tuberosum). Prep. Biochem. Biotechnol. 2015, 45, 696–711. [Google Scholar] [CrossRef]
- Da Silva, O.S.; Gomes, M.H.G.; de Oliveira, R.L.; Porto, A.L.F.; Converti, A.; Porto, T.S. Partitioning and extraction protease from Aspergillus tamarii URM4634 using PEG-citrate aqueous two-phase systems. Biocatal. Agric. Biotechnol. 2017, 9, 168–173. [Google Scholar] [CrossRef]
- Glyk, A.; Scheper, T.; Beutel, S. Influence of different phase-forming parameters on the phase diagram of several PEG–salt aqueous two-phase systems. J. Chem. Eng. Data 2014, 59, 850–859. [Google Scholar] [CrossRef]
- Antov, M.G.; Pericin, D.M.; Dasic, M.G. Aqueous two-phase partitioning of xylanase produced by solid-state cultivation of Polyporus squamosus. Process Biochem. 2006, 41, 232–235. [Google Scholar] [CrossRef]
- Yang, S.; Huang, Z.; Jiang, Z.; Li, L. Partition and purification of a thermostable xylanase produced by Paecilomyces thermophila in solid state fermentation using aqueous two—Phase systems. Process Biochem. 2008, 43, 56–61. [Google Scholar] [CrossRef]
- Poornima, S.; Divya, P.; Karmegam, N.; Karthik, V.; Subbaiya, R. Aqueous two-phase partitioning and characterization of xylanase produced by Streptomyces geysiriensis from low cost lignocellulosic substrates. J. Biosci. Bioeng. 2020, 130, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Concato, J.; Hartigan, J.A. P values: From suggestion to superstition. J. Investig. Med. 2016, 64, 1166–1171. [Google Scholar] [CrossRef]
- Vincente, F.A.; Plazl, I.; Ventura, S.P.M.; Žnidaršič-Plazl, P. Separation and purification of biomacromolecules based on microfluidics. Green Chem. 2020, 22, 4391–4410. [Google Scholar] [CrossRef]




| Salt-H2O-PEG1540 | VPEG solution, mL | pHPEG solution, - | Vsalt solution, mL | pHsalt solution, - | Vbuffer, mL | Vxylanase solution, mL |
|---|---|---|---|---|---|---|
| Salt | ||||||
| sodium sulphate | 1.965 | 7.36 | 1.598 | 6.56 | 0.938 | 0.500 |
| sodium citrate dihydrate | 2.080 | 7.36 | 2.079 | 8.99 | 0.341 | 0.500 |
| sodium formate | 2.730 | 7.36 | 3.053 | 8.84 | 0 | 0.500 |
| potassium sodium tartrate tetrahydrate | 2.319 | 7.36 | 2.350 | 8.33 | 0 | 0.500 |
| ammonium sulphate | 2.040 | 7.36 | 1.694 | 5.01 | 0.766 | 0.500 |
| Salt-H2O-PEG1540 | E, % | K, - | PF, - |
|---|---|---|---|
| Salt | |||
| ammonium sulphate | 43.72 ± 10.23 | 0.78 ± 0.29 | 1.31 ± 0.19 |
| sodium sulphate | No phase formation | ||
| potassium sodium tartrate tetrahydrate | 60.12 ± 8.71 | 1.01 ± 0.36 | 1.36 ± 0.09 |
| sodium citrate dihydrate | 79.63 ± 5.21 | 3.91 ± 0.26 | 1.26 ± 0.25 |
| sodium formate | No phase formation | ||
| Exp. | t, min | γxylanase, mg/mL | wPEG, w/w | Eobserved, % | Epredicted, % |
|---|---|---|---|---|---|
| 1 | 5 | 0.10 | 0.21 | 95.318 ± 0.848 | 96.461 |
| 2 | 15 | 0.10 | 0.21 | 91.227 ± 0.811 | 78.988 |
| 3 | 5 | 0.30 | 0.21 | 106.515 ± 8.088 | 101.102 |
| 4 | 15 | 0.30 | 0.21 | 103.364 ± 7.874 | 96.461 |
| 5 | 5 | 0.20 | 0.20 | 85.500 ± 6.447 | 82.128 |
| 6 | 15 | 0.20 | 0.20 | 88.568 ± 6.678 | 94.737 |
| 7 | 5 | 0.20 | 0.22 | 86.523 ± 6.524 | 94.573 |
| 8 | 15 | 0.20 | 0.22 | 102.886 ± 7.758 | 103.839 |
| 9 | 10 | 0.10 | 0.20 | 72.906 ± 8.145 | 80.429 |
| 10 | 10 | 0.30 | 0.20 | 97.724 ± 3.214 | 91.029 |
| 11 | 10 | 0.10 | 0.22 | 96.517 ± 4.541 | 103.268 |
| 12 | 10 | 0.30 | 0.22 | 104.509 ± 7.065 | 103.786 |
| 13 | 10 | 0.20 | 0.21 | 89.318 ± 6.735 | 86.513 |
| 14 | 10 | 0.20 | 0.21 | 88.568 ± 6.679 | 85.373 |
| 15 | 10 | 0.20 | 0.21 | 88.600 ± 6.681 | 86.870 |
| 16 | 10 | 0.20 | 0.21 | 87.046 ± 6.563 | 86.119 |
| 17 | 10 | 0.20 | 0.21 | 82.432 ± 6.216 | 88.069 |
| Parameter | Influence | p-Value |
|---|---|---|
| β0 | 94.69 | 0.000000 |
| β1 (t) | 3.44 | 0.388259 |
| β2 (t2) | −9.49 | 0.007820 |
| β3 (γxylanase) | 15.21 | 0.048380 |
| β4 (γxylanase2) | 1.45 | 0.787007 |
| β5 (wPEG) | −1.44 | 0.827753 |
| β6 (wPEG2) | 10.78 | 0.074961 |
| β7 (t · γxylanase) | 0.27 | 0.960227 |
| β8 (t · wPEG) | 7.33 | 0.208460 |
| β9 (γxylanase · wPEG) | −13.49 | 0.038038 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Božinović, M.; Vičević, R.; Zekić, N.; Šalić, A.; Jurinjak Tušek, A.; Zelić, B. Intensification of endo-1,4-Xylanase Extraction by Coupling Microextractors and Aqueous Two-Phase System. Processes 2023, 11, 447. https://doi.org/10.3390/pr11020447
Božinović M, Vičević R, Zekić N, Šalić A, Jurinjak Tušek A, Zelić B. Intensification of endo-1,4-Xylanase Extraction by Coupling Microextractors and Aqueous Two-Phase System. Processes. 2023; 11(2):447. https://doi.org/10.3390/pr11020447
Chicago/Turabian StyleBožinović, Marko, Renata Vičević, Nikolina Zekić, Anita Šalić, Ana Jurinjak Tušek, and Bruno Zelić. 2023. "Intensification of endo-1,4-Xylanase Extraction by Coupling Microextractors and Aqueous Two-Phase System" Processes 11, no. 2: 447. https://doi.org/10.3390/pr11020447
APA StyleBožinović, M., Vičević, R., Zekić, N., Šalić, A., Jurinjak Tušek, A., & Zelić, B. (2023). Intensification of endo-1,4-Xylanase Extraction by Coupling Microextractors and Aqueous Two-Phase System. Processes, 11(2), 447. https://doi.org/10.3390/pr11020447










