Numerical Modeling and Economic Analysis of Ultrasonic-Assisted CO2 Absorption Process for Offshore Application
Abstract
:1. Introduction
2. Experimental Testing
2.1. Material
2.2. Experimental Setup
2.3. Experimental Procedure and Analysis
3. Numerical Modeling of The Ultrasonic Absorber
3.1. Kinetic Reactions of CO2 Absorption into PZ + MDEA + Water Blended Solvent
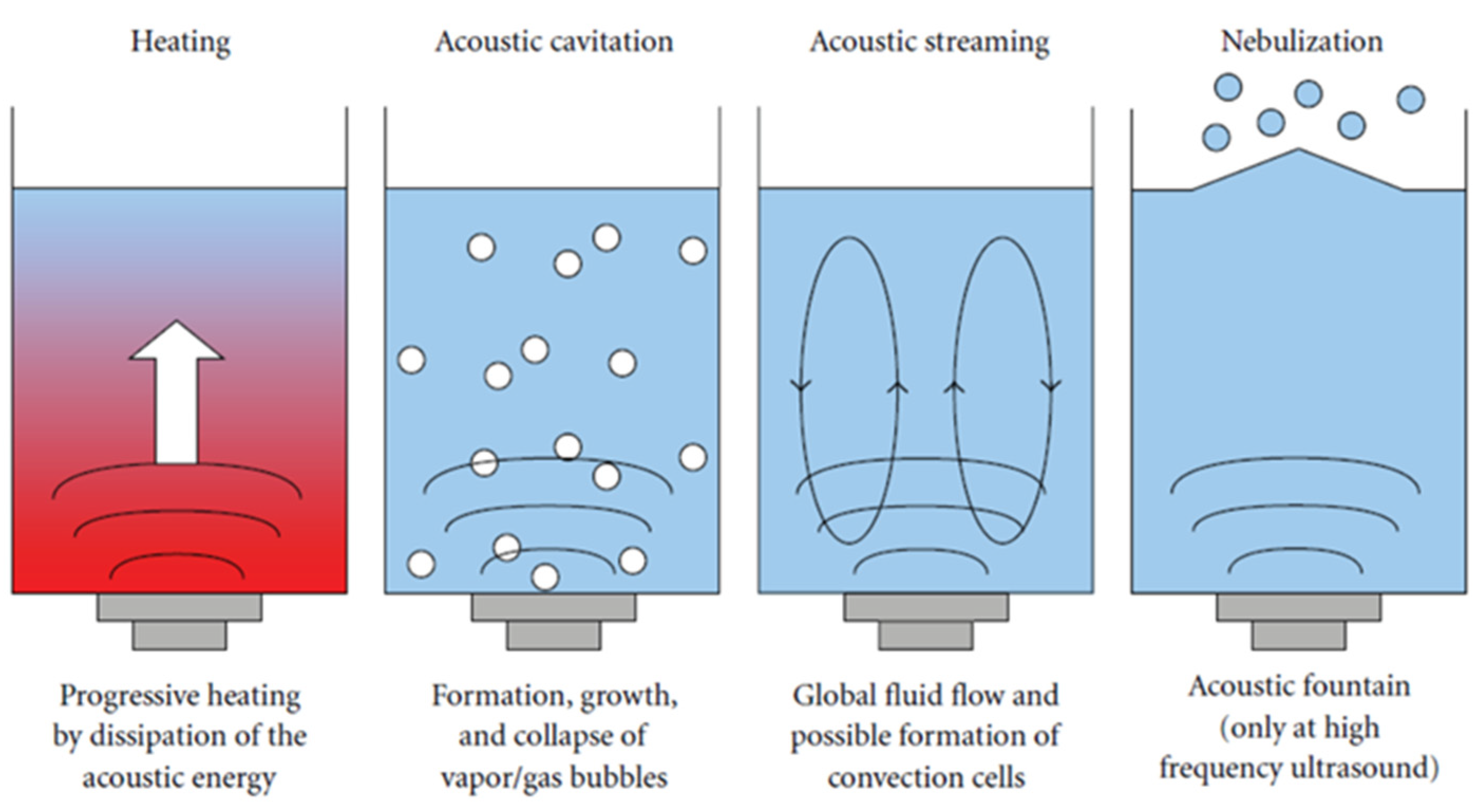
3.2. Modeling of Ultrasonic CO2 Absorption
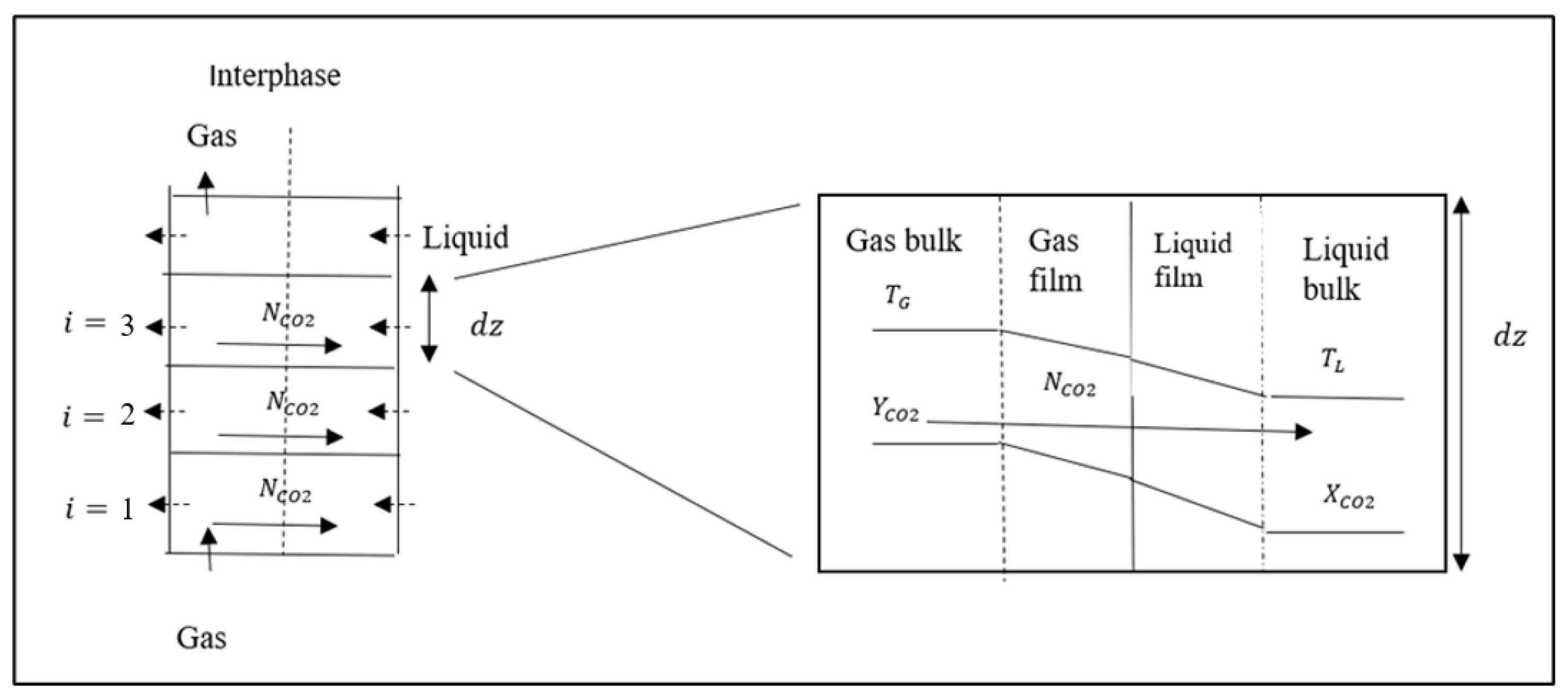
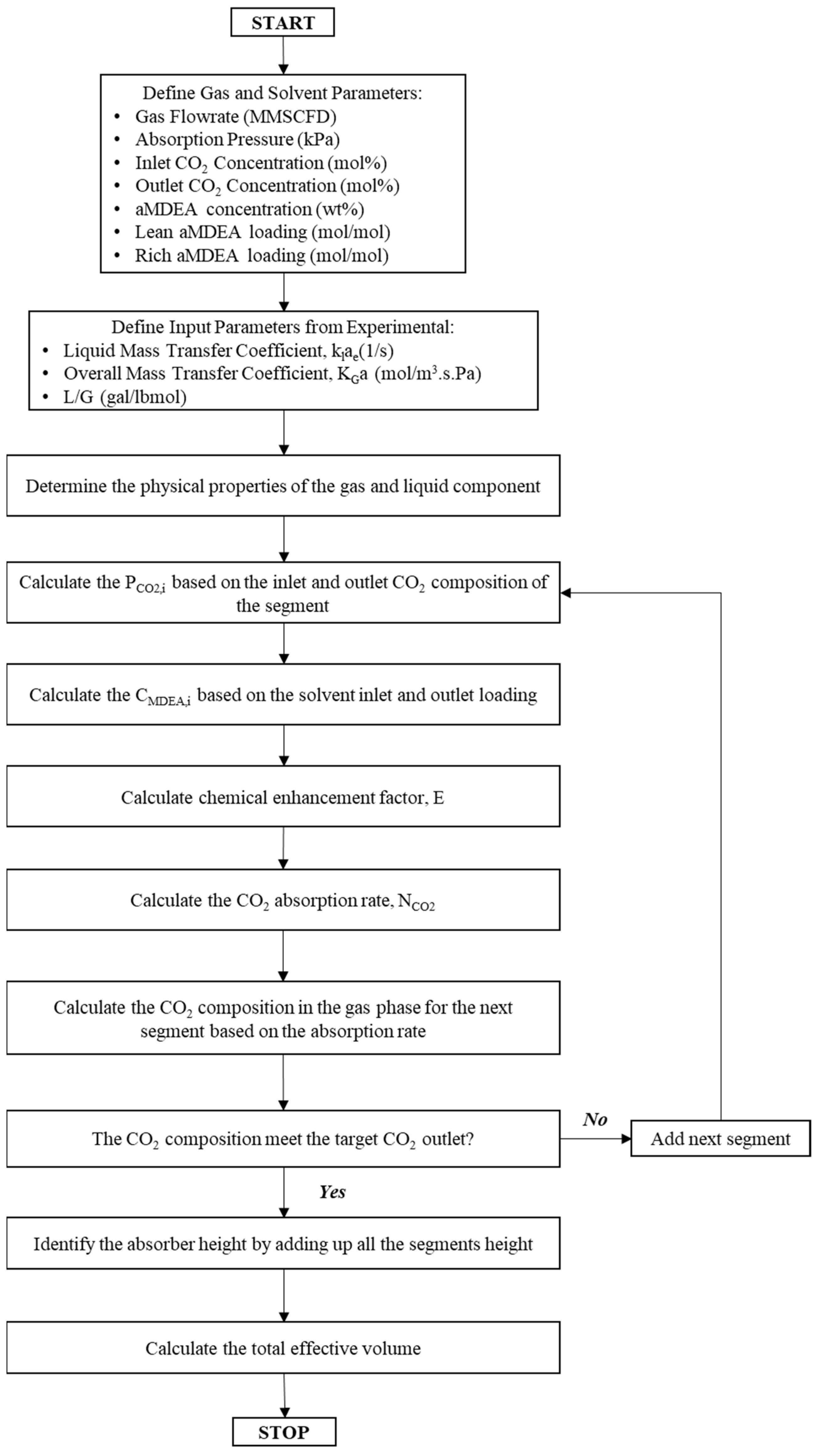
3.3. Modeling of the Ultrasonic Absorber Effective Volume
- The operation is in a steady state.
- The fast absorption reactions occur in the liquid film at the gas-liquid interface.
- The liquid flow rate is constant throughout the absorber.
- The total pressure in the absorber is constant.
- Heat loss to the surroundings is negligible.
- Vaporization of water and MDEA+PZ is not considered in the mass conservation equation.
- The reaction of hydrocarbon and MDEA+PZ is ignored.
3.4. Determination of Ultrasonic Absorber Energy Consumption
4. Process Simulation and Economic Analysis
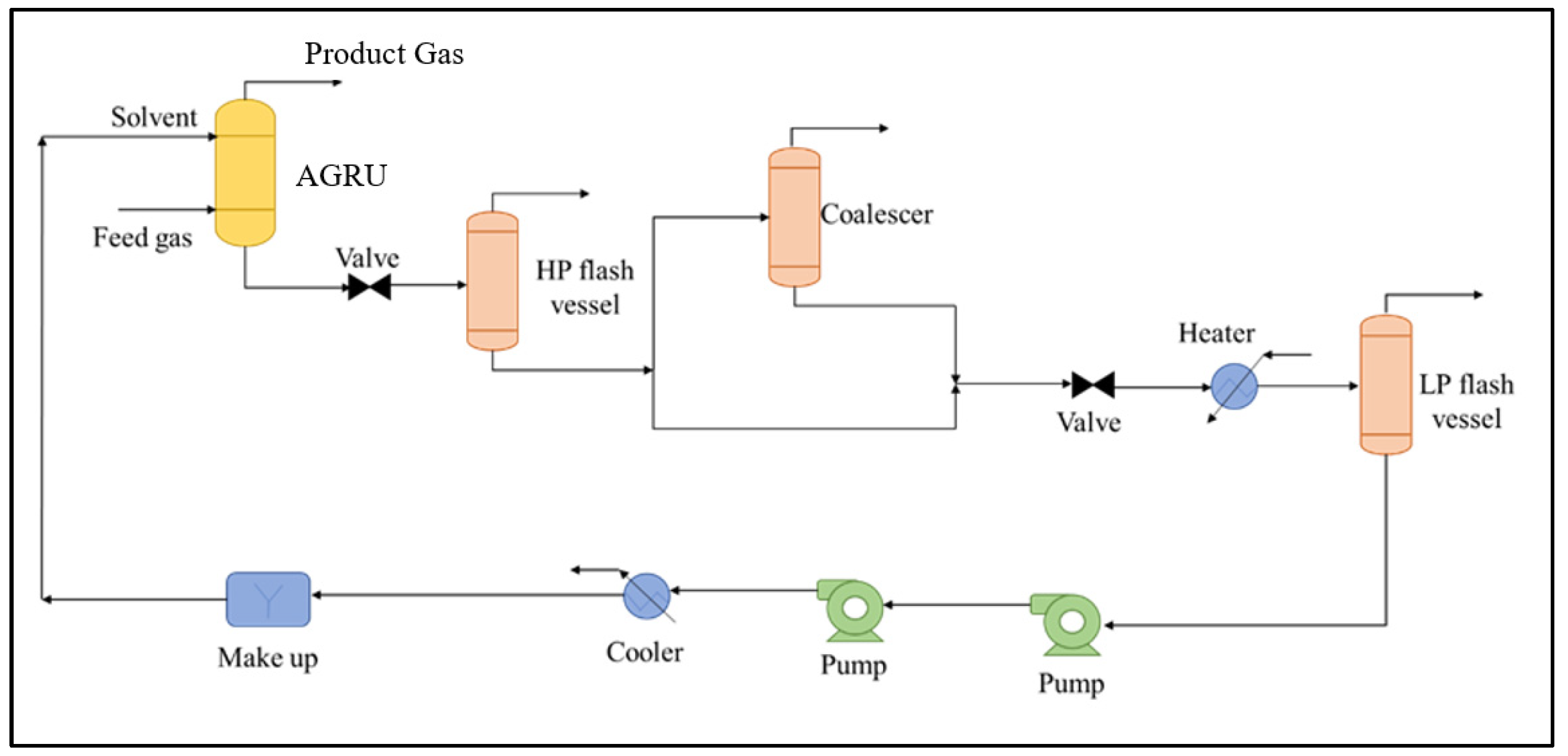
4.1. Process Simulation
4.2. CAPEX Estimation
4.3. OPEX Estimation
4.4. Unit Technical Cost (UTC) Calculation
5. Results and Discussions
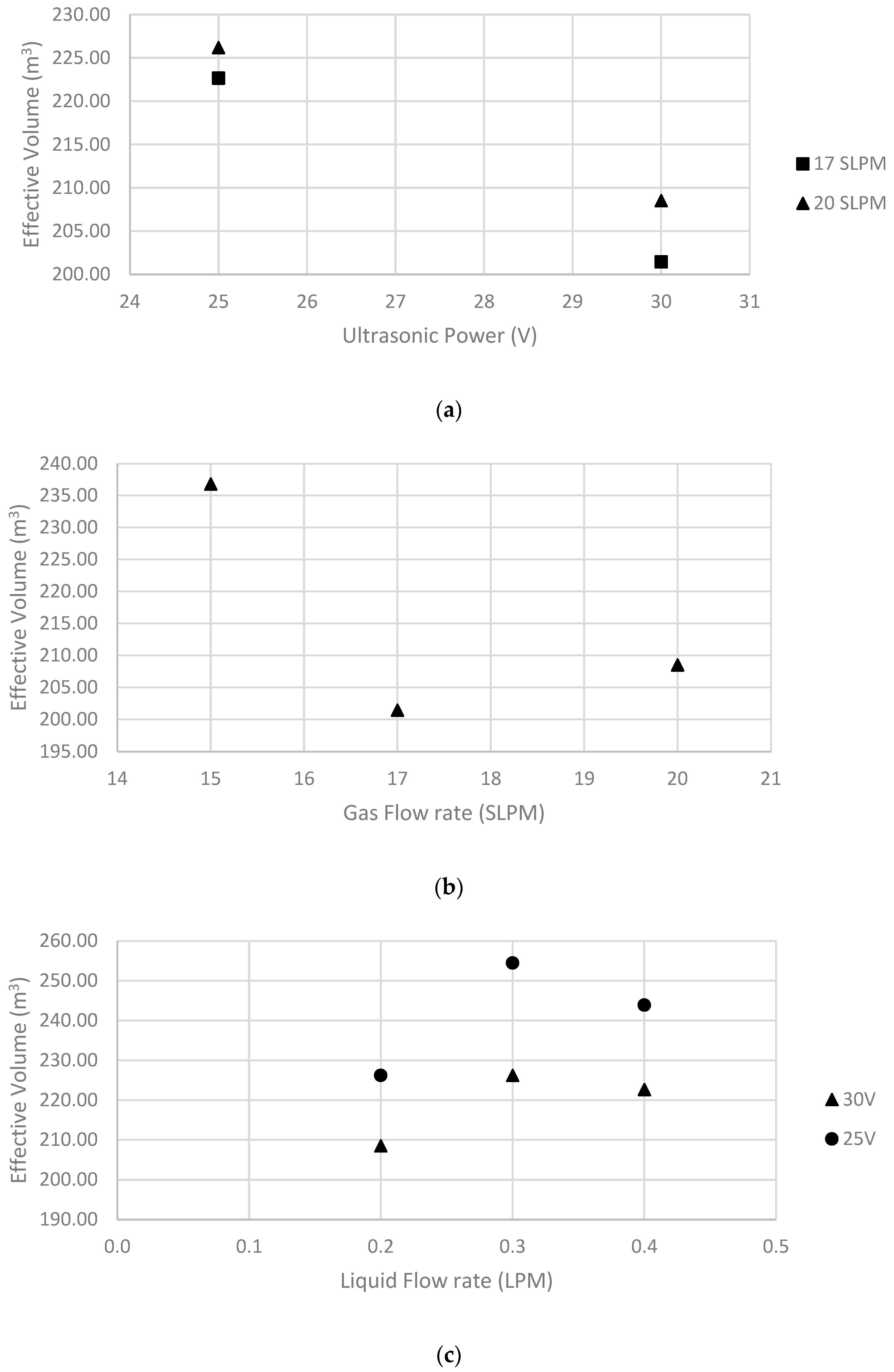
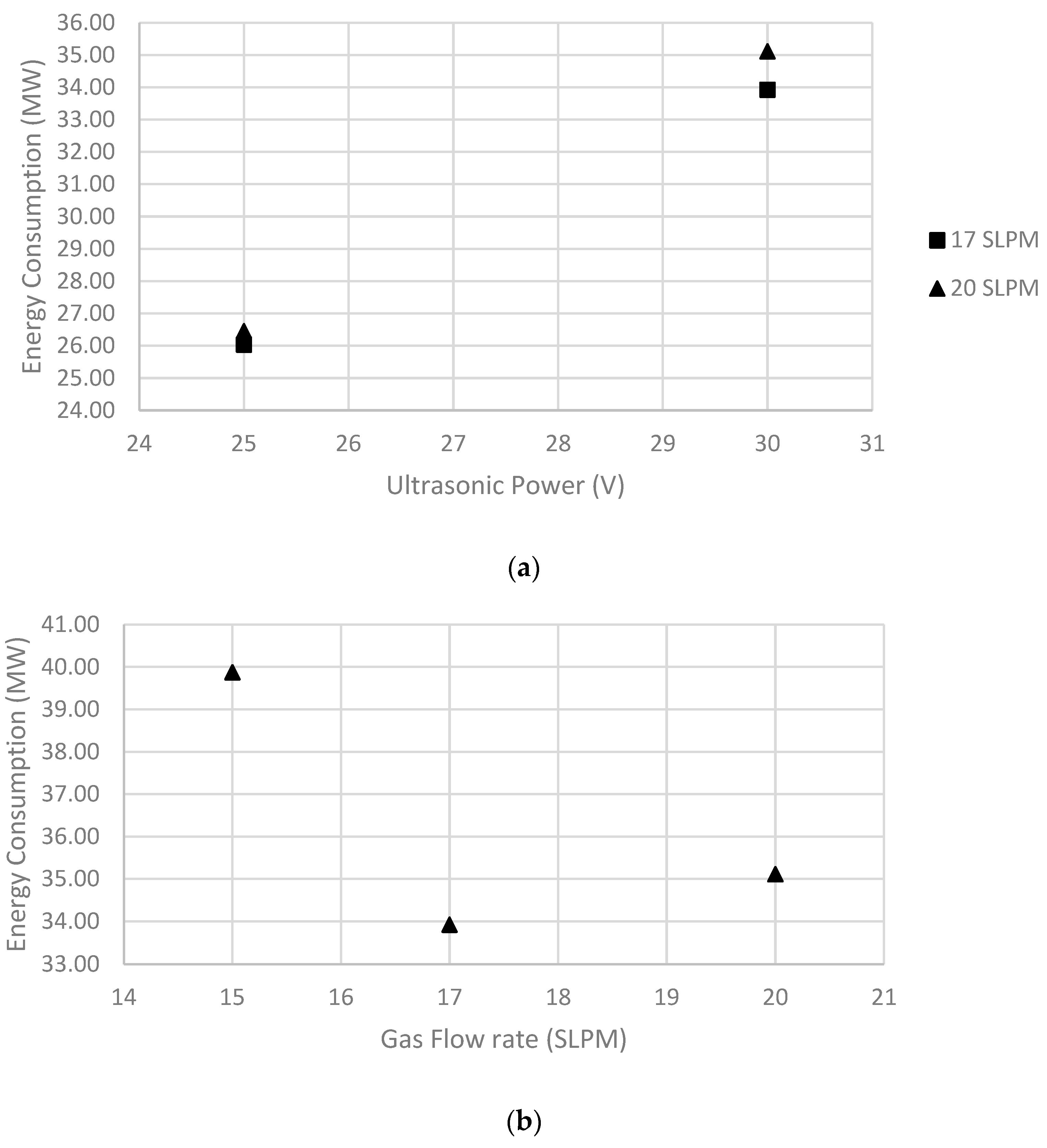
5.1. Effect of Ultrasonic Power
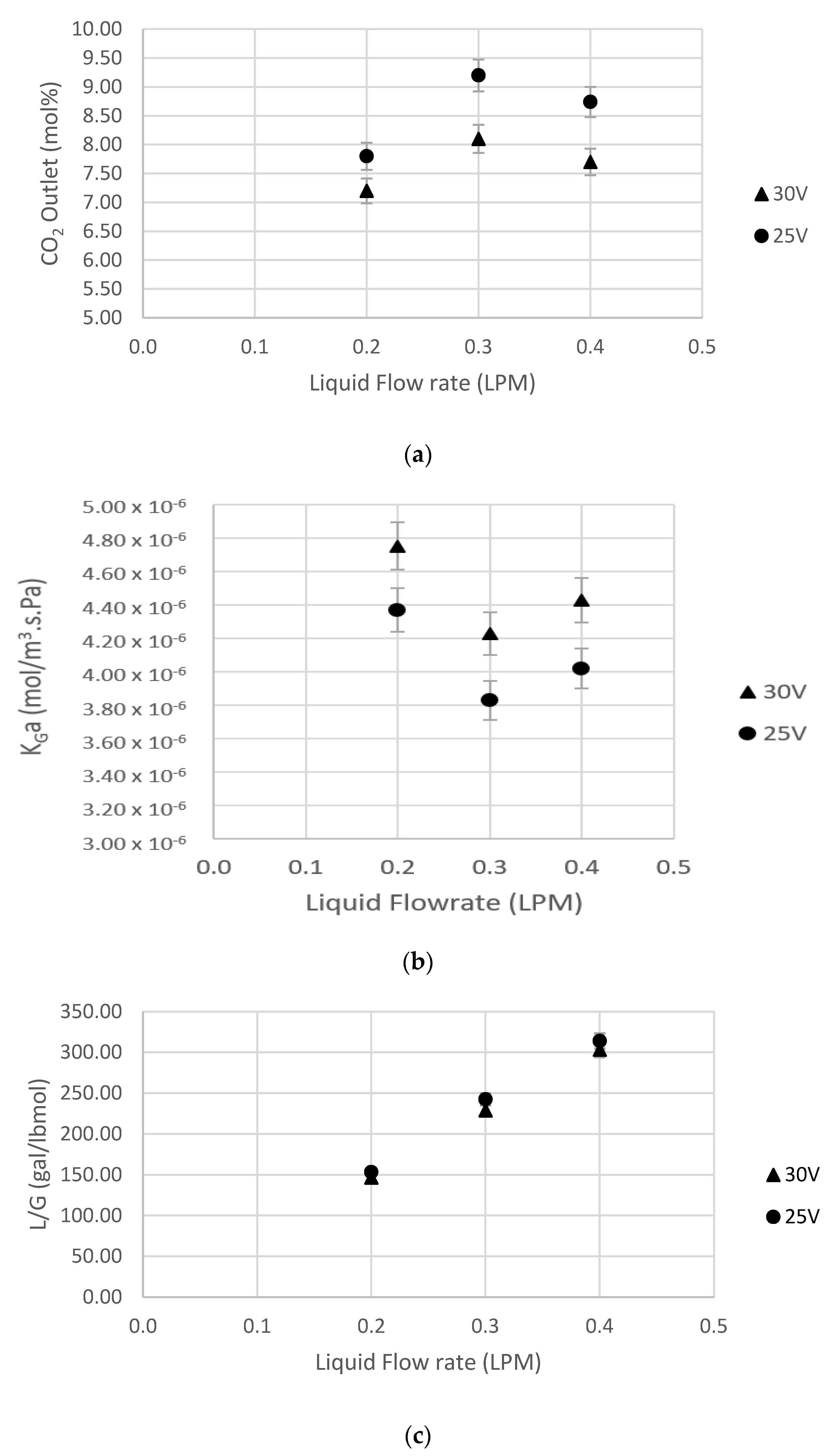
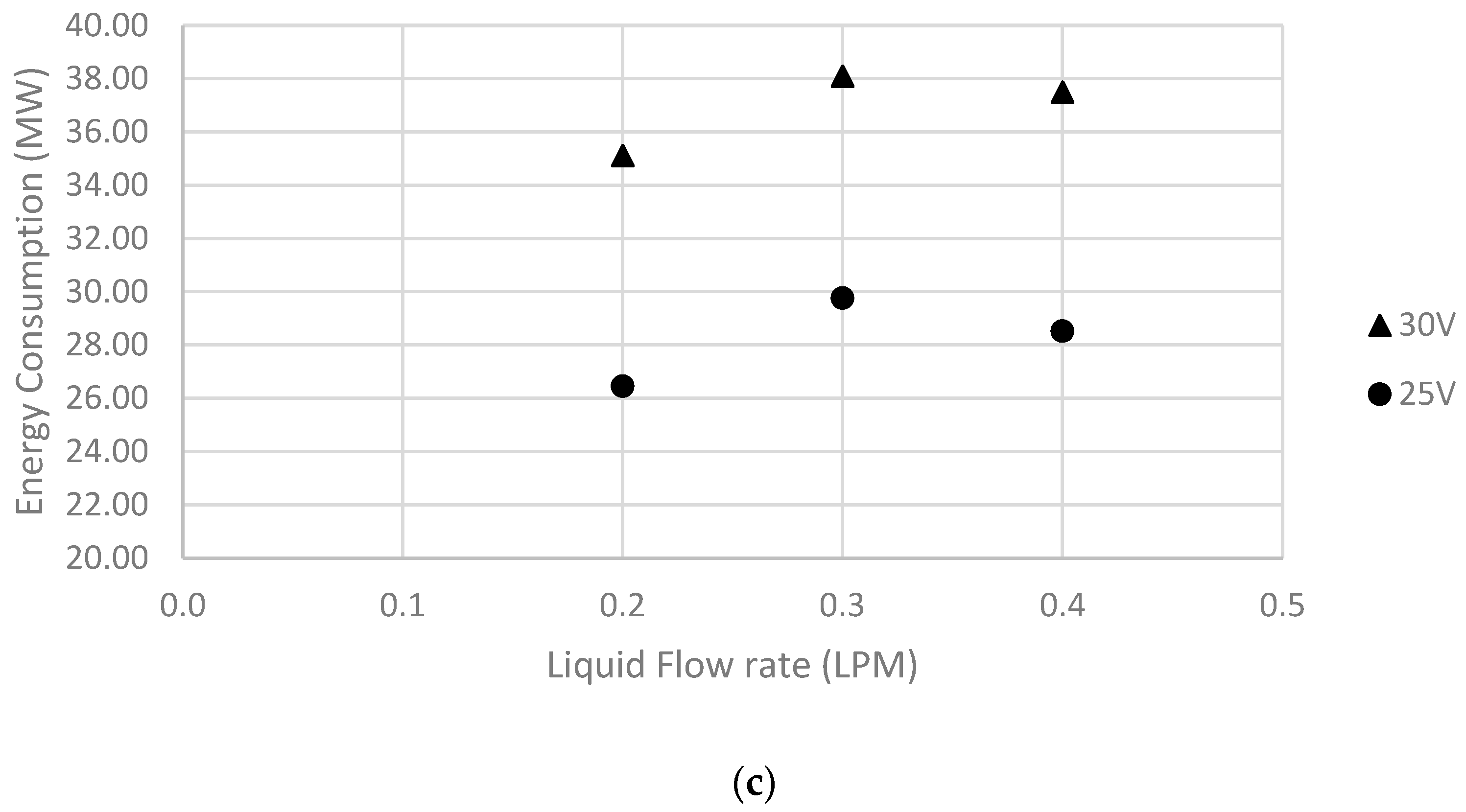
5.2. Effect of Liquid Flow Rate
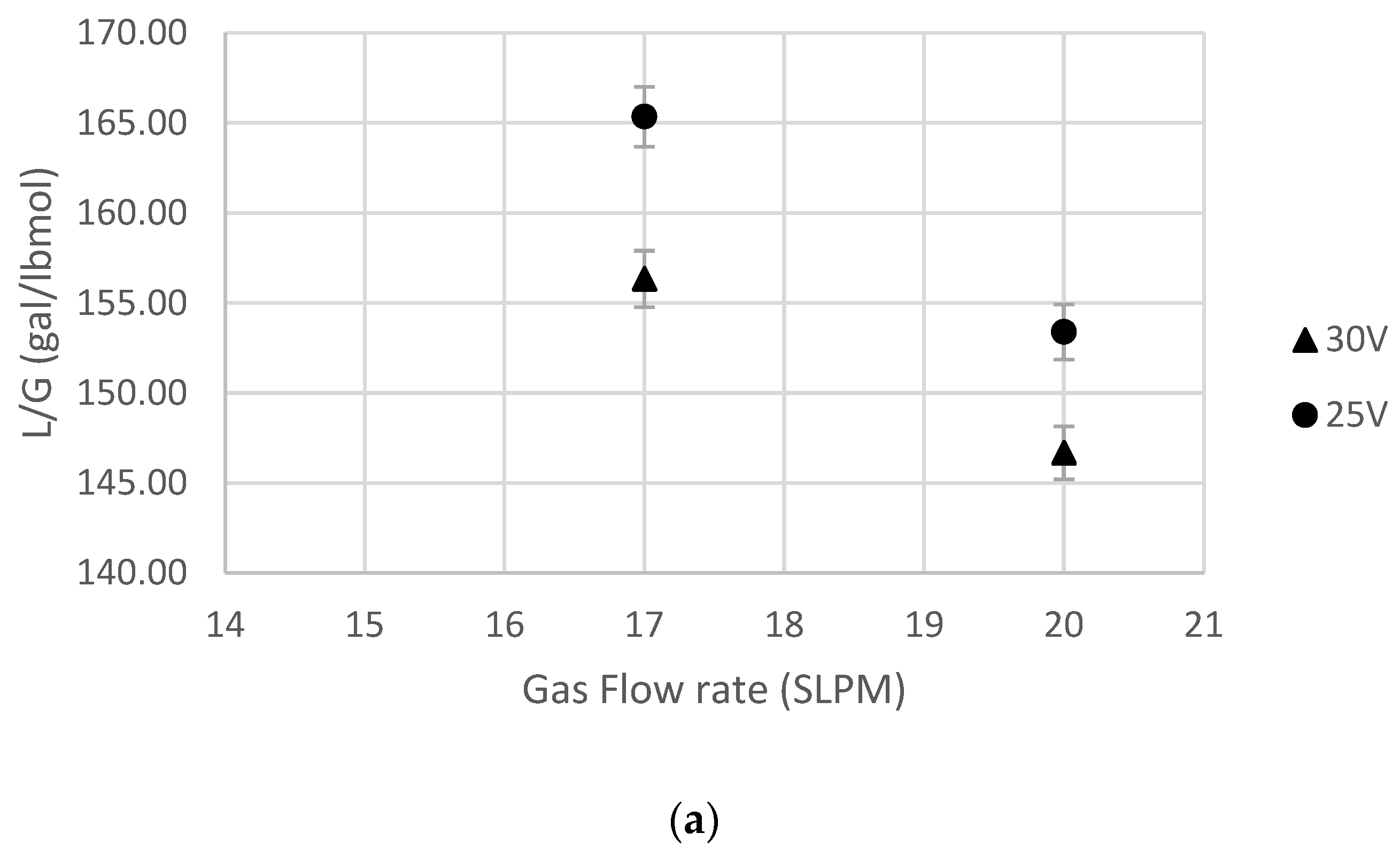
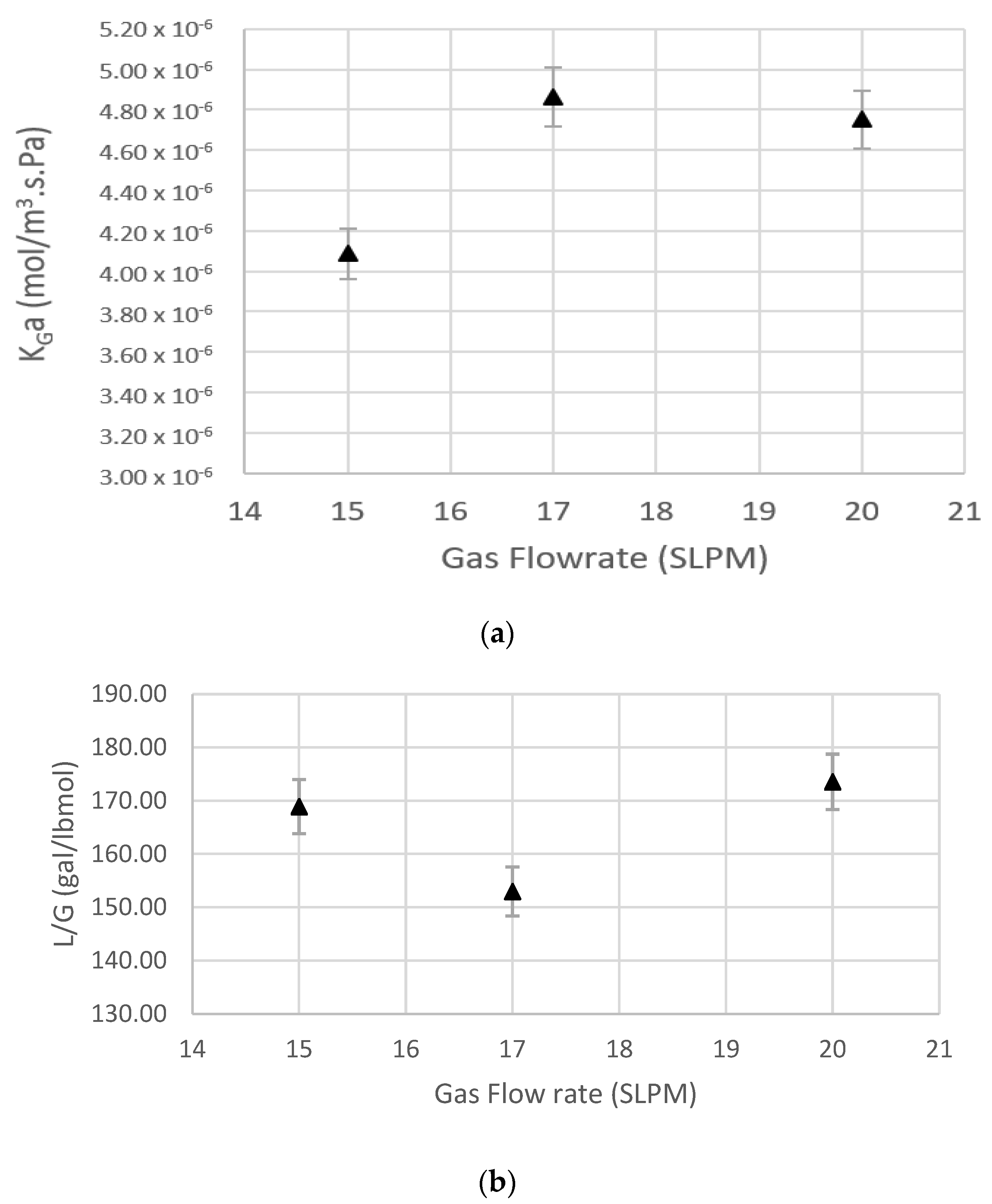
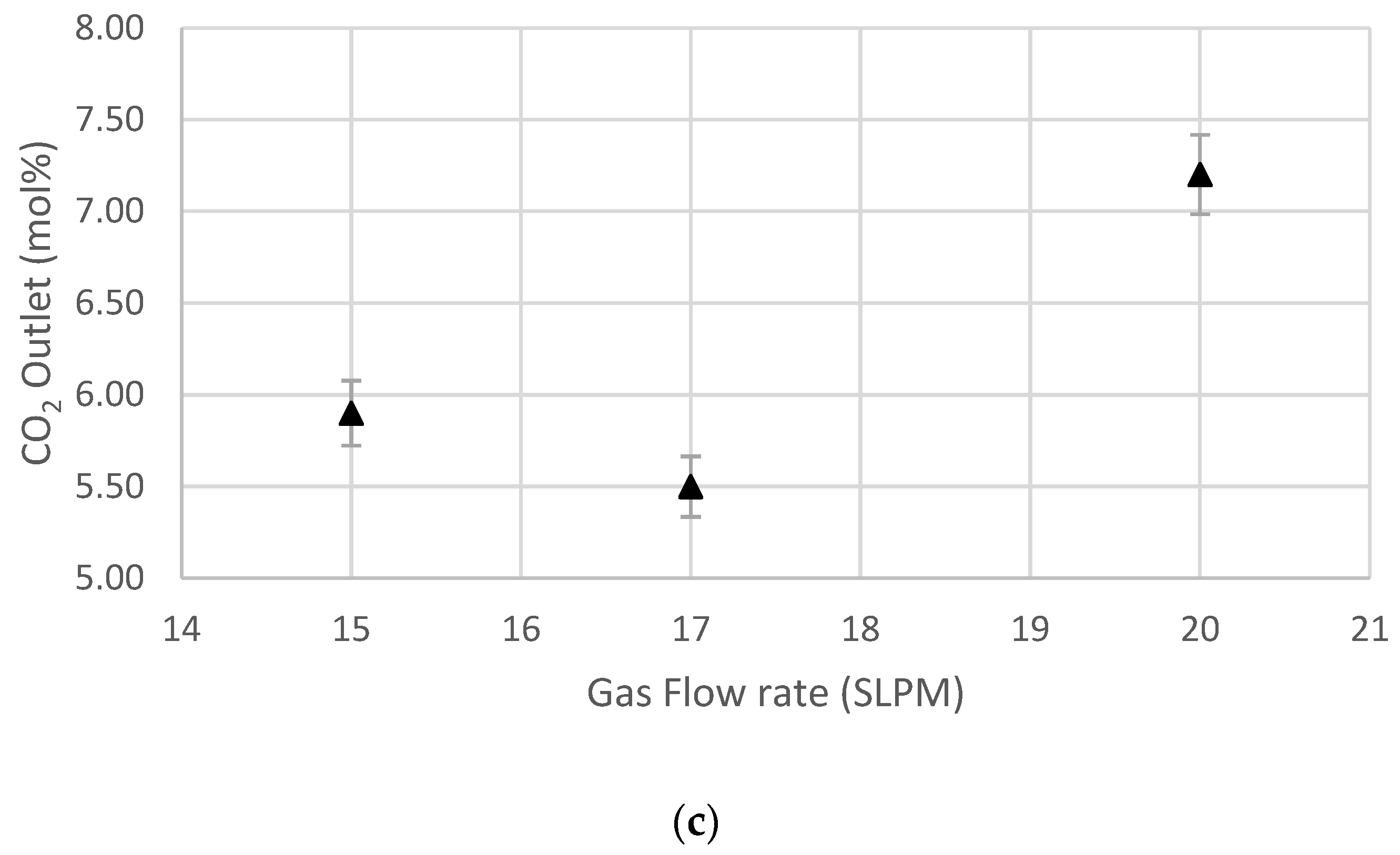
5.3. Effect of Gas Flow Rate
5.4. Prediction of Ultrasonic Absorber Effective Volume and Energy Consumption
5.5. CAPEX Comparison
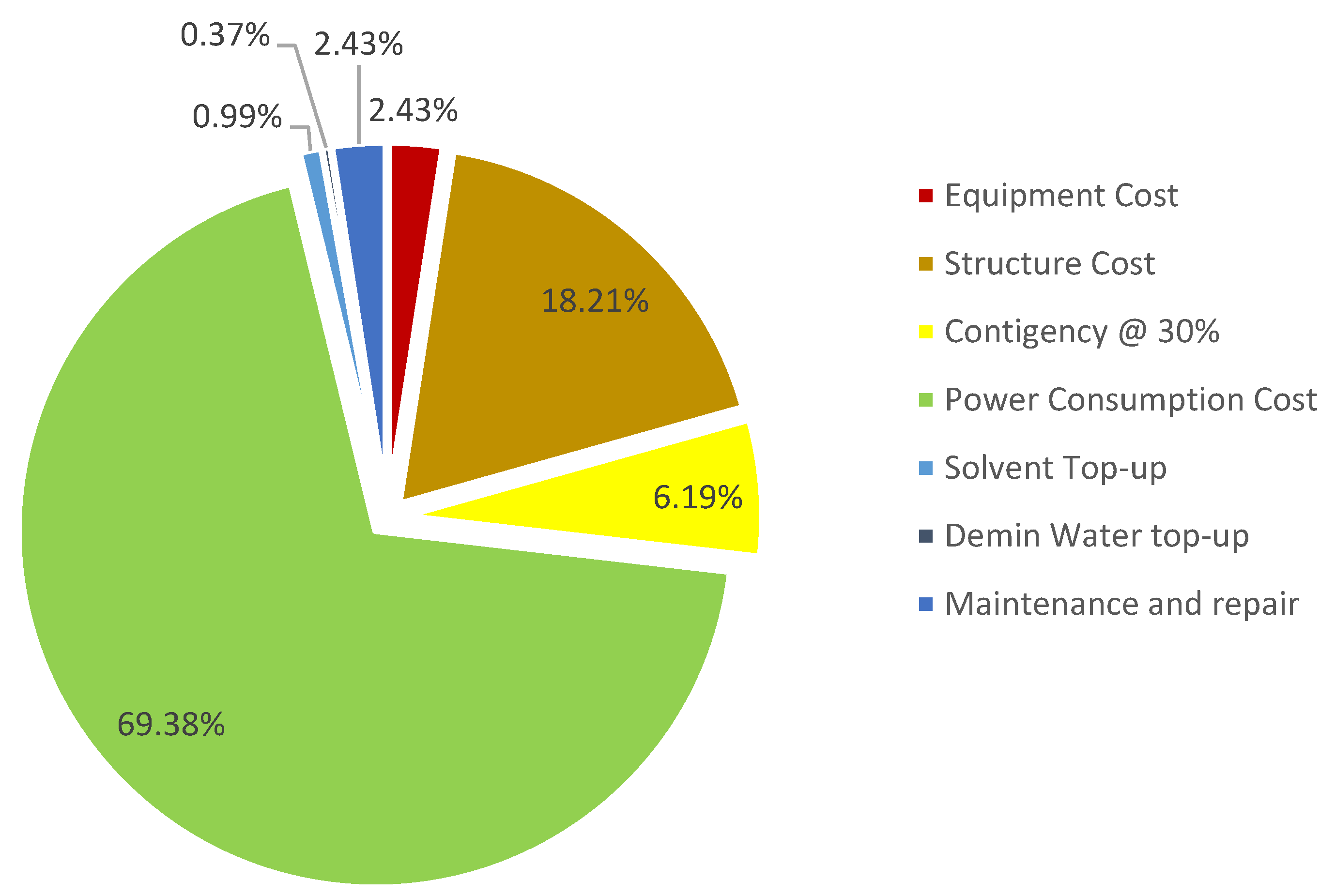
5.6. OPEX Comparison
5.7. UTC Comparison
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| b | Stoichiometric factor |
| CCO2i | CO2 molar concentration at the interphase (mol/L) |
| CMDEA | Free concentration of MDEA (mol/L) |
| CPZ | Free concentration of PZ (mol/L) |
| D | Ultrasonic absorber internal diameter (m) |
| DCO2l | Molecular diffusivity CO2 in the liquid (m2/s) |
| DMDEA | Molecular diffusivity of amine in the liquid (m2/s) |
| EUS | Ultrasonic absorber energy consumption (MW) |
| G | Total gas flow rate per unit cross-sectional area |
| g | Gas phase |
| Ha | Hatta number |
| HCO2 | Henry’s law constant (Pa.m3.mol−1) |
| k2,MDEA | Second-order rate constants for MDEA (m3.kmol−1.s−1) |
| KGa | Mass transfer coefficient (mol/m3.s.Pa) |
| kgae | Gas phase mass transfer coefficient (s−1) |
| kl0 | Liquid physical mass transfer coefficient (ms−1) |
| klae | Liquid phase mass transfer coefficient (s−1) |
| kov | Overall reaction rate constant (s−1) |
| l | Liquid phase |
| N | Number of ultrasonic absorber modules |
| NCO2 | CO2 absorption rate (mol/m3.s) |
| NHCl | Normality of HCl solution (N) |
| NKOH | Normality of KOH solution |
| PCO2 | CO2 partial pressure (Pa) |
| PCO2* | Equilibrium CO2 partial pressure (Pa) |
| PCO2,i | CO2 partial pressure at each stage (Pa) |
| PCO2,i,in | CO2 partial pressure at the inlet of each stage (Pa) |
| PCO2,i,out | CO2 partial pressure at the outlet of each stage (Pa) |
| PT | Total pressure (Pa) |
| PUS | Ultrasound power (V) |
| Q1 | Capacity of the commercial absorber column (m3) |
| Q2 | Capacity of AGRU which the cost was to be determined (m3) |
| R | Power supply circuit resistance (Ω) |
| rov | Overall reaction rate (mol/L.s) |
| UI | Ultrasound intensity (kW/m3) |
| Veff | Effective volume of ultrasonic absorber (m3) |
| VHCl | Volume of HCl used to titrate the sample (mL) |
| VKOH | Volume of KOH used to titrate the sample (mL) |
| VR | Ultrasonic absorber volume (m3) |
| Ws | Weight of the sample for titration (g) |
| Ws,f | Weight of fresh solvent (g) |
| x | Relationship between the capacities |
| yCO2 | Mol ratio of CO2 in the gas phase |
| yCO2* | CO2 equilibrium molar fraction |
| z | Total height of the ultrasonic absorber (m) |
References
- Ahmad, F.; Lau, K.K.; Shariff, A.M. Removal of CO2 from Natural Gas using Membrane Separation System: Modeling and Process Design. J. Appl. Sci. 2010, 10, 1134–1139. [Google Scholar] [CrossRef]
- George, G.; Bhoria, N.; AlHallaq, S.; Abdala, A.; Mittal, V. Polymer membranes for acid gas removal from natural gas. Sep. Purif. Technol. 2016, 158, 333–356. [Google Scholar] [CrossRef]
- Isa, F.; Suleman, H.; Zabiri, H.; Maulud, A.; Ramasamy, M.; Tufa, L.; Shariff, A. An overview on CO2 removal via absorption: Effect of elevated pressures in counter-current packed column. J. Nat. Gas Sci. Eng. 2016, 33, 666–677. [Google Scholar] [CrossRef]
- Nakhjiri, A.T.; Heydarinasab, A.; Bakhtiari, O.; Mohammadi, T. Influence of non-wetting, partial wetting and complete wetting modes of operation on hydrogen sulfide removal utilizing monoethanolamine absorbent in hollow fiber membrane contactor. Sustain. Environ. Res. 2018, 28, 186–196. [Google Scholar] [CrossRef]
- Norouzbahari, S.; Shahhosseini, S.; Ghaemi, A. CO2 chemical absorption into aqueous solutions of piperazine: Modeling of kinetics and mass transfer rate. J. Nat. Gas Sci. Eng. 2015, 26, 1059–1067. [Google Scholar] [CrossRef]
- Marjani, A.; Nakhjiri, A.T.; Adimi, M.; Jirandehi, H.F.; Shirazian, S. Modification of polyethersulfone membrane using MWCNT-NH2 nanoparticles and its application in the separation of azeotropic solutions by means of pervaporation. PLoS ONE 2020, 15, e0236529. [Google Scholar] [CrossRef]
- Zare, A.; Mirzaei, S. Removal of CO2 and H2S using aqueous alkanolamine solusions. World Acad. Sci. Eng. Technol. 2009, 37, 194–203. [Google Scholar]
- Qian, Z.; Xu, L.; Li, Z.; Li, H.; Guo, K. Selective absorption of H2S from a gas mixture with CO2 by aqueous N-methyldiethanolamine in a rotating packed bed. Ind. Eng. Chem. Res. 2010, 49, 6196–6203. [Google Scholar] [CrossRef]
- Pal, P.; AbuKashabeh, A.; Al-Asheh, S.; Banat, F. Role of aqueous methyldiethanolamine (MDEA) as solvent in natural gas sweetening unit and process contaminants with probable reaction pathway. J. Nat. Gas Sci. Eng. 2015, 24, 124–131. [Google Scholar] [CrossRef]
- Vega, F.; Cano, M.; Camino, S.; Fernández, L.M.G.; Portillo, E.; Navarrete, B. Solvents for Carbon Dioxide Capture. In Carbon Dioxide Chemistry, Capture and Oil Recovery; IntechOpen Limited: London, UK, 2018. [Google Scholar]
- Rodríguez, N.; Mussati, S.; Scenna, N. Optimization of post-combustion CO2 process using DEA-MDEA mixtures. Chem. Eng. Res. Des. 2011, 89, 1763–1773. [Google Scholar] [CrossRef]
- Yu, C.-H.; Huang, C.-H.; Tan, C.-S. A Review of CO2 Capture by Absorption and Adsorption. Aerosol Air Qual. Res. 2012, 12, 745–769. [Google Scholar] [CrossRef]
- Tamidi, A.M.; Yasin, N.H. CO2 and H2S co-removal from natural gas using membrane contactor technology. In Proceedings of the SPE Asia Pacific Oil & Gas Conference and Exhibition, Virtual, 17–19 November 2020. [Google Scholar] [CrossRef]
- Ying, J.; Raets, S.; Eimer, D. The Activator Mechanism of Piperazine in Aqueous Methyldiethanolamine Solutions. Energy Procedia 2017, 114, 2078–2087. [Google Scholar] [CrossRef]
- Costa, C.; Di Felice, R.; Moretti, P.; Oliva, M.; Ramezani, R. Piperazine and methyldiethanolamine interrelationships in CO2 absorption by aqueous amine mixtures. Part II—Saturation rates of mixed reagent solutions. Can. J. Chem. Eng. 2020, 98, 2516–2529. [Google Scholar] [CrossRef]
- Xu, G.-W.; Zhang, C.-F.; Qin, S.-J.; Wang, Y.-W. Kinetics Study on Absorption of Carbon Dioxide into Solutions of Activated Methyldiethanolamine. Ind. Eng. Chem. Res. 1992, 31, 921–927. [Google Scholar] [CrossRef]
- Weiland, R.H.; Sivasubramaniam, M.S. Effect of Heat-Stable Salts on Amine. In Proceedings of the Fall Meeting of AIChE, Austin, TX, USA, 7–12 November 2004. [Google Scholar]
- Le Grange, P.; Sheilan, M.; Spooner, B. How to Limit Amine Systems Failures. The Chemical Engineer. Issue October 2017, 916. Available online: https://www.thechemicalengineer.com/features/how-to-limit-amine-systems-failures/ (accessed on 7 August 2023).
- Laugier, F.; Andriantsiferana, C.; Wilhelm, A.M.; Delmas, H. Ultrasound in gas–liquid systems: Effects on solubility and mass transfer. Ultrason. Sonochem. 2008, 15, 965–972. [Google Scholar] [CrossRef] [PubMed]
- Tudela, I.; Sáez, V.; Esclapez, M.D.; Díez-García, M.I.; Bonete, P.; González-García, J. Simulation of the spatial distribution of the acoustic pressure in sonochemical reactors with numerical methods: A review. Ultrason. Sonochem. 2014, 21, 909–919. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Yasuda, K.; Koda, S. Numerical simulation of liquid velocity distribution in a sonochemical reactor. Ultrason. Sonochem. 2013, 20, 452–459. [Google Scholar] [CrossRef]
- Yang, Z.; Goto, H.; Matsumot, M.; Maeda, R. Ultrasonic micromixer for microfluidic systems. In Proceedings of the IEEE Thirteenth Annual International Conference on Micro Electro Mechanical Systems, IEEE, Miyazaki, Japan, 23–27 January 2000; pp. 80–85. [Google Scholar]
- Rahimi, M.; Aghel, B.; Hatamifar, B.; Akbari, M.; Alsairafi, A. CFD modeling of mixing intensification assisted with ultrasound wave in a T-type microreactor. Chem. Eng. Process. Process. Intensif. 2014, 86, 36–46. [Google Scholar] [CrossRef]
- Trujillo, F.J.; Knoerzer, K. A computational modeling approach of the jet-like acoustic streaming and heat generation induced by low frequency high power ultrasonic horn reactors. Ultrason. Sonochem. 2011, 18, 1263–1273. [Google Scholar] [CrossRef]
- Niazi, S.; Hashemabadi, S.H.; Razi, M.M. CFD simulation of acoustic cavitation in a crude oil upgrading sonoreactor and prediction of collapse temperature and pressure of a cavitation bubble. Chem. Eng. Res. Des. 2014, 92, 166–173. [Google Scholar] [CrossRef]
- Tay, W.H.; Lau, K.K.; Shari, A.M. High performance promoter-free CO2 absorption using potassium carbonate solution in an ultrasonic irradiation system. J. CO2 Util. 2017, 21, 383–394. [Google Scholar] [CrossRef]
- Yusof, S.M.M.; Lau, K.K.; Shariff, A.M.; Tay, W.H.; Mustafa, N.F.A.; Lock, S.S.M. Novel continuous ultrasonic contactor system for CO2 absorption: Parametric and optimization study. J. Ind. Eng. Chem. 2019, 79, 279–287. [Google Scholar] [CrossRef]
- Legay, M.; Gondrexon, N.; Le Person, S.; Boldo, P.; Bontemps, A. Enhancement of Heat Transfer by Ultrasound: Review and Recent Advances. Int. J. Chem. Eng. 2011, 2011, 670108. [Google Scholar] [CrossRef]
- Tay, W.H.; Lau, K.K.; Shariff, A.M. High frequency ultrasonic-assisted CO2 absorption in a high pressure water batch system. Ultrason. Sonochem. 2016, 33, 190–196. [Google Scholar] [CrossRef]
- Akbari, M.; Rahimi, M.; Faryadi, M. Gas–liquid flow mass transfer in a T-shape microreactor stimulated with 1.7 MHz ultrasound waves. Chin. J. Chem. Eng. 2017, 25, 1143–1152. [Google Scholar] [CrossRef]
- Abolhasani, M.; Rahimi, M.; Dehbani, M. CFD Modeling of Low, Medium and High Frequency Ultrasound Waves Propagation Inside a Liquid Medium. In Proceedings of the 4th National Conference on CFD Applications in Chemical & Petroleum Industries, Ahwaz, Iran, 16 May 2012. [Google Scholar]
- Laborde, J.; Bouyer, C.; Caltagirone, J.; Gerard, A. Acoustic cavitation field prediction at low and high frequency ultrasounds. Ultrasoonics 1998, 36, 581–587. [Google Scholar] [CrossRef]
- Tamidi, A.M.; Lau, K.K.; Khalit, S.H. A review of recent development in numerical simulation of ultrasonic-assisted gas-liquid mass transfer process. Comput. Chem. Eng. 2021, 155, 107498. [Google Scholar] [CrossRef]
- Abolhasani, M.; Rahimi, M.; Dehbani, M.; Alsairafi, A.A. CFD Modeling of Heat Transfer by 1.7 MHz Ultrasound Waves. Numer. Heat Transfer Part A Appl. 2012, 62, 822–841. [Google Scholar] [CrossRef]
- Parvizian, F.; Rahimi, M.; Faryadi, M.; Abdulaziz, A. Comparison between Mixing in Novel High Frequency Sonoreactor and Stirred Tank Reactor. Eng. Appl. Comput. Fluid Mech. 2012, 6, 295–306. [Google Scholar] [CrossRef]
- Xu, Z.; Yasuda, K.; Liu, X. Simulation of the formation and characteristics of ultrasonic fountain. Ultrason. Sonochem. 2016, 32, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Gogate, P.R.; Pandit, A.B.; Wilhelm, A.M.; Delmas, H. Investigation of induction of air due to ultrasound source in the sonochemical reactors. Ultrason. Sonochem. 2005, 12, 453–460. [Google Scholar] [CrossRef]
- Airgas. Nitrogen Safety Data Sheet. 2021. Available online: https://www.airgas.com/msds/001040.pdf (accessed on 28 July 2023).
- Nakhaei-Kohani, R.; Taslimi-Renani, E.; Hadavimoghaddam, F.; Mohammadi, M.R.; Hemmati-Sarapardeh, A. Modeling solubility of CO2–N2 gas mixtures in aqueous electrolyte systems using artificial intelligence techniques and equations of state. Sci. Rep. 2022, 12, 3625. [Google Scholar] [CrossRef] [PubMed]
- Tamidi, A.M.; Lau, K.K.; Yusof, S.M.M.; Azmi, N.; Zakariya, S.; Patthi, U. Enhancement of CO2 Absorption Process Using High-Frequency Ultrasonic Waves. Sustainability 2023, 15, 11064. [Google Scholar] [CrossRef]
- Nurida, M.Y.; Norfadilah, D.; Aishah, M.R.S.; Phak, C.Z.; Saleh, S.M. Monitoring of CO2 Absorption Solvent in Natural Gas Process Using Fourier Transform Near-Infrared Spectrometry. Int. J. Anal. Chem. 2020, 2020, 9830685. [Google Scholar] [CrossRef]
- Lee, J.; Kim, J.; Kim, H.; Lee, K.S.; Won, W. A new modeling approach for a CO2 capture process based on a blended amine solvent. J. Nat. Gas Sci. Eng. 2019, 61, 206–214. [Google Scholar] [CrossRef]
- Hairul, N.; Shariff, A.; Tay, W.; Mortel, A.; Lau, K.; Tan, L. Modelling of high pressure CO2 absorption using PZ+AMP blended solution in a packed absorption column. Sep. Purif. Technol. 2016, 165, 179–189. [Google Scholar] [CrossRef]
- Donaldson, T.L.; Nguyen, Y.N. Carbon Dioxide Reaction Kinetics and Transport in Aqueous Amine Membranes. Ind. Eng. Chem. Fundam. 1980, 19, 260–266. [Google Scholar] [CrossRef]
- Versteeg, G.F.; van Swaaij, W.P.M. On the kinetics between CO2 and alkanolamines both in aqueous and non-aqueous solutions-II. Tertiary amines. Chem. Eng. Sci. 1988, 43, 587–591. [Google Scholar] [CrossRef]
- Koronaki, I.P.; Prentza, L.; Papaefthimiou, V. Modeling of CO2 capture via chemical absorption processes–An extensive literature review. Renew. Sustain. Energy Rev. 2015, 50, 547–566. [Google Scholar] [CrossRef]
- Rufford, T.; Smart, S.; Watson, G.; Graham, B.; Boxall, J.; da Costa, J.D.; May, E. The removal of CO2 and N2 from natural gas: A review of conventional and emerging process technologies. J. Pet. Sci. Eng. 2012, 94–95, 123–154. [Google Scholar] [CrossRef]
- Haimour, N.; Bidarian, A.; Sandall, O.C. Kinetics of the reaction between carbon dioxide and methyldiethanolamine. Chem. Eng. Sci. 1987, 42, 1393–1398. [Google Scholar] [CrossRef]
- McCabe, W.; Smith, J.; Hariot, P. Unit Operation of Chemical Engineering, 7th ed.; McGraw-Hill Companies, Inc.: Singapore, 2005. [Google Scholar]
- Chan, Z.P.; Li, L.; Kang, G.; Ab Manan, N.; Cao, Y.; Wang, T. Deep CO2 removal using high pressure membrane contactors with low liquid-to-gas ratio. Chem. Eng. Res. Des. 2020, 153, 528–536. [Google Scholar] [CrossRef]
- Wang, C.; Seibert, A.F.; Rochelle, G.T. Packing characterization: Absorber economic analysis. Int. J. Greenh. Gas Control. 2015, 42, 124–131. [Google Scholar] [CrossRef]
- Zeng, Q.; Guo, Y.; Niu, Z.; Lin, W. Mass transfer coefficients for CO2 absorption into aqueous ammonia solution using a packed column. Ind. Eng. Chem. Res. 2011, 50, 10168–10175. [Google Scholar] [CrossRef]
- Aroonwilas, A.; Tontiwachwuthikul, P. High-efficiency structured packing for CO2 separation using 2-amino-2-methyl-1-propanol (AMP). Sep. Purif. Technol. 1997, 12, 67–79. [Google Scholar] [CrossRef]
- Wellek, R.M.; Brunson, R.J.; Law, F.H. Enhancement factors for gas-absorption with second-order irreversible chemical reaction. Can. J. Chem. Eng. 1978, 56, 181–186. [Google Scholar] [CrossRef]
- Technip DSME. Mechanical Data Sheet Bulk Removal Amine Absorber. PETRONAS Floating LNG Project. Front End Engineering Design Report. 2011.
- Orangi, S.; Aromada, S.A.; Razi, N.; Øi, L.E. Simulation and Economic Analysis of MEA+PZ and MDEA+MEA Blends in Post-combustion CO2 Capture Plant. In Proceedings of the First SIMS EUROSIM Conference on Modelling and Simulation, SIMS EUROSIM 2021, and 62nd International Conference of Scandinavian Simulation Society, SIMS 2021, Virtual Conference, Finland, 21–23 September 2021; pp. 317–324. [Google Scholar]
- Jenkins, S. 2021 CEPCI Updates: September (prelim.) and August (Final). 2021. Available online: https://www.chemengonline.com/2021-cepci-updates-september-prelim-and-august-final/?printmode=1#:~:text= (accessed on 18 May 2022).
- Clayton, T.; Baumann, P. Cost-to-Capacity Method: Applications and Considerations. M&TS J. 2014, 30, 49–56. [Google Scholar]
- Ogolo, O. Modification of the unit technical cost equation for the accurate determination of the cost of producing a barrel of oil in relation to the Contractor’s revenue. J. Pet. Sci. Eng. 2021, 198, 108122. [Google Scholar] [CrossRef]
- Aroonwilas, A.; Tontiwachwuthikul, P. Mass Transfer Coefficients and Correlation for CO2 Absorption into 2-Amino-2-methyl-1-propanol (AMP) Using Structured Packing. Ind. Eng. Chem. Res. 1998, 37, 569–575. [Google Scholar] [CrossRef]
- Øi, L.E.; Hansen, P.M.; Henriksen, M. CO2 Absorption Efficiency and Heat Consumption Measured at High Gas to Liquid Ratios in Laboratory Rig. Energy Procedia 2017, 114, 1273–1281. [Google Scholar] [CrossRef]
- Sukor, N.R.; Shamsuddin, A.H.; Indra Mahlia, T.M.; Mat Isa, M.F. Techno-Economic Analysis of CO2 Capture Technologies in Offshore Natural Gas Field. Processes 2020, 8, 350. [Google Scholar] [CrossRef]
- Asadi, J.; Kazempoor, P. Techno-economic analysis of membrane-based processes for flexible CO2 capturing from power plants. Energy Convers. Manag. 2021, 246, 114633. [Google Scholar] [CrossRef]

| Run | Parameters | ||
|---|---|---|---|
| Gas Flow Rate (SLPM) | Liquid Flow Rate (LPM) | Ultrasonic Power (V) | |
| 1 | 20 | 0.2 | 30 |
| 2 | 17 | 0.2 | 30 |
| 3 | 15 | 0.2 | 30 |
| 4 | 17 | 0.2 | 25 |
| 5 | 20 | 0.3 | 30 |
| 6 | 20 | 0.3 | 25 |
| 7 | 20 | 0.4 | 30 |
| 8 | 20 | 0.2 | 25 |
| 9 | 20 | 0.4 | 25 |
| No | Reaction Name | Reaction |
|---|---|---|
| R1 | MDEA catalyzed hydration | |
| R2 | Carbamate formation with PZ | |
| R3 | Bicarbamate formation with PZ | |
| R4 | PZ regeneration reaction |
| Feed Gas | Gas Flow Rate | 10,856 kmol/h @ 218 MMscfd |
| Temperature | 35 °C | |
| Pressure | 59.8 barg | |
| Composition | 19.22 mol% CO2 0.02 mol% H2S 0.06 mol% H2O 80.7 mol% Light and heavy hydrocarbon | |
| Gas Flow Rate | 10,856 kmol/h @ 218 MMscfd | |
| Temperature | 35 °C | |
| Pressure | 59.8 barg | |
| Product Gas | Temperature | 86 °C |
| Pressure | 58.9 barg | |
| Composition | 3.2% CO2 27 ppm H2S | |
| Solvent | Composition | 35 wt% MDEA, 6 wt% PZ, 59 wt% water |
| Lean Amine Loading | 0.2 mol/mol | |
| Rich Amine Loading | 0.36–0.39 mol/mol | |
| Ultrasonic Absorber Properties | No of Modules | 10 |
| Gas Flow rate to each module | 21.8 MMscfd |
| Dimension | Value |
|---|---|
| Inner diameter (ID) | 4.9 m |
| Height | 32.6 m |
| Volume | 614.8 m3 |
| No. of unit | 1 |
| Installed weight | 1210.3 tonne |
| Run | Parameters | Responses | |||||
|---|---|---|---|---|---|---|---|
| Gas Flow Rate (SLPM) | Liquid Flow Rate (LPM) | Ultrasonic Power (V) | Effective Volume (m3) | Energy Consumption (MW) | KGa (×10−6 mol/m3.s.Pa) | L/G (gal/lbmol) | |
| 1 | 20 | 0.2 | 30 | 208.52 | 35.11 | 4.75 | 146.69 |
| 2 | 17 | 0.2 | 30 | 201.45 | 33.92 | 4.86 | 156.35 |
| 3 | 15 | 0.2 | 30 | 236.80 | 39.87 | 4.09 | 181.69 |
| 4 | 17 | 0.2 | 25 | 222.66 | 26.04 | 4.40 | 165.35 |
| 5 | 20 | 0.3 | 30 | 226.19 | 38.09 | 4.23 | 228.79 |
| 6 | 20 | 0.3 | 25 | 254.47 | 29.76 | 3.83 | 242.57 |
| 7 | 20 | 0.4 | 30 | 222.66 | 37.49 | 4.43 | 303.33 |
| 8 | 20 | 0.2 | 25 | 226.19 | 26.45 | 4.37 | 153.40 |
| 9 | 20 | 0.4 | 25 | 243.87 | 28.52 | 4.02 | 313.97 |
| Equipment | Packed Column | Ultrasonic Absorber | ||
|---|---|---|---|---|
| Installed Cost (USD) | Installed Weight (tonne) | Installed Cost (USD) | Installed Weight (tonne) | |
| Cooler | 754,200 | 111.132 | 631,500 | 91.680 |
| Pump A | 1,967,600 | 127.081 | 1,717,400 | 108.337 |
| Coalescer | 385,900 | 59.070 | 364,900 | 53.669 |
| Pump B | 456,400 | 35.205 | 401,800 | 29.883 |
| LP Flash | 622,200 | 76.153 | 553,200 | 67.538 |
| Heater | 548,500 | 83.097 | 479,600 | 72.275 |
| HP Flash | 775,500 | 117.856 | 714,900 | 118.193 |
| AGRU | 11,960,000 | 1210.3 | 15,079,877.8 | 512.85 |
| Total | 17,470,300 | 1819.9 | 19,943,177.8 | 1054.4 |
| Packed Column | Ultrasonic Absorber | |
|---|---|---|
| Installed Equipment Cost (mil USD) | 17.470 | 19.94 |
| Structure cost (mil USD) | 131.05 | 78.61 |
| Contingency @ 30% (mil USD) | 44.56 | 29.57 |
| Total CAPEX (mil USD) | 193.08 | 128.12 |
| Parameters | Packed Column | Ultrasonic Absorber |
|---|---|---|
| Pump (MW) | 8.08 | 6.90 |
| Heater (MW) | 107.30 | 94.14 |
| Absorber (MW) | 0.00 | 26.04 |
| Total Energy (MW) | 115.38 | 127.08 |
| Power Consumption cost (USD/day) | 74,880 | 82,368 |
| Criteria | Packed Column | Ultrasonic Absorber |
|---|---|---|
| Power Consumption (mil USD) | 499.20 | 549.12 |
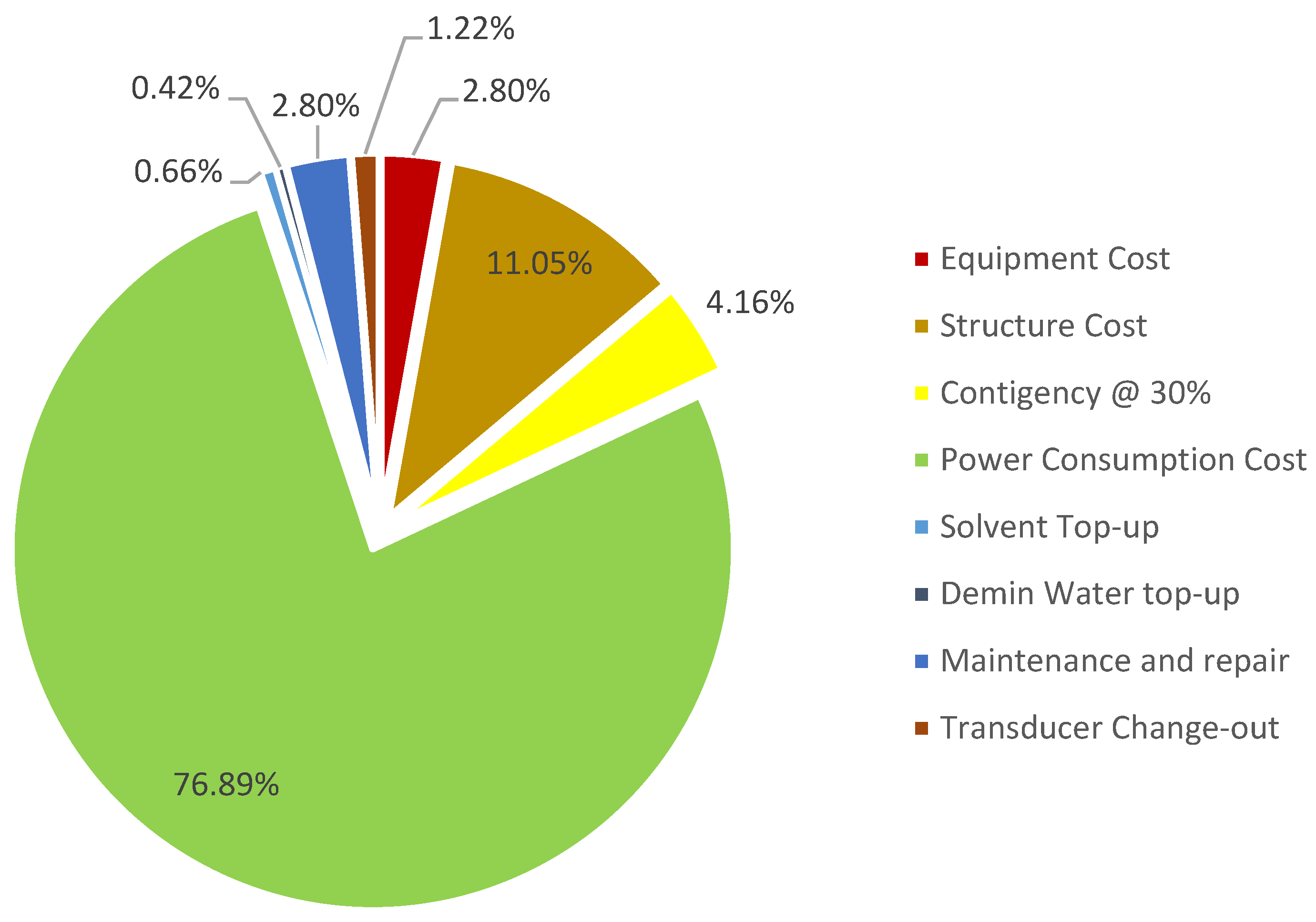
| Solvent Top-up (mil USD) | 7.13 | 4.71 |
| Demin Water top-up (mil USD) | 2.66 | 2.99 |
| Maintenance and repair (mil USD) | 17.47 | 19.94 |
| Transducer Change-out (mil USD) | 0.00 | 8.65 |
| Total OPEX (mil USD) | 526.46 | 585.42 |
| Criteria | Packed Column | Ultrasonic Absorber |
|---|---|---|
| CAPEX (mil USD) | 193.08 | 128.12 |
| OPEX for 20 years (mil USD) | 526.46 | 583.34 |
| UTC (USD/Tonne CO2) | 56.61 | 54.67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohd Tamidi, A.; Lau, K.K.; Ng, L.H.; Mhd Yusof, S.M.; Azmi, N.; Zakariya, S.; Khalit, S.H.; Quek, V.C. Numerical Modeling and Economic Analysis of Ultrasonic-Assisted CO2 Absorption Process for Offshore Application. Processes 2023, 11, 3089. https://doi.org/10.3390/pr11113089
Mohd Tamidi A, Lau KK, Ng LH, Mhd Yusof SM, Azmi N, Zakariya S, Khalit SH, Quek VC. Numerical Modeling and Economic Analysis of Ultrasonic-Assisted CO2 Absorption Process for Offshore Application. Processes. 2023; 11(11):3089. https://doi.org/10.3390/pr11113089
Chicago/Turabian StyleMohd Tamidi, Athirah, Kok Keong Lau, Li Huey Ng, Siti Munirah Mhd Yusof, Nurulhuda Azmi, Shahidah Zakariya, Siti Hajar Khalit, and Ven Chian Quek. 2023. "Numerical Modeling and Economic Analysis of Ultrasonic-Assisted CO2 Absorption Process for Offshore Application" Processes 11, no. 11: 3089. https://doi.org/10.3390/pr11113089
APA StyleMohd Tamidi, A., Lau, K. K., Ng, L. H., Mhd Yusof, S. M., Azmi, N., Zakariya, S., Khalit, S. H., & Quek, V. C. (2023). Numerical Modeling and Economic Analysis of Ultrasonic-Assisted CO2 Absorption Process for Offshore Application. Processes, 11(11), 3089. https://doi.org/10.3390/pr11113089