1. Introduction
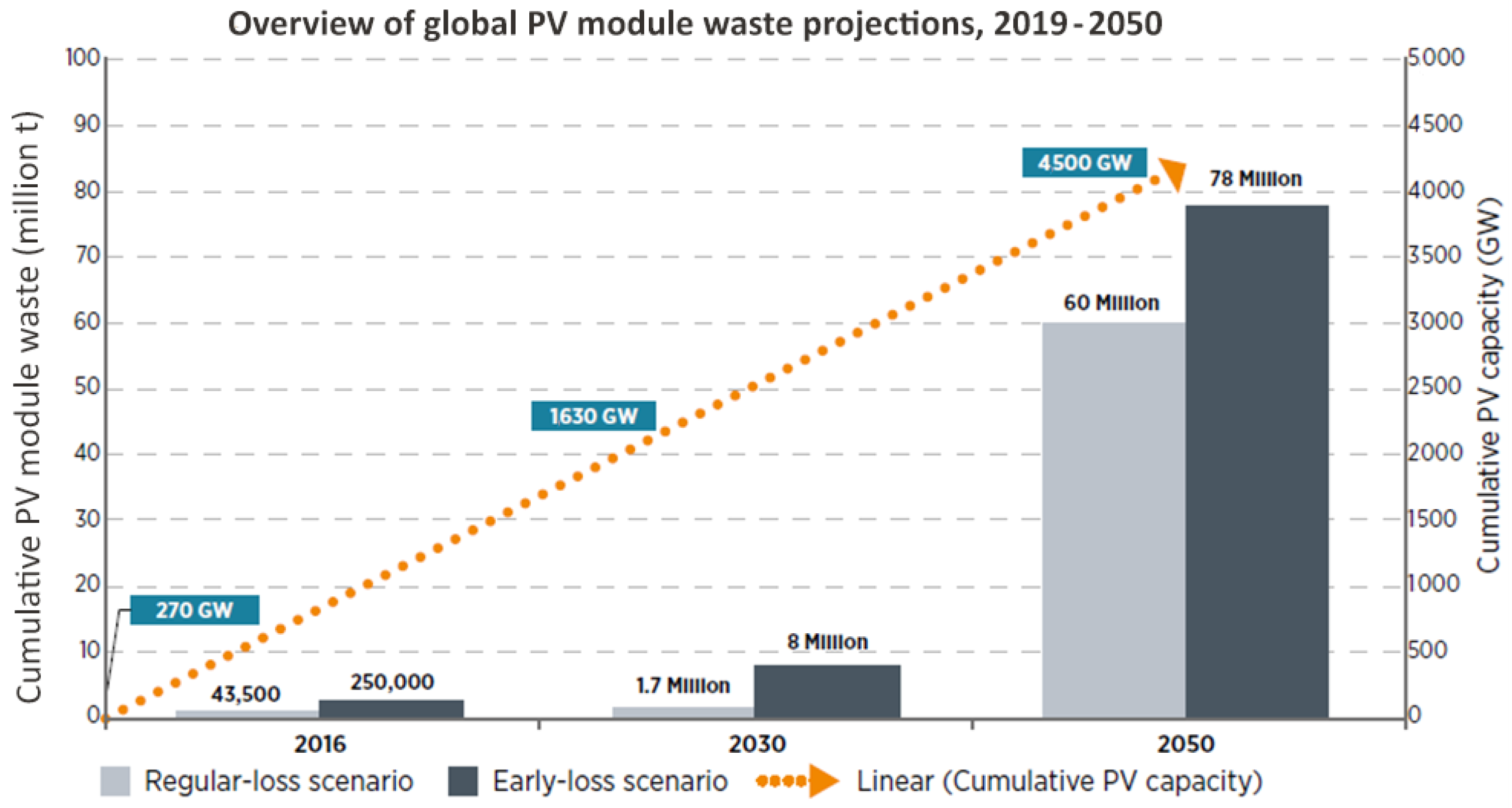
Solar photovoltaic (PV) installations, once they reach the end of their service life, must be properly decommissioned, and all waste must be properly treated and disposed of. In the early boom of photovoltaic plant installation, there was little attention given to the eventual decommissioning. The world’s installed capacity of photovoltaic panels in 2022 was about 1200 GW, while in 2012 it was only 100 GW. This means that the majority of installations are only ten years old or less [
1]. Since the typical guarantee of performance is 20 years and some panels can be expected to operate for perhaps up to 40 years, there is still little drive for commercial disposal, let alone for recycling of the panels at the end of their service (
Figure 1) [
2]. There are a few recycling operations in service, with a concentration in Europe, but the need to be prepared for a massive influx of retired PV modules and associated equipment is obvious. The disposal will have to be ecologically friendly; thus, the focus will have to be on economically viable recycling.
This issue of recycling photovoltaic modules, which have appeared in a large number of installations in the Czech Republic since 2010, is one of the topics of the “Long-term Strategy 2050”. The European Union aims to transform the economies of all member states into a zero-emission future. This vision includes, for example, requirements to increase the share of renewables in member countries to 30% by 2030 [
3], which directly bear on the increased use of photovoltaics. General experience with photovoltaic module disposal and recycling in the Czech Republic can be considered reasonably applicable also to the other member states of the European Union.
Currently, solar energy is the largest energy sector contributor among renewables and is likely to reach the required recycling viability threshold. Many years ago, the idea was already raised that with a greater increase in the sales of PV modules, the need for environmentally sound disposal would arise soon. As of 2012, PV modules have been listed throughout the European Union as electrical equipment creating a specific type of waste. Such waste will have to be disposed of in an appropriate and specified manner. According to estimates of installed PV modules worldwide, up to 8 million tons of this waste can be expected [
2]. In the Czech Republic, the trend of building PV-generating plants became widespread mainly in the 2010s and then again in 2022, when the war in Ukraine started and consumers began to worry about self-sufficiency in electricity generation. The lifetime of PV modules in 2010 was predicted to be twenty years, but tests have shown that it is likely to be much longer, possibly up to forty years. Nevertheless, it is important to prepare for the recycling of these devices. The main reason for recycling PV modules is to recover base metals (mostly aluminum) and precious metals, including silver, indium, germanium, gallium, and silicon. The material composition of silicon crystalline modules is in
Table 1, and the typical values for most thin-film modules are in
Table 2. Aluminum used in the supporting frames is essentially 100% recoverable. The amount of aluminum in a 1 We installed PV module is 0.0107 kg, and the price of aluminum in 1 We is €0.0001. While the amount of precious metal on PV modules is smaller, 0.003 kg/We, the cost is more favorable with 1 We, equal to €0.6086. The expected recovery is about 30% [
4].
Currently, most of the modules installed are silicon PV modules, and therefore, recycling will focus on this type of module. A photovoltaic module consists of a series of photovoltaic cells, PN junctions that upon illumination separate electric charge. For operation, these have to be protected by glass on the front and protected from moisture on the back by plastic. This is often provided using the commercial product Tedlar
®, which is a polyvinyl fluoride polymer registered by DuPont™, Wilmington, DE, USA. The whole system is then vacuum encapsulated by a transparent polymer. To this end, ethylene-vinyl acetate (EVA) polymer is used. The material of the photovoltaic cells is typically semiconductor silicon. Monocrystalline and polycrystalline silicon modules are not difficult to recycle. The modules are first crushed in most recycling lines, and then the individual raw materials are separated. Crushing makes it possible to use existing recycling lines. Most PV modules are still shredded together with other electrical waste, but this does not allow for efficient separation and reuse, especially of silicon wafers, which often account for half of the total value of a PV module [
6,
7].
The new goal is to recover not only the material but preferably the whole silicon cells. Phinikarides et al. [
8] determined the annual decline in PV module performance to be around 0.8% over a total period of 25 years. This decrease is due to degradation processes acting on the whole module, where all the parts are influenced by weathering and degradation processes. Considering the accelerated degradation experiments made by Cotfas et al. [
9] on different types of solar cells, the overall lifespan efficiency drop can be estimated to be 1.5% for multijunction cells and 6% for monocrystalline silicon solar cells. Final efficiency is still a very interesting value applicable to so-called refurbished PV modules, making them attractive for reuse.
The procedures of different recycling companies in recycling silicon modules to obtain whole cells are similar [
10]. First, they remove the aluminum frame, connecting cables, and connection box from the module. Then, the glass that adheres to the EVA film is separated by thermal delamination. The problem is the environmental impact of the thermal removal of the EVA film. The incineration produces dangerous combustion gases. After this delamination, the next step is leaching or etching to obtain the connecting wires. Here, heat treatment can also be used, but this requires considerably more energy. In spite of these steps, it often happens that the individual cells are still connected by interconnecting contacts and thus need to be disconnected from each other [
11].
PV module recycling stems from already known principles and technologies, and much of their disposal and recycling makes use of facilities that are already dedicated to recycling glass and metals [
12]. Mechanical separation of the module into its principal parts can be done with current technology and requires little additional investment. Grinding and further separation will yield crushed cover glass (more than 75% by weight), metals (approximately 8%), and polymers (approximately 10%) [
12]. The used facilities and the procedures vary slightly at different recycling factories, as do the yield and quality of the separated parts. Some facilities already used for metal recycling use mechanical methods to separate the module materials; these methods are grinding, screening, and metal extraction (
Figure 2). Separated crushed glass can be processed further into foam or fiberglass. Metals extracted during this can be offered for further processing to established metal refineries [
12]. In some cases, we can encounter what amounts to the second generation of processing lines that are able to remove the frame and delaminate the protective glass in its entirety. These lines are starting to be installed throughout Europe.
The aim is to find technologies that will allow the reuse of the primary components of solar photovoltaic modules at very low energy and environmental costs. These are called the third generation of PV module recycling lines [
12].
Achieving higher yield and purity of output material inevitably corresponds to higher energy inputs. Electricity consumption in recycling processes ranges anywhere between 50 kWh and 100 kWh per ton of input PV module material for mechanical methods. Recycling metal and glass cullet with a higher yield is based on fine grinding. This calls for about 494 kWh/t [
12]. Currently, there also appears on the energy balance sheet some minor amounts of fossil fuel, the diesel fuel used for the front-end loaders to move material on site. The U.S. Environmental Protection Agency (EPA) gives another take on this recycling effort [
13]. The report states that recycling 100 short tons of glass will save 265 MBtu compared with simple landfill waste disposal. This corresponds to 856 kWh per metric ton when producing glass from raw materials [
12].
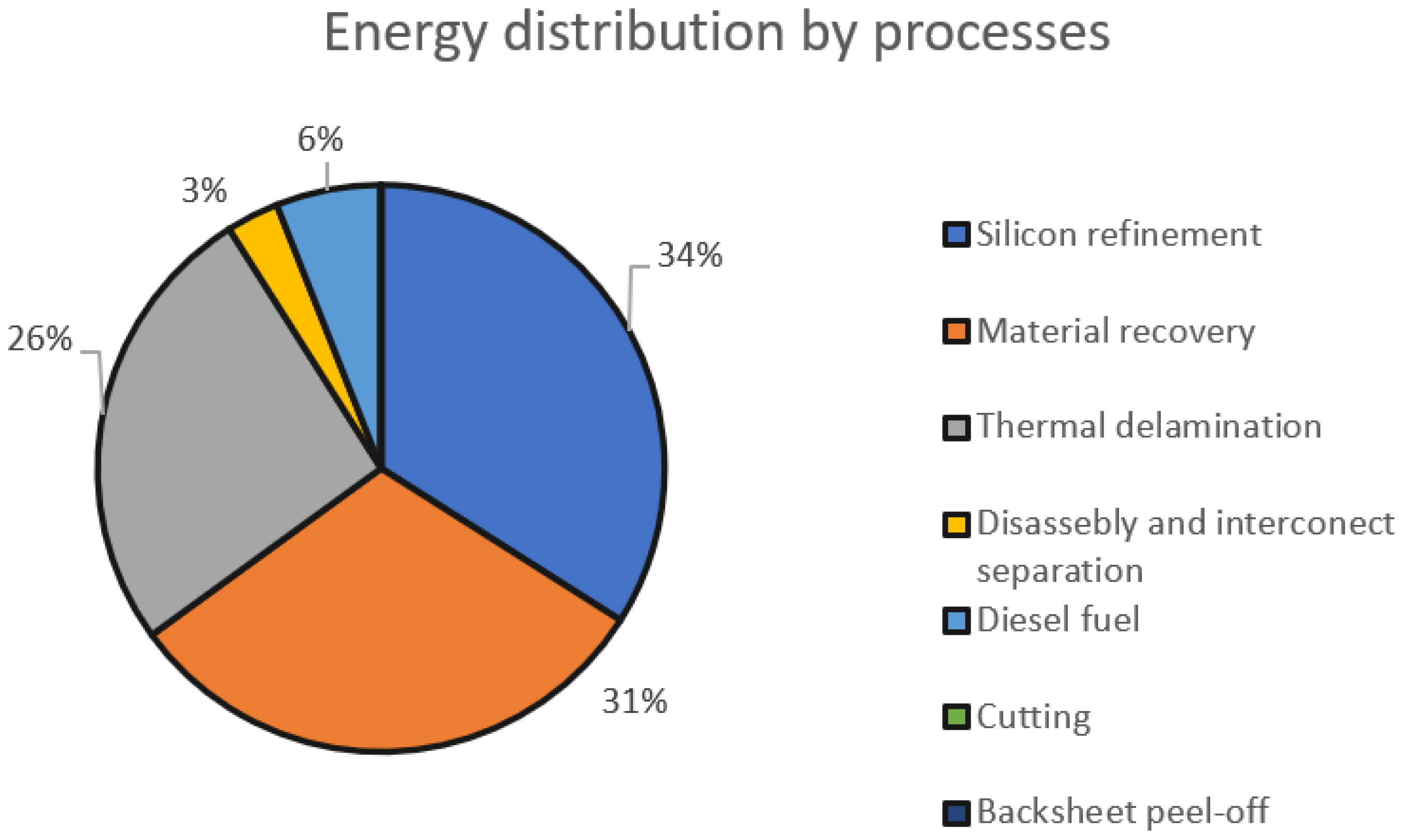
Of the total energy used to recycle PV modules, 26% is used for the thermal delamination of PV modules [
14]. Energy distribution by processes can be seen in
Figure 3.
Chemical Delamination
The photovoltaic assembly of the module is protected from the environment and mainly moisture by encapsulation in a polymer. The most prominent material currently used is (poly)ethylene-vinyl acetate (abbreviated as EVA), which is a binary copolymer of ethylene and vinyl acetate at different ratios [
15,
16,
17]. EVA has suitable optical properties as it is transparent in visible light, and it is also relatively inexpensive. It has been used for this reason in building photovoltaic modules for many years [
16,
17]. Nevertheless, it degrades over the years by yellowing, decreasing the optical transparency [
6]. The degradation of the monomers can also result in the release of small amounts of acetic acid, which then degrades the performance of the PV system sharply [
18,
19,
20].
Since reliable encapsulation is key for the longevity of the modules, the EVA film is applied so that it adheres very well. As a result, the reverse process, to delaminate the EVA film from the glass surface, will be understandably quite difficult. Applied as an embedding medium, its elimination is the crucial factor for the delamination of the module. The often-used and -studied approach is to use heat [
15,
21,
22]. Applied high temperature leads to the decomposition and sometimes outright combustion of the EVA and the back cover polymers (Tedlar).
From our own experiment, we determined that the amount of EVA in the sample was 1.5% by weight. Although it enables the rapid disassembly of the other parts, gaseous products are formed during this process, and the thermal process excludes the possibility of recycling the encapsulation material [
6]. That can be circumvented by the use of chemical agents instead of thermal degradation. EVA has been proven to disintegrate in or interact with a range of dissolvers and solutions. These include agents such as sulfuric acid, lactic acid, xylene, or toluene [
23,
24]. While EVA is somewhat cross linked, it is still reasonable to assume that, at least in part, it could be loosened and then recycled chemically. Recycling of the entire polymerized EVA is probably not likely. This would be due to the partially cross-linked 3D nature of the polymer, increasing the effective molecular weight and thus causing a loss of practical solubility [
25].
Chemical means of recycling photovoltaic modules are a promising approach to recover valuable materials from photovoltaic modules and minimize waste. While this technology has been explored and shows potential, it is important to note that the feasibility and success of chemical recycling depend on various factors, including the composition of the PV modules, the state of technology, and economic considerations.
Some comparison of energy costs in thermal, mechanical, and chemical delamination has already been made, for example, by Wang et al. [
26]. Briand et al. [
27] already analyzed the possibility of the usage of CO
2 for the foaming of EVA in recycling processes. Dias et al. [
25] made experiments and analyses of processed energy when using toluene but only measured energy during the recycling process. Most of these authors have investigated, as we have, the energy intensity of the recycling process itself. We chose to add the energy cost of the solvents themselves to this information and assess the energy cost relative to this input.
In our previous research, it was found that the most straightforward method to separate the PV cell components is to use heat to remove the Tedlar and EVA film layers, for which a relatively high temperature of 540–550 °C is required [
28]. This temperature can be reduced to 340 °C by pressure control [
29]. However, reaching these temperatures is still energy intensive, and the cell surface may oxidize. The chemical process is simpler in this respect, as it only requires a solvent but no heat. The disadvantage is the greater time requirement and the correct choice of the chemical for dissolution.
Part of the study has to be to compare the overall energy consumption of the delamination process. As stated earlier [
12] for thermo-mechanical delamination, the estimated energy consumption is in the range of 50–100 kWh per ton of PV module. To produce toluene, 0.712 to 0.756 kWh per kilogram is needed [
30]. Thus, for chemical delamination to be more energy efficient, the energy consumption of toluene for delamination would have to be less than the energy used for mechanical-thermal delamination. This corresponds to the requirement that no more than 66 kg of toluene should be used per ton of PV module.
3. Evaluation of the Results
Compared with THF and U 6002, toluene was the most effective in dissolving EVA film. However, it cannot be said that satisfactory results were achieved. Toluene only softened and partially dissolved the EVA film layer. In all cases, the EVA film changed its consistency to a gel-like texture. When THF was used, the EVA film had increased adhesion; thus, the subsequent mechanical separation procedure was the most difficult. U 6002 successfully separated the layers but was estimated to have dissolved the least. The last solvent used was cyclohexane, this time at room temperature. After a week, however, there was no reaction with the EVA film or Tedlar.
None of the solvents were able to separate completely the silicon from the EVA film. The most obvious change after the experiment was the cracking of the silicon wafer. The cracking was caused by the change in volume of the EVA film, which absorbed some of the solvent.
If we compare the results of all solvents visually, we can observe the different rates of action. Toluene acted most aggressively; the silicon cell cracked more, probably due to the rapid change in EVA volume. Tetrahydrofuran acted slower in this respect, as the resulting cracked silicon wafer shards were larger. This phenomenon is most apparent in U 6002; here, the solvent acted the slowest, resulting in more EVA film residue around the silicon. The cells themselves are less cracked in this case as there was no rapid change in the volume of the EVA layer.
4. Whole Non-Destructed Sample PV Module
From the results of the separation of the cut parts, it was not clear whether the cracking of the silicon cells was caused only by the swelling of the EVA film or whether the cracking had already occurred during the cutting of the cells with a hand saw. In addition, for a complete module with protective glass, the glass could provide mechanical strength and limit the unintended volume change in the EVA film. For these reasons, it was decided to experiment with an undamaged uncut module.
The experimental procedure was similar to that of the cut parts. The module, stripped of the aluminum frame, was immersed for one week in a solvent at room temperature. The desired result was the complete delamination of the Tedlar layer and protective glass, ideally with minimal cracking of the silicon cells.
4.1. Module Features
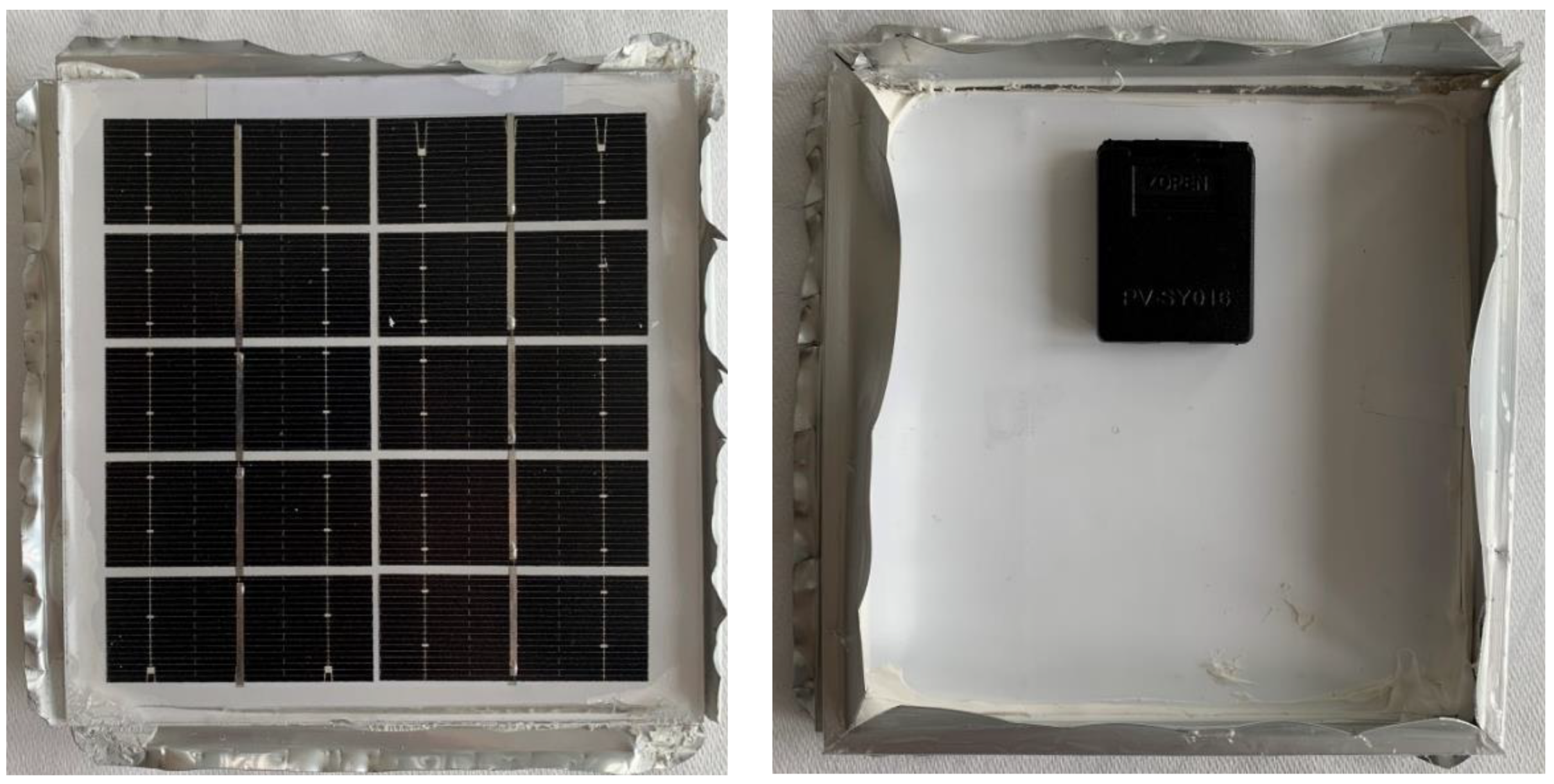
A silicon module (
Figure 8) measuring 125 mm by 135 mm was used for the experiment. It was chosen mainly because of its similar construction to modules used on the roofs of houses and its small size. Similar to these modules, an aluminum frame was used to reinforce the structure and protective glass. Water tightness was ensured by a silicone sealant running around the edges and back. The silicone seal also acted as an adhesive. On the other side of the module, a box with outlets for connection was attached, which was again glued with silicone.
4.2. Preparation for Recycling
The module was stripped (
Figure 9) of its aluminum frame before being immersed in toluene. To remove the frame, the silicone gasket had to be cut off with a knife. A heat gun could be used in the future to facilitate this operation. Similarly, the plastic cover on the back was removed. The glass plate was released from the frame with relatively little force. As the silicon cells provided mechanical strength against bending, it was very unlikely that mechanical damage would occur.
Figure 10 shows the module and all its components. The side view shows the width of the glass compared with the width of the cells, Tedlar, and EVA layer. The EVA film layer in this case was approximately 0.3 mm thick.
After separating the frame and the plastic box, each component of the module was weighed. The weight of the glass, cells, Tedlar, and EVA film was 147 g. The weight of the aluminum frame was 34 g, and the plastic box was 7 g. The next step was to insert it into the container. The container had to be resealable since the toluene used is very volatile and would evaporate quickly. Because of the size of the sample, it was necessary to use a glass baking dish. The sample was placed with the glass facing downwards, so the Tedlar side was the most exposed. A volume of 500 mL of toluene was poured into the container; the container was closed with a lid and sealed with Parafilm.
4.3. Separation Process
After closing and sealing, the container was placed in a fume hood at room temperature, 21 °C. It had to be in the fume hood mainly because of the volatility of the toluene, which evaporated from the container during manipulation. The experiment was originally intended to run for one week. However, after checking, the experiment was extended, and 250 mL of toluene was added at the same time. After one week of exposure, the toluene dissolved the EVA film around the perimeter of the sample toward the center. The Tedlar was not affected by the toluene.
Figure 11 shows the module flooded with toluene. It was evident from the dissolved parts that the cells were cracked around the edges. The experiment was extended for an additional 5 days in anticipation of the complete dissolution of the EVA film even in the center of the specimen. Thus, in total, the sample was in toluene for 12 days (288 h).
4.4. Result of Separation
The result of the experiment after 12 days was that the EVA film was completely dissolved around the edges of the sample, and the damaged silicon cells were washed out of the sample into the container along with the EVA film. Toluene, toward the center of the sample, was unable to penetrate between the silicon cells and the glass plate; thus, the cells are intact in the figure. The removal of the back layer of Tedlar occurred without much problem, as the EVA film was sufficiently softened throughout.
Table 4 shows the parameters of the disassembled module before and immediately after the experiment. The glass plate, EVA film, Tedlar, metal contacts, and silicon cells are counted in the module in the table. At the end of the experiment, the weight of the module was 163 g, up by 16 g before the start. The increase in weight is due to the absorption of toluene by the EVA film. The separated back layer of Tedlar had a weight of 3 g. In our experiment, we achieved toluene consumption corresponding to 108 kg of toluene per one ton of PV modules, which currently means that chemical delamination using toluene is a more energy-intensive process than mechanical-thermal delamination.
5. Evaluation of the Results and Conclusions
The goal was to study possible methods of chemical delamination of PV module encapsulants and compare energy demands vs. thermal decomposition. EVA (ethylene-vinyl acetate) is a copolymer of ethylene and vinyl acetate, with the weight fraction of ethylene being higher; therefore, EVA can be considered non-polar. Thus, only non-polar or somewhat polar solvents were considered in order to disrupt it. Toluene, as the first non-polar solvent, was tried first. Tetrahydrofuran, xylene with 2-ethoxyethylacetate (sold as Czech commercial solvent U 6002), and cyclohexane were also used. Toluene ultimately provided the best results as it was able to penetrate through the laminated layers of the module relatively quickly.
The result of the experiment with a real photovoltaic silicon cell showed the advantages and disadvantages of chemical delamination. At room temperature, toluene was able to completely penetrate between the Tedlar and the silicon cell, so the delamination of the Tedlar was successful. However, toluene was unable to dissolve the bond between the cells and the glass. This was most evident in the middle of the sample, where the cells were not destroyed by the change in volume of the EVA film, but only by its subsequent removal. The overall energy consumption of the delamination process was considered. For thermo-mechanical delamination, the estimated energy consumption was in the range of between 50 and 100 kWh/t of a PV module [
9]. The production of toluene requires 0.712 to 0.756 kWh/kg [
28]. To compete with chemical delamination, no more than 66 kg of toluene should be used per ton of a PV module. So far in our experiment, we achieved a consumption of 108 kg of toluene per 1 ton of PV modules, which unfortunately means that chemical delamination using toluene is a more energy-intensive process than mechanical-thermal delamination. For future studies, we plan to recapture toluene from the processed EVA material.