1. Introduction
Today, the environmental requirements for the quality of hydrocarbon crude products are constantly increasing, which makes it urgent to find the solution to the oil products desulfurization problem. According to the requirements of the Euro-5 quality standard [
1], gasoline and diesel fractions sulfur content should be no more than 10 ppm [
2,
3]. The processing of crude hydrocarbons such as natural gas, gas condensate, and oil results in a wide range of products that should meet these requirements, including the content of sulfur compounds.
According to the International Energy Agency, global energy demand will increase by 30% by 2040, which is mainly due to the dynamic development of China and India [
4]. Despite the fact that the main trend in the development of the energy industry is the use of renewable energy sources, the leading place among all resources is still occupied by oil and natural gas. Therefore, reducing the negative impact of the oil industry on the environment and human health is one of the main challenges of our time. The need to remove sulfur as the most common heteroelement in petroleum products is determined by a number of factors [
5,
6]:
- -
When burning sulfur-containing fuel, sulfur dioxide is formed, which leads to the formation of sulfate aerosol, which, when entering the lungs, causes multiple respiratory diseases;
- -
Interaction of combustion products of sulfur-containing compounds with air moisture leads to acid rain;
- -
Organosulfur compounds deactivate oil-refining catalysts and catalytic converters for controlling automobile exhaust gases;
- -
Hydrogen sulfide and sulfur dioxide formed during processing are corrosive compounds, which negatively affects equipment (processing low-sulfur raw materials causes much less damage to equipment and reduces the cost of the entire process as a whole).
According to the literature data [
7], the content of organosulfur compounds in oil fractions has the following distribution:
- -
Gasoline and kerosene fraction: mercaptans, sulfides or thioethers, disulfides, and thiophene and its alkyl derivatives;
- -
Heavy naphtha, middle distillate: benzothiophene and its alkyl derivatives;
- -
Diesel fraction, middle distillate: dibenzothiophene and the alkyl derivatives of benzo- and dibenzothiophene;
- -
Fuel oil and its distillation products: polycyclic sulfur compounds—dibenzothiophene, benzonaphthothiophene, and phenanthro[4,5-b,c,d]thiophene—and their alkyl derivatives.
Thus, as the boiling point of petroleum distillates increases, the structure of the sulfur-containing compounds included in their composition becomes more complex, and the number of aromatic rings and alkyl substituents increases.
Sulfur compounds are one of the permanent components of gas condensates and oils in most fields. Most of the sulfur is in the form of organosulfur compounds such as mercaptans, sulfides, disulfides, and cyclic sulfides. Small amounts of free sulfur and hydrogen sulfide are also present in the oil. In high-sulfur oils, the total sulfur content can be between 8% and 14% [
8].
Currently, the main industrial process for sulfur removal is catalytic hydrodesulfurization [
9,
10,
11,
12,
13], which is based on the decomposition of organosulfur compounds under the action of hydrogen at a high temperature and pressure, forming hydrogen sulfide, while the hydrocarbon part of the crude molecules is recovered and retained as part of the target liquid products. The hydrogen sulfide formed under these conditions is oxidized to elemental sulfur by the Claus process. Large volumes of the generated sulfur are not used and accumulate, which can lead to environmental problems.
Preliminary desulfurization of gas condensate is very effective. Sulfurs can be removed using oxidative desulfurization, extraction [
14], ozonation, adsorption [
15], and aquathermolysis. The most promising of the above methods are oxidative desulfurization and aquathermolysis. For the gasoline fraction, atmospheric oxygen is the best agent since it is cheap and not hazardous to the environment. This process takes place at atmospheric pressure and temperatures up to 100 °C [
16,
17,
18]. The advantage of oxidative desulfurization is that there is no need to use hydrogen, and the process can be carried out at room temperature and atmospheric pressure, which significantly reduces the cost of purification [
19,
20,
21,
22,
23,
24,
25].
In oxidative desulfurization, sulfur compounds from hydrocarbon fractions are oxidized to the corresponding sulfones and sulfoxides, which are then separated by adsorption or extraction [
19,
20]. The use of adsorption and extraction methods in the desulfurization of crude oil and gas condensate is inappropriate due to the large amount of asphaltenes and resins present in the crude oil, which complicate the adsorptive removal of sulfoxides and sulfones and lead to large losses of extractant and crude oil [
21].
It should be noted that the second stage of extraction of oxidation products from sulfur-containing compounds by adsorption and extraction results in losses of both crude hydrocarbon due to low extraction selectivity and extractant, which generally makes the purification process more expensive. This is why another method of separating sulfides and sulfones—rectification—is of interest [
22]. At the same time, if oxidation is carried out in oil production plants and rectification in oil refineries, the purification scheme can be greatly simplified, as rectification is an integral part of every oil refinery.
Adsorption of N- and S-containing compounds on the surface of the adsorbent occurs with the formation of charge transfer complexes and is suppressed by the presence of aromatic hydrocarbons in the fuel. The maximum degree of removal of nitrogen- and sulfur-containing compounds of 62.5% was achieved by combining oxidation with the process of adsorption of oxidation products on the surface of the adsorbent. In [
23], the use of silica gel for the adsorption [
24] of oxidation products of straight-run diesel fraction and gas oil from catalytic and thermal cracking with hydrogen peroxide in the presence of a mixture of sulfuric and acetic acids made it possible to reduce the sulfur content in the fuel from 482 ppm to 50 ppm.
In [
25], deep eutectic solvents with a high catalytic property based on cyclodextrins were designed, synthesized, and employed as extractants and catalysts at the same time. For extraction combined with oxidative desulfurization of fuel oil, the sulfur removed can reach 99.6%.
As mentioned, the tightening of environmental requirements for the quality of commercial petroleum products poses serious technical and economic challenges for the oil-refining industry. One challenge is the adaptation of modern refineries to work with such petroleum products, which requires detailed information about the distribution and patterns of transformation of the various components included in their composition, mainly heteroatomic compounds and aromatic hydrocarbons. The success of modern methods for identifying sulfur-containing compounds has made it possible to significantly expand the understanding of their composition not only for medium distillates but also for vacuum distillates. The developed approaches allow a deeper study of the reactivity and mechanisms of interaction of these components in the oxidative process. The main problems faced by the commercialization of oxidative desulfurization are the low selectivity of the process in relation to sulfur-containing compounds of real fuels, side reactions leading to losses of olefinic and aromatic hydrocarbons, regeneration and reuse of catalysts and oxidizers, and technological implementation of waste management. Despite the enormous amount of accumulated data, schemes for industrial applications of oxidative desulfurization are still not proposed.
Sulfur-containing compounds were oxidized in gas condensate to the corresponding sulfoxides and sulfones. These compounds have significantly higher boiling points than the initial sulfides. Therefore, with further distillation of the gas condensate, a gasoline fraction purified from sulfur compounds was obtained. At the same time, the boiling points of sulfones exceed 360 °C, which means they will not affect the quality of the gasoline fraction but will concentrate in residual fractions, where the total sulfur content is much higher and the requirements for sulfur content are much softer. This approach simplifies the entire oxidative desulfurization process by eliminating the need for additional extraction purification using often toxic compounds. It is important to note that every oil refinery has a rectification process, which means that using the oxidation process in combination with rectification can significantly simplify the process of oxidative desulfurization. This research work demonstrates for the first time the possibility of purifying the gasoline fraction from sulfur compounds to ultra-low values by oxidizing gas condensate and subsequent rectification.
2. Materials and Methods
A stable gas condensate was obtained from the Karachaganak oil field. With a total sulfur content of 7620 ppm, the source gas condensate was used as the object of study.
The following reagents were used: ammonium heptamolybdate ((NH4)6 Mo7O24·4H2O, AR grade, Sigma Aldrich, Saint Louis, MI, USA), hydrogen peroxide (H2O2, 37%, Laborfarma, Almaty, Kazakhstan), silica gel ASKG, and distilled water according to GOST 6709.
Physical-chemical characteristics of the gas condensate before and after desulfurization and the gasoline and diesel fractions were determined in the Research Laboratory of Complex Analysis of Fuels and Refined Products of the Al-Farabi Kazakh National University. The cold filter plugging point and the pour point of the gas condensate were tested with a Kristall apparatus for measuring low-temperature characteristics of petroleum products in accordance with ISO 9001. The calorific value of the gas condensate was determined with a V08MA K automated calorimeter (LLC “SPECTRO LAB”, Kyiv, Ukraine). The fractional composition of gas condensate and its fractions were determined in accordance with GOST 2177-99 using the ARN-LAB-02 apparatus for analyzing the composition of oil fractions. The sulfur content in all studied samples was determined in accordance with ASTM D 4294-98 by energy-dispersive X-ray fluorescence spectrometry using a Spectroscan S (LLC NPO SPECTRON, St. Petersburg, Russia) device. The analyzer is designed for quantitative determination of the mass fraction of sulfur in the range from 0.0007 to 5.00% wt. in petroleum and distillate petroleum products such as gasoline, kerosene, diesel fuel, lubricating oils, and fuel oil. The elemental composition of the gas condensate and its fraction was carried out with a Vario Micro Cube CHNS analyzer (Elementar-Straße, Langenselbold, Germany). Formed by high-temperature combustion in pure oxygen at a constant furnace temperature of up to 1200 °C, the gases were purified, separated into three adsorption columns, and then quantitatively analyzed. The adsorption columns were heated sequentially, and the retained CO2, H2O, and SO2 were sequentially released, after which they entered the detector to form well-separated peaks. Gaseous N2 was immediately detected by the thermal conductivity detector.
Oxidation of the gas condensate and gasoline fraction with hydrogen peroxide in the presence of the transition metal salt was carried out by the following procedure. First, 50 mL of the gas condensate was poured into the thermostated reactor, and a weighted portion of the catalyst based on the Me (metal):S = 1:100 molar ratio was loaded, whereupon the calculated amount of hydrogen peroxide solution [H2O2:S = (4:1) (moles)] was added. The resulting mixture was stirred for 1–8 h at 20–80 °C. After completion of the oxidation process, the reaction mixture was rinsed with distilled water (50 mL × 2). The organic phase was isolated in a separatory funnel, whereupon the gas condensate was passed through the adsorbent (volume ratio adsorbent:condensate = 1:3) to separate the products of the oxidation of the organosulfur compounds.
Characterization of Gas Condensate and Its Fractions
Gas condensate with an initial sulfur content of 7620 ppm was used as the object of the study (
Table 1).
According to
Table 1, the physicochemical parameters of the source gas condensate, such as density, kinematic viscosity, calorific value, and low-temperature parameters, are similar to those of diesel fractions obtained from oils [
26].
3. Results and Discussion
3.1. Production of Light Distillates by Gas Condensate Rectification
Initially, we carried out rectification of the source gas condensate to obtain light fractions and studied their main physical and chemical characteristics.
For the gas condensate rectification process, 100 mL of crude oil was used, from which the straight-run gasoline fraction (up to 230 °C) was 78%, the diesel fraction (230–315 °C) was 12%, and the residual fraction (above 320 °C) was 10%.
Figure 1 shows the total sulfur content of the source and the fractions after gas condensate rectification. In general, the sulfur content increased as the boiling point of the fraction increased.
As shown in
Figure 1, after rectification of the gas condensate, the total sulfur content was 4880 ppm in the gasoline fraction, 5530 ppm in the diesel fraction, and 7710 ppm in the residue.
Table 2 shows the physicochemical parameters of the straight-run gasoline fraction and diesel fraction obtained from the source unoxidized gas condensate.
As can be seen from
Table 2, the total sulfur content in the straight-run gasoline fraction was 4880 ppm and in the diesel fraction was 5530 ppm.
3.2. Oxidative Desulfurization of Gas Condensate
After physical-chemical study of the gas condensate and its distillation products, the process of oxidative desulfurization of the source gas condensate was investigated.
Gas condensate oxidation was carried out with a hydrogen peroxide oxidation system using ammonium heptamolybdate as a catalyst, which has the ability to form peroxo complexes in the presence of hydrogen peroxide. At the end of the oxidation process, 5 mL of water was added to the reaction mixture to remove the residues of the catalytic oxidation system.
The main advantages of hydrogen peroxide as an oxidizing agent are the high content of active oxygen in its composition (47% per unit mass) and the absence of liquid decomposition products other than water. In addition, this oxidizer is characterized by its commercial availability and relative cheapness. Its disadvantages include corrosiveness and high consumption. The oxidation of organosulfur compounds of petroleum products with hydrogen peroxide is based on a heterogeneous reaction between two immiscible liquids. The oil phase contains organosulfur compounds, and the immiscible polar phase contains the oxidizing agent. The limited mass transfer causes the reaction time to be too high for the implementation of this process in production. In heterophasic oxidation, the rate and completeness of reactions depend on the hydrodynamic regime, i.e., depending on the intensity of stirring, the reaction will occur either at the phase boundary or in the volume of one of the phases. Thus, the rate of the process will be determined either by the true rate of the chemical reaction or by the rate of diffusion. With weak mixing, the process is observed to have a diffusion nature. When a certain speed is reached, the process enters the kinetic region.
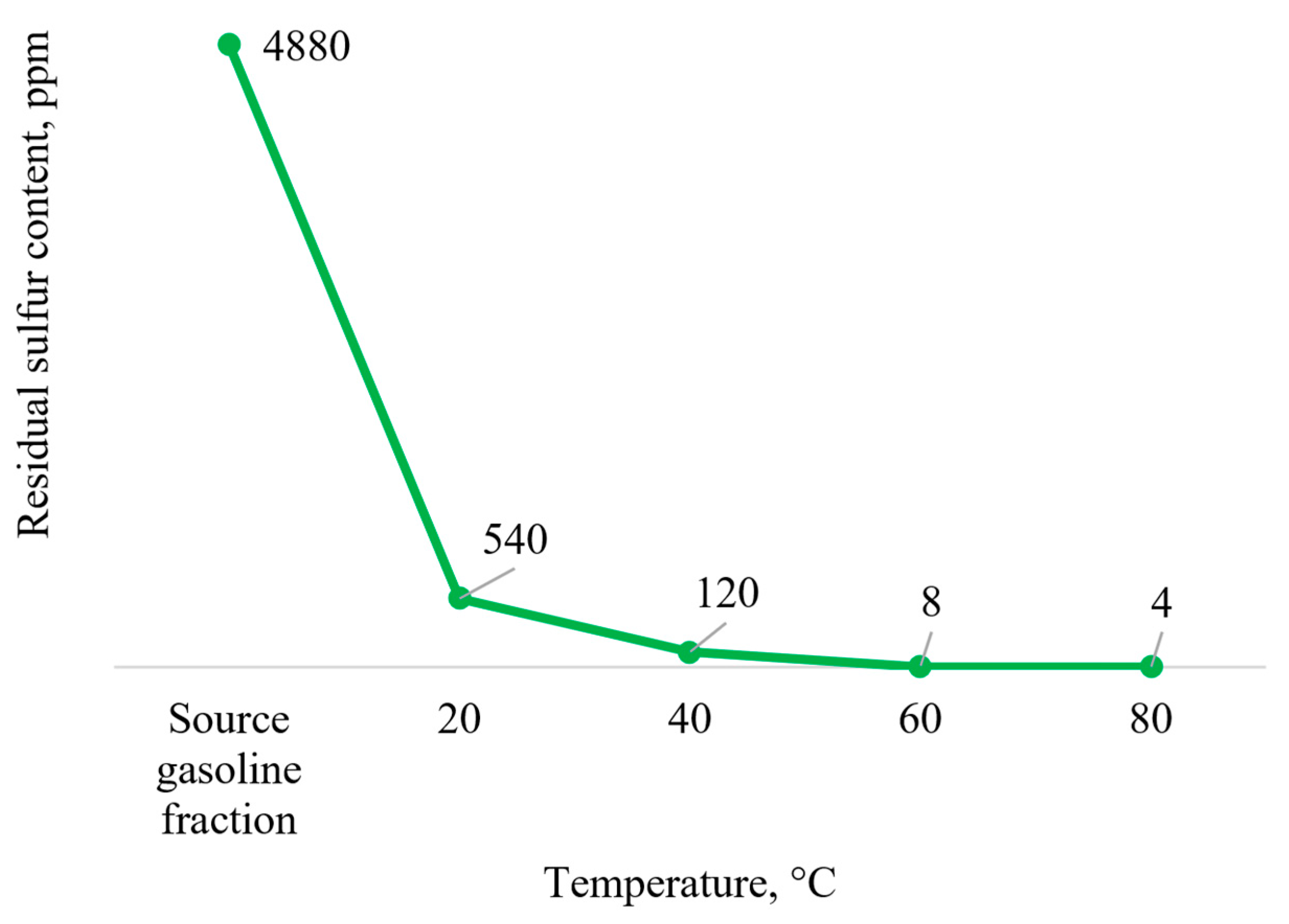
The effect of temperature on the residual sulfur content in the gas condensate was studied. The dependence of the residual sulfur content on the reaction medium temperature is shown in
Figure 2.
Based on the results shown in
Figure 2, we can conclude that the optimum temperature of the reaction medium for the oxidation of sulfur-containing compounds in gas condensate is 60 °C. A further increase of the temperature is undesirable because of the increase in the decomposition rate of hydrogen peroxide, which on the one hand would lead to an unreasonable oxidant consumption and on the other hand would negatively affect the safety of the whole process due to the release of a large volume of gas during oxidant decomposition. At the same time, lowering the temperature below 60 °C would lead to an increase in the residual sulfur content, which appears to be a consequence of a decrease in the rate of oxidation reactions of sulfur-containing substrates.
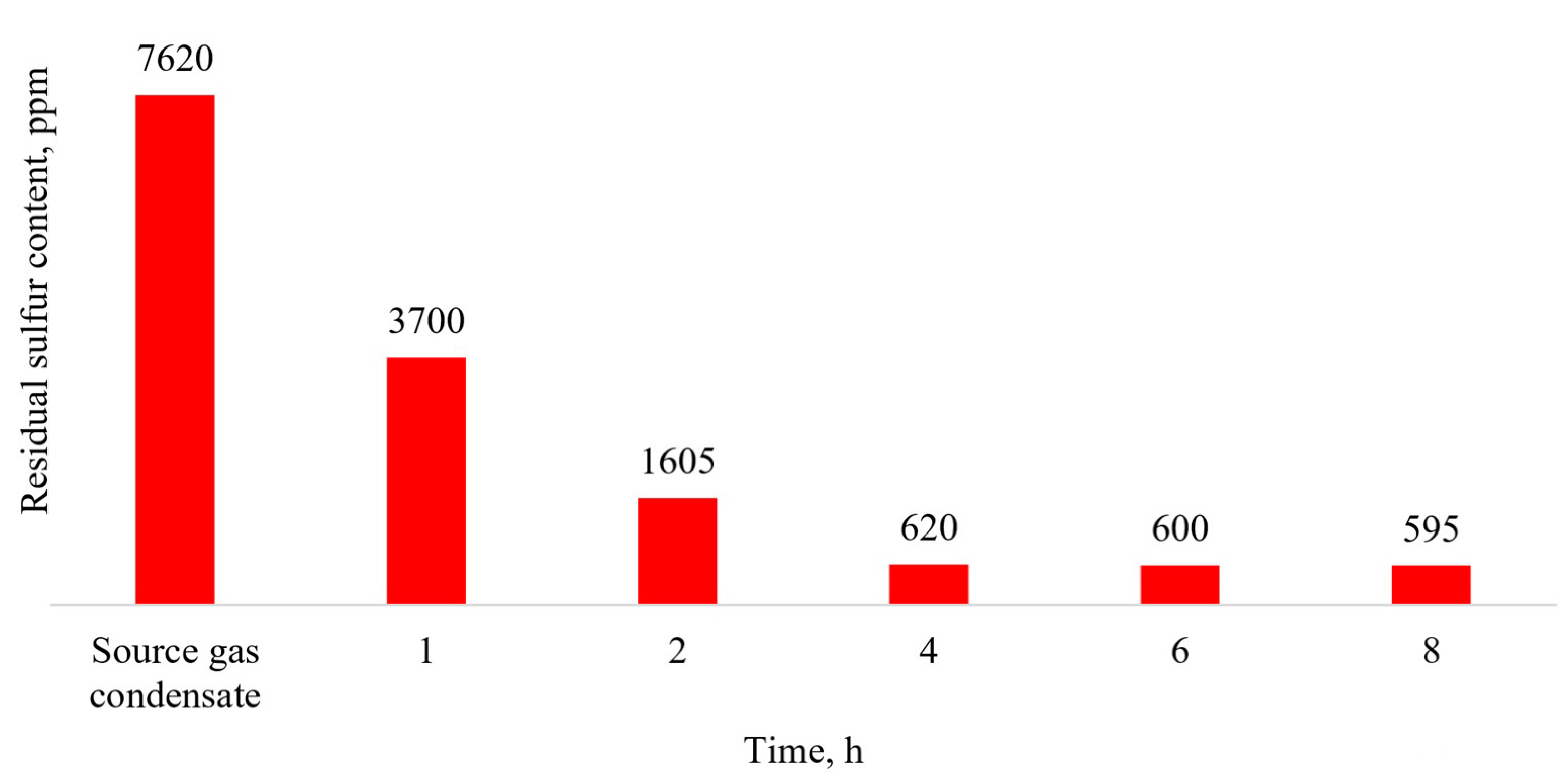
Figure 3 shows the dependence of the gas condensate residual sulfur content on the oxidation reaction time.
The oxidation kinetics (
Figure 3) data show that most of the sulfur compounds oxidized in the first 4 h, and when the reaction time was increased to 6 h, there was only a slight decrease in the total sulfur content of no more than 20 ppm. A further increase in the oxidation time up to 8 h is inappropriate. This characteristic of the dependence of the residual sulfur content on the oxidation time may be caused by a decrease in the hydrogen peroxide concentration with its consumption, which in turn leads to a decrease in the oxidation rate. Another reason may be the presence of branched heteroaromatic compounds (alkyl-substituted benzo- and dibenzothiophenes) in the gas condensate, which, due to the steric difficulties of the sulfur atom, have more difficulty entering into oxidation reactions.
Thus, the proposed catalytic oxidation system consisting of ammonium heptamolybdate and hydrogen peroxide at a temperature of 60 °C and for 4 h of oxidation allows for a reduction in the sulfur content in gas condensate of more than 90%.
As a result of the experiments, the following optimal conditions for selective oxidation of sulfur-containing compounds of gas condensate were selected: 4 h, 60 °C, and a molar ratio of H2O2:S = 4:1 and Mo:S = 1:100, which were further used to obtain low-sulfur light distillates by rectification.
3.3. Oxidative Desulfurization of the Straight-Run Gasoline Fraction
As shown above, the selection of the catalytic oxidation system allows for a significant reduction in the sulfur content of the gas condensate. At the same time, there remains the question of the allocation of the remaining non-oxidized sulfur-containing compounds in the fractions. It should be noted that in addition to the easily oxidizable sulfides and mercaptans, the straight-run gasoline fraction also contains thiophene derivatives which, due to their low electron density, are even more difficult to oxidize than the sulfur compounds in the heavier diesel fraction. Therefore, the process of oxidative desulfurization followed by extraction of the oxidation products of the straight-run gasoline fraction sample obtained from the unoxidized gas condensate was also studied. The results of the experiments on the degree of desulfurization of gasoline fractions depending on the oxidation temperature are shown in
Figure 4.
According to the results obtained at temperatures of 60 °C and 80 °C, it is possible to reduce the sulfur content to very low levels (less than 10 ppm) corresponding to class E-6. This indicates that all major classes of sulfur compounds will undergo oxidation reactions under the selected conditions.
In order to compare the extraction process for the removal of the oxidation products of sulfur compounds in the gasoline fraction using the process of oxidative desulfurization of the gas condensate followed by rectification, the oxidation of the gas condensate was carried out under the conditions described above, under which it was possible to reduce the total sulfur content in the gasoline fraction to values below 10 ppm. In other words, after selecting the conditions that ensured complete oxidation of all major types of sulfur-containing compounds in the gasoline fraction, the gas condensate was oxidized under the above conditions.
The oxidation conditions that allowed the sulfur content to be reduced to very low levels (less than 10 ppm) were also used to purify a gas condensate sample with an initial total sulfur content of 7620 ppm. After the temperature optimization of the oxidation process (60 °C, 4 h, Mo:S = 1:100; H
2O
2:S = 4:1), the gas condensate was washed with water to remove the residual oxidation medium. Further rectification of the obtained gas condensate was carried out with separation of the gasoline fraction. The total sulfur content of the obtained gasoline fraction was 9 ppm, which meets the strict environmental standards for motor fuels. In [
22] using a similar method, a gasoline fraction of 155 ppm was obtained, and in our work, the best results were achieved. Thus, two approaches, oxidative desulfurization of the gasoline fraction and condensate oxidation with distillation, can produce a gasoline fraction with ultra-low sulfur contents of 8 and 9 ppm, respectively. This demonstrates the possibility of using the distillation method in the oxidative desulfurization process for the efficient purification of straight-run distillates. It also reduces the number of stages and reagents required, significantly reducing the cost of the desulfurization process. During the rectification process, oxidized sulfur-containing compounds previously present in the gasoline fraction are converted to heavier fractions.
It can be concluded that the application of the proposed desulfurization process makes it possible to reduce the total sulfur content of the gasoline fraction to the requirements of the Euro 6 standard [
27], which makes the process less costly, less energy consuming, and more environmentally friendly since the process does not require the use of adsorbents and extractants and there are no problems with their disposal.
Table 3 shows the physical-chemical characteristics of the gasoline fraction of the gas condensate in which two different approaches to oxidation product separation were applied.
As shown in
Table 3, the gasoline fraction from the gasoline oxidized with subsequent adsorption by silica gel ASKG and from the oxidized gas condensate with subsequent rectification were analyzed for compliance with all quality indicators (density, octane number, and fractional and elemental composition). The results show that all physical and chemical characteristics were similar and met the requirements of modern environmental standards for motor fuels. At the same time, the properties of two petrol fractions obtained by different oxidation product separation methods were practically identical. The hydrocarbon composition of these fractions is shown in
Table 4.
The results of the chromatography of the samples of the source and purified gas condensate gasoline fraction show that hydrocarbon groups such as paraffins, isoparaffins, and naphthenes were predominate in the petrol samples.
It should be noted that the hydrocarbon group compositions of the source and desulfurized samples of the gasoline fraction were almost identical. This indicates that the process of oxidation of sulfur compounds in the gasoline fraction, as well as the processes of separation of the oxidation products, are highly selective.
4. Conclusions
Thus, for the first time, comparative studies of the oxidative desulfurization process of a straight-run gasoline fraction were carried out using two different approaches: first, oxidation of the sulfur compounds in the gasoline fraction followed by adsorption and second, oxidation of the sulfur compounds in the gas condensate followed by its rectification. The optimum process conditions were chosen to ensure exhaustive oxidation of the sulfur compounds (60 °C, 4 h, Mo:S = 1:100; H2O2:S = 4:1). It is shown that both approaches are equally effective at obtaining the target ultra-low sulfur gasoline fraction, which meets the most stringent modern environmental standards. Detailed physicochemical characterization of the source and purified gasoline fraction samples demonstrates the high selectivity of the purification process.
It is shown for the first time that it is possible to obtain ultra-low sulfur light fractions by oxidation and rectification of the gas condensate. This approach greatly simplifies the oxidative desulfurization process, both economically and environmentally, by eliminating the need to use toxic extractants and the problems associated with their disposal.
A gasoline fraction with a total sulfur content of 9 ppm was obtained. It can be concluded that the application of the proposed desulfurization process makes it possible to reduce the total sulfur content of the gasoline fraction to the requirements of the Euro 6 standard, which makes the process less costly, less energy consuming, and more environmentally friendly since the process does not require the use of adsorbents and extractants and there are no problems with their disposal. It is shown that these selected conditions have virtually no effect on the physical properties of the gas condensate or its fractional composition. The total sulfur content of the gasoline fraction was reduced by 99.8%. It was shown that the properties of the two petrol fractions obtained by different oxidation product separation methods were practically identical.