Nanofluids and Ionic Fluids as Liquid Electrodes: An Overview on Their Properties and Potential Applications
Abstract
:1. Introduction
2. Electrochemical Properties
2.1. Electrical Conductivity Models and Correlations
2.2. Electrical Conductivity Measurement
2.3. Electrochemical Properties
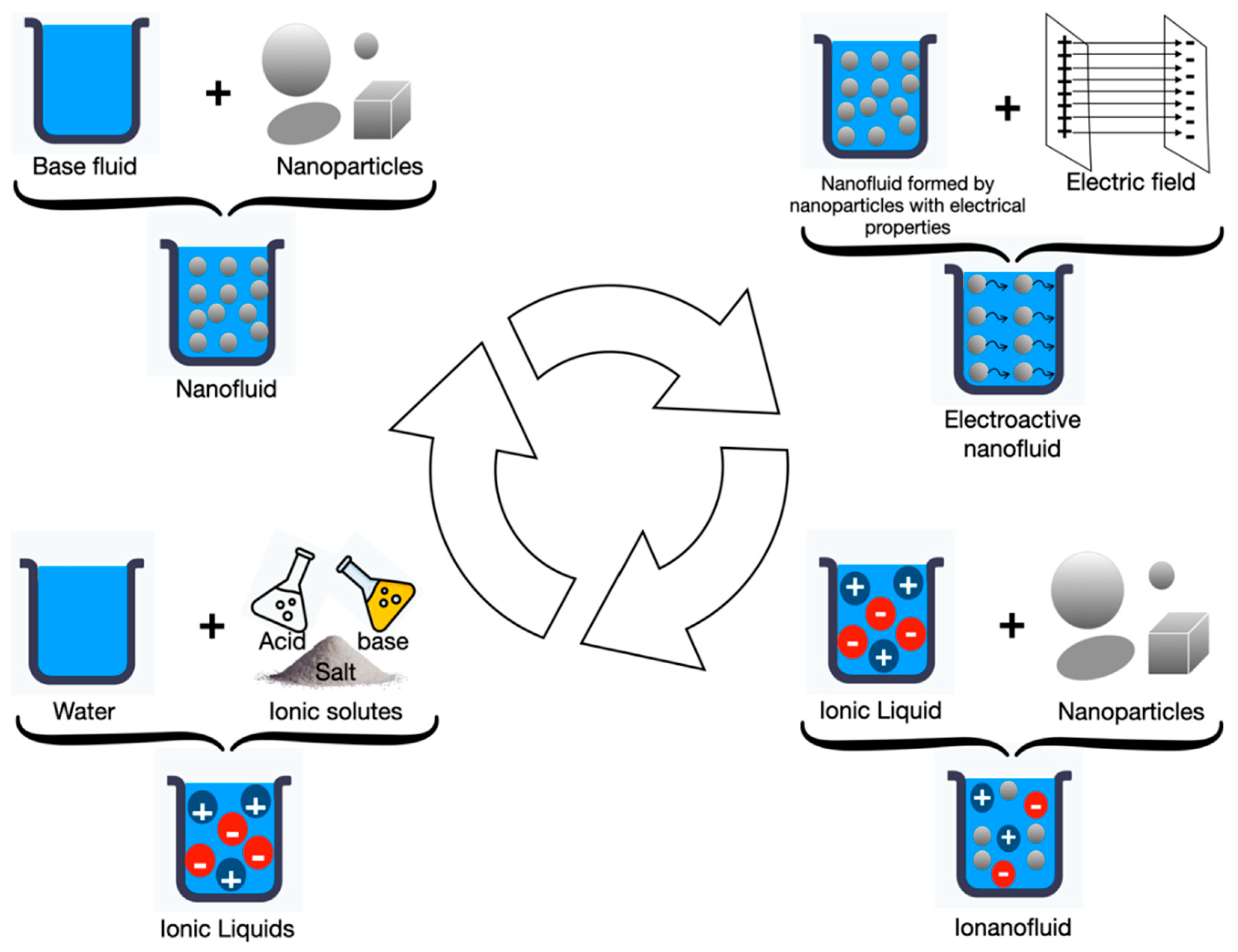
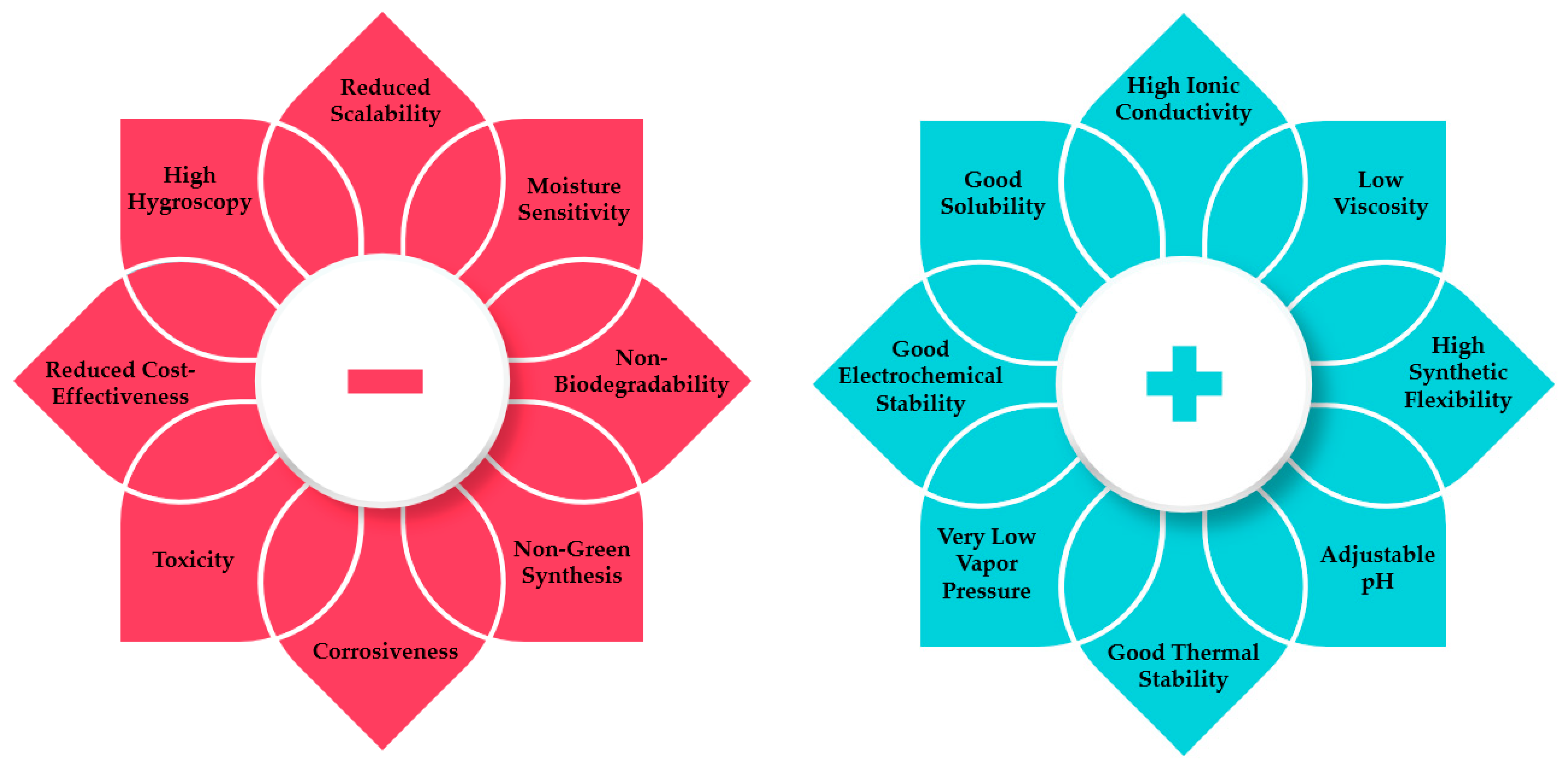
3. Ionic Liquids
4. Electroactive Nanofluids
5. Ionanofluids
6. Electrical-Conductivity-Influencing Factors
6.1. Temperature
6.2. Concentration
6.3. Type of Base Fluid
6.4. Addition of Surfactants
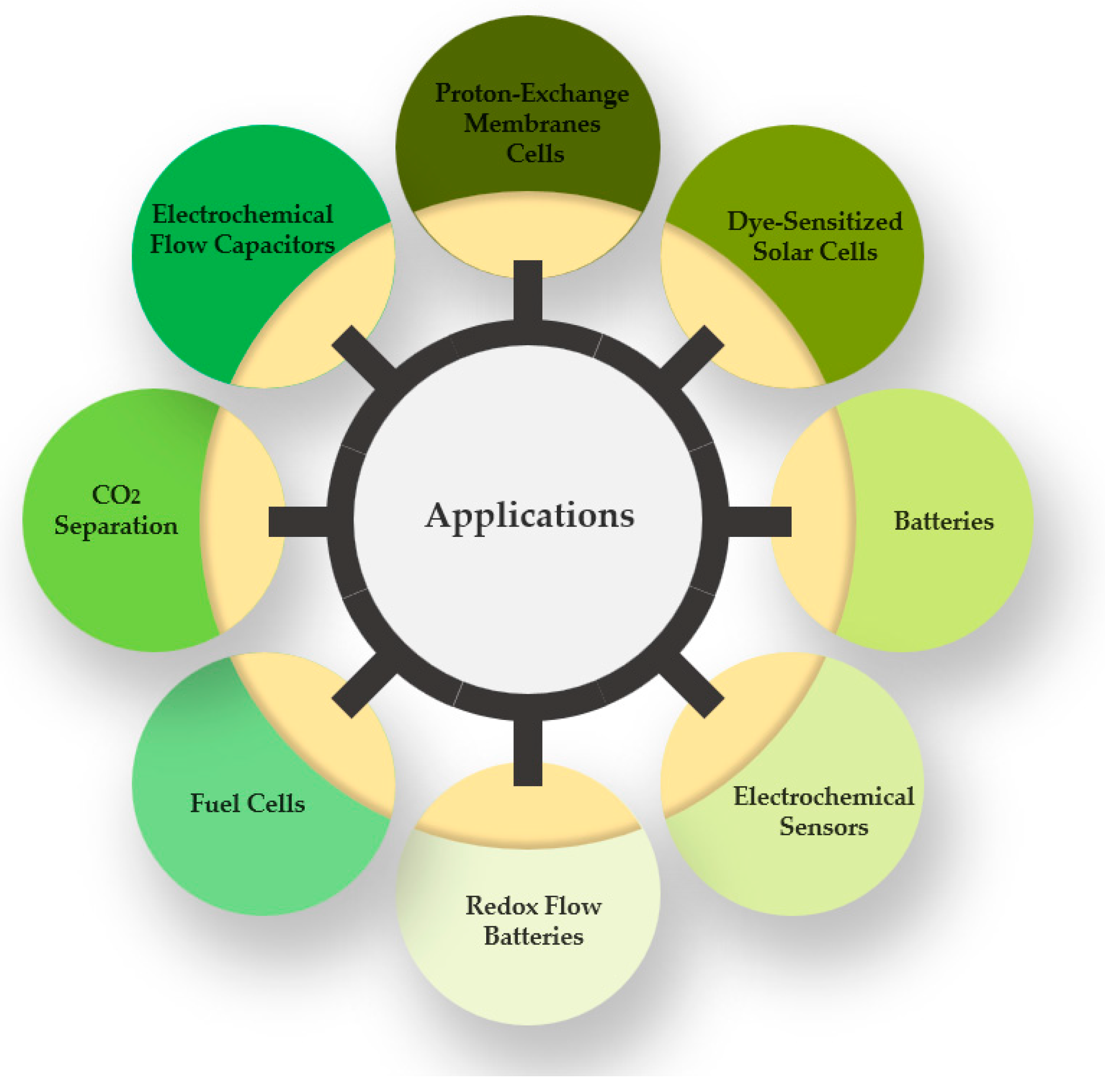
7. Applications
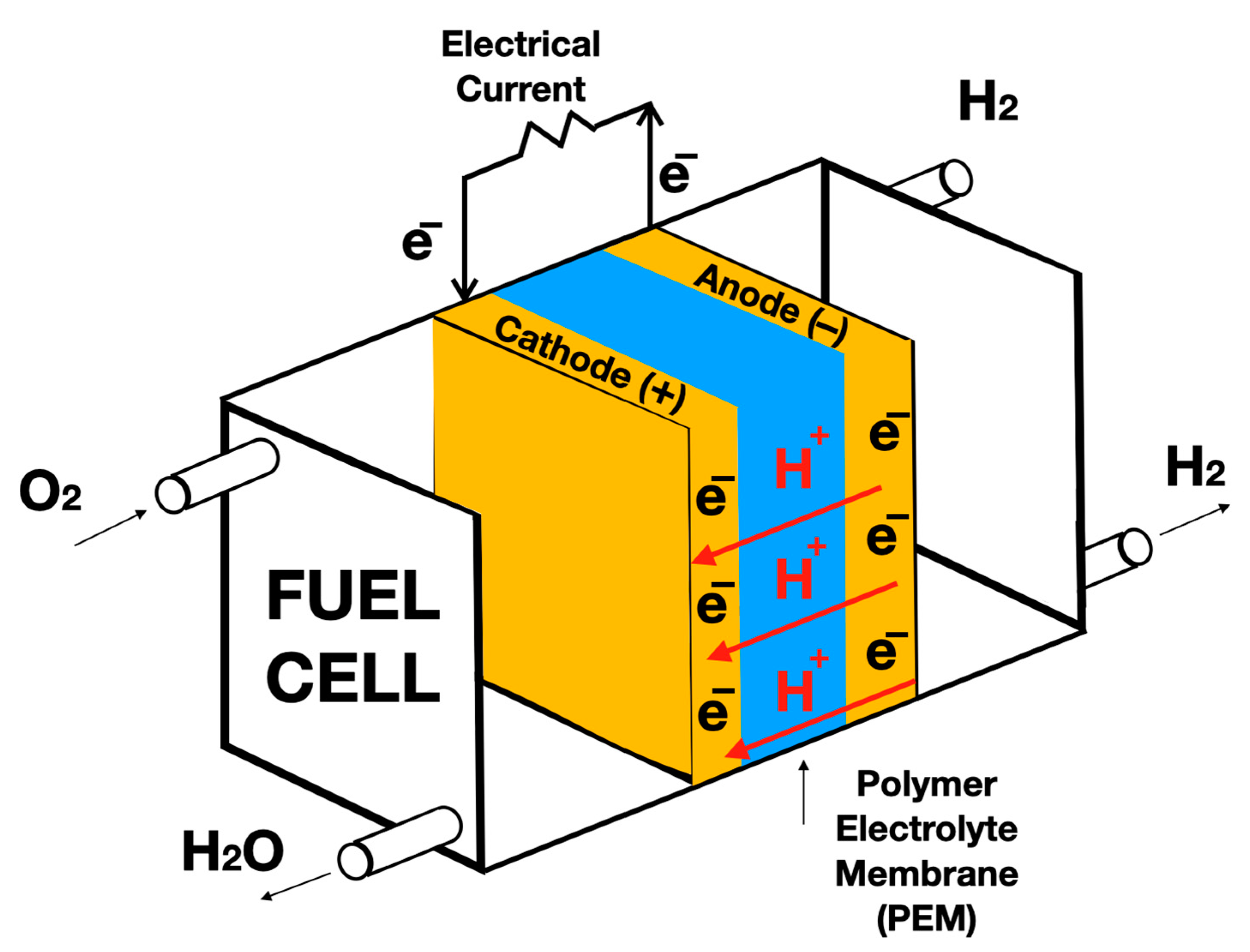
7.1. Fuel Cells
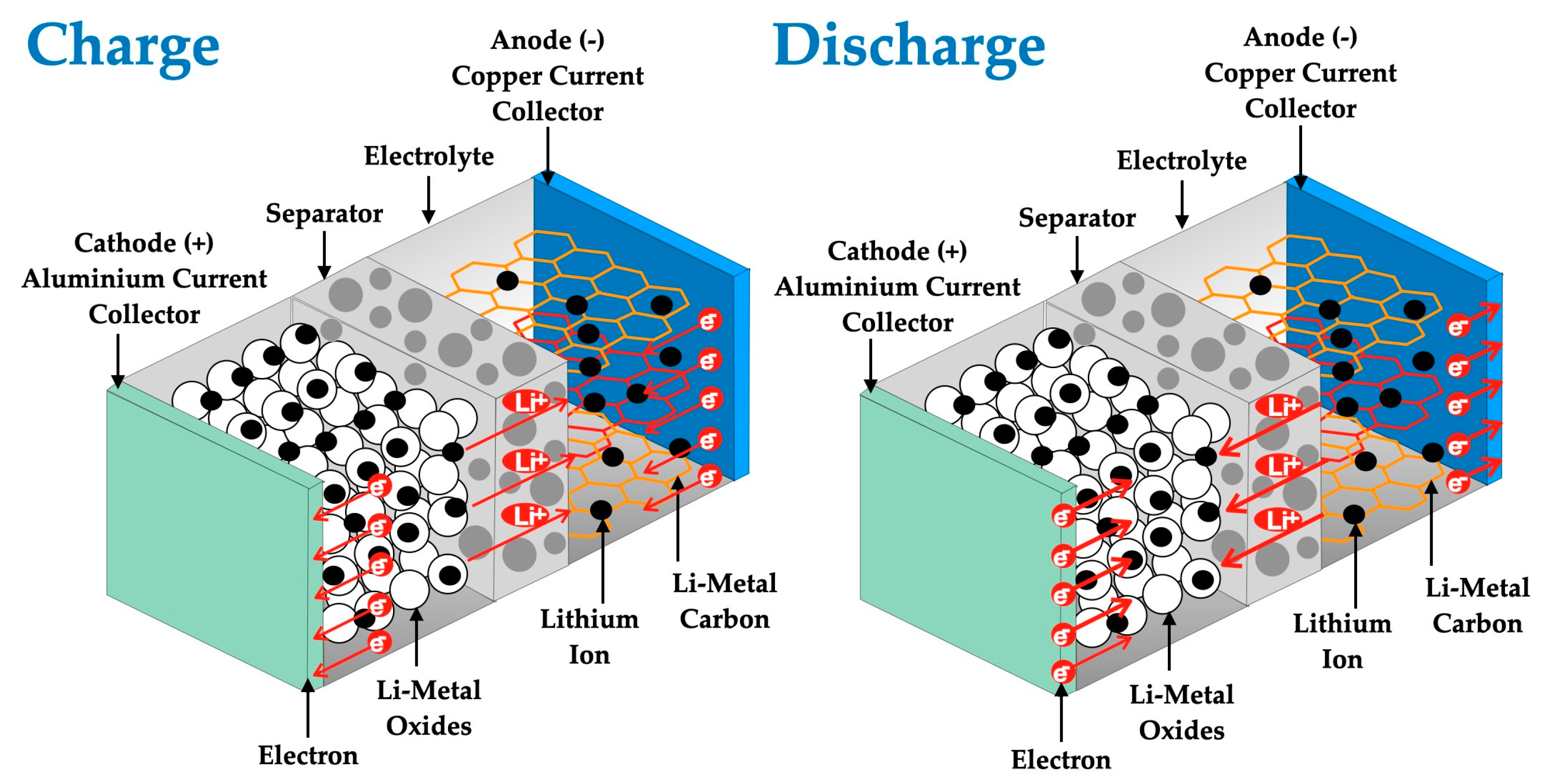
7.2. Batteries
7.3. Redox Flow Batteries
7.4. Electrochemical Flow Capacitors
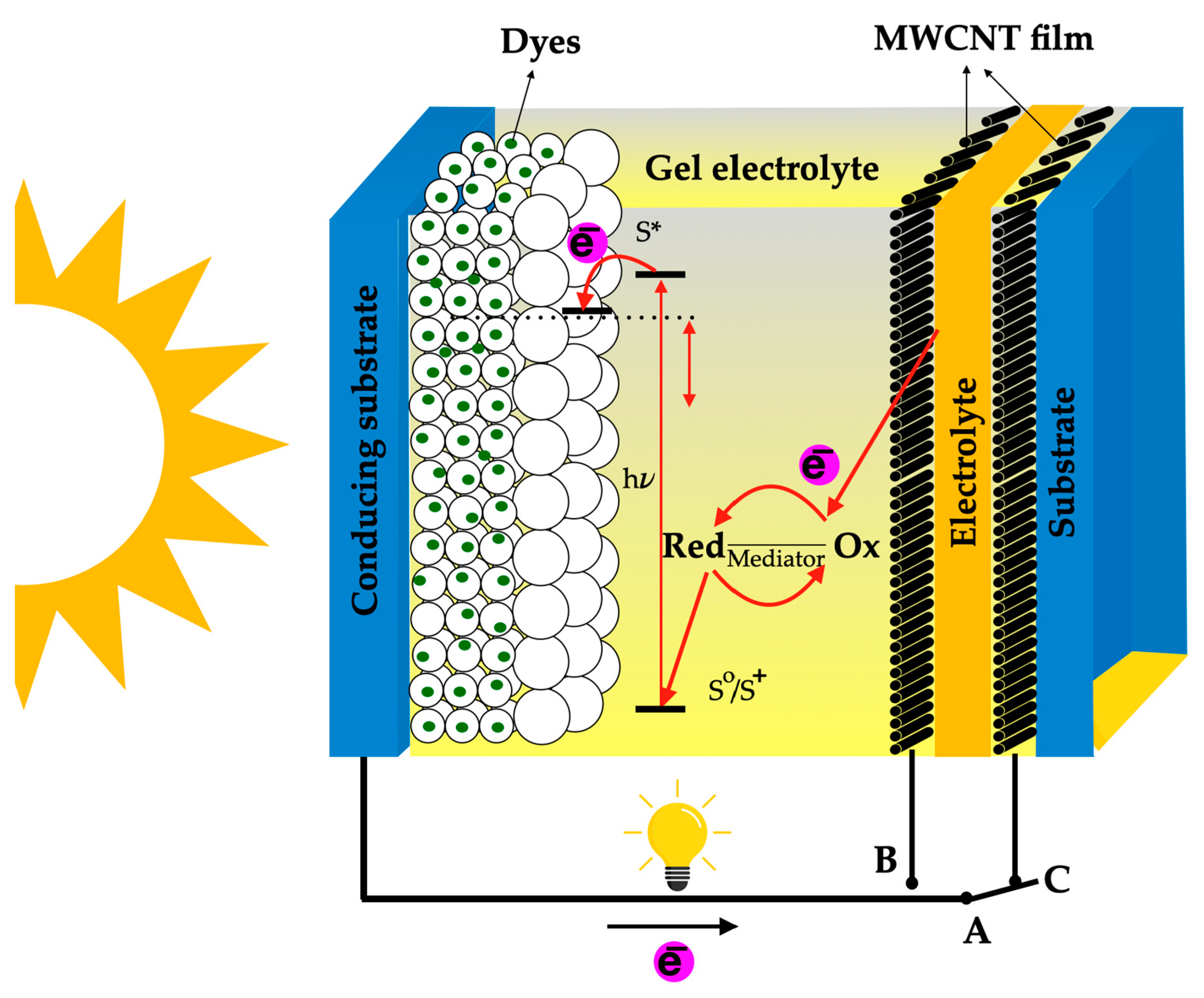
7.5. Dye-Sensitized Solar Cells
7.6. Electrochemical Sensors
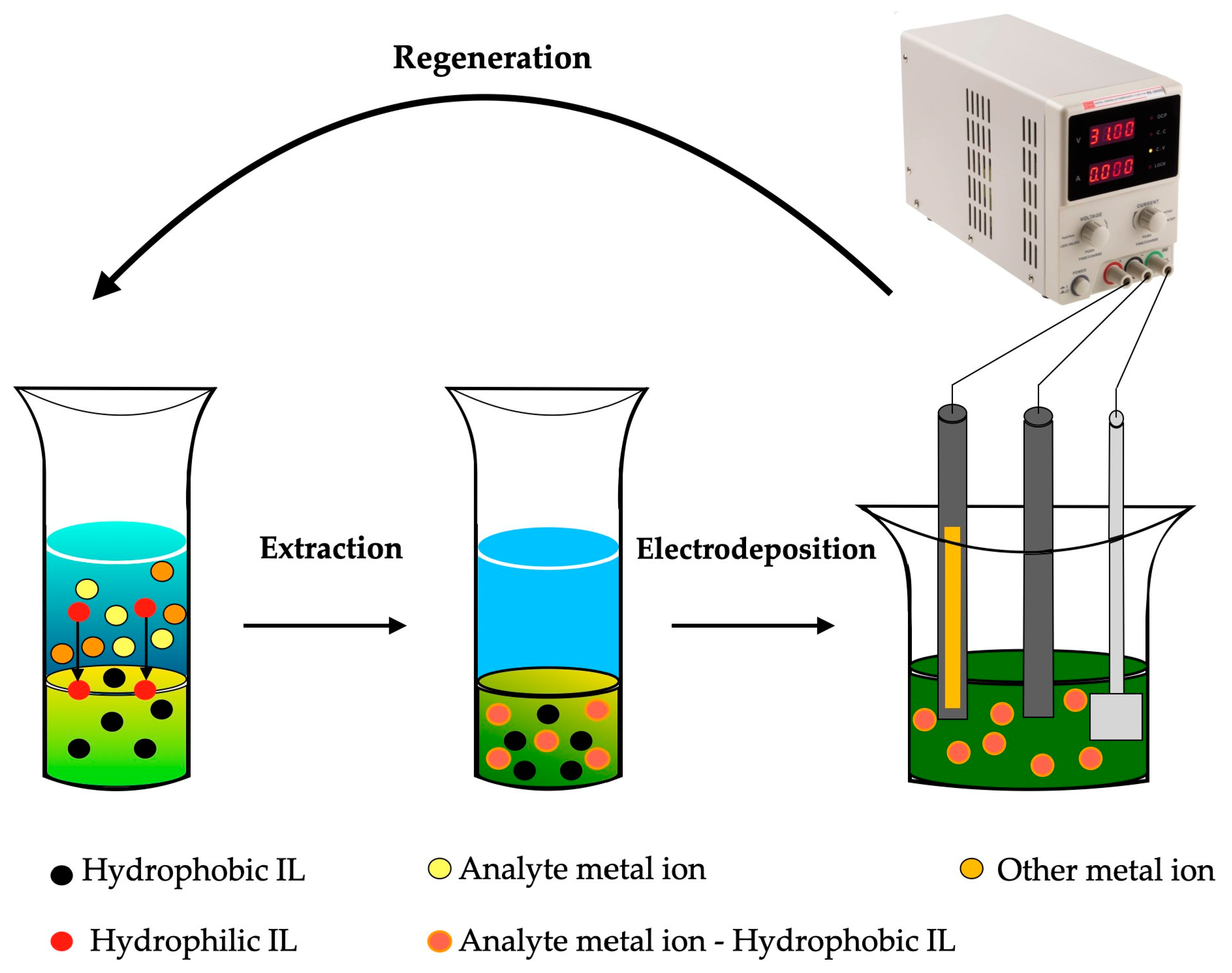
7.7. Metal Electrodeposition
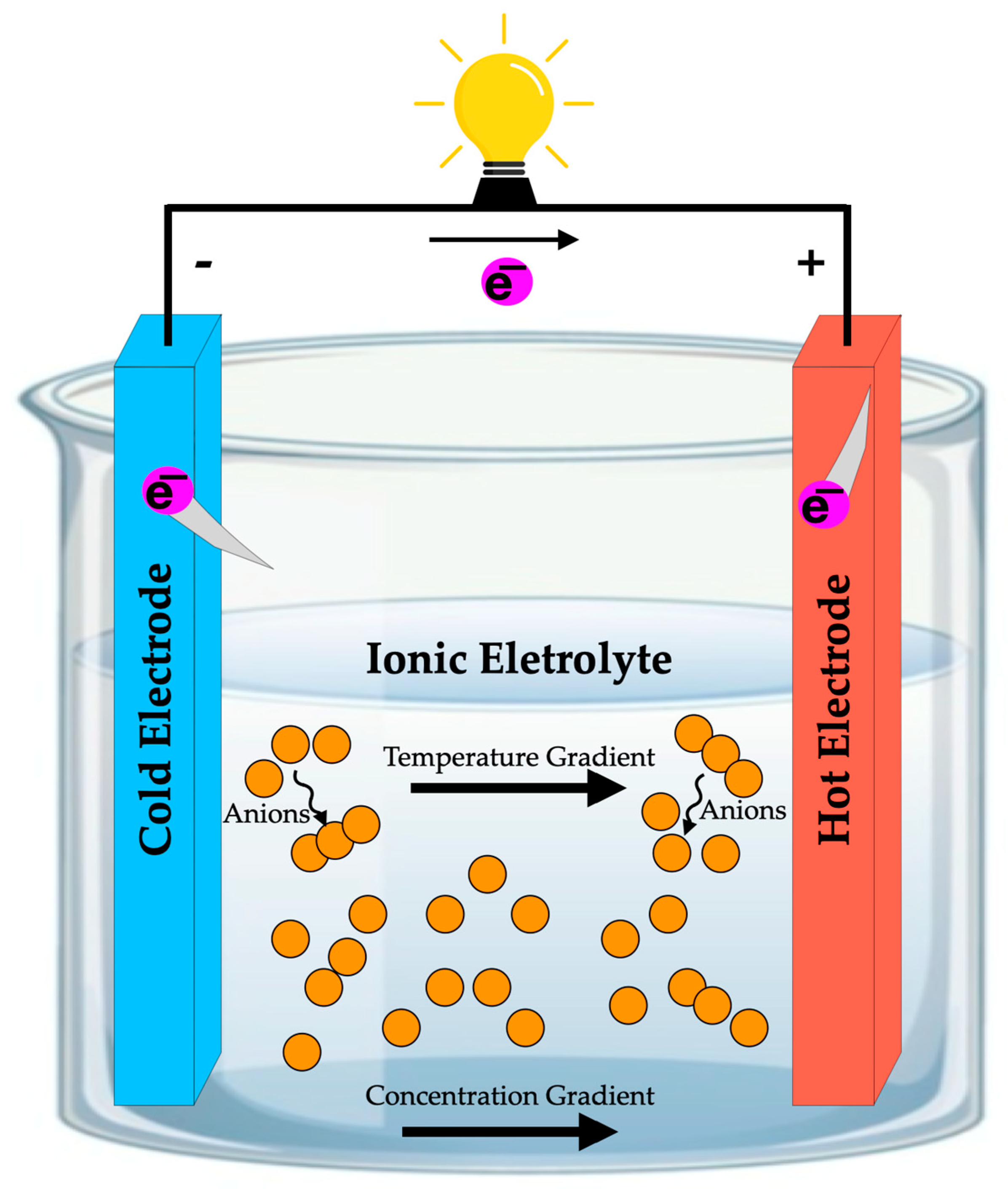
7.8. Thermo-Electrochemical Cells
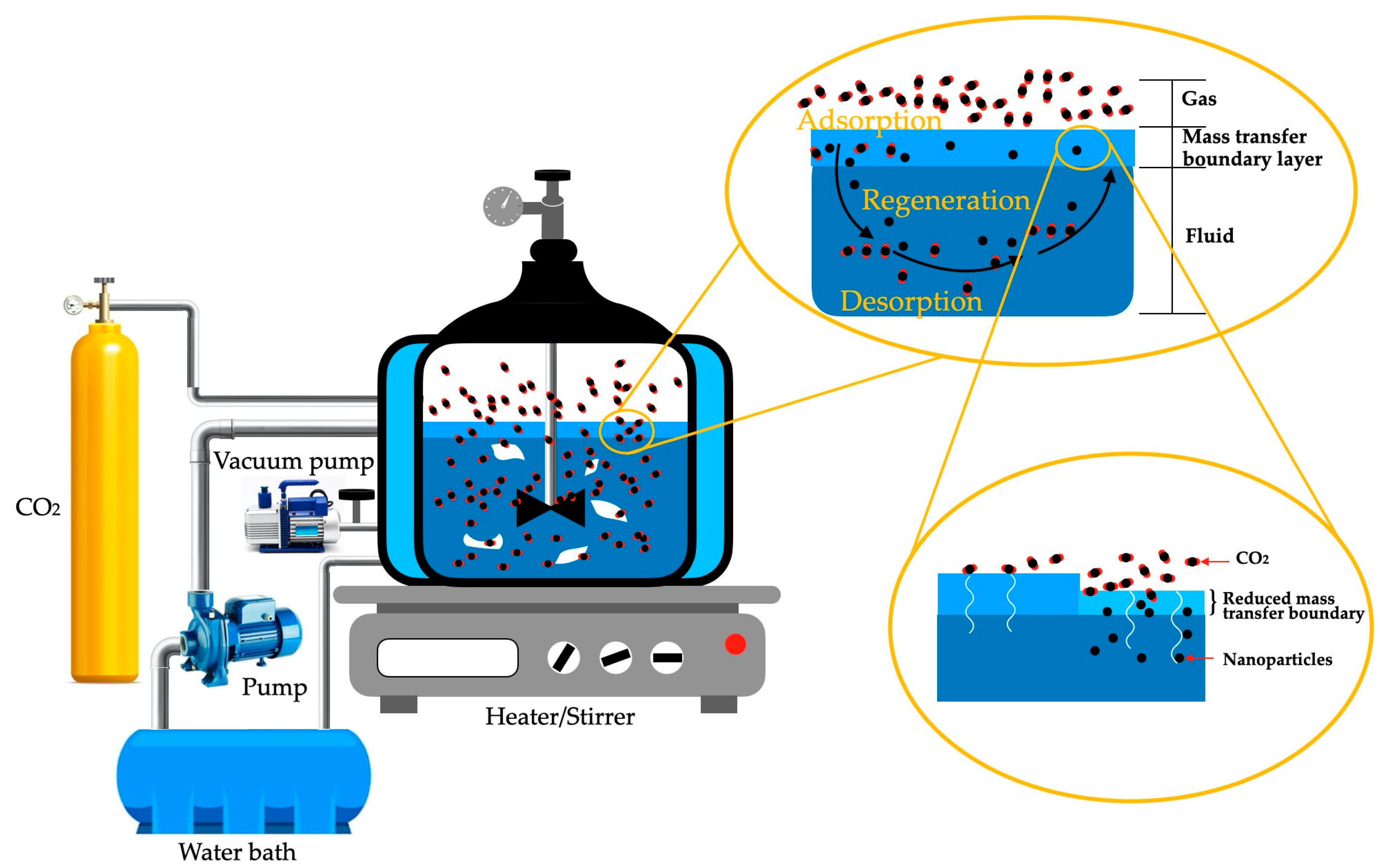
7.9. Carbon Dioxide Capture
8. Recommendations for Further Research Studies
- It is recommended to conduct more research studies on the electrochemical evaluation of the nanofluids with high concentrations of nanoparticles as liquid electrolytes and/or electrofuels to be applied in redox-flow batteries and on further improvement of the surface coating to optimize the electrochemical effectiveness.
- Functionalized nanotubes would reduce the electrical conductivity of nanofluids compared with that attained with non-functionalized prime nanotubes, given that they cause the rupture of the conjugated bond of the nanotubes. Nonetheless, such underlying mechanisms should be further addressed in additional research works on the topic.
- The addition of surfactants and additives may have a considerable impact on the electrochemical properties of the nanofluids, which should be further investigated and sustained by coordinated experimental works.
- Future research work should be focused on retrieving an empirical correlation considering the factors needed for determining the electrical conductivity of hybrid nanofluids in a more accurate way.
- Further research should be performed to infer the long-term electrochemical cycling performance of surface-modified nanomaterials dispersed in ionic liquids, stability of the surface graft, and overall efficiency in redox-flow cells in experiments with diverse concentrations of nanoparticles, ionic conductivities, and flow rates.
- Innovative electrochemical flow capacitors should be further investigated through experimental works using slurries with different nanomaterials, including the incorporation of materials with enhanced faradaic capacitance to be applied in better-performing flow electrochemical devices. Also, more works on the subject are most welcome.
- Regarding redox-flow batteries, the possibility of employing nano-sized pastes with high amounts of nanoparticles dispersed in liquids, thus combining the synergetic effect of the low viscosity of the nanofluids and good flowing features with the higher energy density of the pastes, should be further addressed.
- Further systematic molecular dynamics simulations are recommended for better understand ion mobility and the interactions at the interface between the ionic fluid electrolyte and the solid electrode.
- The development of ionic liquid precursors exhibiting synthesis ease, like, for instance, imidazolium bio-ionic liquids and porous ionic liquids, still needs further advances to obtain a durable super-capacitive activity derived from the charging mechanism of the electric double layer.
- Further attempts should be carried out to integrate the ionic liquid electrolyte and electrode components as individual equipment for manufacturing all-in-one ionic liquid-based capacitor systems.
- Most of the electrodeposition studies involving ionic liquids carried out so far are done at laboratory scale, and the literature findings about practical plating systems are rather scarce. Hence, further works on the scalability of the deposition processes are needed, along with more studies of the lifespan of metallic alloy coatings in the aerospace technological field. Also, there should be proposed ameliorated and innovative testing protocols to accurately assess the quality and lifespan of the coatings in laboratory environments mimicking real-life service conditions.
- Further research is needed on the suitability of the liquid electrodes (e.g., electroactive nanofluids) to be used in reserve batteries [151], in which one of the components is stored in an inactive form and gets activated when called upon. Also, the work performed by the researchers Parekh et al. [152] deserves special attention since it deals with the concept of lithium-ion reserve batteries, and particularly with the in-situ lithiation of lithium-ion free vanadium pentoxide cathode with graphitic anode.
9. Conclusions
- Ionic liquids, ionanofluids, and electroactive nanofluids are very suitable fluids to be applied in electrochemical processes like the ones described in this overview, given that they all possess improved electrochemical properties like enhanced electrical conductivity and capacitance.
- The electrical conductivity of the nanofluids can be influenced by the concentration and morphology of the nanoparticles and operating temperature, among other factors. A temperature increase leads to enhancements in the electrical conductivity mainly due to the Brownian motion increase. Also, a greater amount of solid phase enhances the nanofluid electrical conductivity.
- Most of the research found enhancements in the electrical conductivity of nanofluids derived from increases in the operating temperature and fraction of the nanoparticles. The main rationale behind the electrical conductivity growth at higher concentrations was the clustering of the nanoparticles. At high values of temperature, when the collision rate of the nanoparticles is enhanced owing to the greater kinetic energy, the electrical conductivity will also rise.
- The proposed empirical models and correlations to predict the electrical conductivity of the nanofluids are mostly functions of the temperature and concentration of the nanoparticles. The models are applicable only in a narrow range of concentrations and working temperatures. The temperature and concentration increases exhibited restrictions for increasing the electrical conductivity to a certain limit, beyond which an electrical conductivity decrease occurred. Also, the Maxwell model often could not predict the deviation in electrical conductivity when the nanoparticles were dispersed into the base fluid.
- It was verified that there is usually no considerable impact of the operating temperature on the electrical conductivity of nanofluids at temperatures between 30 °C and 60 °C. Such evidence demonstrates that the underlying mechanisms of electrical conductivity increase may be different from those of the nanofluid thermal conductivity increases.
- Ionic liquids have been gaining attention in automotive and aerospace research and technological areas for the electrodeposition of metals and alloys. Furthermore, the electrochemical stability of a great part of the ionic liquids under real plating scenarios at different current densities and temperatures still requires a better knowledge.
- The cation/anion designability of ionic liquids entails complex van der Walls interactions, electrostatic attractions, and hydrogen-bonding forces, allowing the exploration of ionic liquids in electrolyte and electrode synthesis for supercapacitors. Also, through controlling the pH, alkyl chain substituents, and other factors between the ions, the solid−liquid phase transitions of the ionic liquids are observed and adapt the integrated requirements in only one device.
- Poly(ionic liquid)s in a polymeric state are very promising alternatives to produce task-specific carbonaceous membranes in portable devices for gas separation.
- In the case of inert electrodes, the interfacial ion packing or double-layer capacitance can be increased through an increase in the pore compatibility between the electrode structures and the electrolyte ions of the ionic liquid or, alternatively, by blending the ratios of the ionic liquid eutectic mixtures. When dealing with pseudocapacitive electrodes, the energy storage capability can be strongly enhanced by protic ionic liquid electrolytes and redox-active mediators based on pseudocapacitive hybrid energy storage processes. Furthermore, porous ionic matrices are emerging to mitigate the environmental hazard derived from liquid leakage and corrosion and to be used in compact energy storage devices and systems.
- One of the fundamental concerns related with large-scale metal electrodeposition employing ionic liquids is to attain reproducible deposition with the intended throwing power, which is the ratio between the thickness of the deposit at high current density to thickness of the deposit at low current density of around one.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Souza, R.R.; Gonçalves, I.M.; Rodrigues, R.O.; Minas, G.; Miranda, J.M.; Moreira, A.L.N.; Lima, R.; Coutinho, G.; Pereira, J.E.; Moita, A.S. Recent advances on the thermal properties and applications of nanofluids: From nanomedicine to renewable energies. Appl. Therm. Eng. 2022, 201, 117725. [Google Scholar] [CrossRef]
- Zhu, R.; Feng, J.; Guo, Z. In Situ Observation of Dendrite Behavior of Electrode in Half and Full Cells. J. Electrochem. Soc. 2019, 166, A1107. [Google Scholar] [CrossRef]
- Galiński, M.; Lewandowski, A.; Stępniak, I. Ionic Liquids as Electrolytes. Electrochim. Acta 2006, 51, 5567–5580. [Google Scholar] [CrossRef]
- Dudney, N.J. Composite Electrolytes. Annu. Rev. Mater. Sci. 1989, 19, 103–120. [Google Scholar] [CrossRef]
- Cheng, F.; Liang, J.; Tao, Z.; Chen, J. Functional Materials for Rechargeable Batteries. Adv. Mater. 2011, 23, 1695–1715. [Google Scholar] [CrossRef]
- Hamm, S.C.; Basuray, S.; Mukherjee, S.; Sengupta, S.; Mathai, J.C.; Baker, G.A.; Gangopadhyay, S. Ionic Conductivity Enhancement of Sputtered Gold Nanoparticle-in-Ionic Liquid Electrolytes. J. Mater. Chem. A 2014, 2, 792–803. [Google Scholar] [CrossRef]
- Chen, F.-L.; Sun, I.-W.; Paul Wang, H.; Huang, C.-H. Nanosize Copper Dispersed Ionic Liquids as an Electrolyte of New Dye-Sensitized Solar Cells. J. Nanomater. 2009, 2009, 472950. [Google Scholar] [CrossRef]
- França, J.M.P.; Reis, F.; Vieira, S.I.C.; Lourenço, M.J.V.; Santos, F.J.V.; Nieto de Castro, C.A.; Pádua, A.A.H. Thermophysical Properties of Ionic Liquid Dicyanamide (DCA) Nanosystems. J. Chem. Thermodyn. 2014, 79, 248–257. [Google Scholar] [CrossRef]
- Faizan, M.; Ahmed, R.; Ali, H.M. A Critical Review on Thermophysical and Electrochemical Properties of Ionanofluids (Nanoparticles Dispersed in Ionic Liquids) and Their Applications. J. Taiwan Inst. Chem. Eng. 2021, 124, 391–423. [Google Scholar] [CrossRef]
- Saadmim, F.; Forhad, T.; Sikder, A.; Ghann, W.; MAli, M.; Sitther, V.; Ahammad, A.J.S.; Subhan, M.A.; Uddin, J. Enhancing the Performance of Dye Sensitized Solar Cells Using Silver Nanoparticles Modified Photoanode. Molecules 2020, 25, 4021. [Google Scholar] [CrossRef]
- Adhyaksa, G.W.P.; Baek, S.-W.; Lee, G.I.; Lee, D.K.; Lee, J.-Y.; Kang, J.K. Coupled Near- and Far-Field Scattering in Silver Nanoparticles for High-Efficiency, Stable, and Thin Plasmonic Dye-Sensitized Solar Cells. ChemSusChem 2014, 7, 2461–2468. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, J.C. A Treatise of Electricity and Magnetism, 3rd ed.; Oxford University Press: London, UK, 1892; Volume 1, Chapter IX; pp. 435–449. [Google Scholar]
- Bruggeman, D.A.G. Berechnung Verschiedener Physicalischer Konstanten yon Heterogenen Substanzen. Ann. Phys. 1935, 24, 639–664. [Google Scholar]
- Zyła, G.; Vallejo, J.P.; Fal, J.; Lugo, L. Nanodiamonds—Ethylene Glycol nanofluids: Experimental investigation of fundamental physical properties. Int. J. Heat Mass Trans. 2018, 121, 1201–1213. [Google Scholar] [CrossRef]
- Zyła, G.; Fal, J. Experimental studies on viscosity, thermal and electrical conductivity of aluminum nitride–ethylene glycol (AlN–EG) nanofluids. Thermoch. Acta 2016, 637, 11–16. [Google Scholar] [CrossRef]
- Zyła, G.; Fal, J. Viscosity, thermal and electrical conductivity of silicon dioxide–ethylene glycol transparent nanofluids: An experimental studies. Thermoch. Acta 2017, 650, 106–113. [Google Scholar] [CrossRef]
- Zyła, G.; Fal, J.; Biki’c, S.; Wanic, M. Ethylene glycol based silicon nitride nanofluids: An experimental study on their thermophysical, electrical and optical properties. Phys. E Low-Dimens. Syst. Nanostruct. 2018, 104, 82–90. [Google Scholar] [CrossRef]
- Shen, L.P.; Wang, H.; Dong, M.; Ma, Z.C.; Wang, H.B. Solvothermal synthesis and electrical conductivity model for the zinc oxide-insulated oil nanofluid. Phys. Lett. A 2012, 376, 1053–1057. [Google Scholar] [CrossRef]
- Coelho, M.F.; Rivas, M.A.; Vilão, G.; Nogueira, E.M.; Iglesias, T.P. Permittivity and electrical conductivity of copper oxide nanofluid (12 nm) in water at different temperatures. J. Chem. Thermodyn. 2019, 132, 164–173. [Google Scholar] [CrossRef]
- Hill, A.V. The possible effects of the aggregation of the molecules of hemoglobin on its dissociation curves. J. Physiol. 1910, 40, 4–7. [Google Scholar]
- Anu, K.; Hemalatha, J. Magnetic and electrical conductivity studies of zinc doped cobalt ferrite nanofluids. J. Mol. Liq. 2019, 284, 445–453. [Google Scholar] [CrossRef]
- Ganguly, S.; Sikdar, S.; Basu, S. Experimental investigation of the effective electrical conductivity of aluminum oxide nanofluids. Power Technol. 2009, 196, 326–330. [Google Scholar] [CrossRef]
- Minea, A.A.; Luciu, R.S. Investigations on electrical conductivity of stabilized water based Al2O3 nanofluids. Microfluid. Nanofluid. 2012, 13, 977–985. [Google Scholar] [CrossRef]
- Sundar, L.S.; Shusmitha, K.; Singh, M.K.; Sousa, A.C.M. Electrical conductivity enhancement of nanodiamond–nickel (ND–Ni) nanocomposite based magnetic nanofluids. Int. Commun. Heat Mass 2014, 57, 1–7. [Google Scholar] [CrossRef]
- Chereches, E.I.; Minea, A.A. Electrical conductivity of new nanoparticle enhanced fluids: An experimental study. Nanomaterials 2019, 9, 1228. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.R.; Shabani, B.; Rosengarten, G. Electrical and thermal conductivities of 50/50 water-ethylene glycol based TiO2 nanofluids to be used as coolants in PEM fuel cells. Energy Proc. 2017, 110, 101–108. [Google Scholar] [CrossRef]
- Islam, R.; Shabani, B. Prediction of electrical conductivity of TiO2 water and ethylene glycol-based nanofluids for cooling application in low temperature PEM fuel cells. Energy Proc. 2019, 160, 550–557. [Google Scholar] [CrossRef]
- METTLER TOLEDO Group. CH-8606, A Guide to Conductivity Measurement: Theory and Practice of Conductivity Applications, Nänikon, Switzerland. 2022. Available online: https://static.fishersci.eu/content/dam/fishersci/en_EU/Brands/18162_Mettler_Toledo_New/Guide_Conductivity_EN_30099121B.pdf (accessed on 1 November 2023).
- Ezekwem, C.; Dare, A. Thermal and electrical conductivity of silicon carbide nanofluids. Energy Sources Part A Recovery Util. Environ. Effects 2020. [Google Scholar] [CrossRef]
- Giwa, S.O.; Sharifpur, M.; Meyer, J.P.; Wongwises, S.; Mahian, O. Experimental measurement of viscosity and electrical conductivity of water-based γ-Al2O3/MWCNT hybrid nanofluids with various particle mass ratios. J. Therm. Anal. Calorim. 2021, 143, 1037–1050. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, T.; Zhang, D.; Wang, Q. Experimental investigation of thermal and electrical conductivity of silicon oxide nanofluids in ethylene glycol/water mixture. Int. J. Heat Mass Transf. 2018, 117, 280–286. [Google Scholar] [CrossRef]
- Iglesias, T.P.; Rivas, M.A.; Iglesias, R.; Reis, J.C.R.; Cohelho, F. Electric permittivity and conductivity of nanofluids consisting of 15nm particles of alumina in base Milli-Q and Milli-Ro water at different temperatures. J. Chem. Thermodyn. 2013, 66, 123–130. [Google Scholar] [CrossRef]
- Ponmani, S.; Nagarajan, R.; Sangwai, J.S. Effect of Nanofluids of CuO and ZnO in Polyethylene Glycol and Polyvinylpyrrolidone on the Thermal, Electrical, and Filtration-Loss Properties of Water-Based Drilling Fluids. SPE J. 2016, 21, 405–415. [Google Scholar] [CrossRef]
- Khdher, A.M.; Sidik, N.A.C.; Hamzah, W.A.W.; Mamat, R. An experimental determination of thermal conductivity and electrical conductivity of bio glycol based Al2O3 nanofluids and development of new correlation. Int. Commun. Heat Mass Transf. 2016, 73, 75–83. [Google Scholar] [CrossRef]
- Ijam, A.; Saidur, R.; Ganesan, P.; Golsheikh, A.M. Stability, thermo-physical properties, and electrical conductivity of graphene oxide-deionized water/ethylene glycol based nanofluid. Int. J. Heat Mass Transf. 2015, 87, 92–103. [Google Scholar] [CrossRef]
- Sarojini, K.G.K.; Mnoj, S.V.; Singh, P.K.; Pradeep, T.; Das, S.K. Electrical conductivity of ceramic and metallic nanofluids. Colloids Surf. A Physicochem. Eng. Asp. 2013, 417, 39–46. [Google Scholar] [CrossRef]
- Fal, J.; Barylyak, A.; Besaha, K.; Bobitski, Z.V.; Cholewa, M.; Zawlik, I.; Szmuc, K.; Cebulski, J.; Żyła, G. Experimental Investigation of Electrical Conductivity and Permittivity of SC-TiO2 -EG Nanofluids. Nanoscale Res. Lett. 2016, 11, 375. [Google Scholar] [CrossRef]
- Hadadian, M.; Goharshadi, E.K.; Youssefi, A. Electrical Conductivity, Thermal Conductivity, and Rheological Properties of Gra-phene Oxide-Based Nanofluids. J. Nanoparticle Res. 2014, 16, 2788. [Google Scholar] [CrossRef]
- Bagheli, S.; Fadafan, H.K.; LotfiOrimi, R.; Ghaemi, M. Synthesis and experimental investigation of the electrical conductivity of water-based magnetite nanofluids. Power Technol. 2015, 274, 426–430. [Google Scholar] [CrossRef]
- White, S.B.; Shih, A.J.-M.; Pipe, K.P. Investigation of the Electrical Conductivity of Propylene Glycol-Based ZnO Nanofluids. Nanoscale Res. Lett. 2011, 6, 346. [Google Scholar] [CrossRef]
- Ramalingam, G.; Vignesh, R.; Ragupathi, C.; Magdalane, C.M.; Kaviyarasu, K.; Kennedy, J. Electrical and Chemical Stability of CuS Nanofluids for Conductivity of Water Soluble Based Nanocomposites. Surf. Interfaces 2020, 19, 100475. [Google Scholar] [CrossRef]
- Rodríguez-Palmeiro, I.; Rodríguez-Cabo, B.; Rodil, E.; Arce, A.; Saiz-Jabardo, J.M.; Soto, A. Synthesis and Characterization of Highly Concentrated AgI–[P6,6,6,14]Cl Ionanofluids. J. Nanoparticle Res. 2013, 15, 1881. [Google Scholar] [CrossRef]
- Alizadeh, J.; Keshavarz Moraveji, M. An Experimental Evaluation on Thermophysical Properties of Functionalized Graphene Nanoplatelets Ionanofluids. Int. Commun. Heat Mass Transf. 2018, 98, 31–40. [Google Scholar] [CrossRef]
- Chereches, E.; Minea, A.A. Experimental evaluation of electrical conductivity of ionanofluids based on water–[C2mim][CH3SO3] ionic liquids mixtures and alumina nanoparticles. J. Therm. Anal. Calorim. 2020, 145, 3151–3157. [Google Scholar] [CrossRef]
- Das, L.; Habib, K.; Saidur, R.; Aslfattahi, N.; Yahya, S.M.; Rubbi, F. Improved Thermophysical Properties and Energy Efficiency of Aqueous Ionic Liquid/MXene Nanofluid in a Hybrid PV/T Solar System. Nanomaterials 2020, 10, 1372. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Alexandridis, P. Ionic Liquid and Nanoparticle Hybrid Systems: Emerging Applications. Adv. Colloid Interface Sci. 2017, 244, 54–70. [Google Scholar] [CrossRef] [PubMed]
- Deb, D.; Dutta, B.; Bhattacharya, S. Viscosity Decoupled Charge Transport in Surface Functionalized ZnS Nanoparticle Dispersed Imidazolium Ionanofluids. Mater. Res. Bull. 2019, 116, 22–31. [Google Scholar] [CrossRef]
- Dubal, D.P.; Rueda-Garcia, D.; Marchante, C.; Benages, R.; Gomez-Romero, P. Hybrid Graphene-Polyoxometalates Nanofluids as Liquid Electrodes for Dual Energy Storage in Novel Flow Cells. Chem Rec. 2018, 18, 1076–1084. [Google Scholar] [CrossRef]
- Oster, K.; Hardacre, C.; Jacquemin, J.; Ribeiro, A.P.C.; Elsinawi, A. Ionic liquid-based nanofluids (Ionanofluids) for thermal applications: An experimental thermophysical characterization. Pure Appl. Chem. 2019, 91, 1309–1340. [Google Scholar] [CrossRef]
- De Souza, R.F.; Padilha, J.C.; Gonçalves, R.S.; Dupont, J. Room temperature dialkylimidazolium ionic liquid-based fuel cells. Electrochem. Commun. 2003, 5, 728–731. [Google Scholar] [CrossRef]
- Brennecke, J.F.; Maginn, E.J. Ionic liquids: Innovative fluids for chemical processing. AIChE J. 2001, 47, 2384–2389. [Google Scholar] [CrossRef]
- Asleshirin, S.; Mazaheri, H.; Omidkhah, M.R.; Joshaghani, A.H. Investigation of Thermophysical Properties of Io Nanofluids Containing Multi-Walled Carbon Nanotubes and Graphene. Iran. J. Chem. Chem. Eng. 2022, 41, 380–391. [Google Scholar] [CrossRef]
- Ribeiro, A.P.C.; Vieira, S.I.C.; França, J.M.; Queirós, C.S.; Langa, E.; Lourenço, M.J.V.; Murshed, S.M.S.; de Castro, C.A.N. Thermal Properties of Ionic Liquids and Ionanofluids. In Ionic Liquids: Theory, Properties, New Approaches; Kokorin, A., Ed.; IntechOpen: Rijeka, Croatia, 2011; Chapter 2; pp. 37–60. [Google Scholar] [CrossRef]
- Chen, K.; Xu, B.; Shen, L.; Shen, D.; Li, M.; Guo, L.-H. Functions and performance of ionic liquids in enhancing electrocatalytic hydrogen evolution reactions: A comprehensive review. RSC Adv. 2022, 12, 19452–19469. [Google Scholar] [CrossRef] [PubMed]
- Jónsson, E. Ionic liquids as electrolytes for energy storage applications—A modelling perspective. Energy Storage Mater. 2020, 25, 827–835. [Google Scholar] [CrossRef]
- Bose, P.; Deb, D.; Bhattacharya, S. Ionic liquid based nanofluid electrolytes with higher lithium salt concentration for high-efficiency, safer, lithium metal batteries. J. Power Sources 2018, 406, 176–184. [Google Scholar] [CrossRef]
- Lu, Y.Y.; Das, S.K.; Moganty, S.S.; Archer, L.A. Ionic liquid-nanoparticle hybrid electrolytes and their application in secondary lithium-metal batteries. Adv. Mater. 2012, 24, 4430–4435. [Google Scholar] [CrossRef] [PubMed]
- Moganty, S.S.; Jayaprakash, N.; Nugent, J.L.; Shen, J.; Archer, L.A. Ionic-liquid-tethered nanoparticles: Hybrid electrolytes. Angew. Chem. Int. Ed. 2010, 49, 9158–9161. [Google Scholar] [CrossRef]
- Liu, K.; Wang, Z.; Shi, L.; Jungsuttiwong, S.; Yuan, S. Ionic liquids for high performance lithium metal batteries. J. Energy Chem. 2021, 59, 320–333. [Google Scholar] [CrossRef]
- Lu, Y.; Moganty, S.S.; Schaefera, J.L.; Archer, L.A. Ionic liquid-nanoparticle hybrid electrolytes. J. Mater. Chem. 2012, 22, 4066–4072. [Google Scholar] [CrossRef]
- Jehhef, K.A.; Yasin, N.J. Effect of Ceramic Nanoparticles on Nanofluids Electrical Conductivity. IOP Conf. Ser. Mater. Sci. Eng. 2019, 518, 32002. [Google Scholar] [CrossRef]
- Almeida, C.; Paul, S.; Asirvatham, L.G.; Manova, S.; Nimmagadda, R.; Bose, J.R.; Wongwises, S. Experimental Studies on Thermophysical Electrical Properties of Graphene–Transformer Oil, Nanofluid. Fluids 2020, 5, 172. [Google Scholar] [CrossRef]
- Ezekwem, C.; Dare, A. Thermal and electrical conductivity of Aluminium Nitride nanofluids. Int. J. Nano Dimens. 2020, 11, 1–11. [Google Scholar]
- Sen, S.; Chow, C.-M.; Moazzen, E.; Segre, C.U.; Timofeeva, E. V Electroactive Nanofluids with High Solid Loading and Low Viscosity for Rechargeable Redox Flow Batteries. J. Appl. Electrochem. 2017, 47, 593–605. [Google Scholar] [CrossRef]
- Dubal, D.P.; Gomez-Romero, P. Electroactive Graphene Nanofluids for Fast Energy Storage. 2D Mater. 2016, 3, 31004. [Google Scholar] [CrossRef]
- Rahimi, M.; Molaei Dehkordi, A.; Roberts, E.P.L. Magnetic Nanofluidic Electrolyte for Enhancing the Performance of Polysulfide/Iodide Redox Flow Batteries. Electrochim. Acta 2021, 369, 137687. [Google Scholar] [CrossRef]
- Myures, X.M.; Suresh, S. Nanofluidic Electrolyte Based on Nitrogen Doped Reduced Graphene Oxide as an Electrocatalyst for VO2+/VO2+ in Vanadium Redox Flow Battery. J. Energy Storage 2023, 58, 106387. [Google Scholar] [CrossRef]
- Ajeeb, W.; Murshed, S.M.S. Characterization of Thermophysical and Electrical Properties of SiC and BN Nanofluids. Energies 2023, 16, 3768. [Google Scholar] [CrossRef]
- De Castro, C.A.N.; Murshed, S.M.S.; Lourenço, M.J.V.; Santos, F.J.V.; Lopes, M.L.M.; França, J.M.P. Ionanofluids: New Heat Transfer Fluids for Green Processes Development. In Green Solvents I: Properties and Applications in Chemistry; Mohammad, A., Ed.; Springer: Dordrecht, The Netherlands, 2012; pp. 233–249. [Google Scholar] [CrossRef]
- Ettefaghi, E.; Rashidi, A.; Ahmadi, H.; Mohtasebi, S.S.; Pourkhalil, M. Thermal and rheological properties of oil-based nanofluids from different carbon nanostructures. Int. Commun. Heat Mass Transf. 2013, 48, 178–182. [Google Scholar] [CrossRef]
- Ribeiro, C.A.; Lourenço, A.P.C.; Nieto de Castro, M.J.V. Thermal conductivity of Ionanofluids. In Proceedings of the 17th Symposium on Thermophysical Properties, Boulder, CO, USA, 21–26 June 2009. [Google Scholar]
- Jóźwiak, B.; Boncel, S. Rheology of Ionanofluids—A review. J. Mol. Liq. 2020, 302, 112568. [Google Scholar] [CrossRef]
- Joseph, A.; Xavier, M.M.; Fal, J.; Żyła, G.; Sasi, S.; Radhakrishnan Nair, P.; Padmanabhan, A.S.; Mathew, S. Synthesis and Electrochemical Characterization of Electroactive IoNanofluids with High Dielectric Constants from Hydrated Ferrous Sulphate. Chem. Commun. 2019, 55, 83–86. [Google Scholar] [CrossRef]
- Bakthavatchalam, B.; Habib, K.; Saidur, R.; Aslfattahi, N.; Rashedi, A. Investigation of Electrical Conductivity, Optical Property, and Stability of 2D MXene Nanofluid Containing Ionic Liquids. Appl. Sci. 2020, 10, 8943. [Google Scholar] [CrossRef]
- Deb, D.; Bose, P.; Bhattacharya, S. Gel-polymer electrolytes plasticized with pyrrolidinium-based ionanofluid for lithium battery applications. Ionics 2020, 27, 123–136. [Google Scholar] [CrossRef]
- Nurdin, I. Satriananda. Investigation on Electrical Conductivity Enhancement of Water Based Maghemite (γ-Fe2O3) Nanofluids. Int. J. Mater. Sci. Appl. 2017, 6, 32–36. [Google Scholar] [CrossRef]
- Shirazi, S.F.S.; Gharehkhani, S.; Yarmand, H.; Badarudin, A.; Metselaar, H.S.C.; Kazi, S.N. Nitrogen doped activated carbon/graphene with high nitrogen level: Green synthesis and thermo-electrical properties of its nanofluid. Mater. Lett. 2015, 152, 192–195. [Google Scholar] [CrossRef]
- Akilu, S.; Baheta, A.T.; Kadirgama, K.; Padmanabhan, E.; Sharma, K.V. Viscosity, electrical and thermal conductivities of ethylene and propylene glycol-based β-SiCnanofluids. J. Mol. Liq. 2019, 284, 780–792. [Google Scholar] [CrossRef]
- Zawrah, M.F.; Khattab, R.M.; Girgis, L.G.; El Daidamony, H.; Abdel Aziz Rehab, E. Stability and electrical conductivity of water-base Al2O3 nanofluids for different applications. HBRC J. 2016, 12, 227–234. [Google Scholar] [CrossRef]
- Cherecheş, E.I.; Bejan, D.; Ibanescu, C.; Danu, M.; Minea, A.A. Ionanofluids with [C2mim][CH3SO3] Ionic Liquid and Alumina Nanoparticles: An Experimental Study on Viscosity, Specific Heat and Electrical Conductivity. Chem. Eng. Sci. 2021, 229, 116140. [Google Scholar] [CrossRef]
- Wei, S.; Balakin, B.V.; Kosinski, P. Investigation of nanofluids in alkaline electrolytes: Stability, electrical properties, and hydrogen production. J. Clean. Prod. 2023, 414, 137723. [Google Scholar] [CrossRef]
- Liu, C.; Lee, H.; Chang, Y.-H.; Feng, S.-P. The study of electrical conductivity and diffusion behavior of water-based and ferro/ferricyanide-electrolyte-based alumina nanofluids. J. Colloid Interface Sci. 2016, 469, 17–24. [Google Scholar] [CrossRef]
- O’Brien, R.W. The Electrical Conductivity of a Dilute Suspension of Charged Particles. J. Colloid Interface Sci. 1981, 81, 234–248. [Google Scholar] [CrossRef]
- Glover, B.; Whites, K.W.; Hong, H.; Mukherjee, A.; Billups, W.E. Effective Electrical Conductivity of Functional Single-Wall Carbon Nanotubes in Aqueous Fluids. Synth. Met. 2008, 158, 506–508. [Google Scholar] [CrossRef]
- Mashali, F.; Languri, E.; Mirshekari, G.; Davidson, J.; Kerns, D. Nano diamond nanofluid microstructural and thermo-electrical characterization. Int. Commun. Heat Mass 2019, 101, 82–88. [Google Scholar] [CrossRef]
- Abdolbaqi, M.K.; Azmi, W.H.; Mamat, R.; Sharma, K.V.; Najafi, G. Experimental investigation of thermal conductivity and electrical conductivity of BioGlycol–water mixture based Al2O3 nanofluid. Appl. Therm. Eng. 2016, 102, 932–941. [Google Scholar] [CrossRef]
- Shoghl, S.N.; Jamali, J.; Moraveji, M.K. Electrical conductivity, viscosity, and density of different nanofluids: An experimental study. Exp. Therm. Fluid Sci. 2016, 74, 339–346. [Google Scholar] [CrossRef]
- Esfe, M.H.; Afrand, M. A review on fuel cell types and the application of nanofluid in their cooling. J. Therm. Anal. Calorim. 2020, 140, 1633–1654. [Google Scholar] [CrossRef]
- Zakaria, I.; Mohamed, W.A.N.W.; Azmi, W.H.; Mamat, A.M.I.; Mamat, R.; Daud, W.R.W. Thermo-electrical performance of PEM fuel cell using Al2O3 nanofluids. Int. J. Heat Mass Transf. 2018, 119, 460–471. [Google Scholar] [CrossRef]
- Yu, W.; Xie, H. A Review on Nanofluids: Preparation, Stability Mechanisms, and Applications. J. Nanomater. 2012, 2012, 435873. [Google Scholar] [CrossRef]
- Islam, M.R.; Shabani, B.; Rosengarten, G.; Andrews, J. The Potential of Using Nanofluids in PEM Fuel Cell Cooling Systems: A Review. Renew. Sustain. Energy Rev. 2015, 48, 523–539. [Google Scholar] [CrossRef]
- Manna, I. Synthesis, Characterization and Application of Nanofluid—An Overview. J. Indian Inst. Sci. 2009, 89, 21–33. [Google Scholar]
- Yu, W.; France, D.M.; Choi, S.U.S.; Routbort, J.L. Review and Assessment of Nanofluid Technology for Transportation and Other Applications; Argonne National Lab. (ANL): Argonne, IL, USA, 2007. [Google Scholar] [CrossRef]
- Chen, Z.; Chao, Y.; Li, W.; Wallace, G.G.; Bussell, T.; Ding, J.; Wang, C. Abuse-Tolerant Electrolytes for Lithium-Ion Batteries. Adv. Sci. 2021, 8, 2003694. [Google Scholar] [CrossRef]
- Rana, S.; Thakur, R.C.; Dosanjh, H.S. Ionic liquids as battery electrolytes for lithium-ion batteries: Recent advances and future prospects. Solid State Ionics 2023, 400, 116340. [Google Scholar] [CrossRef]
- Ortiz, M.G.; Čech, O.; Kazda, T.; Čudek, P.; Sedlaříková, M. Synthesis and Characterization of Blended Cathode Materials as Improvement on the Electrochemical Performance in Li-Ion Battery. J. Energy Storage 2021, 42, 103059. [Google Scholar] [CrossRef]
- Li, S.; Li, X.; Liu, J.; Shang, Z.; Cui, X. A low-temperature electrolyte for lithium-ion batteries. Ionics 2015, 21, 901–907. [Google Scholar] [CrossRef]
- Jaguemont, J.; Boulon, L.; Dubé, Y. A comprehensive review of lithium-ion batteries used in hybrid and electric vehicles at cold temperatures. Appl. Energy 2016, 164, 99–114. [Google Scholar] [CrossRef]
- Rueda-Garcia, D.; Cabán-Huertas, Z.; Sánchez-Ribot, S.; Marchante, C.; Benages, R.; Dubal, D.P.; Ayyad, O.; Gómez-Romero, P. Battery and Supercapacitor Materials in Flow Cells. Electrochemical Energy Storage in a LiFePO4/Reduced Graphene Oxide Aqueous Nanofluid. Electrochim. Acta 2018, 281, 594–600. [Google Scholar] [CrossRef]
- Aberoumand, S.; Woodfield, P.; Shi, G.; Kien Nguyen, T.; Nguyen, H.-Q.; Li, Q.; Shabani, B.; Viet Dao, D. Thermo-Electro-Rheological Behaviour of Vanadium Electrolyte-Based Electrochemical Graphene Oxide Nanofluid Designed for Redox Flow Battery. J. Mol. Liq. 2021, 338, 116860. [Google Scholar] [CrossRef]
- Sasi, S.; Murali, A.; Nair, S.V.; Nair, A.S.; Subramanian, K.R.V. The Effect of Graphene on the Performance of an Electrochemical Flow Capacitor. J. Mater. Chem. A 2015, 3, 2717–2725. [Google Scholar] [CrossRef]
- Lee, W.; Lee, J.; Min, S.K.; Park, T.; Yi, W.; Han, S.-H. Effect of Single-Walled Carbon Nanotube in PbS/TiO2 Quantum Dots-Sensitized Solar Cells. Mater. Sci. Eng. B 2009, 156, 48–51. [Google Scholar] [CrossRef]
- Neo, C.Y.; Ouyang, J. Functionalized carbon nanotube-induced viscosity reduction of an ionic liquid and performance improvement of dye-sensitized solar cells. Electrochim. Acta 2012, 85, 1–8. [Google Scholar] [CrossRef]
- Ronkainen, N.J.; Halsall, H.B.; Heineman, W.R. Electrochemical biosensors. Chem. Soc. Rev. 2010, 39, 1747–1763. [Google Scholar] [CrossRef]
- Li, J.; Zhao, F.; Wang, G.; Gui, Z.; Xiao, F.; Zeng, B. Novel composite of multiwalled carbon nanotubes and gold nanoparticles stabilized by chitosan and hydrophilic ionic liquid for direct electron transfer of glucose oxidase. Electroanalysis 2009, 21, 150–156. [Google Scholar] [CrossRef]
- Luo, X.L.; Morrin, A.; Killard, A.J.; Smyth, M.R. Application of nanoparticles in electrochemical sensors and biosensors. Electroanalysis 2006, 18, 319–326. [Google Scholar] [CrossRef]
- Franzoi, A.C.; Vieira, I.C.; Dupont, J.; Scheeren, C.W.; de Oliveira, L.F. Biosensor for luteolin based on silver or gold nanoparticles in ionic liquid and laccase immobilized in chitosan modified with cyanuric chloride. Analyst 2009, 134, 2320–2328. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Chen, Z.; Chen, Y.; Zhan, C.; Zhao, J. New synthesis of self-assembly ionic liquid functionalized reduced graphene oxide-gold nanoparticle composites for electrochemical determination of Sudan I. J. Electroanal. Chem. 2015, 756, 49–55. [Google Scholar] [CrossRef]
- Shang, L.; Zhao, F.; Zeng, B. Highly dispersive hollow PdAg alloy nanoparticles modified ionic liquid functionalized graphene nanoribbons for electrochemical sensing of nifedipine. Electrochem. Acta 2015, 168, 330–336. [Google Scholar] [CrossRef]
- Xiang, C.; Zou, Y.; Sun, L.-X.; Xu, F. Direct electron transfer of cytochrome c and its biosensor based on gold nanoparticles/room temperature ionic liquid/carbon nanotubes composite film. Electrochem. Commun. 2008, 10, 38–44. [Google Scholar] [CrossRef]
- Zhu, C.; Guo, S.; Zhai, Y.; Dong, S. Layer-by-layer self-assembly for constructing a graphene/platinum nanoparticle three-dimensional hybrid nanostructure using ionic liquid as a linker. Langmuir 2010, 26, 7614–7618. [Google Scholar] [CrossRef]
- Bagheri, H.; Afkhami, A.; Shirzadmehr, A.; Khoshsafar, H. A new nano-composite modified carbon paste electrode as a high performance potentiometric sensor for nanomolar Tl(I) determination. J. Mol. Liq. 2014, 197, 52–57. [Google Scholar] [CrossRef]
- Liu, F.; Deng, Y.; Han, X.; Hu, W.; Zhong, C. Electrodeposition of metals and alloys from ionic liquids. J. Alloys Compd. 2016, 654, 163–170. [Google Scholar] [CrossRef]
- Costa JGdRd Costa, J.M.; Almeida Neto, A.F.d. Progress on Electrodeposition of Metals and Alloys Using Ionic Liquids as Electrolytes. Metals 2022, 12, 2095. [Google Scholar] [CrossRef]
- Paduszynski, K.; Domanska, U. Viscosity of Ionic Liquids: An Extensive Database and a New Group Contribution Model Based on a Feed-Forward Artificial Neural Network. J. Chem. Inf. Model. 2014, 54, 1311–1324. [Google Scholar] [CrossRef]
- Tiago, G.A.O.; Matias, I.A.S.; Ribeiro, A.P.C.; Martins, L.M.D.R.S. Application of Ionic Liquids in Electrochemistry—Recent Advances. Molecules 2020, 25, 5812. [Google Scholar] [CrossRef]
- Quijada-Maldonado, E.; Olea, F.; Sepúlveda, R.; Castillo, J.; Cabezas, R.; Merlet, G.; Romero, J. Possibilities and challenges for ionic liquids in hydrometallurgy. Sep. Purif. Technol. 2020, 251, 117289. [Google Scholar] [CrossRef]
- Yavuz, A.; Ozdemir, N.; Yilmaz Erdogan, P.; Zengin, H.; Zengin, G.; Bedir, M. Effect of electrodeposition potential and time for nickel film generation from ionic liquid electrolytes for asymmetric supercapacitor production. Thin Solid Films 2020, 711, 138309. [Google Scholar] [CrossRef]
- Li, W.; Hao, J.; Mu, S.; Liu, W. Electrochemical behavior and electrodeposition of Ni-Co alloy from choline chloride-ethylene glycol deep eutectic solvent. Appl. Surf. Sci. 2020, 507, 144889. [Google Scholar] [CrossRef]
- Alesary, H.F.; Cihangir, S.; Ballantyne, A.D.; Harris, R.C.; Weston, D.P.; Abbott, A.P.; Ryder, K.S. Influence of additives on the electrodeposition of zinc from a deep eutectic solvent. Electrochim. Acta 2019, 304, 118–130. [Google Scholar] [CrossRef]
- Bakkar, A.; Neubert, V. Recycling of cupola furnace dust: Extraction and electrodeposition of zinc in deep eutectic solvents. J. Alloys Compd. 2019, 771, 424–432. [Google Scholar] [CrossRef]
- Yamagami, M.; Higashino, S.; Yamamoto, T.; Ikenoue, T.; Miyake, M.; Hirato, T. Aluminum Electrodeposition in Dry Air Atmosphere: Comparative Study of an Acetamide–AlCl3 Deep Eutectic Solvent and a 1-Ethyl-3-Methylimidazolium Chloride–AlCl3 Ionic Liquid. J. Electrochem. Soc. 2022, 169, 062502. [Google Scholar] [CrossRef]
- Du, C.; Yang, H.; Chen, X.-B.; Wang, L.; Dong, H.; Ning, Y.; Lai, Y.; Jia, J.; Zhao, B. Effect of coordinated water of hexahydrate on nickel platings from choline–urea ionic liquid. J. Mater. Sci. 2018, 53, 10758–10771. [Google Scholar] [CrossRef]
- Wang, Y.-S.; Chen, P.-Y. Electrochemical Study and Electrodeposition of Zn-Ni Alloys in an Imide-Type Hydrophobic Room-Temperature Ionic Liquid: Feasibility of Using Metal Chlorides as the Metal Sources. J. Electrochem. Soc. 2018, 165, D76. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, X.; Zhang, J.; Yang, P.; An, M.; Li, Q. Electrodeposition of Cu-Zn alloy from EMImTfO ionic liquid/ethanol mixtures for replacing the cyanide zincate layer on Al alloy. J. Alloys Compd. 2019, 806, 79–88. [Google Scholar] [CrossRef]
- Yavuz, A.; Yilmaz, N.F.; Artan, M. Fe-Cu Alloy-Based Flexible Electrodes from Ethaline Ionic Liquid. J. Electron. Mater. 2021, 50, 3478–3487. [Google Scholar] [CrossRef]
- Rani, M.A.A.; Hwang, J.; Matsumoto, K.; Hagiwara, R. Poly(vinyl chloride) Ionic Liquid Polymer Electrolyte Based on Bis(fluorosulfonyl)Amide for Sodium Secondary Batteries. J. Electrochem. Soc. 2017, 164, H5031. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, S.; Chi, C.; Hao, J.; Zhao, J.; Xu, Y.; Li, Y. Electrodeposition of a Continuous, Dendrite-Free Aluminum Film from an Ionic Liquid and Its Electrochemical Properties. J. Mater. Sci. Mater. Electron. 2020, 31, 9937–9945. [Google Scholar] [CrossRef]
- Takahashi, H.; Matsushima, H.; Ueda, M. Al Film Electrodeposition from the AlCl3-EMIC Electrolyte under a Magnetic Field. J. Electrochem. Soc. 2017, 164, H5165. [Google Scholar] [CrossRef]
- He, J.-W.; Gu, Y.; Wang, W.-W.; Wang, J.-H.; Chen, Z.-B.; He, H.-Y.; Wu, Q.-H.; Yan, J.-W.; Mao, B.-W. Structures of Solid-Electrolyte Interphases and Impacts on Initial-Stage Lithium Deposition in Pyrrolidinium-Based Ionic Liquids. ChemElectroChem 2021, 8, 62–69. [Google Scholar] [CrossRef]
- Hou, X.; Wang, Z.; Pan, J.; Yan, F. Ionic Liquid Electrolyte-Based Switchable Mirror with Fast Response and Improved Durability. ACS Appl. Mater. Interfaces 2021, 13, 37339–37349. [Google Scholar] [CrossRef]
- Yang, X.; Miao, C.; Sun, Y.; Lei, T.; Xie, Q.; Wang, S. Efficient extraction of gold(I) from alkaline aurocyanide solution using green ionic liquid-based aqueous biphasic systems. J. Taiwan Inst. Chem. Eng. 2018, 91, 176–185. [Google Scholar] [CrossRef]
- Chen, M.; Li, S.; Jin, C.; Shao, M.; Huang, Z. Selective recovery of platinum by combining a novel reusable ionic liquid with electrodeposition. Sep. Purif. Technol. 2021, 259, 118204. [Google Scholar] [CrossRef]
- Shao, M.; Li, S.; Jin, C.; Chen, M.; Huang, Z. Recovery of Pd(II) from Hydrochloric Acid Medium by Solvent Extraction–Direct Electrodeposition Using Hydrophilic/Hydrophobic ILs. ACS Omega 2020, 5, 27188–27196. [Google Scholar] [CrossRef]
- Pölzler, M.; Whitehead, A.H.; Gollas, B. A Study of Zinc Electrodeposition from Zinc Chloride: Choline Chloride: Ethylene Glycol. ECS Trans. 2010, 25, 43. [Google Scholar] [CrossRef]
- Wang, Y.-S.; Yeh, H.-W.; Tang, Y.-H.; Kao, C.-L.; Chen, P.-Y. Voltammetric Study and Electrodeposition of Zinc in Hydrophobic Room-Temperature Ionic Liquid 1-Butyl-1-methylpyrrolidinium Bis((trifluoromethyl)sulfonyl)imide ([BMP][TFSI]): A Comparison between Chloride and TFSI Salts of Zinc. J. Electrochem. Soc. 2017, 164, D39–D47. [Google Scholar] [CrossRef]
- Steichen, M.; Brooks, N.R.; Van Meervelt, L.; Fransaer, J.; Binnemans, K. Homoleptic and heteroleptic N-alkylimidazole zinc(ii)-containing ionic liquids for high current density electrodeposition. Dalton Trans. 2014, 43, 12329–12341. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Würger, A.; Crispin, X. Ionic thermoelectric materials and devices. J. Energy Chem. 2021, 61, 88–103. [Google Scholar] [CrossRef]
- Abraham, T.J.; MacFarlane, D.R.; Baughman, R.H.; Jin, L.; Li, N.; Pringle, J.M. Towards ionic liquid-based thermoelectrochemical cells for the harvesting of thermal energy. Electrochim. Acta 2013, 113, 87–93. [Google Scholar] [CrossRef]
- Cheng, H.; He, X.; Fan, Z.; Ouyang, J. Flexible Quasi-Solid State Ionogels with Remarkable Seebeck Coefficient and High Thermoelectric Properties. Adv. Energy Mater. 2019, 9, 1901085. [Google Scholar] [CrossRef]
- Chen, B.; Chen, Q.; Xiao, S.; Feng, J.; Zhang, X.; Wang, T. Giant Negative Thermopower of Ionic Hydrogel by Synergistic Coordination and Hydration Interactions. Sci. Adv. 2023, 7, eabi7233. [Google Scholar] [CrossRef]
- Li, T.; Zhang, X.; Lacey, S.D.; Mi, R.; Zhao, X.; Jiang, F.; Song, J.; Liu, Z.; Chen, G.; Dai, J.; et al. Cellulose Ionic Conductors with High Differential Thermal Voltage for Low-Grade Heat Harvesting. Nat. Mater. 2019, 18, 608–613. [Google Scholar] [CrossRef] [PubMed]
- Kenarsari, S.D.; Yang, D.; Jiang, G.; Zhang, S.; Wang, J.; Russell, A.G.; Wei, Q.; Fan, M. Review of Recent Advances in Carbon Dioxide Separation and Capture. RSC Adv. 2013, 3, 22739–22773. [Google Scholar] [CrossRef]
- Rheinhardt, J.H.; Singh, P.; Tarakeshwar, P.; Buttry, D.A. Electrochemical Capture and Release of Carbon Dioxide. ACS Energy Lett. 2017, 2, 454–461. [Google Scholar] [CrossRef]
- Lee, J.H.; Hong, J.; Kim, J.H.; Song, D.; Kang, Y.S.; Kang, S.W. Surface Tuned Copper Nanoparticles by 1-Methyl-3-Octylimidazolium Tetrafluoroborate and Its Applications to Facilitated CO2 Transport. Chem. Eng. J. 2014, 235, 252–256. [Google Scholar] [CrossRef]
- He, Z.; Alexandridis, P. Nanoparticles in Ionic Liquids: Interactions and Organization. Phys. Chem. Chem. Phys. 2015, 17, 18238–18261. [Google Scholar] [CrossRef]
- Lee, J.H.; Hong, J.; Kim, J.H.; Kang, Y.S.; Kang, S.W. Facilitated CO2 Transport Membranes Utilizing Positively Polarized Copper Nanoparticles. Chem. Commun. 2012, 48, 5298–5300. [Google Scholar] [CrossRef] [PubMed]
- Hudiono, Y.C.; Carlisle, T.K.; Bara, J.E.; Zhang, Y.; Gin, D.L.; Noble, R.D. A Three-Component Mixed-Matrix Membrane with Enhanced CO2 Separation Properties Based on Zeolites and Ionic Liquid Materials. J. Memb. Sci. 2010, 350, 117–123. [Google Scholar] [CrossRef]
- Willa, C.; Yuan, J.; Niederberger, M.; Koziej, D. When Nanoparticles Meet Poly(Ionic Liquid)s: Chemoresistive CO2 Sensing at Room Temperature. Adv. Funct. Mater. 2015, 25, 2537–2542. [Google Scholar] [CrossRef]
- Tavakoli, A.; Rahimi, K.; Saghandali, F.; Scott, J.; Lovell, E. Nanofluid Preparation, Stability and Performance for CO2 Absorption and Desorption Enhancement: A Review. J. Environ. Manag. 2022, 313, 114955. [Google Scholar] [CrossRef] [PubMed]
- Lucero, R.D. Reserve magnesium anode and zinc/silver oxide batteries. In Linden’s Handbook of Batteries, 4th ed.; Reddy, T.B., Linden, D., Eds.; McGraw-Hill: New York, NY, USA, 2011. [Google Scholar]
- Parekh, M.H.; Palanisamy, M.; Pol, V.G. Reserve lithium-ion batteries: Deciphering in situ lithiation of lithium-ion free vanadium pentoxide cathode with graphitic anode. Carbon 2023, 203, 561–570. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, J.; Souza, R.; Moita, A.; Moreira, A. Nanofluids and Ionic Fluids as Liquid Electrodes: An Overview on Their Properties and Potential Applications. Processes 2023, 11, 3189. https://doi.org/10.3390/pr11113189
Pereira J, Souza R, Moita A, Moreira A. Nanofluids and Ionic Fluids as Liquid Electrodes: An Overview on Their Properties and Potential Applications. Processes. 2023; 11(11):3189. https://doi.org/10.3390/pr11113189
Chicago/Turabian StylePereira, José, Reinaldo Souza, Ana Moita, and António Moreira. 2023. "Nanofluids and Ionic Fluids as Liquid Electrodes: An Overview on Their Properties and Potential Applications" Processes 11, no. 11: 3189. https://doi.org/10.3390/pr11113189
APA StylePereira, J., Souza, R., Moita, A., & Moreira, A. (2023). Nanofluids and Ionic Fluids as Liquid Electrodes: An Overview on Their Properties and Potential Applications. Processes, 11(11), 3189. https://doi.org/10.3390/pr11113189









