Abstract
Maize straw has been widely used for the production of energy through anaerobic digestion, but biogas production can be hindered by a lack of trace elemental nutrients. To address this issue, a lab-scale anaerobic plug flow reactor was continuously operated at 55 °C for 300 days, with a hydraulic retention time of 42 days and an organic loading rate of 2.1 g total solids/(L·day). Results from this study showed that between days 101 and 194, the methane yield slightly decreased from 0.26 ± 0.04 to 0.24 ± 0.03 L/g volatile solids (VS), but significant volatile fatty acid accumulation was observed by reaching up to 2759 ± 261 mg/L. After trace elements were added to the reactor, the methane yield increased to 0.30 ± 0.03 L/g VS, with 53% methane content. Around 62% of the total chemical oxygen demand and volatile solids were broken down into methane. Volatile fatty acid levels dropped and stabilized at around 210 ± 50 mg/L, indicating restored process stability. The addition of trace elements increased the abundance of Firmicutes and decreased Synergistetes in bacteria while simultaneously increasing the abundance of Methanosarcina in archaea. In conclusion, trace element supplementation was experimentally found to be necessary for stable thermophilic anaerobic digestion of maize straw.
1. Introduction
Lignocellulosic biomass, which includes farm and forest waste, energy crops, and grasses, is the most sustainable natural bioresource on Earth [1]. China generated around 8.6 × 108 tons of straw products in 2020, the majority of which was from maize harvests [2]. On average, 580 million tons of processed byproducts are produced annually, of which <40% are exploited [3]. Therefore, it is important to promote the multifaceted exploitation of agricultural products and byproducts. Maize straw can be valorized as a source of sustainable energy through anaerobic digestion (AD). Under anaerobic conditions, the maize straw, which serves as an organic substrate, is converted to biogas, i.e., a mixture of mainly carbon dioxide and methane, and digestate by a wide diversity of micro-organisms [4].
To treat and maximize the value of maize straw, anaerobic digestion has proven to be an effective and promising technology. Hence, it is capable of producing clean energy (biogas) with minimum odor issues, reducing the overall volume of waste that is disposed of, and lowering emissions of greenhouse gases [5]. Nevertheless, aside from overcoming the tough biodegradability of lignocellulosic substrates, including maize straw, the absence of necessary nutrients frequently impairs the efficiency of biogas and methane production during the mono-digestion of maize straw by AD in industrial biogas plants [6,7]. Thus, reactor instability and accumulation of volatile fatty acids (VFAs) have been observed by prior investigations after a specific treatment period and a process failure if no external nutrition and buffering agents were given [8,9]. An alternative method to optimize the nutrient balance of maize straw for AD feedstocks is via supplementation of trace elements. Irrespective of reactor design, trace element deficiency was considered a common cause of failure in AD systems during straw digestion [10,11]. The impacts of deficiency of TEs on the stability of mesophilic anaerobic digestion of maize straw have been reported through long-term operation experiments [12]. However, whether the thermophilic anaerobic system also requires addition of trace elements for maize straw has not been investigated. An adequate quantity of trace elements not only encourages the degradation of VFAs [13] but also increases the effectiveness of VFA conversion [14]. Furthermore, trace elements are crucial for the development of methanogens and are the primary determinant of how effectively anaerobic digestion converts organic matter to methane [15].
Of note, the accumulation of VFAs often leads to system acidification and failure [16]; therefore, it is necessary to determine whether or not trace elements can be added to alleviate the acidification caused by maize straw mono-digestion. In addition to that, long-term mono-digestion of maize straw may also result in biological failure due to the paucity of these essential trace nutrients [16]. The obvious concern is centered on the question of whether or not the anaerobic digestion of maize straw and long-term operation without co-digestion and trace element supplementation would be feasible. In AD, the removal of each ton of chemical oxygen demand (COD) requires 200–450 g of Fe, 6–54 g of Co, and 6–49 g of Ni [17,18]. Based on the industrial-grade metal element prices, the cost of removing each ton of COD ranges from CNY 0.2 to 1.4, making it a low-capital investment method.
Optimizing the levels of trace elements necessary for methane production to simultaneously reduce costs and minimizes the risk of trace element release into the environment, requiring in-depth awareness of the impacts and efficacy of trace elements in anaerobic digestion [19]. Previous researchers have studied the effects of trace elements on different substrates, such as food waste [20], stillage [21], manure [22], and highly concentrated organic wastewater [14], mostly in traditional reactors. Meanwhile, few studies exist on the impact of adding trace elements to straw crops for mono-digestion to preserve long-term biogas yields.
When considering the substrate composition, amount of substrate to be treated, and process economy, a plug flow reactor was used because it is known for its lower cost, more stable performance, and ability to withstand environmental stress [23]. Moreover, researchers have also investigated the effectiveness of plug flow reactors on organic substrates with solid content in the range of 11–14% total solids (TS) [24,25]. Biogas production in a plug-flow reactor may be more favorable than in a typical reactor [26], and the role of trace elements in a plug-flow reactor may be different, but this is unknown at this time. Most studies mainly employ a mesophilic temperature (37 °C) to study the digestion of crop energy, and to the best of our knowledge very few studies have reported on thermophilic anaerobic mono-digestion of crop energy. Microorganisms in anaerobic digestion are extremely responsive to variations in temperature [27], and high temperature could indeed enhance hydrolysis and physical degradation of the substrate, increase the decomposition of organic matter, and boost methane yields during aerobic fermentation [28].
The temperature of the plug flow reactor was set at 55 °C for this investigation of the long-term performance of the anaerobic mono-digestion of maize straw. To this end, the mass balances of some parameters, including COD balance and TS and volatile solids (VS) balance, in biogas production of the maize straw were evaluated. Furthermore, a material flow assessment was carried out to determine the performance of this technology in converting maize straw. The novel contribution of this work lies in investigating the long-term anaerobic mono-digestion of maize straw in a plug flow reactor and assessing the supplementary role of trace elements in the restoration of system acidification.
2. Materials and Methods
2.1. Reactor Design and AD Experiment
The construction and design of a horizontal plug flow reactor for a continuous anaerobic digestion process was carried out. The entire volume of the reactor is 30 L, and the working volume is 21 L. A timer that worked for five minutes every half an hour was used to achieve the intermittent stirring at 60 rpm/min. The thermophilic conditions were maintained by circulating water from the heater water through a water pump (Sensen HQB-2200, China). A thermostatic water bath with a thickness of around three centimeters was used to maintain the temperature at 55 °C. To prevent any further loss of heat, the reactor was shielded with a bubble insulator made of aluminum foil that measured 6 mm in thickness. The reactor was set to a semi-continuous feeding mode, with an input and output of 500 g each day. The reactor’s hydraulic retention time (HRT) was set to 42 days, and, following that, a 300-day experiment was performed. The schematic representation of the plug flow reactor system is illustrated in Figure 1. Biogas production and composition were monitored every day, and the digestate was extracted for the biochemical property analysis every 3 or 5 days.
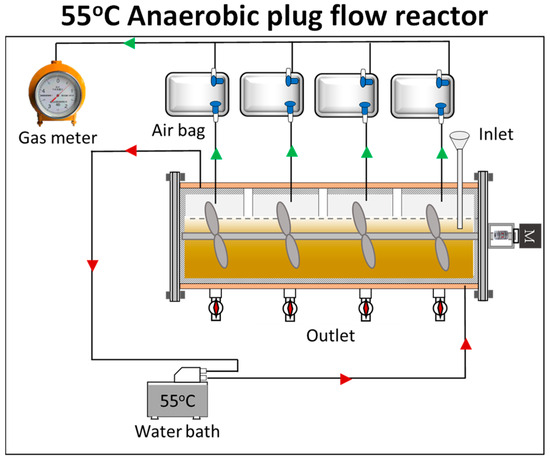
Figure 1.
The schematic representation of the plug flow reactor system.
2.2. Substrate and Inoculum
The maize straw utilized in the aforementioned study was obtained from the China Agricultural University’s Shang Zhuang Experimental Station in Beijing, China. After being returned, the maize straw was initially cut into small segments with a length below 10 cm using a chopping knife and left to air-dry at room temperature. Subsequently, it was introduced into a continuous grinding machine (HBM-103B, 2840 rpm) and a crusher (800A, 35,000 rpm) to be crushed into a mixture of rod-shaped and powdered particles with a length of approximately 1 cm. The maize straw was then sieved through an 18# mesh sieve (1.0 mm size). To prepare the substrate, the maize straw was mixed with a liquid fraction of centrifuged cattle manure digestate from an biogas plant to guarantee that the TS of the mixture was 10–15% and soaked at 55 °C for one day. Table 1 summarizes the most important characteristics of the substrate and the inoculum used during the investigations. Meanwhile, the reactor’s organic loading rate (OLR) was set to 2.1 g TS/(L·day) for long-term operation. The quantitative method of trace metals to be used in this experiment was discussed in a previous study, and the addition of trace elements was conducted in accordance with the same study [12].

Table 1.
The main characteristics of the substrate and the inoculum.
2.3. Analytical Methods
pH, total nitrogen, TS and VS, ammonium nitrogen, and other biochemical parameters were determined according to the American Public Health Association (APHA) methods. Particulate COD (PCOD) is defined as the remaining fraction of total COD (TCOD) after subtracting soluble COD (SCOD). The VFAs and the components of CH4 and CO2 in the biogas were tested by gas chromatography (Shimadzu GC-2010 plus, Kyoto, Japan) and gas chromatography (Shimadzu GC-8A, Japan), respectively.
2.4. Data Analysis
A regression curve was built to characterize the total biogas and methane production data obtained experimentally through a cumulative gas production model referred to per unit of volume of the substrate. The cumulative gas production was only performed for the stable state period. To this end, the Gompertz equation model (1) was used.
where P is the cumulative methane production at time t in mL. P0 is the maximum methane production potential in mL. Rmax is the maximum methane production rate in mL/d. λ is the lag phase duration in days. t is the experimental time in days. e is the constant (approximately 2.7183).
3. Results and Discussion
3.1. Biogas Production
Figure 2 illustrates the daily volumetric biogas yield, gas composition, pH, and VFAs from the reactor during the long-term AD process. The digestibility of the straw was evaluated. The operation parameters and reactor performance are summarized in Figure 2 and Figure 3. The volumetric biogas and methane increased gradually during the initial phase and then increased rapidly from 0.33 ± 0.02 and 0.24 ± 0.03 L/(L·d) to a maximum of 1.12 ± 0.04 and 0.62 ± 0.03 L/(L·d) within 42 days; this might be a result of the thermophilic hydrolytic bacterial population that accelerated the hydrolysis process [29]. This was followed by a gradual reduction in the volume of both biogas and methane production. This must have occurred from a decrease in the supply of convertible organic matter inside the biogas production system as observed by other studies [30]. During this period, a slightly stable period could be achieved, and only a small fluctuation occurred within days 95–136. The system’s biogas production increased drastically again to its maximum, which was then followed by a drastic decrease in production after 193 days of operation. During this phase, methanogenesis might have been inhibited, resulting in the precipitous decline. Therefore, the reactor was no longer being fed on the 194th day for 25 days. In parallel, the VFA concentration (Figure 3c) continued to increase leading to its accumulation, hence causing the system’s instability. The reactor was then fed after supplementing with trace elements [12] for additional days (223–300) to check the stability of the process under this condition. The biogas and methane production were kept stable with an average of 1.25 ± 0.1 and 0.65 ± 0.1 L/(L·d), respectively, at the end of this period. Generally, the actual volumetric biogas and methane produced after supplementation of trace elements were within the ranges of 1.2–1.5 and 0.4–0.8 L/(L·d), respectively. Overall, the volumetric biogas and methane yields increased by 67.2% and 67.7%, respectively, after the addition of trace elements. Our result accords with a study by Zhao et al. [31], who reported that the biogas volume increased by 43.4% when Fe, Co, and Ni were supplemented in the digester. Likewise, Liu et al. [32] reported 61.8%, 55.7%, and 50.8% increments of biogas volume after the addition of Fe, Co, Ni, Mo, and Se in a maize stover substrate. The biogas yield (Figure 2c) also followed a similar pattern to the daily volumetric. The average biogas and methane yields during the first 26 days were 0.44 ± 0.04 L/gVS and 0.26 ± 0.03 L/gVS, respectively. Average biogas yield increased by 4.5% from 26 days to 194 days of operation, while the average methane yield during these periods remained unchanged. After supplementing with trace elements, the average biogas and methane yield increased to 0.58 ± 0.04 L/gVS and 0.30 ± 0.03 L/gVS, which is approximately 32% and 16% increment, respectively. In previous studies on thermophilic AD of maize straw, the methane yield reached levels of 0.18–0.22 L/gVS [33], which were comparable to levels before the addition of trace elements but significantly lower than the methane yield after the addition of trace elements. This further supports the positive effect of adding trace elements to methane production from maize straw. To this end, it can be inferred that trace elements are indeed essential factors in promoting the metabolism of methanogens [34]. The trace elements have a strong connection to the metabolic processes of microorganisms and are among the most important variables in ensuring the reaction’s stability [35]. Reports show that the key trace elements needed to increase the activity of the methanogens studied thus far in the anaerobic digestion were Fe, Co, and Ni [35,36,37], and the absence of them will have an impact on maize straw anaerobic digestion [38,39]. A sufficient quantity of trace elements can activate the activity of relevant enzymes, such as F420, thereby enhancing the conversion of VFAs to methane and alleviating the acidification process in the reactor [12]. As a result, we can conclude that thermophilic mono-digestion of maize straw supplemented with trace elements may provide significant benefits to the economy by increasing biogas generation for a specific quantity of maize straw.
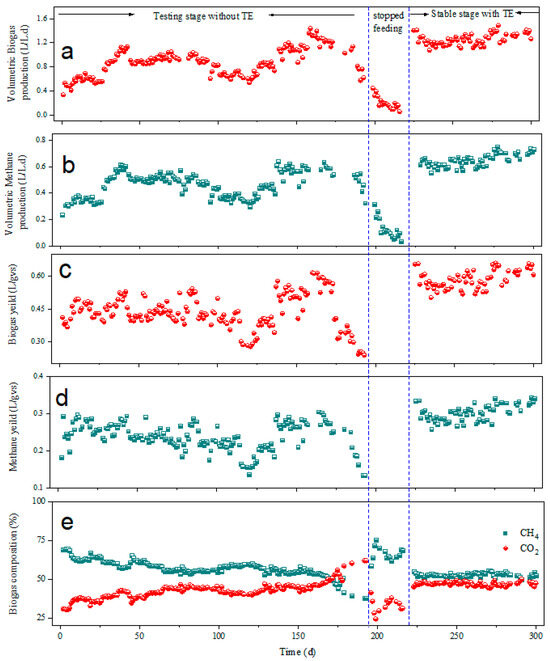
Figure 2.
Gas production during the anaerobic digestion process. (a) Volumetric biogas production; (b) volumetric methane production; (c) biogas yield; (d) methene yield; (e) biogas composition.
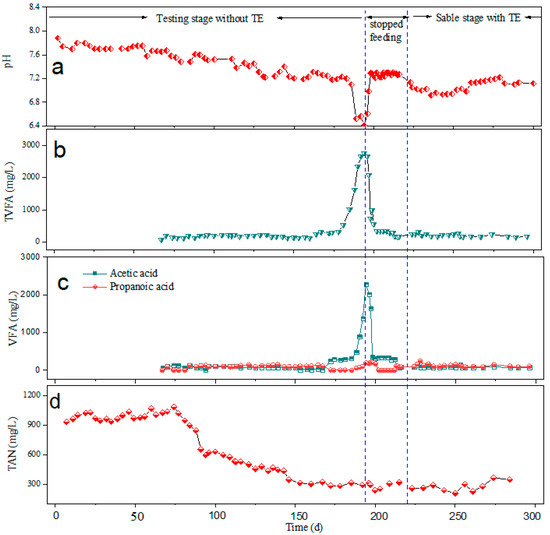
Figure 3.
Stability performance of the reactor. (a) pH; (b) TVFA; (c) VFA; (d) TAN.
Biogas composition is presented in Figure 2e The average methane content in the biogas was 65% during the first 26 days; in the subsequent days, the methane content was 58% and 56% for 27–100 and 101–194 days, respectively. During 169 days of operation, the methane content was within the range of 50–69%. After 169 days of operation, methane decreased rapidly to 37.0 ± 1.5%, while the proportion of CO2 evolved to 62.0 ± 1.8%. This could be attributed to the accumulation of VFAs [40]. The methane content gradually increased when the reactor was not fed for some days, and the methane content measured ranged from 51% to 72%. This may be because the pH was slightly raised, returning to the ideal pH range of 7–8 following a minor reduction [41] for anaerobic digestion. Shortly after re-feeding, alongside supplementation of trace elements the process began to show signs of stability, with the methane content reaching 53 ± 1% by the end of the operation. Examining the variations in digestate pH revealed that pH values slightly fluctuated throughout the operation, though they were within the appropriate pH conditions for anaerobic digestion, showing sufficient buffering abilities [42], until day 189, where the pH dropped below 7.0. Here, the reduction in pH could be related to an accumulation of organic acids caused by methanogens’ failure to convert organics to biogas and methane [43]. After the reactor feeding ceased, the pH returned to around 7.3 and remained stable for a while, and afterwards the addition of trace elements initially decreased the pH again but gradually increased shortly afterwards and remained within the range of 7.00–7.22 until the end of the operation, which could be explained as good bacterial and methanogen cooperation [44].
3.2. Process Stability Performance
Figure 3 shows a closer examination of the alterations in the maize straw digestate’s properties over the entirety of the AD process, such as VFA and TAN values. VFAs (Figure 3b), on the other hand, function as precursors that may indicate the biochemical condition of syntrophic anaerobic consortiums. The predominant VFAs were acetic acid and propionic acid, which are the most prevalent acids in anaerobic digestion, and these comprise more than 90% of TVFA. This observation corroborated the outcomes of other investigations [7,45]. In this study, the VFAs were evaluated after 66 days of operation, and it can be observed that the TVFA was stable and then peaked on the 194th day to a maximum of 2759 ± 261 mg/L. Acetic acid increased up to 2287 ± 183 mg/L, while the concentration of propionic acid was low at 196 ± 20 mg/L, and other acids included were up to 276 ± 14 mg/L. In general, acid inhibition occurs in an anaerobic digestion system when the acetic acid concentration exceeds 2000 mg/L [46]; therefore, in this study, acetate concentrations were accumulated to a level of inhibition. Reports on thermophilic operations reveal that, under conditions of low VFA concentration, a collection of hydrogenotrophic methanogens prevails, while the accumulation of VFA induces the growth of acetolactic methanogens [47]. Another study by Jiang [48] proposes that the specific methanogenic pathway is dependent on the acetate degradation status in the anaerobic digestion reactor. This suggests that, in this study, the growth of acetolactic methanogens was inhibited, hence the accumulation of acetate, whereas the bacterial responsible for propionate degradation were also predominant, leading to their low concentration. Moreover, it has been stated previously that VFAs are closely related to dissolved hydrogen [49,50]. This further implies that dissolved hydrogen directly or partially affected the prevalence of hydrogenotrophic methanogens relative to VFA degradation [47]. This could explain the low biogas and methane production in the system during this period. In other words, the accumulation of VFAs within 194 days induced an episodic partial blockage of the action of hydrolyzing fermentative bacteria. However, after more than 20 days of not feeding the reactor, VFAs dropped to 174 mg/L. It is possible that hydrolysis bacteria activity had resumed, and VFA production by acidogenesis and acetogenesis bacteria had begun. On the contrary, upon the addition of trace elements, it was observed that VFAs were stable until the end of the experiment, which was below 250 mg/L. This phenomenon suggests that adding trace elements might greatly boost the effectiveness of VFA utilization, which may be associated with an increase in methanogenic activity [12,51].
Another aspect to be considered is the TAN. Throughout the experiment, the ammonia concentration remained lower than the stated inhibitory limit of 3500 mg/L [52]. However, the highest TAN concentration occurred on the 19th day, recording 1173 ± 75 mg/L. With trace element supplementation, TAN in the system slightly increased towards the end of the experiment. The observed maximum biogas and methane yield, as well as the stable operation of the anaerobic digestion system, supported non-inhibitory TAN levels in this system.
3.3. The Complete Degradation of Maize Straw in a Feeding Cycle
Another series of investigations recorded the hourly gas output in the digester operating under the same conditions as the long-term but with the addition of trace elements. Degradation studies lasting 24 h within a single feeding cycle are commonly used to assess feedstock decomposition capabilities [53]. In Figure 4, it was discovered that there was no consistency in gas output during 24 h. The maximum amount of gas was produced in the first hours, and there was a surge in gas production at more or less regular intervals. More gas was produced during the first 9 h of anaerobic digestion. The cumulative gas production was determined. Biogas reached 23.6 L, with methane at 12.4 L. No lag phase occurred. Notably, 90% of biogas was generated between hours 1 and 16. Additionally, the composition of biogas changed over time. The hydrolysis and acidification were more active in the first 6 h, and the pH decreased to below 7.0, which significantly increased the CO2 concentration. Nevertheless, the CH4 content was maintained at roughly 55.4% after 9 h of digestion.
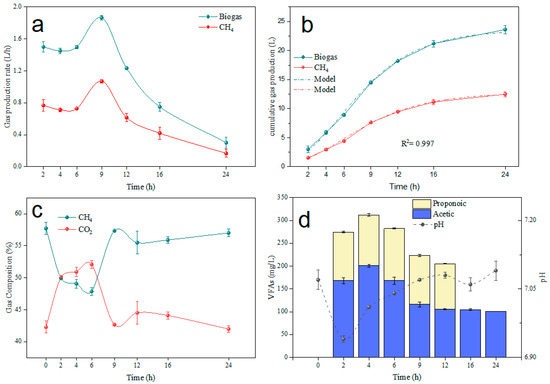
Figure 4.
Biogas production and VFA variations over 24 h. (a) Gas production rate; (b) cumulative gas production; (c) gas composition; (d) VFAs.
In Figure 4d, the VFA concentration increased and eventually decreased to 101 ± 12 mg/L, which is consistent with recent research on food waste [54] and synthetic waste with coffee powder at 55 °C [55]. VFAs accumulated significantly in the first 6 h, reaching a peak of over 300 mg/L, leading to a significant decrease in pH. The accumulation and degradation processes of VFAs were nearly complete around 16 h, supporting more than 85% of the maximum cumulative methane production at this point. These findings indicate that organic matter was decomposed effectively with a single feed cycle.
3.4. Material Flow and Mass Balances in the AD
The mass balances of different parameters, including COD, VS, and TS as well as TS and VS removed, are presented in Figure 5. The variability of TS and VS concentration is frequently connected to a microbial population in the reactor. Thus, this could be largely described as microbial growth and reproduction [42]. Further, changes to TS and VS in AD represent substrates transforming to methane and carbon dioxide via microbial metabolism, resulting in a stable drop in TS and VS [56]. Herein, the change in the absolute mass of the substrate before and after anaerobic treatment, TS, and VS was calculated for the stage of adding trace elements. Moreover, the degradation rates of TS and VS in anaerobic digestion are important indicators with which to evaluate the status of the anaerobic system [57]. Figure 5a depicts the TS and VS variations in the anaerobic digestion of maize straw in this study during the stable period. During this period, the final TS and VS degradation rates were 60.4% and 61.9%, respectively. In other words, a higher TS and VS degradation rate may result in increased methane production. In addition to that, the mass balances of TS and VS bioconversion were calculated based on the fractions of gas, liquid, and solid as represented in Figure 5b. It is clear that the majority of the maize straw’s digestate at this time was solid, with TS and VS results of 59% and 56%, respectively. The liquid digestate was only 8% and 7%, whereas 33% and 37% were converted into biogas of the TS and VS, respectively. The rate of transformation of organic matter per COD was seen when the COD was around 124 g/L and the TS and VS were approximately 12.7% and 11.9%, respectively. This indicates that the amount of bioenergy produced per unit of substrate utilized is higher in the maize straw that has been enriched with trace elements.
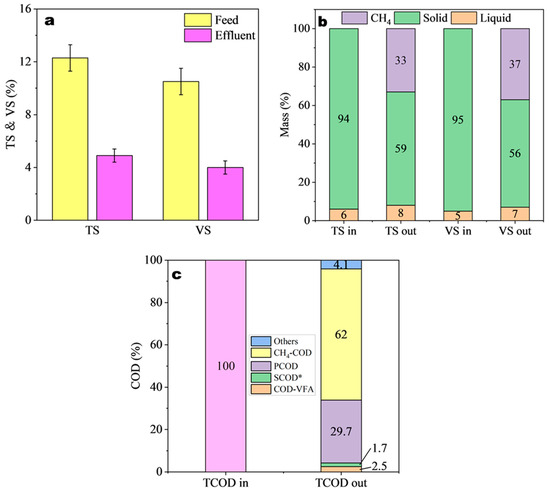
Figure 5.
TS/VS removal, mass balances based on TS/VS, and COD.
The COD mass balance that is displayed in Figure 5c describes the percentage of non-degraded organic matter that was estimated as TCOD, and the mass percentage of the influent was 100% (124 g/L) while the system was in a stable state. The anaerobic process resulted in approximately 62.0% of the TCOD that had been hydrolyzed out being degraded and transformed into methane gas, and this occurred after the operation. It is important to note that the COD of methane gas was determined by applying the Buswell–Mueller formula with a theoretical coefficient of 0.35 L CH4/g COD. This was carried out to arrive at the final result. In the effluent, the levels of PCOD and COD-VFA were 29.7% and 2.5%, respectively. The portion of PCOD that persists after AD may consist of hard-to-degraded lignin and part of cellulose protected by lignin in maize straw. In comparison, there was only 1.7% of SCOD* (excluding COD-VFA) that was still present.
During the stable operation time of the digester, i.e., when trace elements were being introduced, an evaluation of the material flow of the maize substrate was carried out. It can be seen in Figure 6 that the influent was fed at a volume of 0.5 L/d as the substrate. This influent had an initial TCOD concentration of 124 g/L, as well as TS and VS concentrations of 127 and 119 g/L, respectively. The quantities of protein and carbohydrates in the substrate were also examined and found to be around 11.67 and 20.58 g/L, respectively. The organic matter was transformed into biogas at a rate of 25 L/d in the system, which contains 53% methane and 47% carbon dioxide. The effluent had a TCOD concentration that was lower than 50% (50 g/L); the TS and VS concentrations were 50.3 and 45.3 g/L, respectively; and the TVFA concentration was 209 mg/L. At the same time, the quantity of carbohydrates and proteins that were present in the effluent dropped to 3.67 and 14.06 g/L, respectively. During protein degradation in the system, most protein remained in the liquid phase, which was about 10.91 g/L, whereas 3.15 g/L was present in solid form. It was noted that about 68.6% of carbohydrates were removed, while protein decomposition was around 31.79%. Therefore, among the main organic components producing methane, carbohydrates accounted for the largest proportion, while protein was the smallest. Several studies reported that throughout anaerobic digestion, carbohydrates decompose more effectively and faster than protein [53,58], and the rate of hydrolysis constant for carbohydrates is 0.5–2/d, which is greater than the rate constant for protein, that is 0.25–0.8/d [54]. In contrast, because ammonium nitrogen is produced during protein biodegradation [53], it has been established that when ammonium nitrogen concentrations are above 1500 mg/L, inhibitory effects in an AD system can be triggered [59]. Meanwhile, ammonium nitrogen in our study was within 300–1173 mg/L, which is way below the range of the inhibition. Of note, we can strongly infer that a lower protein decomposition rate in this study might be a major factor in the system’s failure to significantly accumulate ammonium nitrogen. The concentration of TAN in the effluent rose significantly to 0.36 g/L, which is attributed to the anaerobic biological conversion of proteins that make up the substrate into amino acids and eventually ammonia [60]. Overall, only 6.52 g/L of protein was degraded (over 31.79%), while 53.0% was dissolved, and the undissolved protein was 15.3%.
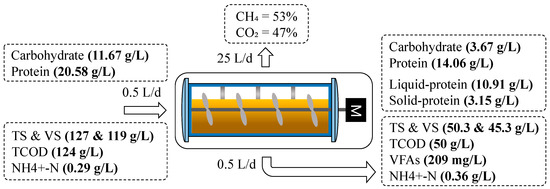
Figure 6.
Material flow during the stable state of AD.
3.5. Microbial Community Analysis and Diversity of Bacteria and Archaea
Microbial communities are shown in Figure 7a,b,c for bacteria phyla level, genus level, and archaea genus level during the acidified and stable stages. The phyla with relative abundances greater than 1% in the substrate during the entire operation were Firmicutes, Coprothermobacterota, Chloroflexi, Synergistetes, and Thermotogae. Synergistetes was dominant during the mono-digestion process. The relative abundance changes in bacterial communities at the genus level showed that seven genera with a relative abundance greater than 1% were detected in the acidified stage, dominated by Acetomicrobium, Defluviitoga, and Candidatus_Caldatribacterium. The addition of trace elements altered the composition of the bacteria. At the phylum level, the dominant bacteria were Firmicutes, Bacteroidetes, Thermotogae, Chloroflexi, and Synergistetes. Firmicutes accounted for the largest proportion followed by Bacteroidetes. Previous studies have stated that the bacteria Bacteroidetes and Clostridia (Firmicutes) are mostly abundant in cellulose substrates as these bacteria are effective in degrading cellulose into organic acids [61]. Moreover, a study reported that biogas yield is correlated with the abundance of Firmicutes, which is consistent with the results of the current work [62]. The significant increase in Firmicutes species indicates that they have good adaptability to environmental changes. The present study found that the increased diversity of bacteria following the addition of trace elements was favorable for methane production in anaerobic digestion.
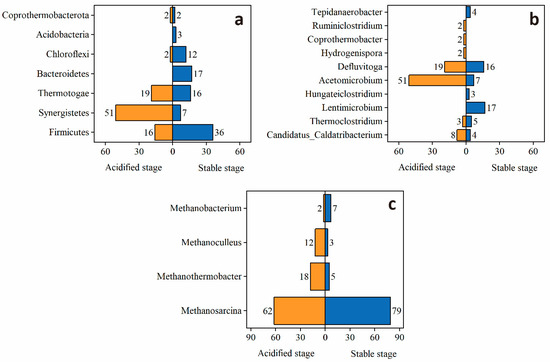
Figure 7.
Bacteria ((a) phylum, (b) genus) and archaea ((c) genus) community of anaerobic digestion in acidified and stable stage.
In the case of Archaea, the diversity of archaea was significantly lower than that of bacteria. Methanosarcina and Methanothermobacter were the major genera during the acidified stage. The results of the current study indicated that the addition of trace elements changed the community composition of the archaea, especially the proportion of Methanothermobacter. Compared with the acidified stage, the proportion of Methanothermobacter decreased from 17.97% to 4.53%, perhaps because Methanothermobacter was not sensitive to the addition of trace elements. Meanwhile, Methanosarcina increased by 17%, perhaps because Methanosarcina was sensitive to the addition of trace elements. Archaea’s fundamental purpose in anaerobic digestion is to produce methane by consuming organic acids, hydrogen, and carbon dioxide. The addition of trace elements to the archaea community did not change dominant archaea. This is comparable to prior research that found that adding Co and Se to a trace-element-deficient substrate did not alter the dominant archaea relative to the control [51].
3.6. The Comparison of Effects of Adding Trace Elements to Maize Straw and Other Substrates
In this study, the purpose of adding trace elements was to enhance the activity of relevant metabolic processes, thereby accelerating the conversion of VFAs to methane. This alleviates acid accumulation, allowing the reactor to operate stably, with a focus on restoration. In some studies involving AD of other substrates, challenges such as methane inhibition due to high ammonia levels and acid accumulation at high OLR may arise. In such cases, supplementation of trace elements can enhance microbial activity, leading to a significant improvement in methane production. For instance, in the AD of chicken manure, which often experiences elevated ammonia concentrations, methane production is inhibited. By adding Co and Ni etc., during continuous operation at an OLR of 3.6 g-VS/(L·d), the methane yield increased by 117% [63]. In comparison, during the stable phase of trace element addition in this study, the methane yield only increased by 16%. The AD of pig and cattle manure showed methane yield increases of only 17–26% [64,65] and 7–25% [66,67], respectively, after adding trace elements, indicating modest improvements possibly due to low loading conditions without methane production inhibition. Therefore, the addition of trace elements tends to have a relatively small impact during stable and normal AD processes. However, when the reactor is experiencing acidification or methane production inhibition, the addition of trace elements often has surprising rescuing and stabilizing effects.
4. Conclusions
Thermophilic anaerobic digestion is often viewed as less stable, especially when treating some ‘pure’ substrates like maize straw. In this study, the long-term operation of a thermophilic anaerobic mono-digestion of maize straw in a plug-flow reactor was examined. The results demonstrated the effectiveness of adding trace elements to maintain process stability. With the introduction of trace elements, accumulated VFAs were converted into methane, then the methane production reached 0.30 ± 0.03 L/gVS, and VFAs remained at a low level. The COD mass balance results further showed that most of the biodegradable TCOD had been converted. A total of 29.7% of TCOD consists of hard-to-degraded PCOD, possibly corresponding to lignin. Additionally, the addition of trace elements increased the abundance of Firmicutes and decreased Synergistetes in bacteria while simultaneously increasing the abundance of Methanosarcina in archaea. These findings suggest that for industrial anaerobic digestion of maize straw, adding trace elements should be considered a mandatory strategy to maintain stability for thermophilic AD.
Author Contributions
Conceptualization, W.Q.; methodology, W.Q.; formal analysis, B.A.F. and L.R.; investigation, L.R. and W.Q.; resources, Y.G., X.F., D.Y., W.Q. and R.D.; writing—original draft preparation, L.R. and B.A.F.; writing—review and editing, J.Z. and W.Q.; supervision, W.Q.; project administration, W.Q.; funding acquisition, W.Q. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partially supported by The 2115 Talent Development Program of China Agricultural University.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
Authors Yanning Gao, Xianli Fu, and Dunyao Yu were employed by the company China Shipbuilding Industry Group Environmental Engineering Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflict of interest.
References
- Nitsos, C.K.; Matis, K.A.; Triantafyllidis, K.S. Optimization of Hydrothermal Pretreatment of Lignocellulosic Biomass in the Bioethanol Production Process. ChemSusChem 2013, 6, 110–122. [Google Scholar] [CrossRef] [PubMed]
- China Biogas Society. The China Biogas Industry Development Report: Peaking Carbon Emissions 2030 and Carbon Neutrality 2060. Available online: https://bookh.yunzhan365.com/zjsx/mtdn/mobile/index.html?hyztg=1 (accessed on 1 November 2023).
- Li, X.; Fang, G.; Chen, L.; Guo, R.; Zou, D.; Liu, Y. Optimization of Thermally Activated Persulfate Pretreatment of Corn Straw and Its Effect on Anaerobic Digestion Performance and Stability. Biomass Bioenergy 2021, 154, 106216. [Google Scholar] [CrossRef]
- Pavlostathis, S.G.; Giraldo-Gomez, E. Kinetics of Anaerobic Treatment: A Critical Review. Crit. Rev. Environ. Control 1991, 21, 411–490. [Google Scholar] [CrossRef]
- Khanh Nguyen, V.; Kumar Chaudhary, D.; Hari Dahal, R.; Hoang Trinh, N.; Kim, J.; Chang, S.W.; Hong, Y.; Duc La, D.; Nguyen, X.C.; Hao Ngo, H.; et al. Review on Pretreatment Techniques to Improve Anaerobic Digestion of Sewage Sludge. Fuel 2021, 285, 119105. [Google Scholar] [CrossRef]
- Laera, A.; Shakeri Yekta, S.; Hedenström, M.; Buzier, R.; Guibaud, G.; Dario, M.; Esposito, G.; van Hullebusch, E.D. A Simultaneous Assessment of Organic Matter and Trace Elements Bio-Accessibility in Substrate and Digestate from an Anaerobic Digestion Plant. Bioresour. Technol. 2019, 288, 121587. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Li, Z.; Ran, W.; Yuan, H.; Li, X. Performance and Microbial Community Dynamics in Anaerobic Co-Digestion of Chicken Manure and Corn Stover with Different Modification Methods and Trace Element Supplementation Strategy. Bioresour. Technol. 2021, 325, 124713. [Google Scholar] [CrossRef] [PubMed]
- Fahlbusch, W.; Hey, K.; Sauer, B.; Ruppert, H. Trace Element Delivery for Biogas Production Enhanced by Alternative Energy Crops: Results from Two-Year Field Trials. Energy Sustain. Soc. 2018, 8, 38. [Google Scholar] [CrossRef]
- Hinken, L.; Urban, I.; Haun, E.; Urban, I.; Weichgrebe, D.; Rosenwinkel, K.-H. The Valuation of Malnutrition in the Mono-Digestion of Maize Silage by Anaerobic Batch Tests. Water Sci. Technol. 2008, 58, 1453–1459. [Google Scholar] [CrossRef]
- Yao, Y.; Zhou, J.; An, L.; Kafle, G.K.; Chen, S.; Qiu, L. Role of Soil in Improving Process Performance and Methane Yield of Anaerobic Digestion with Corn Straw as Substrate. Energy 2018, 151, 998–1006. [Google Scholar] [CrossRef]
- Gu, Y.; Chen, X.; Liu, Z.; Zhou, X.; Zhang, Y. Effect of Inoculum Sources on the Anaerobic Digestion of Rice Straw. Bioresour. Technol. 2014, 158, 149–155. [Google Scholar] [CrossRef]
- Ren, L.; Hou, Z.; Gao, Y.; Fu, X.; Yu, D.; Lin, M.; Dong, R.; Qiao, W. Maintaining the Long-Term Stability of Anaerobic Digestion of Maize Straw in a Continuous Plug Flow Reactor by Verifying the Key Role of Trace Elements. Waste Biomass Valorization 2023, 14, 2103–2113. [Google Scholar] [CrossRef]
- Liu, X.L.; Wang, J.; Song, Y.H.; Zeng, P. Response Surface Optimization of Trace Element Requirement for the Production of Volatile Fatty Acids from Excess Sludge. Adv. Mater. Res. 2014, 878, 663–669. [Google Scholar] [CrossRef]
- Moestedt, J.; Nordell, E.; Shakeri Yekta, S.; Lundgren, J.; Martí, M.; Sundberg, C.; Ejlertsson, J.; Svensson, B.H.; Björn, A. Effects of Trace Element Addition on Process Stability during Anaerobic Co-Digestion of OFMSW and Slaughterhouse Waste. Waste Manag. 2016, 47, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Bi, S.; Westerholm, M.; Qiao, W.; Mahdy, A.; Xiong, L.; Yin, D.; Fan, R.; Dach, J.; Dong, R. Enhanced Methanogenic Performance and Metabolic Pathway of High Solid Anaerobic Digestion of Chicken Manure by Fe2+ and Ni2+ Supplementation. Waste Manag. 2019, 94, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Thamsiriroj, T.; Nizami, A.S.; Murphy, J.D. Why Does Mono-Digestion of Grass Silage Fail in Long Term Operation? Appl. Energy 2012, 95, 64–76. [Google Scholar] [CrossRef]
- Qiang, H.; Lang, D.-L.; Li, Y.-Y. High-Solid Mesophilic Methane Fermentation of Food Waste with an Emphasis on Iron, Cobalt, and Nickel Requirements. Bioresour. Technol. 2012, 103, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Takashima, M.; Shimada, K.; Speece, R.E. Minimum Requirements for Trace Metals (Iron, Nickel, Cobalt, and Zinc) in Thermophilic and Mesophilic Methane Fermentation from Glucose. Water Environ. Res. 2011, 83, 339–346. [Google Scholar] [CrossRef]
- Zandvoort, M.H.; van Hullebusch, E.D.; Fermoso, F.G.; Lens, P.N.L. Trace Metals in Anaerobic Granular Sludge Reactors: Bioavailability and Dosing Strategies. Eng. Life Sci. 2006, 6, 293–301. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, B.; Li, A.; Zhang, L.; Li, R.; Yang, T.; Xing, W. Mechanism of Process Imbalance of Long-Term Anaerobic Digestion of Food Waste and Role of Trace Elements in Maintaining Anaerobic Process Stability. Bioresour. Technol. 2019, 275, 172–182. [Google Scholar] [CrossRef]
- Schmidt, T.; Nelles, M.; Scholwin, F.; Pröter, J. Trace Element Supplementation in the Biogas Production from Wheat Stillage—Optimization of Metal Dosing. Bioresour. Technol. 2014, 168, 80–85. [Google Scholar] [CrossRef]
- Wang, K.; Yun, S.; Xing, T.; Li, B.; Abbas, Y.; Liu, X. Binary and Ternary Trace Elements to Enhance Anaerobic Digestion of Cattle Manure: Focusing on Kinetic Models for Biogas Production and Digestate Utilization. Bioresour. Technol. 2021, 323, 124571. [Google Scholar] [CrossRef] [PubMed]
- Lansing, S.; Martin, J.F.; Botero, R.B.; da Silva, T.N.; da Silva, E.D. Methane Production in Low-Cost, Unheated, Plug-Flow Digesters Treating Swine Manure and Used Cooking Grease. Bioresour. Technol. 2010, 101, 4362–4370. [Google Scholar] [CrossRef] [PubMed]
- Adl, M.; Sheng, K.C.; Xia, Y.; Gharibi, A.; Xiang, C. Examining a Hybrid Plug-Flow Pilot Reactor for Anaerobic Digestion of Farm-Based Biodegradable Solids. Int. J. Environ. Res. 2012, 6, 335. [Google Scholar]
- Cantrell, K.; Ducey, T.; Ro, K.; Hunt, P.G. Livestock Waste-to-Bioenergy Generation Opportunities. Bioresour. Technol. 2008, 99, 7941–7953. [Google Scholar] [CrossRef]
- Das, A.; Mondal, C.; Chatterjee, S. Time-lag models for continuous stirred tank and plug flow digesters for biogas production. Energy Fuels 2016, 30, 10404–10416. [Google Scholar] [CrossRef]
- Liu, Y.; Wachemo, A.C.; Yuan, H.; Li, X. Anaerobic Digestion Performance and Microbial Community Structure of Corn Stover in Three-Stage Continuously Stirred Tank Reactors. Bioresour. Technol. 2019, 287, 121339. [Google Scholar] [CrossRef]
- Kim, S.-H.; Han, S.-K.; Shin, H.-S. Feasibility of Biohydrogen Production by Anaerobic Co-Digestion of Food Waste and Sewage Sludge. Int. J. Hydrogen Energy 2004, 29, 1607–1616. [Google Scholar] [CrossRef]
- Sukkar, K.A.; Al-Zuhairi, F.K.; Dawood, E.A. Evaluating the Influence of Temperature and Flow Rate on Biogas Production from Wood Waste via a Packed-Bed Bioreactor. Arab. J. Sci. Eng. 2021, 46, 6167–6175. [Google Scholar] [CrossRef]
- Adou, E.; Alle, O.; Kouakou, A.; Kopoin, A.; Drogui, P.; Tyagi, R. Anaerobic Mono-Digestion of Wastewater from the Main Slaughterhouse in Yamoussoukro (Côte d’Ivoire): Evaluation of Biogas Potential and Removal of Organic Pollution. J. Environ. Chem. Eng. 2020, 8, 103770. [Google Scholar] [CrossRef]
- Zhaomeng, C.; Guangming, Z.; Bibo, Z.; Wei, Q.; Panyue, Z.; Tianjue, H.; Xiaoyun, J. Study on Improvement of Trace Activators and Energy Production in Anaerobic Digestion of Municipal Organic Refuse. J. Nanhua Univ. 2004, 18, 12–16. [Google Scholar]
- Liu, C.; Yuan, H.; Zou, D.; Liu, Y.; Zhu, B.; Li, X. Improving Biomethane Production and Mass Bioconversion of Corn Stover Anaerobic Digestion by Adding NaOH Pretreatment and Trace Elements. BioMed Res. Int. 2015, 2015, 125241. [Google Scholar] [CrossRef][Green Version]
- Li, J.; Li, X.; Wachemo, A.C.; Chen, W.; Zuo, X. Determining Optimal Temperature Combination for Effective Pretreatment and Anaerobic Digestion of Corn Stalk. Int. J. Environ. Res. Public. Health 2022, 19, 8027. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-J.; Park, S.-J.; Cha, I.-T.; Min, D.; Kim, J.-S.; Chung, W.-H.; Chae, J.-C.; Jeon, C.; Rhee, S.-K. Metabolic versatility of toluene-degrading, iron-reducing bacteria in tidal flat sediment, characterized by stable isotope probing-based metagenomic analysis. Environ. Microbiol. 2014, 16, 189–204. [Google Scholar] [CrossRef] [PubMed]
- Hoban, D.J.; Van Den Berg, L. Effect of iron on conversion of acetic acid to methane during methanogenic fermentations. J. Appl. Microbiol. 1979, 47, 153–159. [Google Scholar]
- Ram, M.; Singh, L.; Suryanarayana, M.; Alam, S. Effect of Iron, Nickel and Cobalt on Bacterial Activity and Dynamics During Anaerobic Oxidation of Organic Matter. Water Air Soil Pollut. 2000, 117, 305–312. [Google Scholar] [CrossRef]
- Taylor, G.; Pirt, S. Nutrition and Factors Limiting the Growth of a Methanogenic Bacterium (Methanobacterium Thermoautotrophicum). Arch. Microbiol. 1977, 113, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Fermoso, F.G.; Bartacek, J.; Jansen, S.; Lens, P.N.L. Metal Supplementation to UASB Bioreactors: From Cell-Metal Interactions to Full-Scale Application. Sci. Total Environ. 2009, 407, 3652–3667. [Google Scholar] [CrossRef]
- Khatri, S.; Wu, S.; Kizito, S.; Zhang, W.; Li, J.; Dong, R. Synergistic Effect of Alkaline Pretreatment and Fe Dosing on Batch Anaerobic Digestion of Maize Straw. Appl. Energy 2015, 158, 55–64. [Google Scholar] [CrossRef]
- Gaby, J.; Zamanzadeh, M.; Horn, S. The Effect of Temperature and Retention Time on Methane Production and Microbial Community Composition in Staged Anaerobic Digesters Fed with Food Waste. Biotechnol. Biofuels 2017, 10, 302. [Google Scholar] [CrossRef]
- Drosg, B. Process Monitoring in Biogas Plants; IEA Bioenergy: Paris, France, 2013; pp. 1–38. [Google Scholar]
- Wu, L.-J.; Qin, Y.; Hojo, T. Upgrading of Anaerobic Digestion of Waste Activated Sludge by Temperature-Phased Process with Recycle. Energy 2015, 87, 381–389. [Google Scholar] [CrossRef]
- Gashaw, A. Anaerobic Co-Digestion of Biodegradable Municipal Solid Waste with Human Excreta for Biogas Production: A Review. Am. J. Appl. Chem. 2014, 2, 55. [Google Scholar] [CrossRef]
- Sun, C.; Xia, A.; Fu, Q.; Huang, Y.; Lin, R.; Murphy, J.D. Effects of Pre-Treatment and Biological Acidification on Fermentative Hydrogen and Methane Co-Production. Energy Convers. Manag. 2019, 185, 431–441. [Google Scholar] [CrossRef]
- Chi, X.; Li, J.; Wang, X.; Zhang, Y.; Leu, S.-Y.; Wang, Y. Bioaugmentation with Clostridium Tyrobutyricum to Improve Butyric Acid Production through Direct Rice Straw Bioconversion. Bioresour. Technol. 2018, 263, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, O.P.; Visvanathan, C. Bio-Energy Recovery from High-Solid Organic Substrates by Dry Anaerobic Bio-Conversion Processes: A Review. Rev. Environ. Sci. Biotechnol. 2013, 12, 257–284. [Google Scholar] [CrossRef]
- Hori, T.; Haruta, S.; Ueno, Y.; Ishii, M.; Igarashi, Y. Dynamic Transition of a Methanogenic Population in Response to the Concentration of Volatile Fatty Acids in a Thermophilic Anaerobic Digester. Appl. Environ. Microbiol. 2006, 72, 1623–1630. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Wu, Z.; Yao, J.; Wandera, S.M.; Algapani, D.E.; Dong, R.; Qiao, W. Enhancing the Performance of Thermophilic Anaerobic Digestion of Food Waste by Introducing a Hybrid Anaerobic Membrane Bioreactor. Bioresour. Technol. 2021, 341, 125861. [Google Scholar] [CrossRef]
- Ahring, B.K. Methanogenesis in Thermophilic Biogas Reactors. Antonie Van Leeuwenhoek 1995, 67, 91–102. [Google Scholar] [CrossRef]
- Cord-Ruwisch, R.; Mercz, T.I.; Hoh, C.-Y.; Strong, G.E. Dissolved Hydrogen Concentration as an On-Line Control Parameter for the Automated Operation and Optimization of Anaerobic Digesters. Biotechnol. Bioeng. 1997, 56, 626–634. [Google Scholar] [CrossRef]
- Munk, B.; Lebuhn, M. Process Diagnosis Using Methanogenic Archaea in Maize-Fed, Trace Element Depleted Fermenters. Anaerobe 2014, 29, 22–28. [Google Scholar] [CrossRef]
- Qiao, W.; Takayanagi, K.; Niu, Q.; Shofie, M.; Li, Y.Y. Long-Term Stability of Thermophilic Co-Digestion Submerged Anaerobic Membrane Reactor Encountering High Organic Loading Rate, Persistent Propionate and Detectable Hydrogen in Biogas. Bioresour. Technol. 2013, 149, 92–102. [Google Scholar] [CrossRef]
- Al-Wahaibi, A.; Osman, A.I.; Al-Muhtaseb, A.H.; Alqaisi, O.; Baawain, M.; Fawzy, S.; Rooney, D.W. Techno-Economic Evaluation of Biogas Production from Food Waste via Anaerobic Digestion. Sci. Rep. 2020, 10, 15719. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Qiao, W.; Wang, Y.; Zou, T.; Lin, M.; Dong, R. Balancing Acidogenesis and Methanogenesis Metabolism in Thermophilic Anaerobic Digestion of Food Waste under a High Loading Rate. Sci. Total Environ. 2022, 824, 153867. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Westerholm, M.; Qiao, W.; Yin, D.; Bi, S.; Jiang, M.; Dong, R. Impact of Temperature and Substrate Concentration on Degradation Rates of Acetate, Propionate and Hydrogen and Their Links to Microbial Community Structure. Bioresour. Technol. 2018, 256, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wachemo, A.C.; Yuan, H.; Zuo, X.; Li, X. Evaluation of System Stability and Anaerobic Conversion Performance for Corn Stover Using Combined Pretreatment. Waste Manag. 2019, 97, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Lan, Y.; Zhu, J.; Wachemo, A.C.; Li, X.; Yu, L. Effect on Anaerobic Digestion Performance of Corn Stover by Freezing–Thawing with Ammonia Pretreatment. Chin. J. Chem. Eng. 2019, 27, 200–207. [Google Scholar] [CrossRef]
- Yang, G.; Zhang, P.; Zhang, G.; Wang, Y.; Yang, A. Degradation Properties of Protein and Carbohydrate during Sludge Anaerobic Digestion. Bioresour. Technol. 2015, 192, 126–130. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Yu, M.; Wu, C.; Wang, Q.; Gao, M.; Huang, Q.; Liu, Y. A Comprehensive Review on Food Waste Anaerobic Digestion: Research Updates and Tendencies. Bioresour. Technol. 2018, 247, 1069–1076. [Google Scholar] [CrossRef]
- Li, H.; Tan, F.; Ke, L.; Xia, D.; Wang, Y.; He, N.; Zheng, Y.; Li, Q. Mass Balances and Distributions of C, N, and P in the Anaerobic Digestion of Different Substrates and Relationships between Products and Substrates. Chem. Eng. J. 2016, 287, 329–336. [Google Scholar] [CrossRef]
- Flint, H.J.; Bayer, E.A.; Rincon, M.T.; Lamed, R.; White, B.A. Polysaccharide Utilization by Gut Bacteria: Potential for New Insights from Genomic Analysis. Nat. Rev. Microbiol. 2008, 6, 121–131. [Google Scholar] [CrossRef]
- Abendroth, C.; Vilanova, C.; Günther, T.; Luschnig, O.; Porcar, M. Eubacteria and Archaea Communities in Seven Mesophile Anaerobic Digester Plants in Germany. Biotechnol. Biofuels 2015, 8, 87. [Google Scholar] [CrossRef]
- Molaey, R.; Bayrakdar, A.; Recep, S.; Alli, B. Anaerobic digestion of chicken manure: Mitigating process inhibition at high ammonia concentrations by selenium supplementation. Biomass Bioenergy 2018, 108, 439–446. [Google Scholar] [CrossRef]
- Liang, Y.G.; Li, X.J.; Zhang, J.; Zhang, L.G.; Cheng, B. Effect of microscale ZVI/magnetite on methane production and bioavailability of heavy metals during anaerobic digestion of diluted pig manure. Environ. Sci. Pollut. Res. 2017, 24, 12328–12337. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Yang, F.; Huang, W.; Lei, Z.; Zhang, Z.; Huang, W. Combined effect of zero valent iron and magnetite on semi-dry anaerobic digestion of swine manure. Bioresour. Technol. 2022, 346, 126438. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Gu, J.; Wang, X.; Peng, H.; Bao, J. Effects of nano-zerovalent iron on antibiotic resistance genes during the anaerobic digestion of cattle manure. Bioresour. Technol. 2019, 289, 121688. [Google Scholar] [CrossRef]
- Singh, D.; Malik, K.; Sindhu, M.; Kumari, N.; Rani, V.; Mehta, S.; Malik, K.; Ranga, P.; Sharma, K.; Dhull, N.; et al. Biostimulation of Anaerobic Digestion Using Iron Oxide Nanoparticles (IONPs) for Increasing Biogas Production from Cattle Manure. Nanomaterials 2022, 12, 497. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).