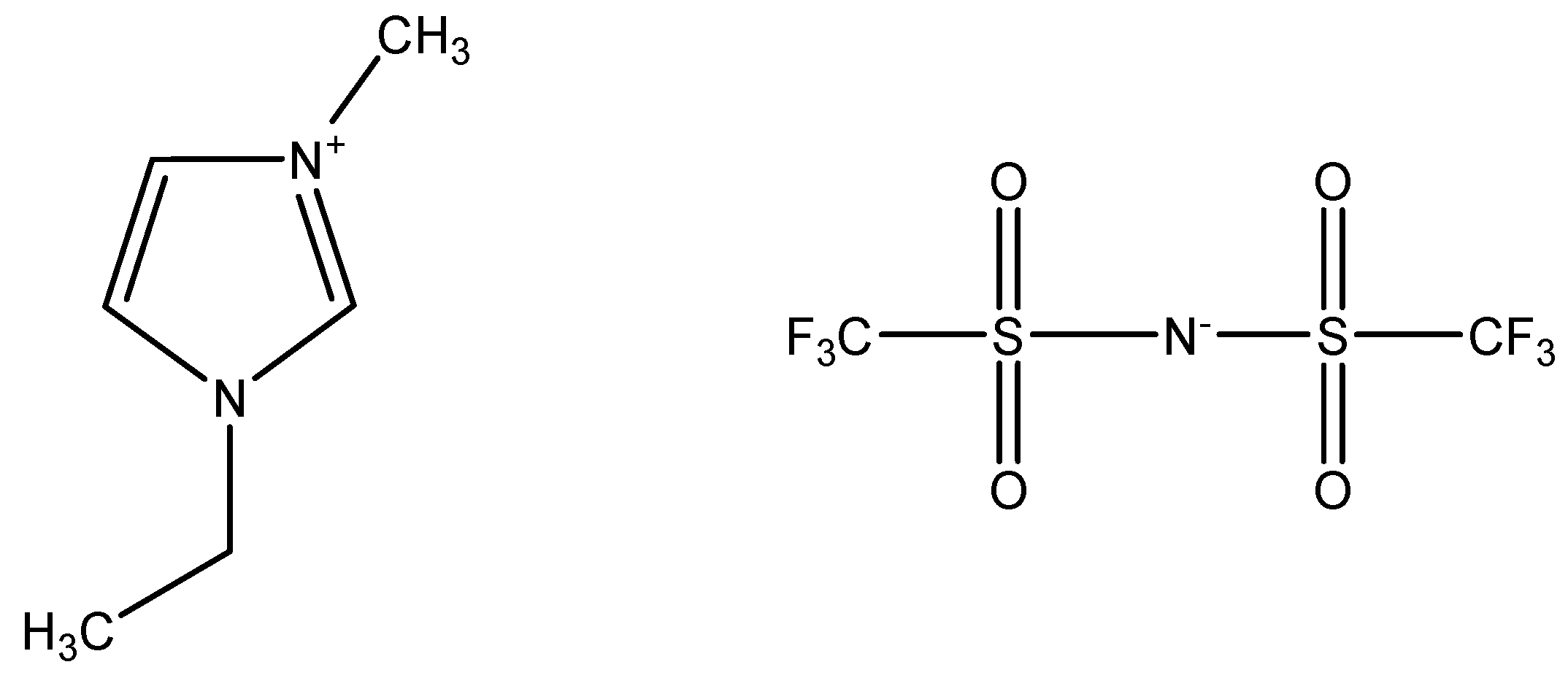
Thermal Safety of 1-Ethyl-3-Methylimidazolium Bis(trifluoromethylsulfonyl)imide for Construction-Related Safety Process
Abstract
:1. Introduction
2. Experimental Methods
2.1. Sample
2.2. Thermogravimetric Analysis
3. Reaction Mode and Heat Exchange Calculation
3.1. Integrating Activation Energy and Pre-Exponential Factor to Complete the Kinetic Triplet: Determining the Reaction Mechanism
3.2. Utilizing the Kinetic Triplet for Estimating Heat Transfer between External Environment and Material
4. Results and Discussion
4.1. Evaluate Thermokinetic of [EMIM][Tf2N]
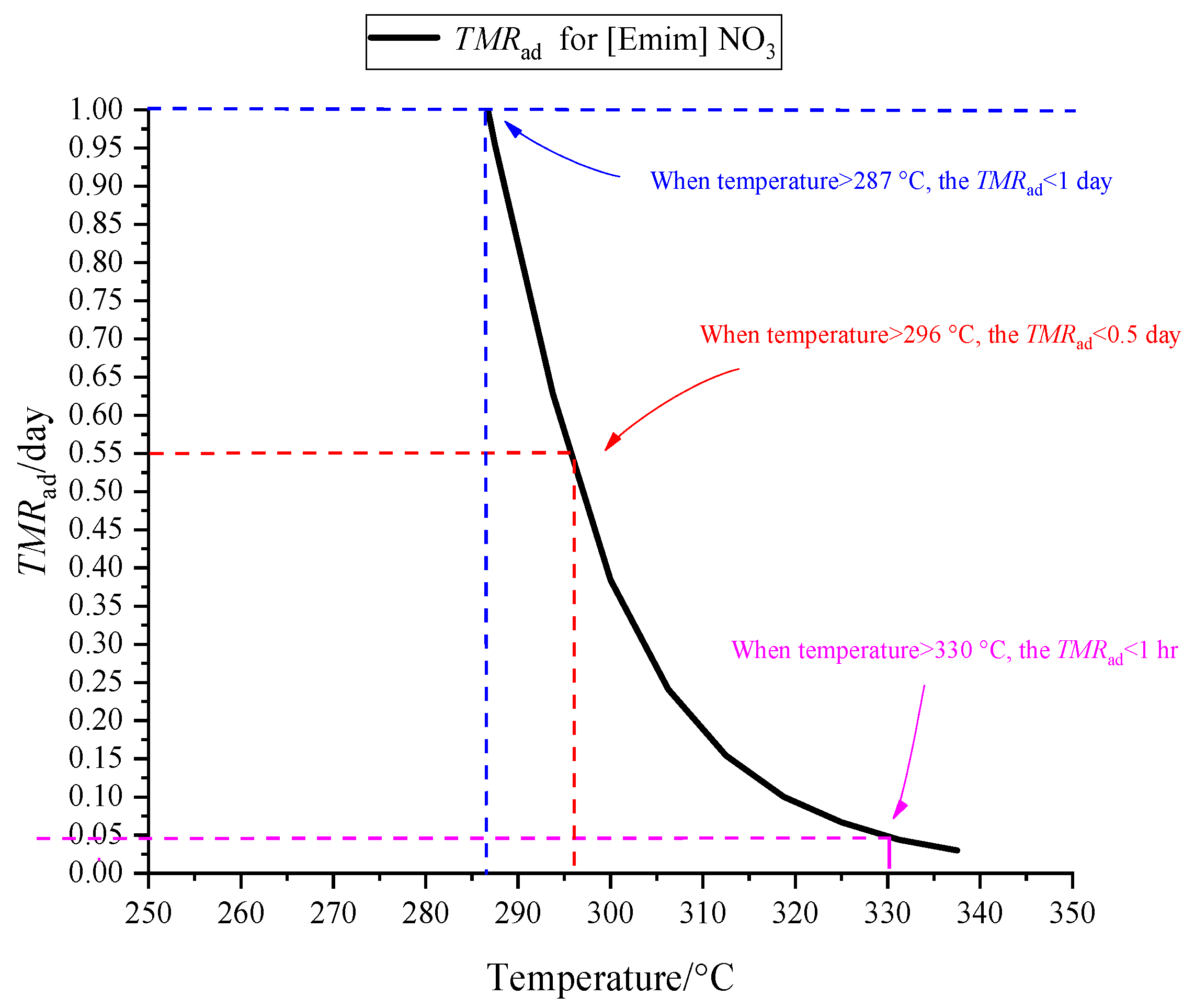
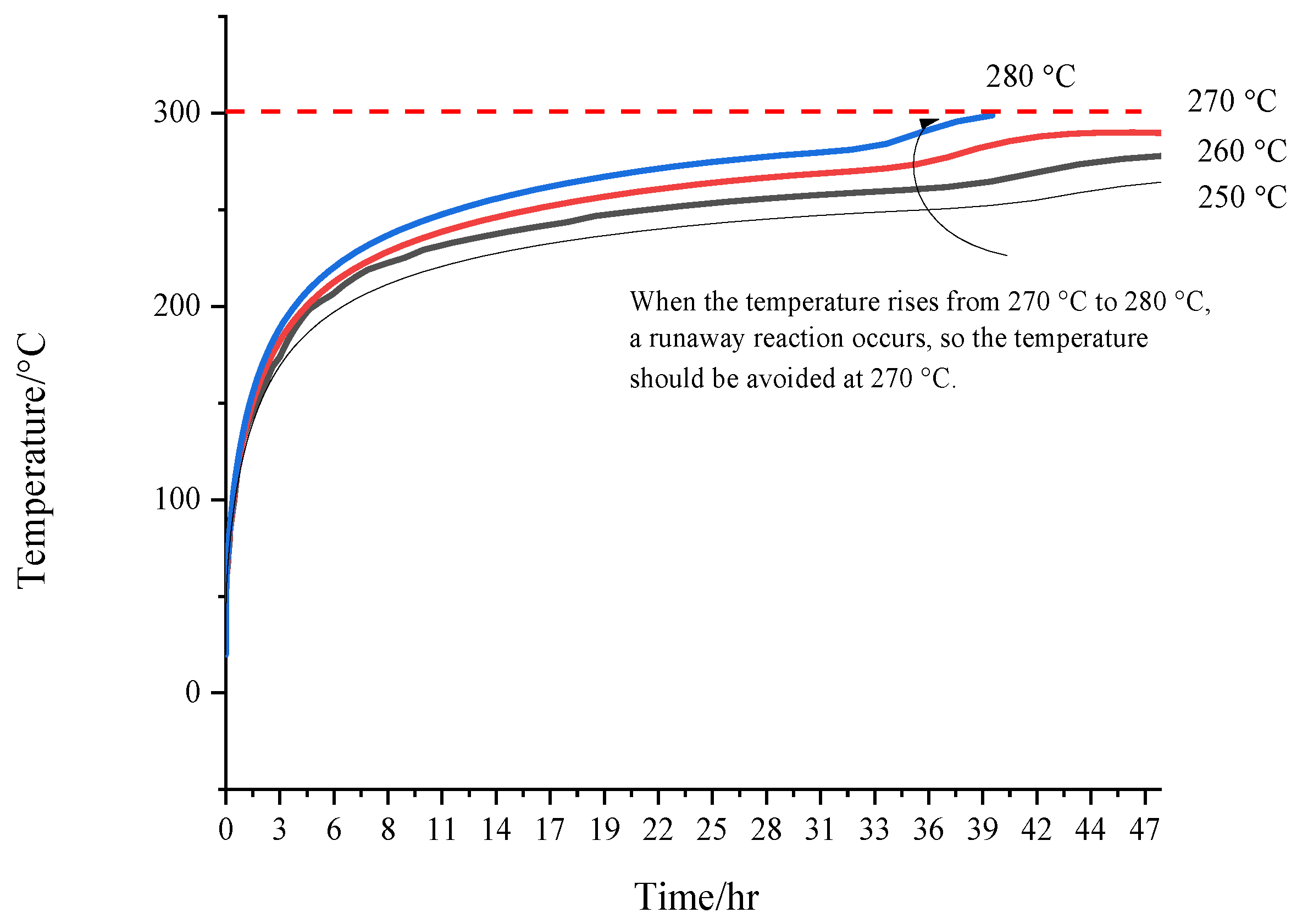
4.2. Evaluating Storage and Transport Hazard Parameters for [EMIM][Tf2N]
5. Conclusions
6. Future Prospects
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meng, J.; Pan, Y.; Ran, Z.; Li, Y.; Jiang, J.; Wang, Q.; Jiang, J. Thermal hazard and decomposition kinetics of 1-butyl-2,3-dimethylimidazolium nitrate via TGA/DSC and FTIR. J. Loss Prev. Process Ind. 2021, 72, 104562. [Google Scholar] [CrossRef]
- Tsai, C.-F.; Wen, I.J.; Liu, S.-H.; Cao, C.-R.; Zhang, H. Evaluation of thermal hazard characteristics of four low temperature reactive azo compounds under isothermal conditions. J. Loss Prev. Process Ind. 2021, 71, 104453. [Google Scholar] [CrossRef]
- Liu, K.-H.; Xiao, Y.; Zhang, H.; Pang, P.; Shu, C.-M. Inhibiting effects of carbonised and oxidised powders treated with ionic liquids on spontaneous combustion. Process Saf. Environ. Prot. 2022, 157, 237–245. [Google Scholar] [CrossRef]
- Zaitsau, D.H.; Abdelaziz, A. The study of decomposition of 1-ethyl-3-methyl-imidazolium bis(trifluoromethylsulfonyl)imide by using Termogravimetry: Dissecting vaporization and decomposition of ILs. J. Mol. Liq. 2020, 313, 113507. [Google Scholar] [CrossRef]
- Liu, S.-H.; Chen, C.-C.; Zhang, B.; Wu, J.-H. Fire and explosion hazards of 1-ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide. RSC Adv. 2020, 10, 22468–22479. [Google Scholar] [CrossRef]
- Yu, C.-F.; Liu, S.-H.; Xia, R.; Wu, K.-F. Studies on the thermal stability and decomposition kinetics of 1-ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide via density functional theory and experimental methods. J. Mol. Liq. 2022, 360, 119422. [Google Scholar] [CrossRef]
- Chengula, P.J.; Pogrebnaya, T.; Pogrebnoi, A. Ionic liquids based on 1-ethyl-3-methylimidazolium cation and anions of tetrafluoroborate and bis(trifluoromethylsulfonyl)imide: Structural and thermodynamic properties by DFT study. J. Mol. Liq. 2020, 299, 112209. [Google Scholar] [CrossRef]
- Safarov, J.; El-Awady, W.A.; Shahverdiyev, A.; Hassel, E. Thermodynamic Properties of 1-Ethyl-3-methylimidazolium Bis(trifluoromethylsulfonyl)imide. J. Chem. Eng. Data 2011, 56, 106–112. [Google Scholar] [CrossRef]
- Mu, T.; Han, B. Structures and Thermodynamic Properties of Ionic Liquids; Springer: Berlin/Heidelberg, Germany, 2014; Volume 15, pp. 107–139. [Google Scholar] [CrossRef]
- Li, X.; Curnow, O.J.; Choi, J.; Yip, A. Recent advances in the imidazolium-based ionic liquid-templated synthesis of microporous zeolites. Mater. Today Chem. 2022, 26, 101133. [Google Scholar] [CrossRef]
- Barrulas, R.V.; Zanatta, M.; Casimiro, T.; Corvo, M.C. Advanced porous materials from poly(ionic liquid)s: Challenges, applications and opportunities. Chem. Eng. J. 2021, 411, 128528. [Google Scholar] [CrossRef]
- Tang, Y.; Cao, P.; Li, W.; He, M.; Dai, Z.; Xiong, Y. Redox-responsive poly(ionic liquid) microgels explored as the building blocks for supramolecular assembly. Polymer 2021, 220, 123575. [Google Scholar] [CrossRef]
- Jayakumar, M.; Venkatesan, K.A.; Srinivasan, T.G.; Vasudeva Rao, P.R. Electrochemical behavior of ruthenium (III), rhodium (III) and palladium (II) in 1-butyl-3-methylimidazolium chloride ionic liquid. Electrochim. Acta 2009, 54, 6747–6755. [Google Scholar] [CrossRef]
- Maton, C.; De Vos, N.; Stevens, C.V. Ionic liquid thermal stabilities: Decomposition mechanisms and analysis tools. Chem. Soc. Rev. 2013, 42, 5963–5977. [Google Scholar] [CrossRef] [PubMed]
- Burnham, A.K. Computational aspects of kinetic analysis.: Part D: The ICTAC kinetics project–multi thermal history model fitting methods and their relation to isoconversional methods. Thermochim. Acta 2000, 355, 165–170. [Google Scholar] [CrossRef]
- Brown, M.E.; Maciejewski, M.; Vyazovkin, S.; Nomen, R.; Sempere, J.; Burnham, A.; Opfermann, J.; Strey, R.; Anderson, H.L.; Kemmler, A.; et al. Computational aspects of kinetic analysis: Part A: The ICTAC kinetics project-data, methods and results. Thermochim. Acta 2000, 355, 125–143. [Google Scholar] [CrossRef]
- Opfermann, J. Kinetic analysis using multivariate non-linear regression. I. Basic concepts. J. Therm. Anal. Calorim. 2000, 60, 641–658. [Google Scholar] [CrossRef]
- Di, S.I.; Marotta, R.; Andreozzi, R.; Caprio, V. Kinetic and chemical characterization of thermal decomposition of dicumylperoxide in cumene. J. Hazard. Mater. 2011, 187, 157–163. [Google Scholar] [CrossRef]
- Wang, S.Y.; Kossoy, A.A.; Yao, Y.D.; Chen, L.P.; Chen, W.H. Kinetics-based simulation approach to evaluate thermal hazards of benzaldehyde oxime by DSC tests. Thermochim. Acta 2017, 655, 319–325. [Google Scholar] [CrossRef]
- Liu, S.H.; Lin, C.P.; Shu, C.M. Thermokinetic parameters and thermal hazard evaluation for three organic peroxides by DSC and TAM III. J. Therm. Anal. Calorim. 2011, 106, 165–172. [Google Scholar] [CrossRef]
- Liu, S.H.; Shu, C.M. Advanced technology of thermal decomposition for AMBN and ABVN by DSC and VSP2. J. Therm. Anal. Calorim. 2015, 121, 533–540. [Google Scholar] [CrossRef]
- Liu, S.H.; Hou, H.Y.; Shu, C.M. Thermal hazard evaluation of the autocatalytic reaction of benzoyl peroxide using DSC and TAM III. Thermochim. Acta 2015, 605, 68–76. [Google Scholar] [CrossRef]
- Liu, S.H.; Cao, C.R.; Lin, W.C.; Shu, C.M. Experimental and numerical simulation study of the thermal hazards of four azo compounds. J. Hazard. Mater. 2019, 365, 164–177. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Liu, N. Kinetic analysis based on the kinetic compensation effect and optimization calculation. Thermochim. Acta 2020, 690, 178686. [Google Scholar] [CrossRef]
- Liu, H.; Ahmad, M.S.; Alhumade, H.; Elkamel, A.; Sammak, S.; Shen, B. A hybrid kinetic and optimization approach for biomass pyrolysis: The hybrid scheme of the isoconversional methods, DAEM, and a parallel-reaction mechanism. Energy Convers. Manag. 2020, 208, 112531. [Google Scholar] [CrossRef]
- Luo, L.; Guo, X.; Zhang, Z.; Chai, M.; Rahman, M.M.; Zhang, X.; Cai, J. Insight into Pyrolysis Kinetics of Lignocellulosic Biomass: Isoconversional Kinetic Analysis by the Modified Friedman Method. Energy Fuels 2020, 34, 4874–4881. [Google Scholar] [CrossRef]
- Kossoy, A.A.; Koludarova, E.Y. Specific features of kinetics evaluation in calorimetric studies of runaway reactions. J. Loss Prev. Process Ind. 1995, 8, 229–235. [Google Scholar] [CrossRef]
- Liu, S.H.; Zhang, B. Using thermal analysis technology to assess the thermal stability of 1,3-dimethylimidazolium nitrate. Process Saf. Environ. Prot. 2019, 124, 181–186. [Google Scholar] [CrossRef]
- Andreozzi, R.; Caprio, V.; Somma, I.D.; Sanchirico, R. Kinetic and safety assessment for salicylic acid nitration by nitric acid/acetic acid system. J. Hazard. Mater. 2006, 134, 1–7. [Google Scholar] [CrossRef]
- Gonzales, N.O.; Levin, M.E.; Zimmerman, L.W. The reactivity of sodium borohydride with various species as characterized by adiabatic calorimetry. J. Hazard. Mater. 2007, 142, 639–646. [Google Scholar] [CrossRef]
- Kossoy, A.A.; Akhmetshin, Y.G. Simulation-based approach to design of inherently safer processes. Process Saf. Environ. Prot. 2012, 90, 349–356. [Google Scholar] [CrossRef]
- Kossoy, A.A.; Belokhvostov, V.M.; Koludarova, E.Y. Thermal decomposition of AIBN: Part D: Verification of simulation method for SADT determination based on AIBN benchmark. Thermochim. Acta 2015, 621, 36–43. [Google Scholar] [CrossRef]
- Kossoy, A.A.; Akhmetshin, Y.G. Identification of kinetic models for the assessment of reaction hazards. Process Saf. Prog. 2007, 26, 209–220. [Google Scholar] [CrossRef]
- ChemInform Saint Petersburg (CISP) Ltd., Thermal Safety Series. Available online: http://www.cisp.spb.ru (accessed on 5 May 2023).
- Bridges, N.J.; Visser, A.E.; Fox, E.B. Potential of Nanoparticle-Enhanced Ionic Liquids (NEILs) as Advanced Heat-Transfer Fluids. Energy Fuels 2011, 25, 4862–4864. [Google Scholar] [CrossRef]
- Paul, T.C.; Tikadar, A.; Mahamud, R.; Salman, A.S.; Morshed, A.M.; Khan, J.A. A critical review on the development of ionic liquids-based nanofluids as heat transfer fluids for solar thermal energy. Processes 2021, 9, 858. [Google Scholar] [CrossRef]
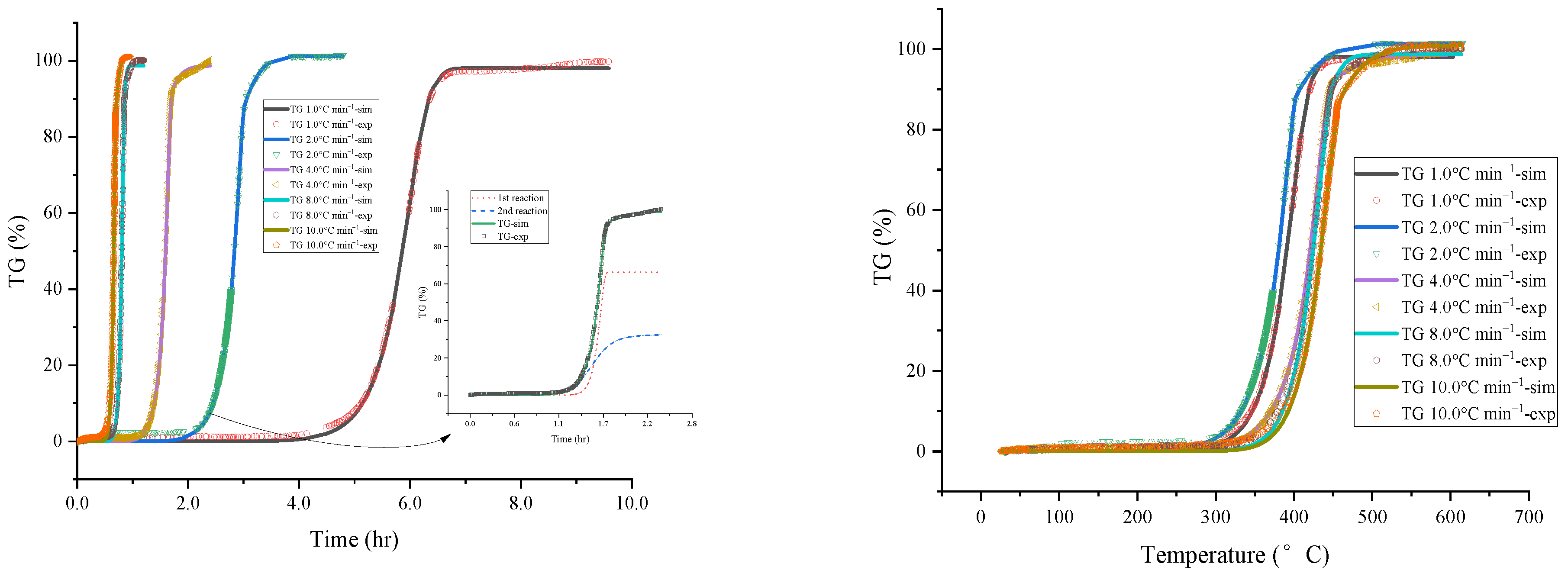
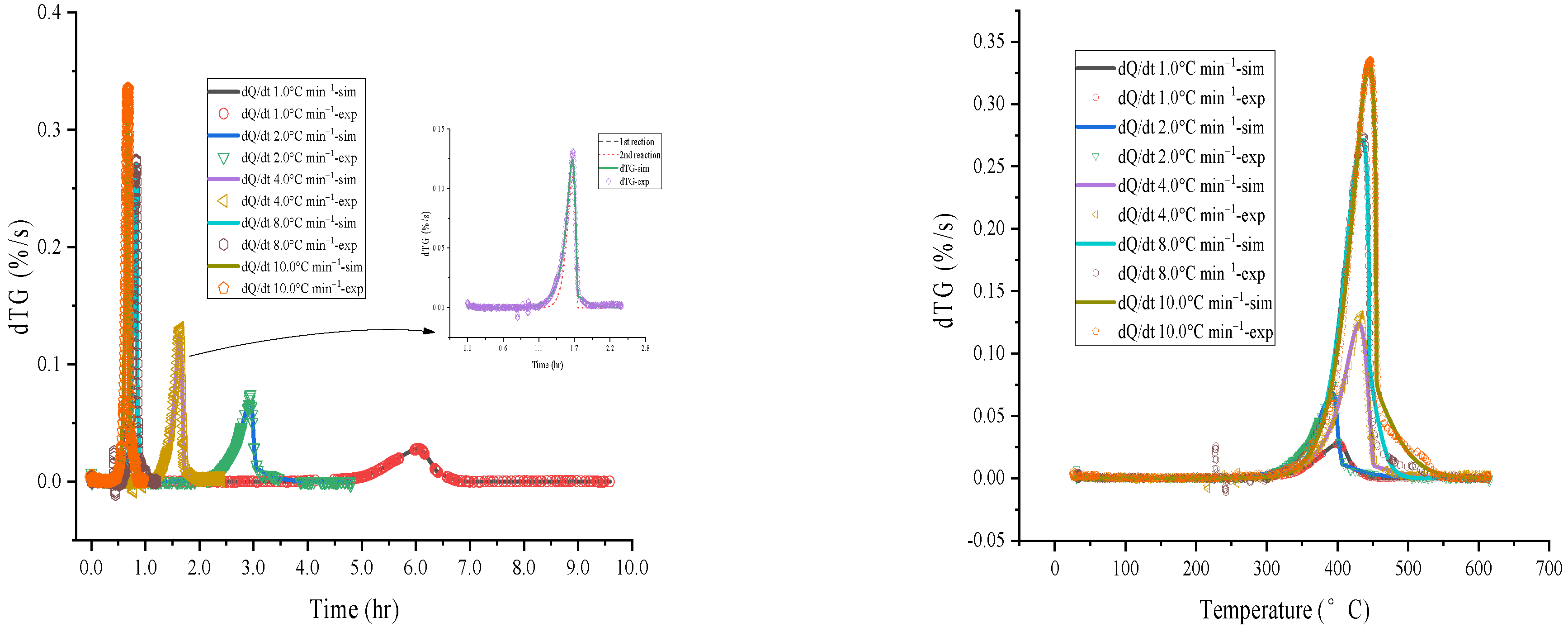

| Stage 1 | |||||
|---|---|---|---|---|---|
| 1.0 °C min−1 | 2.0 °C min−1 | 4.0 °C min−1 | 8.0 °C min−1 | 10.0 °C min−1 | |
| ln (k0)/ln (s−1) (±0.01) | 29.74 | 29.01 | 30.76 | 32.25 | 29.25 |
| Ea/kJ mol−1 (±0.1) | 167.9 | 169.6 | 183.1 | 223.7 | 202.6 |
| n/Dimensionless (±0.01) | 1.13 | 1.44 | 1.56 | 1.69 | 1.44 |
| Stage 2 | |||||
| ln (k0)/ln (s−1) (±0.01) | 9.56 | 8.98 | 10.43 | 10.87 | 10.92 |
| Ea/kJ mol−1 (±0.1) | 97.2 | 97.3 | 94.6 | 106.4 | 102.6 |
| n/Dimensionless (±0.01) | 1.03 | 1.33 | 1.70 | 1.30 | 1.74 |
| The range of parameters in this study Stage 1 ln (k0)/ln (s−1): 29.7423–32.2564 Ea/kJ mol−1: 167.9841–223.7765 n/Dimensionless: 1.1345–1.6920 Stage 2 ln (k0): 8.9837–10.9268 Ea/kJ mol−1: 97.0284–106.4358 n/Dimensionless: 1.0358–1.7468 | The range of parameters from Zaitsau et al. [4]: ln (k0)/ln (s−1): 14–16 (Isoconversional approach) Ea/kJ mol−1: 240 ± 15 (at α = 0.2) to 270 ± 15 (Isoconversional approach) n/Dimensionless: No correlation calculation The range of parameters from Liu et al. [5]: ln (k0)/ln (s−1): No correlation calculation Ea/kJ mol−1: 165.2 ± 6.6 and 163.9 ± 6.0 (FWO and Starink methods) 210.4 ± 5.9 (ASTM E698 method) n/Dimensionless: No correlation calculation The range of parameters from [6]: ln (k0)/ln (s−1): 15.4–49.6 (Coats-Redfern method for different f(α)) Ea/kJ mol−1: 25.7–211.4 (Coats-Redfern method for different f(α)) 142.4 (at α = 0.1) to 204.2 (at α = 0.8); (Friedman); 136.3 (at α = 0.1) to 198.7 (at α = 0.8) (KAS); 140.6 (at α = 0.1) to 199.9 (at α = 0.8) (FWO) n/Dimensionless: No correlation calculation | ||||
| Material | Size/cm | Shell Thickness /mm | Filling Height/cm | Density/g cm–3 | Specific Heat Capacity /J g−1 K−1 | Thermal Conductivity Coefficient /W m−1 K−1 | |
|---|---|---|---|---|---|---|---|
| 50 kg Drum | [EMIM][Tf2N] | R × H 23 × 63 | 7.0 | 35 | 0.75 | 1.7 | 0.3 |
| Physical parameters for [EMIM][Tf2N]. | |||||||
| Surface temperature/°C: 25 (room temperature) | |||||||
| Density/g cm–3: 1.37 | |||||||
| Specific heat capacity/J g−1 K−1: 1.53 | |||||||
| Thermal conductivity coefficient/W m−1 K−1: 0.165 | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hung, L.-C.; Pan, N.-H. Thermal Safety of 1-Ethyl-3-Methylimidazolium Bis(trifluoromethylsulfonyl)imide for Construction-Related Safety Process. Processes 2023, 11, 2966. https://doi.org/10.3390/pr11102966
Hung L-C, Pan N-H. Thermal Safety of 1-Ethyl-3-Methylimidazolium Bis(trifluoromethylsulfonyl)imide for Construction-Related Safety Process. Processes. 2023; 11(10):2966. https://doi.org/10.3390/pr11102966
Chicago/Turabian StyleHung, Li-Chi, and Nai-Hsin Pan. 2023. "Thermal Safety of 1-Ethyl-3-Methylimidazolium Bis(trifluoromethylsulfonyl)imide for Construction-Related Safety Process" Processes 11, no. 10: 2966. https://doi.org/10.3390/pr11102966
APA StyleHung, L.-C., & Pan, N.-H. (2023). Thermal Safety of 1-Ethyl-3-Methylimidazolium Bis(trifluoromethylsulfonyl)imide for Construction-Related Safety Process. Processes, 11(10), 2966. https://doi.org/10.3390/pr11102966






