A New Method for Measuring the Effective Length of Acid-Fracturing Fractures
Abstract
:1. Introduction
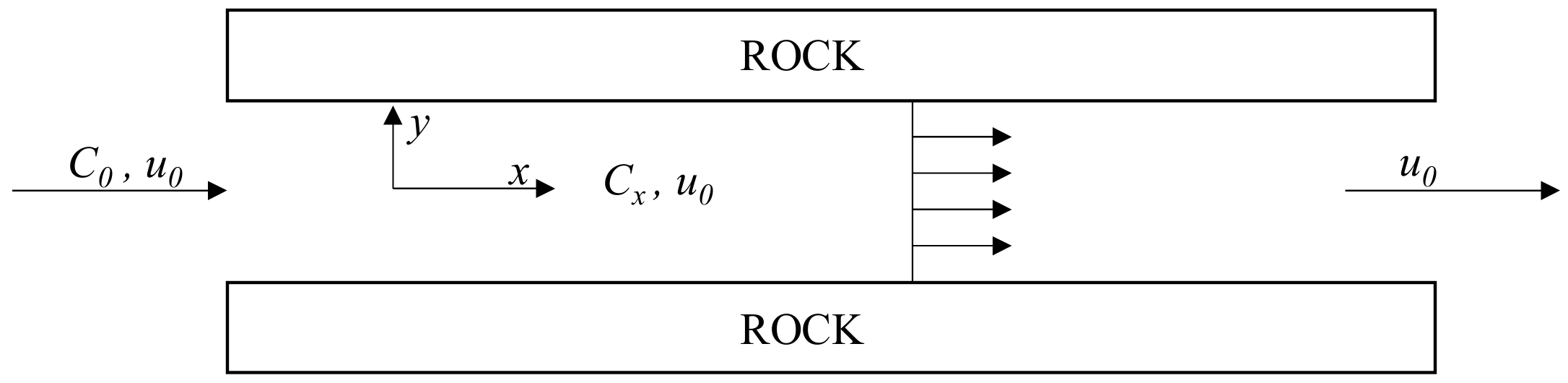
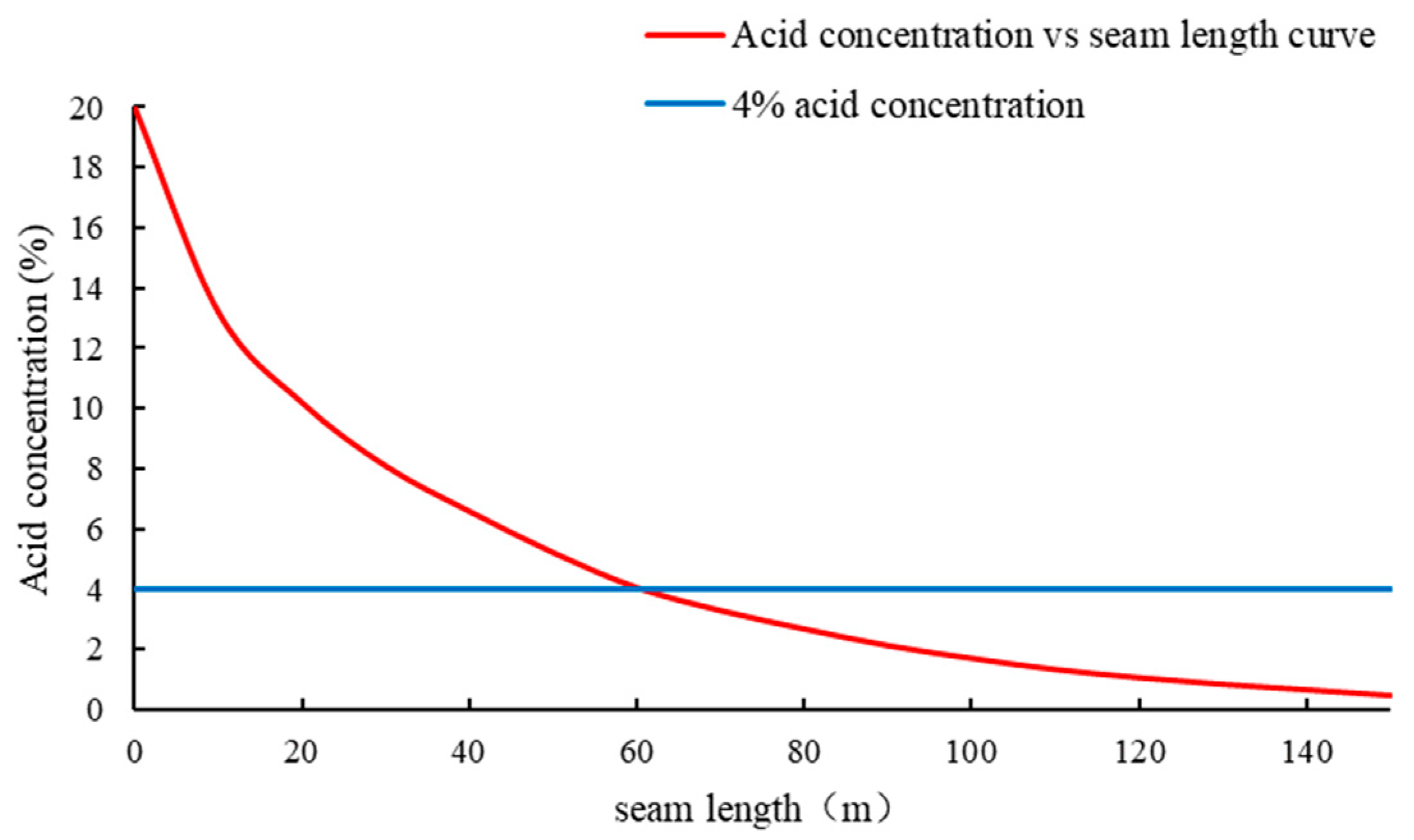
2. Calculation Model of Acid Concentration Distribution in Acid-Fracturing Fracture
3. Experimental Design
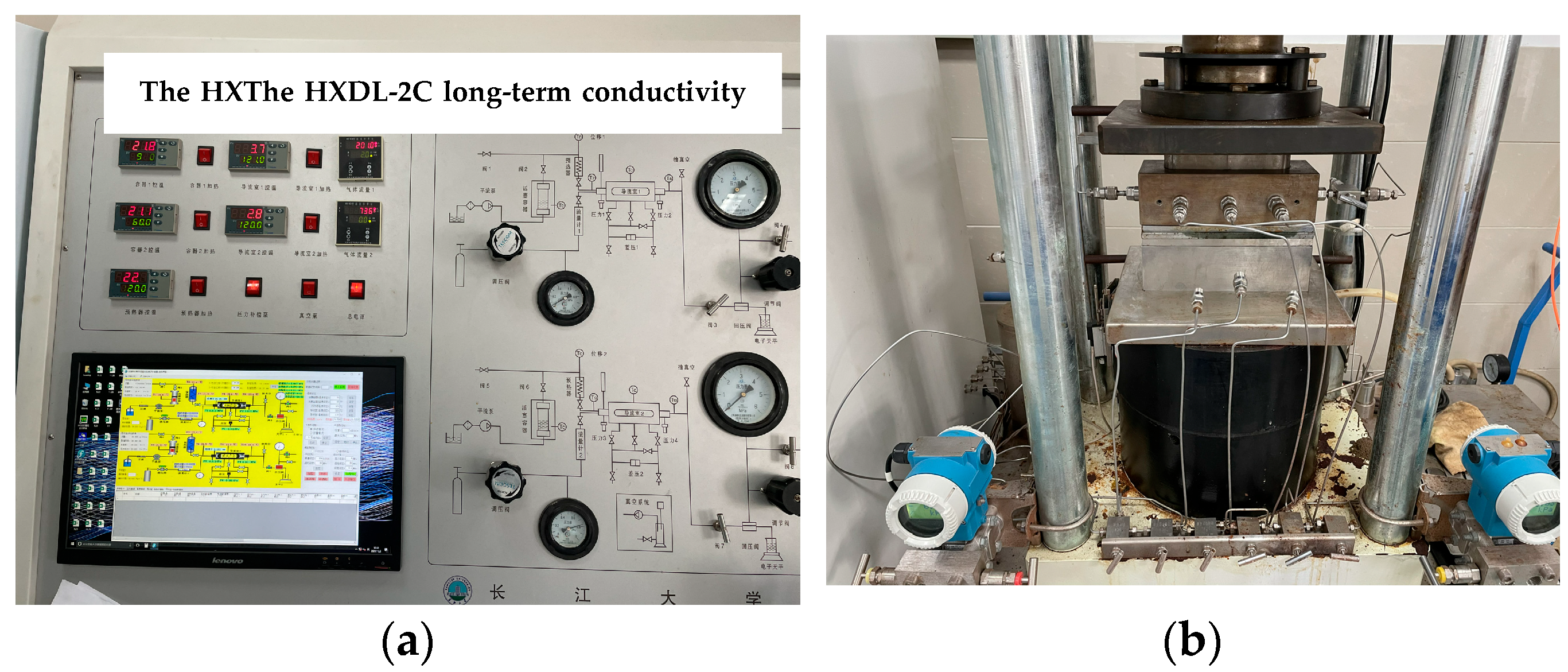
3.1. Experimental Setup
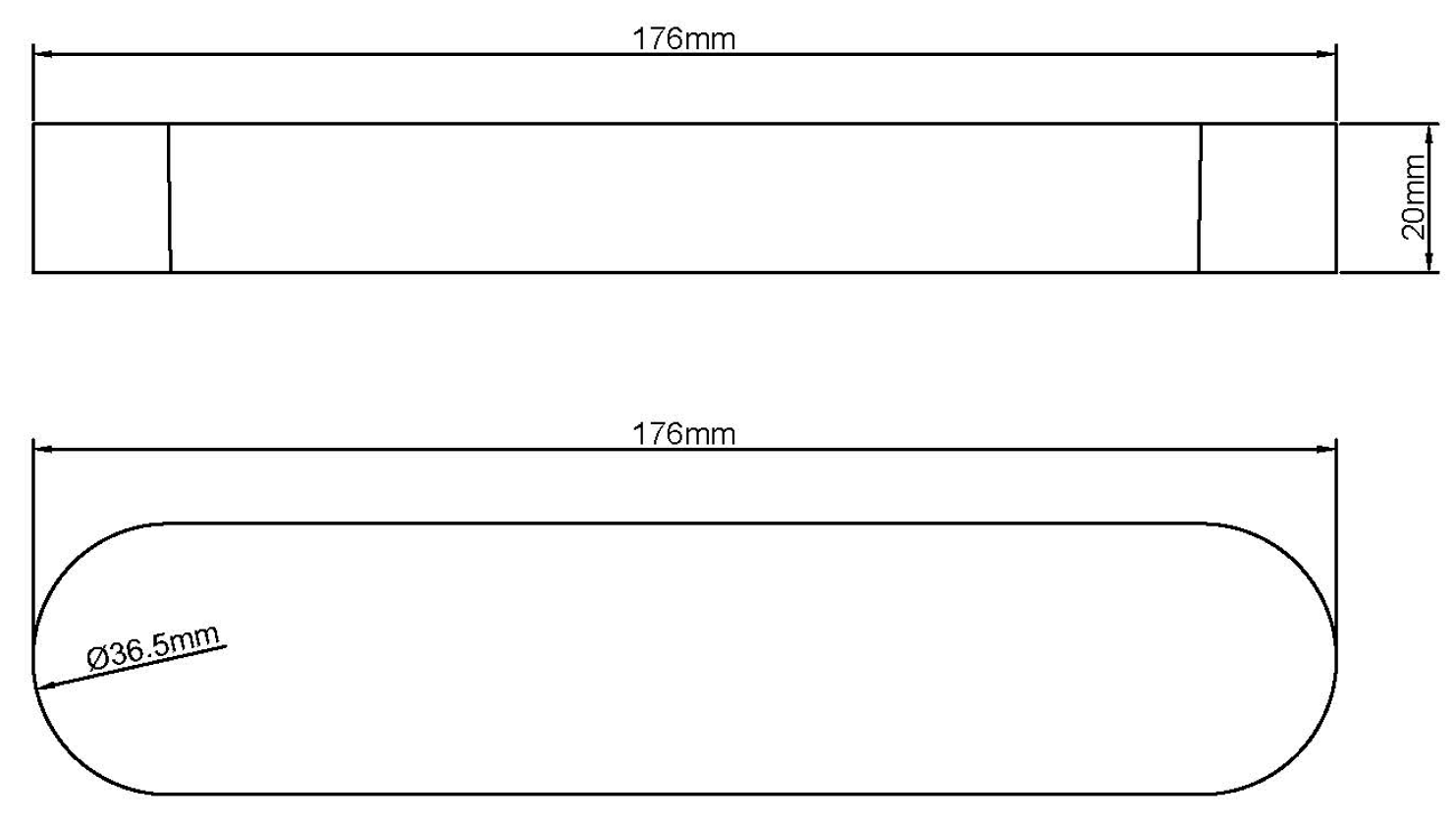
3.2. Experimental Materials
3.3. Test Methods
3.4. Experimental Parameter Design
4. Case Studies
4.1. Experimental Parameters
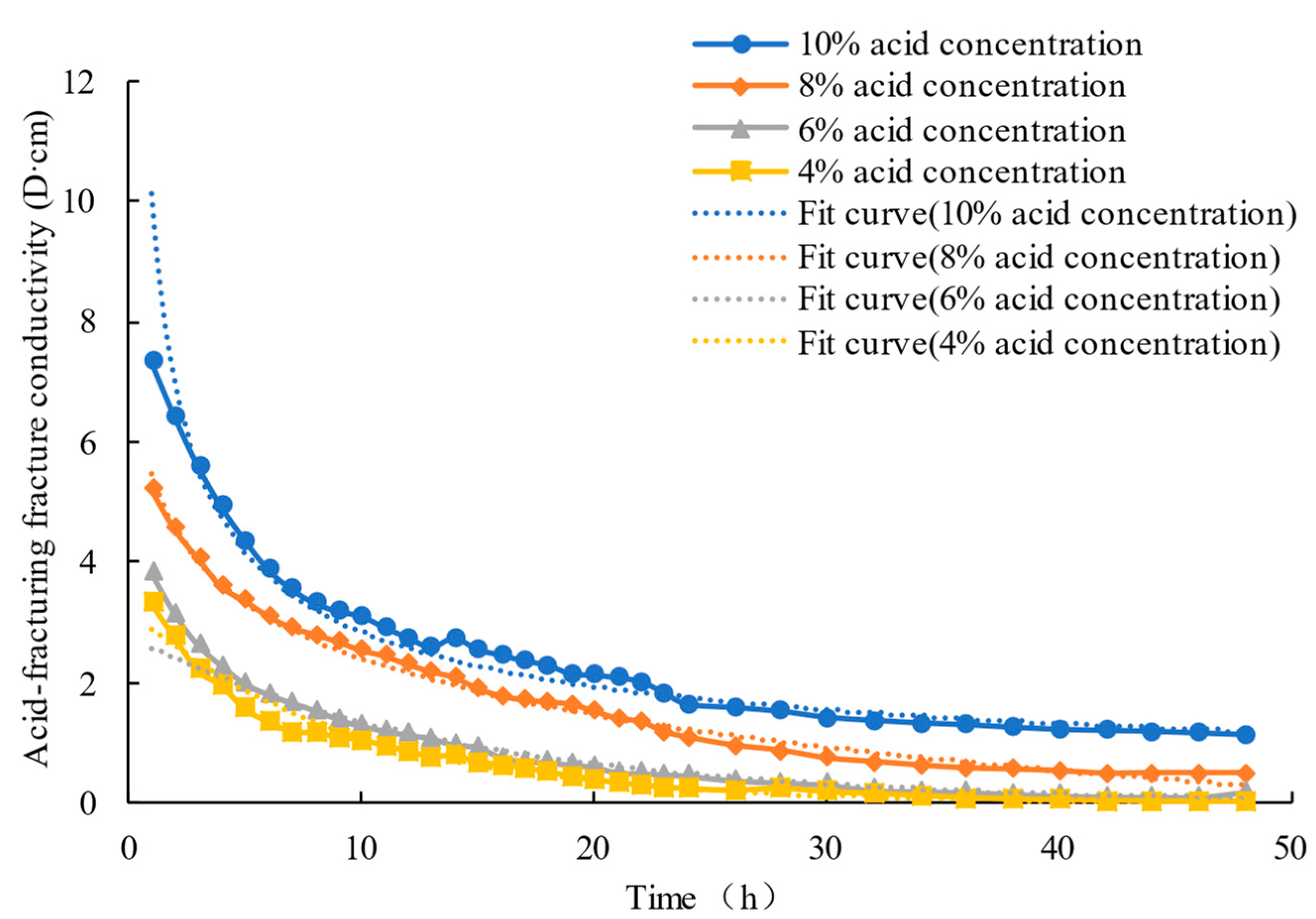
4.2. Experimental Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Qun, L.E.; Yun, X.U.; Zhanwei, Y.A.; Bo, C.A.; Xin, W.A.; Lang, Z.H.; Huifeng, L.I.; Minjie, X.U.; Liwei, W.A.; Li, S. Progress and development directions of stimulation techniques for ultra-deep oil and gas reservoirs. Pet. Explor. Dev. 2021, 48, 221–231. [Google Scholar]
- Yini, L.I.U.; Mingyi, H.U.; Zhang, S. Types, structural evolution difference and petroleum geological significance of Cambrian–Ordovician carbonate platforms in Gucheng–Xiaotang area, Tarim Basin, NW China. Pet. Explor. Dev. 2022, 49, 1019–1032. [Google Scholar]
- Li, Q.; Wang, F.; Wang, Y.; Forson, K.; Cao, L.; Zhang, C.; Zhou, C.; Zhao, B.; Chen, J. Experimental investigation on the high-pressure sand suspension and adsorption capacity of guar gum fracturing fluid in low-permeability shale reservoirs: Factor analysis and mechanism disclosure. Environ. Sci. Pollut. Res. 2022, 29, 53050–53062. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Han, Y.; Liu, X.; Ansari, U.; Cheng, Y.; Yan, C. Hydrate as a by-product in CO2 leakage during the long-term sub-seabed sequestration and its role in preventing further leakage. Environ. Sci. Pollut. Res. 2022, 29, 77737–77754. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhou, F.; Mou, J.; Xu, G.; Zhang, S.; Li, Z. A new method to improve long-term fracture conductivity in acid fracturing under high closure stress. J. Pet. Sci. Eng. 2018, 171, 760–770. [Google Scholar] [CrossRef]
- Aljawad, M.S.; Aljulaih, H.; Mahmoud, M.; Desouky, M. Integration of field, laboratory, and modeling aspects of acid fracturing: A comprehensive review. J. Pet. Sci. Eng. 2019, 181, 106158. [Google Scholar] [CrossRef]
- Li, X.; He, Y.; Yang, Z.; Zhu, J.; Li, F.; Song, R. Fully coupled model for calculating the effective acid penetration distance during acid fracturing. J. Nat. Gas Sci. Eng. 2020, 77, 103267. [Google Scholar]
- Basu, S.; Ali, M.Y.; Farid, A.; Berteussen, K.A.; Mercado, G. A microseismic experiment in Abu Dhabi, United Arab Emirates: Implications for carbonate reservoir monitoring. Arab. J. Geosci. 2014, 7, 3815–3827. [Google Scholar] [CrossRef]
- Solovyov, Y.V.; Alekseev, B.G.; Abramov, A.A.; Shalaginov, S.L.; Toksubayeva, A.S.; Mayorova, E.S.; Shestakov, A.G.; Serkerov, B.; Ishberdina, G.; Rylov, R.; et al. Microseismic monitoring of non-proppant acid hydraulic fracturing in horizontal well. In Horizontal Wells. Problems and Prospects 2015; European Association of Geoscientists & Engineers: Moscow, Russia, 2015; Volume 2015, pp. 1–5. [Google Scholar]
- Zhang, N.; Liu, B.; Fa, W.; Dai, X.M. Research on Application of Microseismic Monitoring and Interpretation Technology. In International Field Exploration and Development Conference; Springer Nature: Singapore, 2021; pp. 3084–3091. [Google Scholar]
- Zhang, N.L.; Zhao, L.; Luo, Z. Simulation of acid fracturing effective distance in fissured carbonate reservoir. Electron. J. Geotech. Eng. 2017, 22, 4317–4331. [Google Scholar]
- Zhang, R.; Hou, B.; Zhou, B.; Liu, Y.; Xiao, Y.; Zhang, K. Effect of acid fracturing on carbonate formation in southwest China based on experimental investigations. J. Nat. Gas Sci. Eng. 2020, 73, 103057. [Google Scholar]
- Liu, F.; Li, L.; Zhou, C.; Ma, Y.; Chen, W.; Zeng, R.; Fu, Y.; Hu, Q.; He, T.; Guan, W. An Experimental Test Method for Effective Length of Acid-Etched Fractures. CN112983372A, 18 June 2021. [Google Scholar]
- Gu, Y.; Yi, X.; Dai, Y.; Zhang, Y.; Zhou, C. A new method to determine the effective distance of acid-rock reaction. Sci. Technol. Eng. 2015, 15, 34–37. [Google Scholar]
- Luo, Z.; Chen, X.; Zhao, L.; Xiao, Y.; Lu, X.; Miao, W.; Liu, H. Large-scale acid fracturing based on a large-scale conductivity apparatus. ACS Omega 2021, 6, 6559–6570. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Li, L. Calculation of effective flow distance of acid in fractures. Pet. Explor. Dev. 1981, 65–75. [Google Scholar]
- Al-Ameri, A.; Gamadi, T. Optimization of acid fracturing for a tight carbonate reservoir. Petroleum 2020, 6, 70–79. [Google Scholar] [CrossRef]
- Aljawad, M.S.; Desouky, M.; Sølling, T.I.; Amao, A.O.; Al-Ramadan, K. Improving carbonate rock hardness by consolidating additives to sustain long term fracture conductivity. J. Pet. Sci. Eng. 2020, 195, 107897. [Google Scholar] [CrossRef]
- Desouky, M.; Aljawad, M.S.; Solling, T.; Abduljamiu, A.; Norrman, K.; Alshehri, D. Improving long-term hydraulic fracture conductivity by alteration of rock minerals. J. Pet. Sci. Eng. 2021, 196, 108046. [Google Scholar]
- Samarkin, Y.; Amao, A.; Aljawad, M.S.; Solling, T.; Al-Ramadan, K.; AlTammar, M.J.; Alruwaili, K.M. Conductivity Enhancement of Fractured Carbonates through High-Temperature Diammonium Hydrogen Phosphate Consolidation: A Preliminary Study. SPE J. 2023, 1–17. [Google Scholar] [CrossRef]
- Kozhevnikov, E.V.; Turbakov, M.S.; Gladkikh, E.A.; Riabokon, E.P.; Poplygin, V.V.; Guzev, M.A.; Qi, C.; Kunitskikh, A.A. Colloid migration as a reason for porous sandstone permeability degradation during coreflooding. Energies 2022, 15, 2845. [Google Scholar] [CrossRef]
- Turbakov, M.S.; Kozhevnikov, E.V.; Riabokon, E.P.; Gladkikh, E.A.; Poplygin, V.V.; Guzev, M.A.; Jing, H. Permeability Evolution of Porous Sandstone in the Initial Period of Oil Production: Comparison of Well Test and Coreflooding Data. Energies 2022, 15, 6137. [Google Scholar] [CrossRef]


| Parameter Class | Value |
|---|---|
| Fresh acid concentration | 20% |
| Hydrogen ion mass transfer coefficient | 6.532 × 10−9 m2/min |
| Experimental fracture width | 0.002 m |
| Experimental fracture height | 0.0365 m |
| Dynamic fracture height | 50 m |
| Average fracture width of dynamic fracture | 0.008 m |
| Construction capacity | 5 m3/min |
| Construction time | 60 min |
| Acid density | 1.136 g/cm3 |
| Combined filtration coefficient | 1.3 × 10−3 m/min1/2 |
| Acid concentration (%) | 10 | 8 | 6 | 4 | 2 |
| Acid injection flow (mL/min) | 456 | 456 | 456 | 456 | 456 |
| Acid dosage (mL) | 6539 | 6158 | 5469 | 4104 | 211 |
| Calcium chloride mass (g) | 1130 | 1277 | 1323 | 1134 | 680 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, W.; Li, S.; Zhang, J.; Wang, L.; Feng, Y.; Liao, Y. A New Method for Measuring the Effective Length of Acid-Fracturing Fractures. Processes 2023, 11, 3084. https://doi.org/10.3390/pr11113084
Xu W, Li S, Zhang J, Wang L, Feng Y, Liao Y. A New Method for Measuring the Effective Length of Acid-Fracturing Fractures. Processes. 2023; 11(11):3084. https://doi.org/10.3390/pr11113084
Chicago/Turabian StyleXu, Wenjun, Shengxiang Li, Jianpeng Zhang, Lei Wang, Yan Feng, and Yuanai Liao. 2023. "A New Method for Measuring the Effective Length of Acid-Fracturing Fractures" Processes 11, no. 11: 3084. https://doi.org/10.3390/pr11113084
APA StyleXu, W., Li, S., Zhang, J., Wang, L., Feng, Y., & Liao, Y. (2023). A New Method for Measuring the Effective Length of Acid-Fracturing Fractures. Processes, 11(11), 3084. https://doi.org/10.3390/pr11113084







