1. Introduction
CO
2 injection has become a common and mature technology for increasing production. The phase state properties of CO
2 can easily change with temperature and pressure, exhibiting high solubility in crude oil in reservoirs. In recent years, many oil fields around the world have entered a period of high water cut development. CO
2 flooding technology is an effective technical means for tertiary oil recovery in high water cut reservoirs. Using existing well patterns to inject CO
2 is an economical and feasible development method at low oil prices. For some special low-permeability reservoirs, especially high-pressure and low-permeability reservoirs, water injection is difficult. Long-term water injection will cause irreversible damage to a reservoir to a certain extent, and the suspended solid-phase particles and oil pollution carried by the injected water can easily form blockages in reservoirs [
1,
2,
3]. The rocks in the eighth area of Jinglou Oilfield are mainly brown, brown fine sandstone, and siltstone. The mineral composition is mainly quartz, followed by feldspar. The porosity of the reservoir is 16.51% to 36.67%, with an average of 25.8%. The permeability is 0.066–7.935 μm
2, with an average of 1.339 μm
2. Due to the imperfect well pattern and the increase in under-injection wells, the formation pressure has been gradually increasing since the development of the eighth district. Therefore, the use of CO
2 flooding technology is an important technology for the effective development of the eighth district.
CO
2 injection has become a common and mature technology for increasing production. The phase state properties of CO
2 can easily change with temperature and pressure, exhibiting high solubility in crude oil in reservoirs. Therefore, it can be used as an efficient displacement agent for oil displacement in reservoir development [
4,
5]. After CO
2 is injected into a reservoir, due to the dissolution of carbonate cements and the change in wettability, a large number of clay particles are released into the fluid. With the migration of the fluid, the throat of the rock pores is blocked, the flow of the pores is hindered, and the permeability is reduced [
6,
7]. In order to clarify the damage mechanism of particle migration and plugging in the low-permeability reservoirs of Changqing Oilfield, Zhang Dongliang systematically constructed a comprehensive research method for assessment of the damage mechanism of particle migration and plugging in low-permeability reservoirs, which takes rock mineral analysis—pore structure description—particle size characterization as the static level, takes the quantitative evaluation experiment of water flooding macroscopic damage as the dynamic level, and takes the microscopic changes in pore throat–mineral analysis of produced fluid as the supporting level. It has been found that clay minerals can easily produce particle bridging and plugging at pore throats [
8]. However, when CO
2 enters a reservoir, it will react with water, rock, and crude oil. Carbonate minerals can react with carbonate solution under acidic conditions. Carbonate minerals will be subject to certain dissolution in the reaction. The dissolution of minerals may increase the pore space of the reservoir and change the porosity and permeability of the reservoir. To a certain extent, this can increase the permeability of the reservoir and improve the recovery rate [
9].
Based on the collection and analysis of field data from the target oil area, the field sampling was carried out. Using displacement experiments, supplemented by SEM and XRD, particle migration and plugging experiments caused by different concentrations of CO2 displacement and evaluation experiments of CO2-aqueous solution on rock dissolution were carried out. In the process of CO2 flooding, CO2 is injected into the formation and reacts with water and rock to produce particle migration and dissolution. This phenomenon is compared and analyzed, and the advantages of CO2 flooding are further explained, which has important theoretical and practical significance for improving the recovery rate of water injection wells.
2. Experimental Materials and Conditions
2.1. Experimental Materials and Instruments
Kerosene, consisting of light and medium crude components and free of asphaltenes, was used in this study. Ground raw oil at the Jinglou 8 wellhead area of Henan Oilfield was collected: crude oil density was 0.8956~0.9024 g/cm
3 and the viscosity of ground degassed crude oil was 21.71~31.27 mPa·s at 70 °C. The content of gum asphalt was 15.24~36.39%, wax was 14.90~31.32%, sulfur was 0.03~0.22%, and the freezing point was −5~27 °C. For the simulation formation water, which was obtained from Beijing Huayuan Gas Chemical company Ltd. (Beijing, China), the total salinity was about 8700 mg/L, the content of Cl
− was 2900~3600 mg/L, and the CO
2 purity was 99.999%. This was extracted from the natural core column with a diameter of 25 mm at Henan Oilfield; the physical parameters are shown in
Table 1. Cores and lithic fragments were taken from the field of Henan Oilfield and washed with anhydrous ethanol + benzene (1:3). After high-temperature and high-pressure washing with oil and salt, the fragments were dried in the laboratory.
An ISCO-260D high-precision displacement pump was obtained from Teledyne ISCO, Lincoln, NE, USA. A Hitachi SU8010 cold field emission scanning electron microscope was acquired from Changsha Kemei Analytical Instrument Company Ltd. (Changsha, China). An X-ray diffractometer was obtained from Rigaku Corporation, Tokyo, Japan.
2.2. Experimental Method
2.2.1. Experimental Study on the Particle Migration and Blockage Law Caused by CO2 Flooding under Different Conditions
According to the industry standard SY/T 6315-2017,
Determination method of relative permeability in high temperature and oil displacement efficiency of heavy oil reservoirs [
10], the core was vacuumed and pressurized to saturate the formation water, and the amount of pumping required was recorded. The core was taken out and weighed after saturation. Displacement experiments under different conditions were carried out after calculating the pore volume of the core.
The formation water flooding was carried out at a certain flow rate, and the initial water phase permeability of the core was measured after the differential pressure flow had stabilized. CO2 flooding was carried out at a certain flow rate; the core was dried after the pressure had stabilized or became 20 times greater than the pore volume. The dry core, after flooding, was vacuumed to saturate the formation water, the pore volume of the core was calculated, and the water phase permeability of the core after flooding was measured. The experimental results on the physical properties of the core were influenced by particle migration and blockage caused by inorganic precipitation and particles produced by loose cementation.
- 2.
Oil-bearing conditions
Strata water flooding was carried out at a certain flow rate, and the initial water phase permeability of the core was measured after the differential pressure flow rate had stabilized. The crude oil taken from the oilfield site after dewatering and degassing was used to displace the core until no water was produced from it. Then, the amount of water produced was recorded and bound water saturation was calculated. Under the formation temperature and pressure conditions, the dead saturated formation crude oil was displaced by live oil until the gas–oil ratio at the outlet end was consistent with the gas–oil ratio of the compound fluid. CO2 flooding was carried out at an injection speed of 0.1 mL/min, and the pressure changes and the amount of oil produced at the outlet end under different injection rates were recorded until the displacement multiple reached more than 20 times the pore volume. After CO2 flooding, the core was cleaned with n-heptane, re-saturated after drying, and the pore volume and water phase permeability were measured. The experimental results are the results of porosity and permeability under the influence of asphaltene precipitation and inorganic precipitation. The core was washed with toluene+alcohol, dried, and saturated again; then, its pore volume and water phase permeability were measured for a second time. The experimental results are the outcomes of porosity and permeability under the influence of particle migration of the formation itself.
2.2.2. Evaluation Experiment of Rock Corrosion by CO2-Aqueous Solution
In this study, the research objects were rock debris and the formation water taken from the target reservoir block. The dissolution experiment of the CO
2-aqueous solution on the rock was carried out using a high-temperature and high-pressure reaction kettle, assisted by SEM and XRD devices. The changes in surface morphology, mineral composition, and quality were caused by CO
2–water–rock interactions under the simulated conditions of formation temperature and formation pressure. The corrosion evaluation of the CO
2-aqueous solution on the rock was carried out. According to the national standard GB/T 7477-87,
Determination of total calcium and magnesium in water quality by EDTA titrimetric method [
11], the ion concentration of the solution was determined before and after the reaction. According to the industry standard JB/T 6842-1993,
the test method of scanning electron microscope [
12], and SY/T 5163-2018,
The clay minerals in sedimentary rocks and X-ray diffraction analysis experiments method of common clay minerals [
13], SEM and XRD experiments were carried out. Ultimately, the dissolution mechanism of the CO
2-aqueous solution on the rock was clarified.
2.2.3. Evaluation Experiment of the Oil–Water Relative Permeability Curve after CO2 Flooding
The unsteady-state method is based on the principle of one-dimensional two-phase oil–water displacement and describes the relationship between the distribution of oil–water saturation with time and distance during the displacement process [
14]. Based on the unsteady state method, the oil–water two-phase relative permeability curve was measured to clarify the oil–water two-phase seepage law before and after CO
2 flooding in the target reservoir and the mechanism of CO
2 flooding for step-down augmented injection. The oil–water displacement experiment was carried out using the core at an invariable speed. By recording the changes in pressure, oil production, and water production at both ends of the core with time under the formation temperature (50 °C) and 8 MPa pressure conditions, the relationship between water saturation and the relative permeability of oil and water phases was calculated. The determination method of the oil–water two-phase permeability curve refers to the industry standard GBT 28912-2012,
Determination method of relative permeability of two fluids in rock [
15].
3. Experimental Results and Discussion
3.1. The Law of Particle Migration and Blockage Caused by the CO2 Flooding Process
After CO2 is injected into a reservoir, it will undergo complex physical and chemical reactions with reservoir crude oil, rock, and formation water; destroy the equilibrium state of colloidal asphaltene–crude oil in the crude oil system; cause the precipitation of asphaltene particles; and block the pore throat. At the same time, CO2 dissolves in the formation water to form carbonic acid, which reacts with clay minerals in the rocks. Moreover, carbonate and bicarbonate generated by carbonic acid hydrolysis may also react with metal ions in the formation water to form inorganic precipitates. The resulting double precipitates migrate under the action of fluid, which may cause irreversible damage to the reservoir, resulting in an increase in crude oil seepage resistance and a decrease in oil well productivity. In addition, the loose cementation of rock may also induce particle migration when the fluid flow rate is too large, thus blocking the pore throat.
In summary, there may be three kinds of blockages after CO2 injection: organic matter precipitation and deposition or migration and blockage; inorganic-scale blockage; and the loose particles in the formation itself being released and transported, causing blockages.
In order to determine the law of particle migration and plugging in the process of CO
2 flooding, the mechanism of particle migration and plugging was analyzed by CO
2 flooding of the core obtained by field sampling, and the change in reservoir physical properties before and after displacement were recorded [
16].
3.1.1. Mechanism of Particle Migration and Plugging after CO2 Flooding
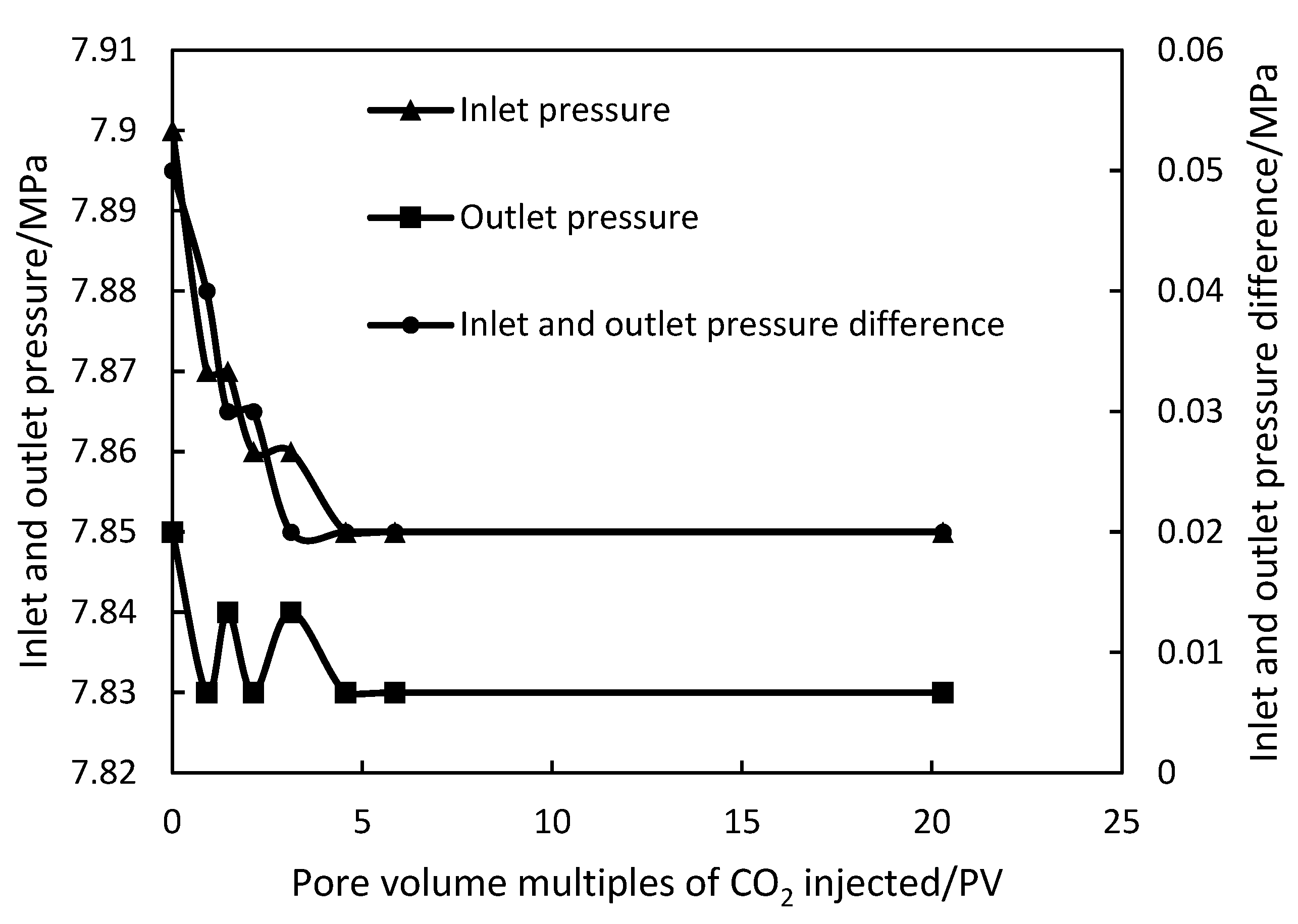
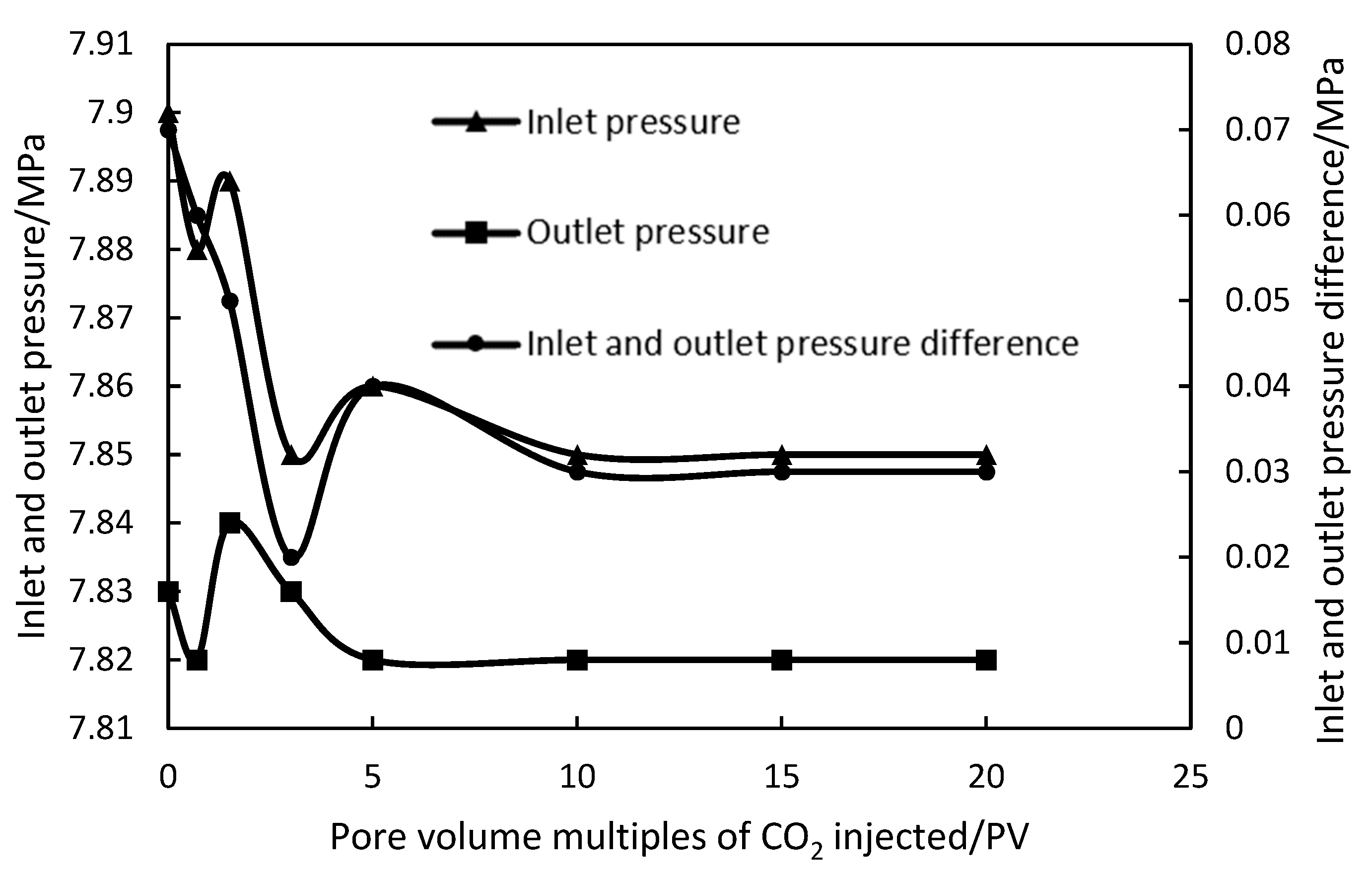
Under the conditions of 50 °C and 8 MPa, the target core was injected with CO
2 flooding 20.28 PV at different injection rates. The experimental results are shown in
Figure 1 and
Figure 2.
At the injection rate of 0.1 mL/min, the injection pressure and outlet pressure of the CO2 fluctuated greatly at the initial stage of the injection process. The pressure difference between the inlet and outlet was 0.05 MPa. With the increase in CO2 injection amount, the pressure difference decreased rapidly and finally stabilized at 0.02 MPa. Under the 1 mL/min injection rate conditions, the injection pressure and outlet pressure fluctuated greatly in the initial stage of the CO2 injection process, and the inlet and outlet pressure difference was 0.07 MPa. With the increase in CO2 injection volume, the pressure difference decreased and finally stabilized at 0.03 MPa. By comparing the changes in injection pressure and outlet pressure under different injection speeds, it can be seen that the injection of CO2 under different injection speeds can effectively reduce the injection pressure, thus achieving the purpose of reducing the pressure and increasing the injection.
3.1.2. The Change in Core Physical Properties before and after CO2 Flooding under Non-Oil Conditions
After CO
2 flooding, the core was vacuumed to saturate the formation water, and the influence of particle migration and blockage caused by inorganic precipitation and particles produced by loose cementation on the physical properties of the core after flooding was measured. The experimental results are presented in
Table 2.
From
Table 2, it can be seen that after CO
2 flooding, both the porosity and permeability of the core increased. The porosity increased from 30.567% to 30.665%, an increase of about 0.32%, and the growth rate was very small; the permeability increased from 73.315 mD to 103.826 mD, a large increase of about 41.6%. In the process of CO
2 flooding, due to the reaction of the CO
2–water with the rock, the porosity and permeability increase, indicating that there is a mineralization reaction in the actual injection process, resulting in the formation of inorganic substances; the reactants do not remain in the pores, resulting in pore blockage. From the growth rate of the porosity and permeability, in which the porosity increases slightly while the permeability increases greatly, it is clear that there is particle migration in the core under oil-free conditions, but it can be discharged at the core scale, indicating that the number of movable inorganic-scale fragments and particles is small under unbound conditions and accumulation phenomena cannot be generated, which does not affect the seepage capacity.
3.1.3. Changes in Core Physical Properties before and after CO2 Flooding under Oil-Bearing Conditions
After CO
2 flooding, the core was vacuumed to saturate the formation water, and the influence of particle migration and blockage caused by inorganic precipitation and particles produced by loose cementation on the physical properties of the core after flooding was measured. The experimental results are shown in
Table 3.
After CO2 flooding, the core was washed and dried with n-heptane; then, the saturated water was re-drained. The measured core porosity decreased from 30.665% to 30.214%, with a loss of about 1.47%, and the permeability decreased from 106.826 mD to 58.310 mD, with a loss of about 43.8%. n-heptane can be fully mixed with other components in crude oil and the remaining fluid in the core can be removed; the decreases in porosity and permeability are caused by asphaltene, inorganic-scale fragments, and particle residues. Combined with the results of the oil-free experiments, the actual residual should be greater than the measured pore volume reduction, and the decrease in permeability is mainly caused by inorganic precipitation and asphaltene deposition, indicating that the blockage is very serious. Using toluene and anhydrous ethanol to clean the core, the measured porosity increased from 30.214% to 30.362%, which was about 0.49% higher than that before cleaning, and the permeability increased from 58.310 mD to 72.475 mD, which was about 24.3%. Asphaltene can be washed out after toluene and anhydrous ethanol cleaning; therefore, the recovery of core porosity and permeability is caused by the removal of asphaltene. By comparing the damage degree of permeability before and after cleaning, it was found that the final permeability decreased from 103.826 mD to 72.475 mD, a loss of about 30.2%. At this time, the decreases in porosity and permeability were caused by inorganic-scale fragments and particle residues. Compared with the results of non-oil-bearing experiments, the movable particles under oil-bearing conditions are more easily bound by asphaltenes and are not easily produced; thus, they remain in the pores and cause blockages, affecting the fluid seepage capacity.
3.2. The Experimental Results and Analysis of CO2–Water Solution Dissolution
From the experiments on particle migration and blockage caused by CO
2-alternating-water flooding under different conditions, it was found that decreases in porosity and permeability after cleaning with n-heptane and toluene + anhydrous ethanol are caused by inorganic-scale fragments and particle residues. When CO
2 enters the reservoir, it dissolves and forms carbonic acid in the formation water, gradually dissociating H
+. With the increase in H
+ concentration, the acidity in the solution gradually increased, and the rock minerals in the reservoir made contact with it. Dissolution occurred to form non-precipitates that can dissolve in water, which plays a role in unblocking or increasing the porosity and permeability of a reservoir. When the amount of CO
2 dissolved in water reaches the extreme value, it precipitates again and blocks the pores, affecting the ultimate recovery factor and storage efficiency of CO
2 flooding [
16].
In order to observe the effect of the dissolution of rock minerals and CO2 on the reservoir, the influence of particle migration on the reservoir was analyzed further. By detecting the change in ion concentration after the interaction between rock and CO2, and utilizing SEM and XRD devices, dissolution experiments under the action of CO2-aqueous solution were carried out.
3.2.1. XRD Experiments before and after the Dissolution of Rock by CO2-Aqueous Solution
In order to observe the dissolution effect of the CO
2-aqueous solution on the rock after CO
2 displacement, the dried core powder was put into the reactor, CO
2 was injected to increase the formation pressure, and the temperature was raised to the formation temperature. The dissolution experiment was carried out and XRD was used to perform the scanning electron microscopy experiment before and after the dissolution of the cleaned core powder. XRD results before and after the dissolution experiment of the CO
2-aqueous solution and the core powder are shown in
Table 4 and
Table 5.
From the XRD results, it can be observed that the content of potassium feldspar increased from 13% to 15%, the content of plagioclase increased from 42% to 47%, the content of ankerite decreased from 2% to 1%, the content of calcite decreased from 3% to 1%, and the content of illite decreased from 19% to 18%. The content of quartz decreased from 33% to 28%, and the content of chlorite decreased from 50% to 46%. The decrease in chlorite was the most significant, indicating that the reaction of the CO
2-aqueous solution was mainly chlorite, and the reaction equation of the chlorite and CO
2-aqueous solution was:
. From the equation, the dissolution of chlorite will release ions such as Ca
2+, Mg
2+, and Fe
2+, which exist in large quantities in the whole system, providing ingredients for the precipitation of carbonate minerals and providing key minerals for CO
2–water–rock interactions. The increase in the kaolinite and illite–montmorillonite mixed layer indicates that CO
32− and HCO
3− ions exist in large quantities in the whole system, and Mg
2+ and Fe
2+ ions will precipitate to form new substances [
17].
3.2.2. EDS Experiment before and after Rock Dissolution Caused by the CO2-Aqueous Solution
In order to further prove the composition of particles and the ion distribution in the whole system, the mineral elements of rock samples before and after dissolution were identified and counted by EDS technology, and the changes in rock element content before and after dissolution were compared and summarized. The EDS experimental results before and after the dissolution of the rock by the CO
2-aqueous solution are shown in
Figure 3 and
Table 6.
From the comparison results in
Table 6, the mass content of MgO increased from 1.38% to 3.98% after the reaction between the CO
2–water and the rock, which increased by 2.6%. The mass content of CaO increased from 2.48% to 3.45%, an increase of 0.97%. The mass content of Fe
2O
3 increased from 5.05% to 13.96%, an increase of 8.91%. From the results in
Table 5, it can be seen that the reaction of the CO
2-aqueous solution is mainly chlorite, indicating that the reaction mainly generates FeCO
3, while FeCO
3 is unstable and will decompose into FeO and CO
2. FeO is unstable, and it can react with O
2 in the air to form Fe
2O
3. Additionally, it shows that there are many Fe
2O
3 compounds in the particles, which cause blockages at the pore throat and cause certain damage to the reservoir.
3.2.3. Changes in Ion Concentration after Interactions between Rocks and CO2
In order to observe the effect of the dissolution of rock minerals and CO
2 in the reservoir through the change in ion concentration after the reaction of rock and CO
2, we further analyzed the influence of particle migration on the reservoir. We injected 60 mL of simulated formation water into the high-pressure reactor. After CO
2 injection, the ion concentration of the solution at different pressures and temperatures was sampled and detected, and the amplitudes of changes in different ion concentrations were analyzed to determine the effect of particle migration on the seepage capacity of the reservoir after CO
2 flooding.
Table 7 shows the ion composition of the water produced after CO
2 flooding.
It can be seen from the ion composition of the water produced before and after CO2 flooding that the possible reactions in the rock are Ca2+, Mg2+, and Fe2+ reacting with CO32− to form CaCO3, MgCO3, and FeCO3, respectively. The composition of calcite in the rock is CaCO3; therefore, the participation of CO2 in the reaction will dissolve CaCO3 and increase the content of Ca2+ in the solution. The ion concentration of the water produced after CO2 displacement can be seen. The concentration of Ca2+ in the displaced water increased from 1637.6 mg/L to 4215.6 mg/L, a large increase of 157.43%; the concentration of Mg2+ decreased from 4108.0 mg/L to 2927.6 mg/L, a decrease of about 43.33%. The concentration of Fe2+ decreased from 1135.6 mg/L to 562.5 mg/L, a decrease of about 50.47%. Due to the instability of FeCO3 in the reaction process, it can decompose into FeO and CO2, which will reduce the concentration of Fe2+; FeO is also unstable. FeO will react with O2 in the air to generate Fe2O3, which also shows that there is Fe2O3 in the particles. Mg2+ can directly combine with CO32− and exists in the form of MgCO3; thus, the concentration of Mg2+ decreases. It can be seen from the ion composition of the produced water after displacement that most of the dissolved parts in the rock are calcite, and the precipitated components are mainly Fe2O3 and MgCO3. According to the changing range of each ion after displacement, although the migration of MgCO3 and Fe2O3 will block the pore throat in the rock, the dissolution of CaCO3 by CO2 improves the seepage capacity of the fluid, and the dominant seepage channel becomes larger, thus improving the water injection capacity and water injection effect of CO2 flooding to subsequent water flooding to a certain extent.
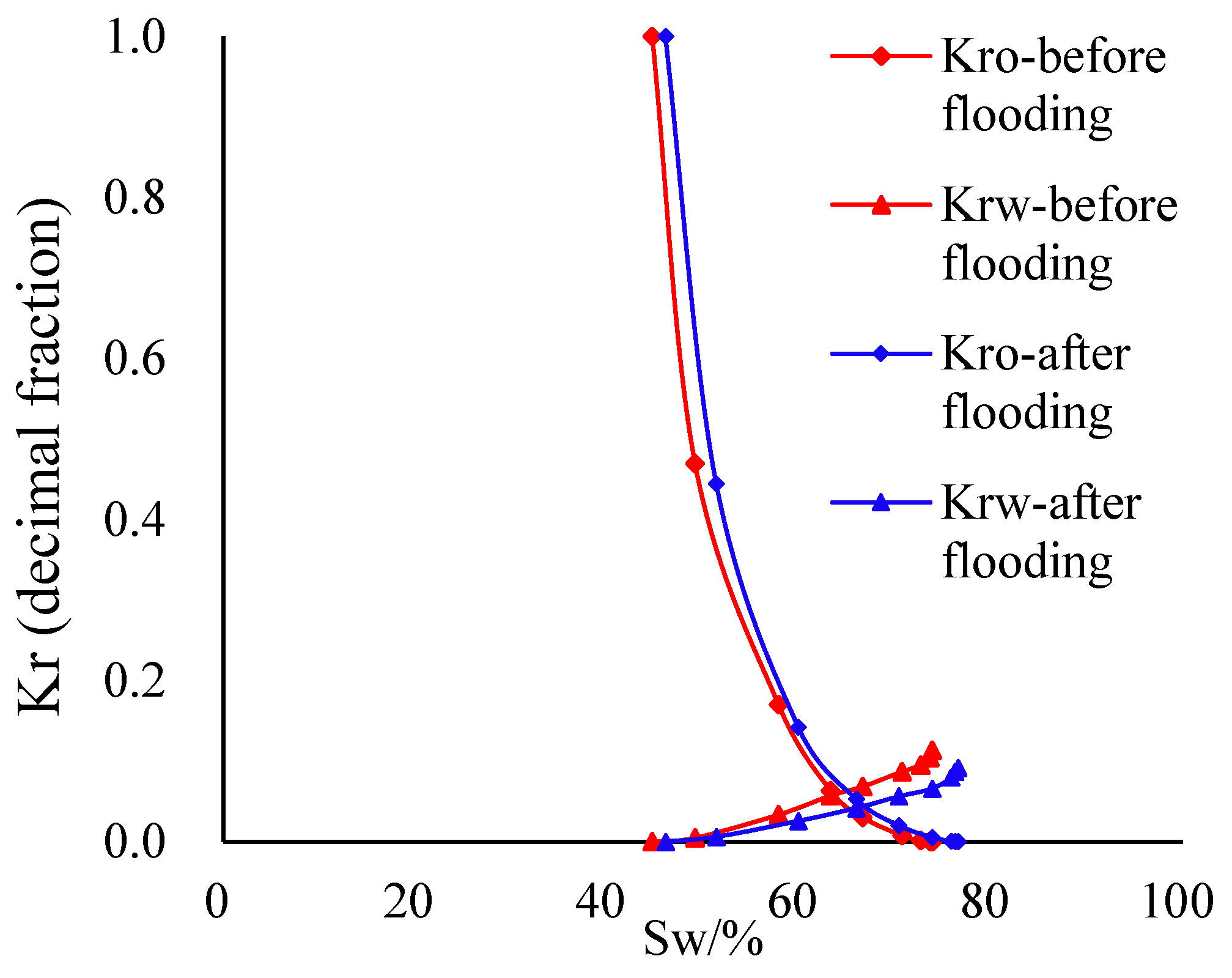
3.3. Evaluation of the Oil–Water Relative Permeability Curve after CO2 Flooding
In order to further study the influence of CO
2 flooding on the water injection capacity and water injection effect of the core, the relative permeability curve of the target core was tested after washing the oil with toluene + anhydrous, and the oil–water two-phase relative permeability curves before and after displacement were compared. The results are shown in
Figure 3.
By comparing the influence of CO2 flooding on the core relative permeability curve under different permeability values, it was found that the core two-phase relative permeability curve shifted to the right, and the bound water saturation increased, the irreducible oil saturation decreased, the two-phase co-permeability zone increased, and the oil displacement efficiency increased. This shows that the CO2–water–rock dissolution during CO2 flooding increases the pore space and seepage channel and improves the injection capacity of injected water. However, compared with the flow capacity of crude oil, the increase in the water injection capacity is relatively small.
The characteristic parameters of the relative permeability curve before and after CO
2 flooding were statistically sorted; the summary results are shown in
Table 8.
When compared with the characteristic parameters before CO2 flooding, after CO2 flooding the irreducible water saturation increases, the residual oil saturation decreases, and the relative permeability of the water phase endpoint under conditions of residual oil saturation increases slightly, indicating that the hydrophilicity of the rock increases after CO2 flooding, and the seepage capacity of the water phase increases after CO2 flooding; this is conducive to determining the water injection capacity of water flooding at later periods. Although particle migration blocks the pore throat of the rock and affects the seepage capacity of the reservoir, CO2 flooding has a greater dissolution effect on the reservoir. Overall, the occurrence of dissolution improves the seepage capacity of the fluid, and the dominant seepage channel becomes larger, which improves the existence conditions of particle migration blockage. At the same time, it also increases the movable space volume of the fluid and improves the water wetting degree of the rock, so as to improve the water injection capacity and water injection effect of the subsequent water flooding after CO2 flooding to a certain extent, which is conducive to improving the efficiency of water flooding.
4. Conclusions
In this study, on the basis of a full investigation, combined with the characteristics of the target reservoir and the analysis of the development characteristics, an assessment of the particle migration and plugging law caused by different conditions after CO2 flooding was carried out and the characteristics of the relative permeability curve before and after the displacement were evaluated. The following conclusions were obtained in this study.
(1) CO2 flooding reduces the injection pressure when CO2 is injected, indicating that CO2 flooding can reduce pressure and increase injection. Moreover, particle migration caused by CO2 flooding generally does not cause blockages under oil-free conditions; however, under oil-containing conditions, particle migration at the inorganic scale and organic scale will be bound by crude oil during the migration process and can easily accumulate into clusters, thus blocking the pore throat of the rock.
(2) The CO2-aqueous solution has a certain dissolution effect on the rock. The possible reactions in the rock are Ca2+, Mg2+, and Fe2+ reacting with CO32− to form CaCO3, MgCO3, and FeCO3, respectively. FeCO3 further forms Fe3O4, and secondary precipitation occurs, resulting in clogging problems. However, it can be seen from the ion concentration of the produced water after CO2 flooding that the concentration of Ca2+ in the produced water increases greatly and the concentrations of Mg2+ and Fe2+ decrease slightly, indicating that, although there is a certain MgCO3 and Fe3O4 blockage, the overall dissolution improves the seepage capacity of the fluid and the dominant seepage channel becomes larger, which can improve the water injection capacity and water injection effect of CO2 flooding to a certain extent.
(3) The variation characteristics of relative permeability curves before and after CO2 flooding show that the two-phase relative permeability curves of the core shift to the right, and the irreducible water saturation increases, the residual oil saturation decreases, the two-phase co-permeability zone increases, and the oil displacement efficiency increases. This further demonstrates that, although the CO2–water–rock reaction caused by CO2 flooding causes the blockage of particle migration, the induced dissolution increases the pore space and seepage channel as a whole and improves the injection capacity of injected water.
Author Contributions
Conceptualization, Y.L.; methodology, Y.L. and M.F.; writing—original draft, Y.L.; writing—review and editing, M.F. and C.W.; resources, C.W. and S.X.; supervision, F.M. and Y.S. All authors have read and agreed to the published version of the manuscript.
Funding
The authors acknowledge the financial support from the Study on Optimization Methods for the Characterization, Identification, and Regulation of Gas Channeling Channels in CO2 Flooding in Low-permeability Reservoirs, a project supported by the National Natural Science Foundation of China (grant No. 52104018).
Data Availability Statement
All data generated or analyzed during this study are included in this published article.
Conflicts of Interest
Author Yanlai Shen was employed by the company CNOOC Ningbo Daxie Petrochemical Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships.
References
- Shi, L. Damage characteristics of CO2 huff and spit asphaltene deposition on tight sandstone reservoir. Oilfield Chem. 2022, 39, 343–348. [Google Scholar]
- Zheng, W. Experimental study on solid phase deposition law of CO2 flooding in low permeability reservoir. Pet. Geol. Recovery Effic. 2021, 28, 131–136. [Google Scholar]
- Liu, L.; Ma, Y.; Pi, Y.; Liu, J.; Bai, M. Effect of CO2 flooding on rock properties under different formation water salinity. Oilfield Chem. 2020, 37, 665–668. [Google Scholar]
- Ding, S.; Xi, Y.; Liu, G.; Liu, Q.; Yu, H. Adaptability of different injection methods of CO2 flooding to enhanced oil recovery and geological storage in low permeability reservoirs. Pet. Geol. Recovery Effic. 2023, 30, 104–111. [Google Scholar]
- Zhao, Y.; Liu, K.; Wang, W.; Yao, Z. Prediction method of enhanced oil recovery by CO2 flooding in low permeability reservoirs. Energy Environ. Prot. 2022, 44, 95–101. [Google Scholar]
- Zhao, L.; Jiang, E.; Wang, S.; Luo, Q.; Li, B.; Zhu, D.; Bai, H. Effect of carbon dioxide injection on mineral and pore structure of low permeability reservoir. Oilfield Chem. 2021, 38, 659–664. [Google Scholar]
- Li, L.; Ju, B.; Jiang, H.; Di, P. Oil particle migration and its influence on reservoir physical properties. China Int. Energy Resour. 2011, 16, 50–54. [Google Scholar]
- Zhang, D.; Rao, L.; Cai, X. Mechanism of particle migration plugging damage in low permeability reservoir in Changqing Oilfield. Fault Block Oil Gas Field 2023, 30, 441–447. [Google Scholar]
- Yuan, Z.; Liao, X.; Zhao, X.; Chen, Z. Effect of dissolution on reservoir physical properties during CO2 displacement in sandstone reservoir. Pet. Geol. Recovery Effic. 2020, 27, 97–104. [Google Scholar]
- SY/T 6315-2017; Determination of High Temperature Relative Permeability and Displacement Efficiency of Heavy Oil Reservoirs. National Energy Board: Beijing, China, 2017.
- GB/T 7477-87; Water Quality—Determination of Total Calcium And Magnesium—EDTA Titration. State Environmental Protection Administration: Beijing, China, 1987.
- JB/T 6842-1993; Test Methods for Scanning Electron Microscopy. Shanghai Institute of Optical Technology Electronics: Shanghai, China, 1993.
- SY/T 5163-2018; Methods for Quantitative Analysis of Total Clay Minerals and Common Non-Clay Minerals in Sedimentary Rocks by X-ray Diffraction. National Energy Administration: Beijing, China, 2018.
- Peng, C.; Xue, X.; Wang, F.; Shi, G. Experimental data processing method of oil-water relative permeability by unsteady state method. Pet. Geol. Oilfield Dev. Daqing 2018, 37, 74–78. [Google Scholar]
- GBT 28912-2012; Method for Determination of Relative Permeability of Two-Phase Fluids in Rocks. National Petroleum Standardization Technical Committee: Beijing, China, 2012.
- Sun, Z. Experimental study on asphaltene deposition under CO2 flooding. Oilfield Chem. 2010, 27, 374–376. [Google Scholar]
- Qian, K.; Yang, S.; Huang, F.; Dou, H.; Wang, Q. Damage and wettability of low permeability reservoir induced by asphaltene precipitation during CO2 injection. Oilfield Chem. 2020, 37, 536–541. [Google Scholar]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).